Abstract
Increases in cell death by programmed (ie., apoptosis, autophagy) or non-programmed mechanisms (ie., necrosis) occur during tissue injury, and may contribute to the etiology of several pulmonary or vascular disease states. The low molecular weight stress protein heme oxygenase-1 (HO-1) confers cytoprotection against cell death in various models of lung and vascular injury by inhibiting apoptosis, inflammation, and cell proliferation. HO-1 serves a vital metabolic function as the rate-limiting step in the heme degradation pathway and in the maintenance of iron homeostasis. The transcriptional induction of HO-1 occurs in response to multiple forms of chemical and physical cellular stress. The cytoprotective functions of HO-1 may be attributed to heme turnover, as well as to beneficial properties of its enzymatic reaction products: biliverdin-IXα, iron, and carbon monoxide (CO). Recent studies have demonstrated that HO-1 or CO inhibits stress-induced extrinsic and intrinsic apoptotic pathways in vitro. A variety of signaling molecules have been implicated in the cytoprotection conferred by HO-1/CO, including autophagic proteins, p38 mitogen activated protein kinase, signal transducer and activator of transcription proteins, nuclear factor-κB, phosphatydylinositol-3-kinase/Akt, and others. Enhanced HO-1 expression or the pharmacological application of HO end-products affords protection in preclinical models of tissue injury, including experimental and transplant-associated ischemia/reperfusion injury, promising potential future therapeutic applications.
Must not all things at the last be swallowed up in death?
-Plato
Introduction
Heme oxygenase-1 (HO-1) provides an inducible defense mechanism that can be activated ubiquitously in cells and tissues in response to noxious stimuli, conferring cellular protection against injury inflicted by such stimuli [1–3]. HO-1 serves a vital metabolic function as the rate-limiting step in the oxidative catabolism of heme-b, to generate equimolar carbon monoxide (CO), biliverdin-IXα (BV), and ferrous iron; the BV generated is subsequently converted to bilirubin-IXα (BR) by NADPH biliverdin reductase (BVR)[4]. The essential role of HO-1 in stress adaptation has been demonstrated by the phenotype of ho-1−/− (null) mice and in a unique case of human HO-1 deficiency. The ho-1−/− mice exhibit altered tissue iron distribution, increased susceptibility to pulmonary ischemia/reperfusion (I/R) injury, but paradoxical resistance to hyperoxic lung injury [5–8]. The HO-1 deficient child exhibited extensive endothelial cell damage, anemia, hypobilirubinemia, aberrant tissue iron deposition, and increased inflammatory indices [9]. Furthermore, vascular endothelial cells derived from ho-1−/− mice or the HO-1 deficient child exhibited enhanced susceptibility to oxidative stress in vitro [6, 9].
HO-1 expression can confer cytoprotection in many lung and vascular injury and disease models [1–3, 8]. The cytoprotective effects of HO-1 are related to end-product formation (Figure 1) [1, 10–11], though non-catalytic functions of the protein have also been proposed [12]. The pharmacological application of CO and BV/BR can mimic the HO-1 dependent cytoprotection in many injury models [1, 10–11]. Tissue protection generally involves inhibition of apoptosis and related cell death pathways, although inhibition of inflammation and/or cell proliferation may also be involved, depending on the specific injury model [1]. In recent years, intensive investigation has focused on potential therapeutic tools to manipulate apoptosis in order to alter the outcome of pulmonary or vascular diseases. Such approaches have involved the use of agonists or inhibitors of specific signal transduction components, (eg., mitogen activated protein kinases, MAPK), or antioxidants, thus far with limited clinical efficacy [13–14]. In this regard, the targeted manipulation of HO-1 or of its end-products remains a promising experimental and translational strategy. This review will focus on the role of HO-1/CO in modulating cell death mechanisms in tissue injury and pathology models. The specific roles of HO-1 in inflammation and carcinogenesis have been reviewed elsewhere [15–16]. Recent works examining the therapeutic potential of HO-1/CO in models of oxidative stress, I/R injury, cigarette smoke exposure, and other forms of acute and chronic lung or vascular injuries will be described.
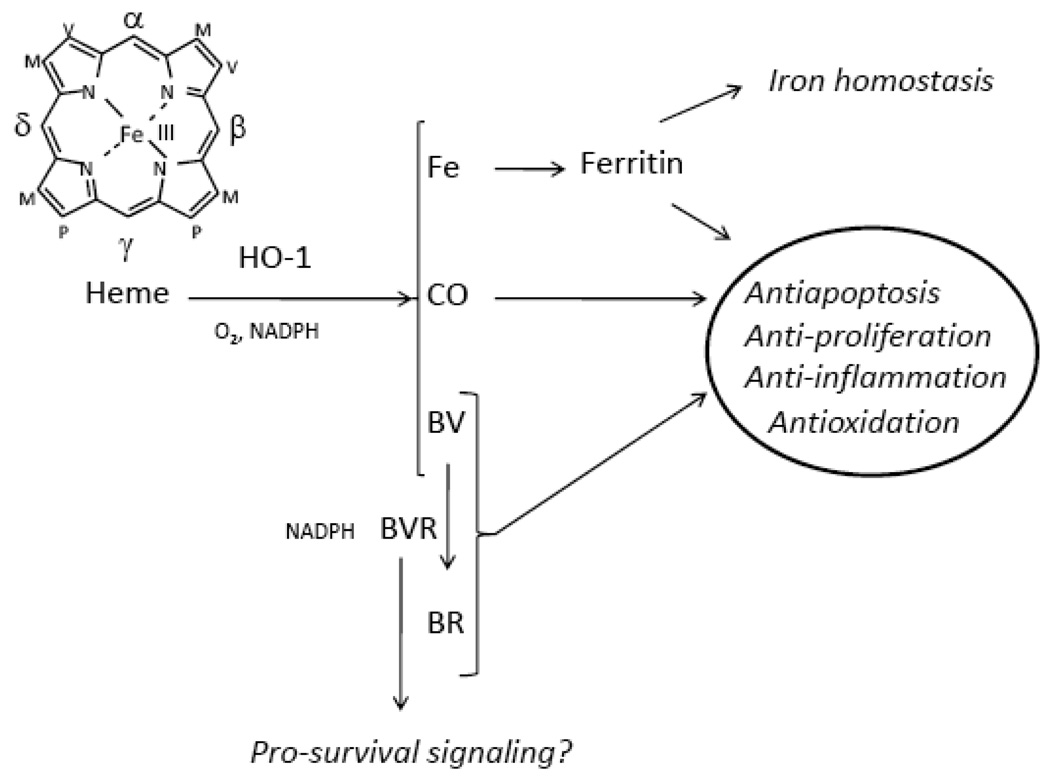
Figure 1. Relationship between the HO-1 system and cytoprotective functions.
Heme oxygenase activity catalyzes the rate-limiting step in the oxidative catabolism of heme-b, to generate equimolar quantities of carbon monoxide (CO), biliverdin-IXα (BV), and ferrous iron. The reaction requires three moles of molecular oxygen (O2) and reducing equivalents from NADPH: ferrihemoprotein (cytochrome p-450) reductase. The biliverdin-IXα generated is rapidly converted to bilirubin-IXα (BR) by NADPH biliverdin reductase (BVR). The iron generated in the HO reaction induces a compensatory elevation in the synthesis of ferritin, which sequesters the iron. The HO system provides cellular and tissue protection through the concerted action of these end-products, through anti-inflammatory, antiapoptotic, anti-proliferative, or anti-oxidative effects. Non-catalytic functions of BVR in the regulation of pro-survival cell signaling pathways have also been proposed.
Mechanisms of Cell Death
Just like the multi-cellular organisms they constitute, cells must die. The method of cell death may be traumatic, resulting from acute, accidental, or non-physiological injury (ie., necrosis), or may arise as the consequence of genetic programs, (ie., apoptosis or Type I programmed cell death). Apoptosis provides essential homeostatic functions in regulating growth and development of organs, and in tissue responses to injurious stimuli, such as exposure to xenobiotics or adverse environmental conditions [17]. Disruption of apoptosis may lead to tumorigenesis or autoimmune disease, whereas excessive apoptosis may cause organ failure [18–20]. The role of apoptosis in the pathogenesis of specific diseases remains controversial, but has been implicated in cigarette smoke (CS)-induced chronic obstructive pulmonary disease (COPD), oxidative lung injury, pulmonary I/R injury, transplant-associated I/R injury, and others [20–23].
Apoptosis is distinguished from necrosis on the basis of morphological and biochemical features [17, 24]. Apoptosis requires the activation of proteases (eg., caspases) and nucleases within an intact plasma membrane, resulting in organelle decomposition [25]. On the other hand, necrosis is characterized by membrane damage and leakage of cytosol into the extracellular space, which may promote local inflammation and damage to surrounding tissues [24]. The cardinal biochemical features of apoptosis include DNA fragmentation, mitochondrial dysfunction, and increased expression or activation of pro-apoptotic (eg., Bax, Bid) relative to anti-apoptotic (eg., Bcl-2, Bcl-Xl) proteins of the Bcl-2 family [25–27].
Cells can initiate apoptosis by two distinct pathways, a receptor-dependent “extrinsic” pathway and an “intrinsic” pathway involving mitochondrial dysfunction [28–30] (Figure 2). In response to stimuli, (ie., nutrient deprivation, DNA-damaging agents), proapoptotic Bcl-2 family proteins such as Bax initiate the intrinsic pathway by permeabilizing the outer mitochondrial membrane, thereby releasing cytochrome c (Cyt-c) from the inter-membrane space [31–32]. Cytosolic Cyt-c forms a complex with apoptotic protease-activating factor-1, and dATP which activates caspase-9, and its downstream caspase-3, resulting in the activation of the apoptosis [3].
Figure 2. Inhibition of apoptosis by HO-1 and its enzymatic products.
Cells may undergo apoptosis by two major pathways, an extrinsic receptor-dependent pathway and an intrinsic (mitochondrial) apoptosis pathway, in response to various acute injurious stimuli. In the intrinsic pathway, environmental stress may cause direct mitochondrial damage, or promote signaling pathways dependent on ROS generation, resulting in the activation of Bax. Bax translocates to the mitochondrial outer membrane, where it oligomerizes or forms complexes with other proapoptotic Bcl-2 related proteins such as Bid or Bad. Bax oligomers form pores in the outer mitochondrial membrane, which facilitate the release of pro-apoptotic molecules such as cytochrome-c. Cytochrome c forms a complex with Apaf-1 and caspase-9, leading to caspase-9 activation. In extrinsic apoptosis, a death inducing ligand such as Fas ligand (FasL), initiates the death program upon interacting with its corresponding receptor (ie., Fas).These interactions lead to the recruitment and activation of caspase-8 in a death-inducing signal complex (DISC). Caspase-8 activation can lead to caspase-3 activation, or to the activation of Bid. Bid assists in the activation and mitochondrial translocation of Bax. Bid activation also results in the release of cytochrome c from the mitochondria. This diagram indicates those points in the apoptotic pathway at which HO-1 and its enzymatic products have been shown to inhibit cell death. Each number corresponds with an individual reference, and the shape of each symbol indicates which effector molecule has been implicated: HO-1, CO, BV/BR or ferritin/iron.
In contrast, the extrinsic pathway initiates when a death ligand, (ie., FasL) interacts with its cell surface receptor (i.e., Fas), forming a death-inducing signal complex (DISC)[28, 33]. Activation of Fas triggers the rapid recruitment of FADD (Fas-associated death domain protein) and caspase-8, resulting in the activation of caspase-8. Active caspase-8 cleaves Bid into truncated Bid (tBid), which translocates to the mitochondrial membrane, assists in Bax activation, and triggers Cyt-c release [33]. Thus, the extrinsic and intrinsic pathways converge at the mitochondria, and share similar features downstream of Bax activation and Cyt-c release.
Recent studies indicate that autophagy, a regulated pathway for internal organelle or protein degradation influences the outcome of cell death pathways [17, 34]. During autophagy, cytoplasmic double membrane-bound vesicles (ie., autophagosomes) sequester damaged organelles for delivery to the lysosome where they are degraded and recycled [35–37]. The Bcl-2 interacting protein Beclin 1 and the microtubule-associated protein-1 light chain-3 (LC3), represent major regulators of autophagosome formation in human cells [39–40]. Autophagy plays a central role in cellular adaptation to environmental stress, such as oxidative stress, serum starvation, endoplasmic reticulum stress, hypoxia, and infection [34–38]. Activation of autophagy is regarded as a survival mechanism during nutrient deprivation, whereas excessive autophagy may promote cell death under certain conditions [37, 41]. Recent studies suggest that autophagic proteins may cross-regulate the initiation of the apoptotic program in response to stress [42–44]. Though increased autophagosome formation is frequently observed in dying cells, the casual relationship between autophagy and cell death remains a matter of current controversy [45]. The role of HO-1 in regulating specific pathway elements of autophagy and apoptosis will be discussed in subsequent sections.
Heme Oxygenase-1: biochemical properties and subcellular localization
HO-1, the inducible HO isozyme, is the major inducible low molecular weight (32–34 kDa) stress protein of mammalian cells and tissues [46]. Heme oxygenase has a major constitutively expressed isozyme, heme oxygenase-2 (HO-2) [47]. HO-1 and HO-2 represent the products of two distinct genes [48]. Although HO-1 and HO-2 both catalyze heme-b to biliverdin, the two enzymes differ in primary structure and biochemical/biophysical properties [48–49]. A highly conserved sequence of 24 amino acid residues corresponding to the heme catalytic domain has been identified in common to both HO-1 and HO-2 [50]. Furthermore, both HO-1 and HO-2 hare similar hydrophobic regions at the carboxyl-terminus that anchor the proteins in cellular membranes [51–53]. A gene potentially encoding a third HO isozyme, ho-3 was cloned in the rat, though further analysis has suggested that the rat ho-3 genes represent processed pseudogenes, and to date, no natural protein has been found [54–55].
The expression of HO-1 occurs at very low levels in most tissues under physiological conditions, with the exception of the spleen, the site of erythrocyte hemoglobin turnover, where high levels of HO-1 are constitutively present. In contrast, HO-2 is expressed under basal conditions in most tissues, including testes, spleen, liver, kidney, brain, and vasculature (Reviewed in [1]).
HO-1 is an integral membrane component of the smooth endoplasmic reticulum (ER), evidenced by its abundance in microsomal membrane preparations. Recent studies have identified possible subcellular compartmentalization of HO-1 beyond ER membranes [56–61]. For example, a nuclear localization of HO-1 has been described [56]. These studies have suggested that HO-1 undergoes a proteolytic truncation of the carboxyl terminal hydrophobic region to facilitate nuclear entry. Despite apparent lack of heme catalytic activity of the truncated nuclear HO-1, recent work suggests that HO-1 may have a non-catalytic function in regulating nuclear transcription factor activities [12, 56]. Increased nuclear localization of HO-1 has been associated with several clinical disease conditions, as highlighted by recent studies in prostate cancer [57], suggesting a possible role for nuclear HO-1 accumulation in malignancy.
Furthermore, HO-1 and functional HO activity can be detected in detergent-resistant fractions (lipid rafts) of plasma membrane. Sucrose density gradient fractionation revealed the presence of HO-1 in low-density caveolin-1 containing fractions (caveolae). Within this compartment, caveolin-1 was shown to bind to HO-1 and negatively regulate HO activity [58]. Caveolin-1 regulates a number of signaling-related molecules in caveolae, (ie., endothelial nitric oxide synthase)[59]. The regulation of HO activity in this compartment may represent a mechanism for the controlled production of CO for signaling purposes [58]. However, the functional significance of HO-1 in plasma membrane lipid rafts remains unclear at present.
HO-1 also localizes in part to the mitochondria as shown in pulmonary epithelial cells [60] and in rat hepatocytes [61]. In epithelial cells, the expression of HO-1 protein and activity in the mitochondria increases in response to toxic stimuli, including cigarette smoke, bacterial lipopolysaccharide, and hemin [60]. While the mitochondria play central roles in the regulation of apoptosis, it remains unclear whether mitochondria-localized HO-1 modulates the apoptotic program by acting on specific components of the mitochondria. In conclusion, the specificity and function of the novel compartmentalization (ie., nucleus, mitochondria, lipid raft) of HO-1 remains unclear, but further study may reveal additional mechanisms relevant to the anti-apoptotic or cytoprotective potential of HO-1.
Regulation of HO-1 transcription
The transcriptional upregulation of the ho-1 gene, and subsequent de novo synthesis of the corresponding protein occurs in response to elevated levels of its natural substrate heme, and to a multiplicity of endogenous factors including NO, cytokines, heavy metals, hormones, and growth factors (Reviewed in [1]). Many agents that induce HO-1 are associated with oxidative stress in that they (I) directly or indirectly promote the intracellular generation of reactive oxygen species (ROS), (II) fall into a class of electrophilic antioxidant compounds which includes plant-derived polyphenolic substances [62–64], or (III) complex intracellular reduced glutathione and other thiols [65–66]. HO-1 expression responds to pro-inflammatory stress, associated with the production of ROS from activated inflammatory cells [67]. Furthermore, HO-1 expression responds to changes in ambient O2 tension, such as hyperoxia (increased pO2) or hypoxia (decreased pO2), both which can modulate mitochondrial ROS production. The regulation of HO-1 by hypoxia can vary in a cell type and species-specific manner, with transcriptional repression rather than induction reported in human cell types [68].
Because expression of HO-1 is regulated by a wide array of compounds in a cell-type and inducer-specific fashion, defining unifying transcriptional regulatory mechanisms has been challenging [69]. Two enhancer regions located at approximately −4kb and −10kb relative to the ho-1 transcriptional start site have been identified in the mouse gene [70–71]. The dominant sequence element of the enhancers is the stress-responsive element (StRE), which is structurally and functionally similar to Maf response element (MARE) and the antioxidant response element (ARE)[69, 72]. Several transcriptional regulators bind these sequences, including nuclear factor erythroid 2-related factor-2 (Nrf2) and Bach1. These are both members of the Cap’n’collar/basic-leucine zipper family capable of heterodimerizing with small Maf proteins and regulating MARE-driven genes. Nrf2 contains a transcription activation domain and positively regulates HO-1 transcription, whereas Bach1 competes with Nrf2 and represses transcription [73–76]. Mice lacking the gene for Bach1 have dramatic increases in HO-1 expression in the heart, lung and liver, indicating a role for Bach1 in tonic suppression of HO-1 transcription. Conversely, inhibition of Nrf2 gene function results in impairment of transcriptional responses to known inducers of HO-1 [73–74, 77–79]. Thus, HO-1 expression depends upon changes in the balance of activity of Nrf2 and Bach1. Heme can relieve Bach1 of DNA binding activity through complex formation, promote the nuclear export of Bach1 and inhibit the proteasomal degradation of Nrf2, thus enhancing HO-1 expression [73–74, 79–82]. The Kelch-like ECH-associated protein (Keap1) inhibits HO-1 expression by sequestering Nrf2 in the cytoplasm. Keap1 facilitates the targeting of Nrf2 by Cullin 3-based E3 ubiquitin ligase complex, which marks Nrf2 for proteasomal degradation [83–86]. Under basal conditions, Keap1 binds to Nrf2 and sequesters it in the cytoplasm, which results in a lower accumulation of Nrf2 in nucleus and reduced transcription of the HO-1 gene [85, 87–88]. When cells are exposed to electrophiles or oxidants, Nrf2 dissociates from Keap1, translocates to the nucleus, and binds to target genes [89–90]. In Keap1 null mice, Nrf2 constitutively increases in the nucleus and inappropriately stimulates its target genes, leading to growth retardation and early death. However, the phenotypic abnormalities can be reversed by reduction of Nrf2 abundance in the Keap1-null mutant background [91]. Nrf-2 knockout mice were more susceptible to tobacco smoke-induced lung apoptosis and emphysema relative to wild type mice, illustrating a critical role for Nrf2 in the regulation of adaptive responses to stress [92].
While the Nrf2 system has been characterized extensively in the mouse as the major stress inducible operator of the ho-1 gene, many additional cis-acting promoter elements have been identified in the context of the mouse, rat, and human ho-1 upstream regulatory regions which may also contribute to ho-1 regulation in a cell type an inducer-specific fashion. Examples of elements that respond in an inducer-specific or selective fashion include those responsive to hyperthermia [93] or hypoxia [94] which represent the targets of distinct DNA-binding proteins such as heat shock factors (HSF) and hypoxia-inducible factor-1 (HIF-1), respectively.
While the human ho-1 promoter contains regions analogous to the upstream enhancer regions described in the mouse, further studies have revealed additional complexities specific to the human ho-1 promoter. The human ho-1 gene contains an additional 10 bp sequence element, termed the cadmium responsive element (CdRE), occurring within the –4kB enhancer region but extrinsic to the StRE sequence homologies, which mediates CdCl2 induction of the gene [95]. Furthermore, the human −4kb enhancer region contains a binding site preferentially targeted by cyclic AMP responsive element binding factor (CREB) [96]. A region at −9.5 kb corresponds to a binding site for the early growth response-1 (Egr-1) factor, which mediates the response to metalloporphyrin induction [97]. A number of proximal promoter elements have also been identified as potentially important in human ho-1 gene regulation [98–100]. These include E-box motifs potentially recognized by the upsteam stimulatory factor (USF), and/or basic helix loop helix (bHLH) proteins [101–102], ETS binding sites (EBS) [103], as well as binding sites for nuclear factor-kappa B (NF-κB) [104], STAT-3 [105], activator protein-2 (AP-2) [104], and HSF-1[99]. An upstream region contains potential binding sites for hepatocyte nuclear factors (HNE1 and HNE4), AP-1, STAT-x, c-Rel, and GATA-X [106–107]. These studies reveal the complex nature of ho-1 gene regulation, which potentially involves the coordinated interaction of multiple transcription factors. The functional significance of all of these factors however, remains incompletely understood [10, 65, 98].
A microsatellite (GT)n dinucleotide length polymorphism has been described in the promoter region of the human ho-1 gene, which can result in the impaired transcriptional regulation and decreased expression of HO-1 in individuals that carry the long (L) allele [ie., (GT)n ≥30] of this polymorphism [108]. Multiple human genetic epidemiology studies have examined the association between this length polymorphism in the human ho-1 gene, and COPD-related traits. The “Long” allele of the GT repeat (≥25, ≥30, ≥32, or ≥33 repeats, depending on study) has been associated with COPD [109], emphysema [108], COPD severity [110], lung function decline in COPD [111] and lung function decline in the general population [112–114]. For example, in a retrospective study of French smokers the L allele [(GT)n ≥ 33] was associated with decreased lung function parameters relative to non-carriers. The greatest decline in lung function was observed in heavy smokers that carried the L allele [112]. However, the association with lung function decline was not replicated in COPD subjects in the National Heart, Lung, and Blood Institute Lung Health Study (115), while other studies have demonstrated associations with different alleles [ie., (GT)n ≥30] of the GT repeat [116]. Thus additional studies are required before a consensus can be realized. Interestingly, lymphoblastoid cells cultured from homozygous carriers of the L allele displayed increased susceptibility to oxidant (H2O2)-mediated apoptosis in vitro [117]. These studies suggest that a genetically-dependent downregulation of HO-1 expression may arise in subpopulations, possibly linked to increased susceptibility to oxidative stress-related disease.
Heme Oxygenase-1/CO Confers Protection Against Cell Death in Pulmonary/Vascular Cell Injury
Cytokine mediated apoptosis
The antiapoptotic effects of CO were originally described in vitro, and several potential mechanisms were implicated. Exogenous CO was shown to inhibit tumor necrosis factor-α (TNFα)-initiated apoptosis in mouse fibroblasts [118] and endothelial cells [119]. In fibroblasts, an anti-apoptotic effect was also observed with HO-1 overexpression [118]. In endothelial cells, the inhibitory effect of CO on TNFα-induced apoptosis could be abolished with the selective p38α/β MAPK inhibitor, SB203580, or a p38 MAPK dominant negative mutant, implying a critical role for the p38 MAPK pathway [119]. Furthermore, a role for NF-κB and the downstream activation of anti-apoptotic genes was also described [120]. In mouse lung endothelial cells, protection against TNFα/actinomycin-D induced cell death involved p38β MAPK activation, leading to upregulation of heat shock factor-1 and expression of the 70-kDa heat shock protein [116]. The cytoprotective effects of CO were diminished in heat shock factor-1 knockout (hsf1−/−) mice, indicating a role of the heat shock response in CO-mediated cytoprotection [121].
In cultured vascular smooth muscle cells, CO inhibited cytokine (TNFα, IL1-β, INF γ)-induced apoptosis, dependent on activation of soluble guanylate cyclase (sGC), whereas p38 MAPK was not implicated in this cell type [122–123]. Despite numerous reports of antiapoptotic activity of HO-1/CO in cultured cells, evidence for pro-apoptotic effects for HO-1/CO have also been described. For example, Thom et al. reported induction of apoptosis in cultured endothelial cells with CO application [124]. Liu et al. reported that adenoviral-directed expression of HO-1 in smooth muscle cells promoted apoptosis in a dose-dependent fashion [125]. Though the reasons for these contrasting observations are unclear, they are consistent with the notion that HO-1/CO can modulate apoptosis in vascular cells with the outcome dependent on specific experimental conditions, cell type, or nature of inducing stimuli.
Hyperoxia-induced lung cell death
Hyperoxia, or high O2 tension, induces the expression of HO-1 in several cell types including endothelial cells and fibroblasts [126–127]. HO-1 overexpression or CO treatment protected A549 alveolar epithelial cells against cell killing by exposure to hyperoxia [128, 129]. Treatment with the p38 MAPK inhibitor or transient transfection with dominant negative mutants of p38β or mitogen activated protein kinase kinase-3 (MKK3) abolished the cytoprotective effect of CO against hyperoxia [129]. Recent studies have also implicated the signal transducer and activator of transcription protein-3 (STAT3), as a mediator of CO-dependent antiapoptotic protection in hyperoxic lung cell injury [130].
CO (250 ppm) specifically inhibited the initiation and propagation of extrinsic apoptosis pathways in mouse lung endothelial cells subjected to hyperoxia [131]. CO co-treatment inhibited the hyperoxia-induced DISC formation as well as downstream caspase-8 and Bid activation. Antiapoptotic cytoprotection culminated with the inhibition of caspase-9/3 activation and protection against cell death. CO also inhibited hyperoxia-induced ROS production, as well as the activation of the DISC, and its plasma membrane assimilation. Furthermore the protective effects of CO in this model depended on the downregulation of the ERK1/2 MAPK pathway, which was shown to regulate NADPH oxidase-dependent ROS production in this cell type during hyperoxia [131].
Hypoxia/reoxygenation induced lung cell death
Hypoxia/reoxygenation (H/R) or anoxia/reoxygenation (A/R) are commonly used as in vitro models of the oxygen flux that occurs during I/R injury, though these models do not recapitulate the systemic and hemodynamic stress components of I/R injury in vivo. H/R and A/R protocols cause apoptosis in cultured endothelial cells. In vitro experiments using pulmonary endothelial cells demonstrated that exogenously applied CO at low concentrations inhibited A/R-induced apoptosis. The antiapoptotic effect of CO involved inhibition of Fas/FasL expression, and other apoptosis-related factors including caspases (-3,-8,-9), mitochondrial Cyt-c release, Bcl-2 proteins, and poly (ADP-ribose) polymerase (PARP) cleavage [132]. CO activated the p38α MAPK isoform and its upstream kinase (MKK3), whereas it inhibited ERK and JNK activation [132–133]. The antiapoptotic effects of CO in A/R exposed endothelial cells were inhibited in cells from mkk3−/− mice or by treatment with a p38α/β MAPK-specific inhibitor [132]. Further studies revealed that CO activated the phosphatydylinositol-3 kinase (PI3K)/Akt pathway, which mediated the activation of the p38 MAPK pathway and activated the STAT3 pathway. Increased phosphorylation and DNA binding activity of STAT3 in response to CO was accompanied by suppression of STAT-1 activation. STAT3 mediated both the inhibition of Fas expression and the inhibition of caspase-3 activation in response to CO treatment; whereas a dominant negative mutant of STAT3 increased endothelial cell apoptosis in response to A/R and antagonized the effects of CO [134].
HO-1 in cytoprotection against cigarette smoke-induced cell death
Cigarette smoke (CS) contains over 4,500 distinct chemical species. Since CS contains ROS, NO and other free radicals, electrophilic substances and heavy metals, and furthermore can trigger the intracellular production of ROS and deplete natural antioxidants, CS is generally regarded as an oxidative cellular stress [135]. While animals in inhalation studies are typically exposed to mainstream or side stream CS, cell culture studies typically utilize aqueous cigarette smoke extract (CSE) [136]. Though some reports have suggested that CSE causes primarily necrotic cell death in bronchial epithelial cells [137], apoptotic phenotypes have also been observed depending on cell model and experimental conditions [60, 138]. Lower dose and shorter kinetics of CSE exposure generally favor apoptosis [60].
We found that CSE activated the extrinsic apoptotic pathway in human epithelial cells (Beas-2B) and primary human lung fibroblasts. CSE induced assimilation of the Fas-dependent DISC at the cell surface plasma membrane, as the result of mobilization of pre-formed complexes in the Golgi apparatus. CSE induced DISC formation resulted in activation of caspase-8, and subsequent activation of downstream caspases. The primary involvement of an extrinsic pathway in CSE-induced cell death is supported by reduced cell death in Fas−/− fibroblasts exposed to CSE [139].
The induction of HO-1 by CS or CSE was observed in a number of pulmonary and non-pulmonary cell types, including fibroblasts, alveolar macrophages, and epithelial cells [60, 140–142]. In our recent studies, human bronchial epithelial cells (Beas-2B) subjected to CSE responded with a time- and dose-dependent upregulation of HO-1 protein and enzymatic activity. HO-1 protected against CSE-induced cell death and preserved cellular ATP levels which were depleted by the CSE treatment. This protection afforded by HO-1 at high concentrations of CSE, as well as preservation of cellular ATP levels suggest a general anti-necrotic effect of HO-1 against CSE-exposure [60]. Anti-necrotic protection of HO-1 was also described in HO-1 expressing epithelial cells subjected to direct NO exposure, in this case attributed to decreased membrane lipid peroxidation [143].
At lower, apoptosis inducing, concentrations of CSE, expression of HO-1 was also shown to inhibit the activation of the extrinsic pathway in Beas-2B cells by inhibiting the formation of the DISC and subsequent activation of downstream capsases -8,-9,-3 [144]. Interestingly, HO-1 expression also inhibited the expression of autophagic regulator proteins, LC3B and Beclin 1. Further experimentation revealed that these autophagic proteins were critical mediators of the initiation of extrinsic apoptosis in these cells in response to CSE exposure. Infection with siRNA specific for either LC3B or Beclin 1 inhibited CSE-induced activation of DISC formation and caspase-8 activation in Beas-2B cells. These experiments suggest that the complex interplay of signaling molecules affected by HO-1 include not only regulators of apoptosis pathways, but also of autophagic pathways. In the case of CSE-induced cell death, enhanced autophagy correlated with increased cell death, therefore the homeostatic function of HO-1 is consistent with the downregulation of both pathways. These experiments suggest that inducible stress responses such as HO-1 act as a first-line defense against oxidative cell injury, which can reduce the progression of the cell toward stress-inducible autophagy as well as apoptosis [144]. The direct mechanisms by which HO-1 downregulates Beclin 1 or LC3B expression in epithelial cells, and the overall significance of HO-1 in the regulation of autophagy in general remain unclear.
Endoplasmic reticulum stress
The endoplasmic reticulum (ER) is a vital organelle involved in the protein secretory pathway and lipid biosynthesis. Various stress conditions, including alterations in intracellular calcium homeostasis, inhibition of protein glycosylation, and/or accumulation of misfolded proteins, can induce an ER stress response, characterized by increased synthesis of ER stress proteins. Excessive ER stress and ER dysfunction can promote apoptotic cell death, through the activation of caspases (ie., caspase-12, -9, -8) [145–146]. Recent studies identify HO-1 as a critical component of the ER stress response in smooth muscle cells (SMC) [146–147]. HO-1 expression responded to transcriptional induction by canonical ER stress-inducing chemicals (ie., thapsigargin, brefeldin-A, homocysteine, proteosome inhibitors) in SMC [147]. Exogenous application of CO inhibited apoptosis induced by ER stress-inducing agents in SMC, which was associated with the downregulated expression of the propapoptotic protein GADD153 [147]. In human endothelial cells an antiapoptotic mechanism of HO-1/CO against ER stress was demonstrated involving the activation of p38 MAPK and downregulation of the proapoptotic C/EBP homologous protein (CHOP) [148]. Pharmacological induction of HO-1 or application of the CO-releasing molecule (RuCO) downregulated CHOP and protected against thapsigargin-induced apoptosis. CO also activated protein kinase R–like endoplasmic reticulum kinase (PERK), resulting in Nrf-2 activation and nuclear translocation. Activation of Nrf-2 in turn led to an induction of HO-1 which contributed to the cytoprotective response [148]. These results, demonstrate that HO-1/CO confers antiapoptotic cytoprotection against apoptotic signals originating from ER compartment stress.
Role of biliverdin/bilirubin in cytoprotection
The bile pigments, biliverdin (BV) and bilirubin (BR) generated from the action of HO and BVR activities respectively, have also been implicated in both toxicity and cytoprotection, depending on the dose and model system. The overproduction of BR in neonates is neurotoxic, thus strategies to remove BR (phototherapy) or inhibit its formation (competitive inhibitors of HO activity) are implemented clinically. BV and BR have been demonstrated to have antioxidant properties in in vitro model systems which include the inhibition of LDL oxidation, protein damage and peroxidation of phospholipid micelles. Albumin bound BR acts as a co-antioxidant with α-tocopherol. The relative significance of BR as a circulating antioxidant in vivo remains unclear due to the existence of multiple circulating antioxidants [149]. BR has been shown to confer cytoprotection against oxidative or nitrosative stress conditions in various cultured cells, including endothelial, vascular smooth muscle, HeLa cells, cardiomyocytes, and neural cells (Reviewed in [150]). Addition of BR to cultured HeLa cells has been shown to protect against H2O2 dependent cytotoxicity [151]. Moreover, various in vivo studies investigating disease models such as I/R, transplantation, pulmonary fibrosis, or organ injury have demonstrated a reduction of cell death when BR or BV were administered or were present at elevated levels (Reviewed in [15]). BV adjuvant therapy has been shown to protect rat liver grafts from I/R injury. In this model, BV suppressed activation of apoptosis (Cyt-c release) and inhibited JNK activation [152]. In addition to possible antioxidative effects, BR, has been shown to inhibit the proliferation of vascular smooth muscle cells [153–154]. Exogenous BV administration inhibited neointimal hyperplasia associated with vascular balloon injury in rats [153–154], and hyperbilirubinemic animals also displayed increased resistance to vascular injury [153].
Recent studies have suggested that BVR, in addition to its metabolic capacity in the conversion of BV to BR, may have additional functions in the activation of cellular signaling pathways (Reviewed in [155]). Experiments using siRNA approaches support a role for BVR in cytoprotection. For example, siRNA directed knockdown of BVR increased total cellular ROS production in HeLa cells [151]. The siRNA directed knockdown of BVR increased apoptosis in response to sodium arsenite treatment, as evidenced by increased Cyt-c release and PARP cleavage, and other biochemical markers [156]. siRNA directed knockdown of BVR augmented cardiomyocyte apoptosis in response to H/R stress in vitro [157]. The antiapoptotic protection of BVR was related to not only its metabolic activity, but also to activation of the PI3K/Akt pathway, through an intramolecular interaction with PI3K [157].
Role of iron in cytotoxicity and protection
Despite the essential requirement for iron in various cellular processes, such as heme synthesis, DNA replication, mitochondrial function, and oxygen sensing, iron potentially poses a toxic insult to cells by catalyzing free radical generating reactions. Iron loading sensitizes endothelial cells to oxidant-mediated cell killing [158]. On the other hand, complete deprivation of iron leads to apoptosis, as shown by proapoptotic effects of metal chelator treatments, suggesting that at least a minimal amount of iron is needed to serve for vital cellular processes [159]. Nevertheless, the role of intracellular iron in the regulation of apoptosis remains incompletely understood. Though HO activity is generally associated with cellular protection, HO activity releases ferrous iron from heme, with potential deleterious effects. Evidence from in vitro models, describes a potential toxic threshold for HO-1 overexpression, related to iron accumulation [160–161]. For example, hyperoxia sensitivity was observed at high levels of HO-1 overexpression, whereas hyperoxia-resistance at moderate levels of HO-1 expression [160–161]. Similarily, HO-2 overexpressing clones displayed short-term hypersensitivity to UVA radiation in the presence of heme loading, relative to wild type cells [162]. The apparent toxicity of HO-1 or HO-2 in these models was likely related to gene overdosing and/or substrate load conditions resulting in a transient rise in intracellular iron under pro-oxidant conditions.
The antioxidative protection afforded by HO activity cannot be explained alone by the conversion of heme in exchange for increased intracellular iron, which is potentially toxic. This antioxidant protection of HO with respect to iron metabolism is possible by further coupling with proteins that either promote the sequestration or export of the liberated iron. HO-1 has been associated with iron efflux pathways [163], and also promotes the synthesis of ferritin, which eventually neutralizes potentially reactive iron [164]. Iron release from HO activity leads to post-translational release of translational repression of ferritin, therefore ferritin synthesis is increased [164]. The HO-1 dependent increase in ferritin was linked to cytoprotection in several models of oxidative stress, including UVA radiation and photosensitizer-mediated oxidative stress [164–165]. Ferritin overexpression also reduced apoptosis in a model of I/R injury after liver transplantation [166]. Thus ferritin appears to confer cytoprotection against oxidative challenge, and potentially plays an intermediate role in HO-mediated antiapoptotic protection. Furthermore, an ATP-dependent iron pump has been shown to colocalize with HO-1 in the microsomal membrane. This pump also might facilitate the exocytosis of HO-derived iron [167]. A possible antiapototic mechanism of intracellular HO-derived iron has recently been proposed, whereby HO-derived iron activates NF-κB which in turn mediates an antiapoptotic pathway [168]. These studies, taken together, suggest that HO-derived iron contributes to phenotypic effects of HO activation, and cannot be discounted in therapeutic applications of HO-1, though these processes remain incompletely understood.
Cytoprotective actions of HO-1 in preclinical models of lung injury
Pulmonary I/R
Pulmonary I/R caused by temporal clamping of the pulmonary artery induced the biochemical features of apoptosis in rodent lungs, including increased expression of Fas, FasL, activation of caspases (3-,8-,9-), modulation of Bcl2-related proteins, PARP cleavage, and Cyt-c release [132]. The protective effect of CO pretreatment on mice subjected to lung I/R injury in vivo was associated with the inhibition of apoptosis markers, including caspase-3 activation, and depended on activation of p38α MAPK [133]. Homozygous ho-1 null mice (ho-1−/−) were highly susceptible to lung I/R injury. CO inhalation compensated for the HO-1 deficiency in ho-1−/− mice, and improved survival following lung I/R [7]. The protection provided by CO involved the stimulation of fibrinolysis, by the cGMP-dependent inhibition of plasminogen activator inhibitor-1, a macrophage–derived activator of smooth muscle cell proliferation [7]. CO also inhibited fibrin deposition and improved circulation in ischemic lungs [169]. These protective effects were recently associated with downregulated expression of early growth response-1 (Egr-1), a multifunctional transcription factor, with the concomitant downregulation of Egr-1 target genes, many which contribute to inflammatory or pro-thrombotic processes. The downregulation of Egr-1 depended on the enhancement of cGMP signaling by CO treatment, leading to the inhibition of the ERK1/2 MAPK pathway [169]. Recent in vivo studies indicate that ho-1 knockdown using siRNA dramatically increased apoptosis during pulmonary I/R [170].
Transplantation
One of the best studied models of I/R injury is organ transplantation. Generally, these models involve cold preservation of organs followed by warm reperfusion of the grafts. The transplanted organs are therefore at risk of injury during both preservation and reperfusion phases. The involvement of HO-1 in chronic graft dysfunction due to immunologic rejection has also been well studied [23], but this subject is beyond the scope of this current review. During the ischemic stage, ATP depletion can lead to altered intracellular electrolyte balance, hypoxanthine accumulation and activation of cytotoxic enzymes, resulting in cell damage or death. Upon reperfusion and reintroduction of oxygen, ROS are generated, potentially extending cellular injury, and promoting cell death by both necrosis and apoptosis [171]. HO-1 is consistently upregulated in models of transplantation [123], and there is ample evidence that HO-1 confers protection in this setting. The bulk of experimental evidence has been derived from rodent I/R transplantation models, where HO-1 induction pharmacologically or by gene transfer has been shown to provide cytoprotection in transplanted heart [172], liver [173], kidney [174], small bowel [175], pancreatic islets [176], and muscular flap [177]. In addition, a recent report correlating donor HO-1 polymorphisms with post-transplant renal graft function suggests that HO-1 function may contribute to clinical disease outcome [178].
The mechanism by which HO-1 provides protection in transplant-associated models of I/R injury appears to be multifactorial. Hemoglobin, the major source of extracellular heme protein, is known to contribute to I/R injury [179]. Heme contributes to oxidative injury, which may trigger programmed cell death, and thus increased catabolism of heme by HO-1 may attenuate injury. Scavenging of ROS by BV and/or BR may protect cells from oxidative injury. Increased ferritin synthesis may promote iron redistribution and neutralization in the setting of transplantation [172]. CO may also contribute to protection by regulating vascular tone and platelet aggregation [180–182], and more importantly, through direct inhibition of apoptosis.
In addition to manipulation of HO-1, the direct application of CO has also been shown to confer protection in I/R transplantation models, including vascular transplantation [182], cardiac [181], small intestinal [183], kidney, [184], liver [185], and lung transplantation [186]. During orthotopic left lung transplantation in rats, the transplanted lungs developed severe intra-alveolar hemorrhage and intravascular coagulation. In the presence of continuous CO exposure (500 ppm), histologic analysis showed markedly improved graft preservation, with reduction in hemorrhage, fibrosis, and thrombosis after transplantation. Furthermore, CO reduced lung cell apoptosis and downregulated lung and proinflammatory cytokine and growth factor production (IL-6, macrophage inflammatory protein-1α, macrophage migration inhibitory factor, platelet-derived growth factor), which were induced during transplantation [186]. CO treatment also conferred protection during cardiovascular transplantation. When transplant recipients of aortic grafts were maintained in a CO environment (250 ppm) with preconditioning, these animals displayed significantly less intimal hyperplasia, and reduced accumulation of leukocytes, macrophages, and T cells in the graft [182]. Bubbling of CO through the organ buffer prevented cold I/R injury during subsequent intestinal transplantation, implicating the ex vivo application of CO as an alternative therapeutic strategy [187]. In recent studies, inclusion of the water soluble carbon monoxide releasing molecule (CORM)-3 in the preservation fluid improved cardiac functional parameters following cardiac transplantation [188]. Several studies have recently examined the mechanisms underlying the antiapoptotic effects of CO in transplantation models. The antiapoptotic effect of CO during transplantation of pancreatic islets or intestinal grafts involved activation of a cGMP-dependent pathway [189–192]. The induction of Hsp-70 potentially contributed to the antiapoptotic effect of CO after liver transplantation [185]. The inhibition of apoptosis and inflammation are currently believed to represent the major mechanisms by which CO protects transplanted organs from dysfunction and failure [192], though improvement of blood circulation by CO within the reperfused transplanted organ cannot be discounted [182, 192–193].
The pharmacological application of BR or BV also has been documented to confer tissue protection in I/R transplantation models, including liver [194], kidney [195], and cardiac [188] transplantation. BV provided tissue protection in an ex vivo model of cold hepatic I/R injury. Furthermore, inclusion of BV in the perfusate increased the survivability of rats undergoing liver transplantation by preserving liver function [194]. This protection afforded by BV occurred in association with the decreased expression of pro-inflammatory markers, including neutrophil influx, proinflammatory cytokine expression, and inducible NOS (iNOS) [194]. In the isolated perfused kidney model, inclusion of BR in the perfusate protected against warm I/R–induced tissue injury and preserved renal function [195]. BV treatment also improved the survivability of rat cardiac allografts by reducing leukocyte infiltration and inhibiting T-cell proliferation [196]. The combined application of BV and CO provided a synergistic tissue protection against transplant-associated cold I/R injury of heart and kidney grafts, under conditions where little protection was observed with either agent alone [197]. In conclusion, protection by HO-1 and its end products BV/BR and CO in I/R injury [167–168, 172–174, 176, 186], as well as other models of oxidative injury [198] has in many cases been correlated in part, with the inhibition of apoptosis, in a complex interplay of protective mechanisms that also may include anti-inflammatory [199] and antiproliferative [182] effects.
Conclusions
There now exists ample evidence that the HO-1 system, as well as the pharmacological application of HO pathway end products, provides robust protection against cellular stress. While considerable progress has been made in defining the therapeutic potential of HO-1 in vitro and in preclinical models of organ injury and disease, the clinical benefit of therapies involving HO-1 have yet to been demonstrated in humans. Of the preclinical disease models exploring medical applications for HO-1, transplantation is perhaps the best developed. Transplantation is in many ways the ideal clinical setting for exploiting the antiapoptotic function of HO-1, since the time of injury is predictable and well defined, and the opportunity exists to apply the therapy not only to the patient but to the donor organ as well. Nevertheless, the use of HO-1 as a treatment strategy has yet to be adopted. Concern for toxicity from gene therapy or from pharmacologic use of BV or CO has delayed progress in the clinical arena, although enthusiasm for further research in this area remains strong. The development of non-toxic inducers of HO-1 or Nrf2 activation remains a viable strategy [89]. Further progress in gene therapy approaches that allow tissue or cell type specific gene delivery may also facilitate progress in this area. As an alternative to inhalation of CO, pharmacological application of CO with the transition-metal carbonyl CO-releasing molecules, (CORMs) may provide an additional therapeutic avenue [200]. Whether direct application of CO by either method will provide a safe modality for the treatment of human disease requires further research directed at understanding the pharmacokinetics and toxicology of CO application in humans. Further progress awaits the completion of several ongoing clinical trials in transplantation and sepsis.
Acknowledgments
This work was supported in part by NIH grant P01-HL70807 (A.M.K. Choi and S. Ryter), and by R01-HL60234, R01-HL55330, R01-HL079904, awarded to A. M. K. Choi
List of Abbreviations
- A/R
anoxia/reoxygenation
- BR
Bilirubin
- BV
Biliverdin
- BVR
biliverdin reductase
- cGMP
- CO
carbon monoxide
- CORM
carbon monoxide releasing molecule
- COPD
chronic obstructive pulmonary disease
- CS
cigarette smoke
- CSE
cigarette smoke extract
- Cyt-c
cytochrome-c
- DISC
death inducing signaling complex
- HO-1
heme oxygenase-1
- HO-2
heme oxygenase-2
- H/R
hypoxia/reoxygenation
- IL
interleukin
- I/R
ischemia/reperfusion
- JNK
c-Jun NH2-terminal kinase
- Keap1
Kelch-like ECH-associated protein
- LC3
microtubule-associated protein-1 light chain-3
- MAPK
mitogen activated protein kinase
- MKK3
mitogen activated protein kinase kinase-3
- NF-κB
nuclear factor kappa-B
- Nrf2
NF-E2 related factor-2
- PARP
poly (ADP-ribose) polymerase
- PI3K/Akt
phosphatydylinositol-3 kinase/Akt
- ROS
reactive oxygen species
- sGC
soluble guanylate cyclase
- siRNA
small interfering ribonucleic acid
- STAT3
signal transducer and activator of transcription-3
- TNFα
tumor necrosis factor-alpha.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 2.Morse D, Choi AM. Heme oxygenase-1: the “emerging molecule” has arrived. Am. J. Respir. Cell Mol. Biol. 2002;27:8–16. doi: 10.1165/ajrcmb.27.1.4862. [DOI] [PubMed] [Google Scholar]
- 3.Ryter S, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol. Cell. Biochem. 2002;234–235:249–263. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J. Biol. Chem. 1969;244:6388–6394. [PubMed] [Google Scholar]
- 5.Poss KD, Tonegawa S. Heme oxygenase-1 is required for mammalian iron reutilization. Proc. Natl. Acad. Sci. USA. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase-1 deficient cells. Proc. Natl. Acad. Sci. USA. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita T, Toda K, Karimova A, Yan SF, Naka Y, Yet SF, Pinsky DJ. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat. Med. 2001;7:598–604. doi: 10.1038/87929. [DOI] [PubMed] [Google Scholar]
- 8.Dennery PA, Visner G, Weng YH, Nguyen X, Lu F, Zander D, Yang G. Resistance to hyperoxia with heme oxygenase-1 disruption: role of iron. Free Radic. Biol. Med. 2003;34:124–133. doi: 10.1016/s0891-5849(02)01295-9. [DOI] [PubMed] [Google Scholar]
- 9.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryter SW, Kim HP, Nakahira K, Zuckerbraun BS, Morse D, Choi AM. Protective functions of heme oxygenase-1 and carbon monoxide in the respiratory system. Antioxid. Redox Signal. 2007;9:2157–2173. doi: 10.1089/ars.2007.1811. [DOI] [PubMed] [Google Scholar]
- 11.Ryter S, Morse D, Choi AM. Carbon monoxide and bilirubin: potential therapies for pulmonary/vascular injury and disease. Am. J. Respir. Cell Mol. Biol. 2007;36:175–182. doi: 10.1165/rcmb.2006-0333TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin QS, Weis S, Yang G, Zhuang T, Abate A, Dennery PA. Catalytic inactive heme oxygenase-1 protein regulates its own expression in oxidative stress. Free Radic. Biol. Med. 2008;44:847–855. doi: 10.1016/j.freeradbiomed.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirkham P, Rahman I. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol. Ther. 2006;111:476–494. doi: 10.1016/j.pharmthera.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Mercer BA, D'Armiento JM. Emerging role of MAP kinase pathways as therapeutic targets in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2006;1:137–150. doi: 10.2147/copd.2006.1.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 16.Jozkowicz A, Was H, Dulak J. Heme oxygenase-1 in tumors: is it a false friend? Antioxid. Redox Signal. 2007;9:2099–2117. doi: 10.1089/ars.2007.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L, Kroemer G. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–1243. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- 18.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 19.Rashedi I, Panigrahi S, Ezzati P, Ghavami S, Los M. Autoimmunity and apoptosis-therapeutic implications. Curr. Med. Chem. 2007;14:3139–3151. doi: 10.2174/092986707782793952. [DOI] [PubMed] [Google Scholar]
- 20.Park JW, Ryter SW, Choi AM. Functional significance of apoptosis in chronic obstructive pulmonary disease. COPD. 2007;4:347–353. doi: 10.1080/15412550701603775. [DOI] [PubMed] [Google Scholar]
- 21.Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 22.Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc. Am. Thorac. Soc. 2006;3:350–356. doi: 10.1513/pats.200601-001TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soares MP, Bach FH. Heme oxygenase-1 in organ transplantation. Front. Biosci. 2007;12:4932–4945. doi: 10.2741/2439. [DOI] [PubMed] [Google Scholar]
- 24.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 25.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu. Rev. Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 26.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 27.Huang DC, Strasser A. BH3-Only proteins-essential initiators of apoptotic cell death. Cell. 2000;103:839–842. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 28.Nagata S. Fas ligand-induced apoptosis. Annu. Rev. Genet. 1999;33:29–55. doi: 10.1146/annurev.genet.33.1.29. [DOI] [PubMed] [Google Scholar]
- 29.Wang K, Grossm A, Waksman G, Korsmeyer SJ. Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol. Cell Biol. 1998;18:6083–6089. doi: 10.1128/mcb.18.10.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou JC. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem. J. 2000;345:271–278. [PMC free article] [PubMed] [Google Scholar]
- 31.Riedl SJ, Salvesen GS. The apoptosome: signaling platform of cell death. Nat. Rev. Mol. Cell Biol. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- 32.Saelens X, Festjens N, Vande WL, van Gurp M, van Loo G, Vandenabeele P. Toxic proteins released from mitochondria in cell death. Oncogene. 2004;23:2861–2874. doi: 10.1038/sj.onc.1207523. [DOI] [PubMed] [Google Scholar]
- 33.Walsh CM, Luhrs KA, Arechiga AF. The “fuzzy logic” of the death-inducing signaling complex in lymphocytes. J. Clin. Immunol. 2003;23:333–353. doi: 10.1023/a:1025313415487. [DOI] [PubMed] [Google Scholar]
- 34.Kelekar A. Autophagy. Ann. NY Acad. Sci. 2006;1066:259–271. doi: 10.1196/annals.1363.015. [DOI] [PubMed] [Google Scholar]
- 35.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat. Rev. Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 36.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiffin R, Bandyopadhyay U, Cuervo AM. Oxidative stress and autophagy. Antioxid. Redox. Signal. 2006;8:152–162. doi: 10.1089/ars.2006.8.152. [DOI] [PubMed] [Google Scholar]
- 38.Cuervo AM. Autophagy: in sickness and in health. Trends Cell Biol. 2004;14:70–77. doi: 10.1016/j.tcb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 40.He H, Dang Y, Dai F, Guo Z, Wu J, She X, Pei Y, Chen Y, Ling W, Wu C, Zhao S, Liu JO, Yu L. Post-translational modifications of three members of the human MAP1LC3 family and detection of a novel type of modification for MAP1LC3B. J. Biol. Chem. 2003;278:29278–29287. doi: 10.1074/jbc.M303800200. [DOI] [PubMed] [Google Scholar]
- 41.Galluzzi L, Vicencio JM, Kepp O, Tasdemir E, Maiuri MC, Kroemer G. To die or not to die: that is the autophagic question. Curr. Mol. Med. 2008;8:78–91. doi: 10.2174/156652408783769616. [DOI] [PubMed] [Google Scholar]
- 42.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat. Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 44.Crighton D, Wilkinson S, O'Prey J, Syed N, Smith P, Harrison PR, Gasco M, Garrone O, Crook Y, Ryan KM. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121–134. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 45.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc. Natl. Acad. Sci. USA. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trakshel GM, Kutty RK, Maines MD. Purification and characterization of the major constitutive form of testicular heme oxygenase. J. Biol. Chem. 1986;261:11131–11137. [PubMed] [Google Scholar]
- 48.Cruse I, Maines MD. Evidence suggesting that the two forms of heme oxygenase are products of different genes. J. Biol.Chem. 1988;263:3348–3353. [PubMed] [Google Scholar]
- 49.Rotenberg MO, Maines MD. Isolation, characterization, and expression in Escherichia coli of a cDNA encoding rat heme oxygenase-2. J. Biol. Chem. 1990;265:7501–7506. [PubMed] [Google Scholar]
- 50.Rotenberg MO, Maines MD. Characterization of a cDNA-encoding rabbit brain heme oxygenase-2 and identification of a conserved domain among mammalian heme oxygenase isozymes: possible heme-binding site? Arch. Biochem. Biophys. 1991;290:336–344. doi: 10.1016/0003-9861(91)90549-x. [DOI] [PubMed] [Google Scholar]
- 51.Ishikawa K, Sato M, Yoshida T. Expression of rat heme oxygenase in Esherichia coli as a catalytically active, full length form that binds to bacterial membranes. Eur. J. Biochem. 1991;202:161–165. doi: 10.1111/j.1432-1033.1991.tb16357.x. [DOI] [PubMed] [Google Scholar]
- 52.Shibahara S, Muller RM, Taguchi H, Yoshida T. Cloning and expression of cDNA for rat heme oxygenase. Proc. Natl. Acad. Sci. USA. 1985;82:7865–7869. doi: 10.1073/pnas.82.23.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCoubrey WK, Maines MD. Domains of rat heme oxygenase-2: the amino terminus and histidine 151 are required for heme oxidation. Arch. Biochem. Biophys. 1993;302:402–408. doi: 10.1006/abbi.1993.1231. [DOI] [PubMed] [Google Scholar]
- 54.McCoubrey WK, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur. J. Biochem. 1997;247:725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- 55.Hayashi S, Omata Y, Sakamoto H, Higashimoto Y, Hara T, Sagara Y, Noguchi M. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene. 2004;336:241–250. doi: 10.1016/j.gene.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Lin Q, Weis S, Yang G, Weng YH, Helston R, Rish K, Smith A, Bordner J, Polte T, Gaunitz F, Dennery PA. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J. Biol. Chem. 2007;282:20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- 57.Sacca P, Meiss R, Casas G, Mazza O, Calvo JC, Navone N, Vazquez E. Nuclear translocation of haeme oxygenase-1 is associated to prostate cancer. Br. J. Cancer. 2007;97:1683–1689. doi: 10.1038/sj.bjc.6604081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim HP, Wang X, Galbiati F, Ryter SW, Choi AMK. Caveolae compartmentalization of heme oxygenase-1 in endothelial cells. FASEB J. 2004;18:1080–1089. doi: 10.1096/fj.03-1391com. [DOI] [PubMed] [Google Scholar]
- 59.Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin-1 isoforms in cardiac myocytes and endothelial cells. J. Biol. Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- 60.Slebos DJ, Ryter SW, van der Toorn M, Liu F, Guo F, Baty CJ, Karlsson JM, Watkins SC, Kim HP, Wang X, Lee JS, Postma DS, Kauffman HF, Choi AM. Mitochondrial localization and function of heme oxygenase-1 in cigarette smoke-induced cell death. Am. J. Respir. Cell Mol. Biol. 2007;36:409–417. doi: 10.1165/rcmb.2006-0214OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Converso DP, Taille C, Carreras MC, Jaitovich A, Poderoso JJ, Boczkowski J. HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism. FASEB J. 2006;20:1236–1238. doi: 10.1096/fj.05-4204fje. [DOI] [PubMed] [Google Scholar]
- 62.Scapagnini G, Foresti R, Calabrese V, Giuffrida Stella AM, Green CJ, Motterlini R. Caffeic acid phenethyl ester and curcumin: a novel class of heme oxygenase-1 inducers. Mol. Pharmacol. 2002;61:554–556. doi: 10.1124/mol.61.3.554. [DOI] [PubMed] [Google Scholar]
- 63.Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, De Galarreta CM, Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 64.Foresti R, Hoque M, Monti D, Green CJ, Motterlini R. Differential activation of heme oxygenase-1 by chalcones and rosolic acid in endothelial cells. J. Pharmacol. Exp. Ther. 2005;312:686–693. doi: 10.1124/jpet.104.074153. [DOI] [PubMed] [Google Scholar]
- 65.Saunders EL, Maines MD, Meredith MJ, Freeman ML. Enhancement of heme oxygenase-1 synthesis by glutathione depletion in chinese hamster ovary cells. Arch. Biochem. Biophys. 1991;288:368–373. doi: 10.1016/0003-9861(91)90208-z. [DOI] [PubMed] [Google Scholar]
- 66.Lautier D, Luscher P, Tyrrell RM. Endogenous glutathione levels modulate both constitutive and UVA radiation/hydrogen peroxide inducible expression of the human heme oxygenase gene. Carcinogenesis. 1992;13:227–232. doi: 10.1093/carcin/13.2.227. [DOI] [PubMed] [Google Scholar]
- 67.Rizzardini M, Carelli M, Cabello Porras MR, Cantoni L. Mechanisms of endotoxin-induced haem oxygenase mRNA accumulation in mouse liver: Synergism by glutathione depletion and protection by N-acetylcysteine. Biochem. J. 1994;304:477–483. doi: 10.1042/bj3040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shibahara S, Han F, Li B, Takeda K. Hypoxia and heme oxygenases: oxygen sensing and regulation of expression. Antioxid. Redox Signal. 2007;9:2209–2225. doi: 10.1089/ars.2007.1784. [DOI] [PubMed] [Google Scholar]
- 69.Alam J, Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am. J. Respir. Cell Mol. Biol. 2007;36:166–174. doi: 10.1165/rcmb.2006-0340TR. [DOI] [PubMed] [Google Scholar]
- 70.Alam J, Cai J, Smith A. Isolation and characterization of the mouse heme oxygenase-1 gene. Distal 5' sequences are required for induction by heme or heavy metals. J. Biol. Chem. 1994;269:1001–1009. [PubMed] [Google Scholar]
- 71.Alam J, Camhi S, Choi AM. Identification of a second region upstream of the mouse heme oxygenase-1 gene that functions as a basal level and inducer-dependent transcription enhancer. J. Biol. Chem. 1995;270:11977–11984. doi: 10.1074/jbc.270.20.11977. [DOI] [PubMed] [Google Scholar]
- 72.Inamdar NM, Ahn YI, Alam J. The heme-responsive element of the mouse heme oxygenase-1 gene is an extended AP-1 binding site that resembles the recognition sequences for MAF and NF-E2 transcription factors. Biochem. Biophys. Res. Commun. 1996;221:570–576. doi: 10.1006/bbrc.1996.0637. [DOI] [PubMed] [Google Scholar]
- 73.Igarashi K, Sun J. The heme-Bach1 pathway in the regulation of oxidative stress response and erythroid differentiation. Antioxid. Redox Signal. 2006;8:107–118. doi: 10.1089/ars.2006.8.107. [DOI] [PubMed] [Google Scholar]
- 74.Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, Taketo MM, Yamamoto M, Igarashi K. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 76.Oyake T, Itoh K, Motohashi H, Hayashi N, Hoshino H, Nishizawa M, Yamamoto M, Igarashi K. Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Mol. Cell Bio. 1996;16:6083–6095. doi: 10.1128/mcb.16.11.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 78.Ishii T, Itoh K, Ruiz E, Leake DS, Unoki H, Yamamoto M, Mann GE. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ. Res. 2004;94:609–616. doi: 10.1161/01.RES.0000119171.44657.45. [DOI] [PubMed] [Google Scholar]
- 79.Sun J, Brand M, Zenke Y, Tashiro S, Groudine M, Igarashi K. Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc. Natl. Acad. Sci. U S A. 2004;101:1461–1466. doi: 10.1073/pnas.0308083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suzuki H, Tashiro S, Sun J, Doi H, Satomi S, Igarashi K. Cadmium induces nuclear export of Bach1, a transcriptional repressor of heme oxygenase-1 gene. J. Biol. Chem. 2003;278:49246–49253. doi: 10.1074/jbc.M306764200. [DOI] [PubMed] [Google Scholar]
- 81.Stewart D, Killeen E, Naquin R, Alam S, Alam J. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J. Biol. Chem. 2003;278:2396–2402. doi: 10.1074/jbc.M209195200. [DOI] [PubMed] [Google Scholar]
- 82.Alam J, Killeen E, Gong P, Naquin R, Hu B, Stewart D, Ingelfinger JR, Nath KA. Heme activates the heme oxygenase-1 gene in renal epithelial cells by stabilizing Nrf2. Am. J. Physiol. Renal Physiol. 2003;284:F743–F752. doi: 10.1152/ajprenal.00376.2002. [DOI] [PubMed] [Google Scholar]
- 83.Itoh K, Wakabayashi N, Katoh Y, Ishii T, O'Connor T, Yamamoto M. Keapl regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells. 2003;8:379–391. doi: 10.1046/j.1365-2443.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 84.Kobayashi M, Itoh K, Suzuki T, Osanai H, Nishikawa K, Katoh Y, Takagi Y, Yamamoto M. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells. 2002;7:807–820. doi: 10.1046/j.1365-2443.2002.00561.x. [DOI] [PubMed] [Google Scholar]
- 85.Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal. 2006;8:76–87. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- 86.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kang MI, Kobayashi A, Wakabayashi N, Kim SG, Yamamoto M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc. Natl. Acad. Sci. USA. 2004;101:2046–2051. doi: 10.1073/pnas.0308347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zipper LM, Mulcahy RT. The Keap1 BTB/POZ dimerization function is required to sequester Nrf2 in cytoplasm. J. Biol. Chem. 2002;277:36544–36552. doi: 10.1074/jbc.M206530200. [DOI] [PubMed] [Google Scholar]
- 89.Jeong WS, Jun M, Kong AN. Nrf2: a potential molecular target for cancer chemoprevention by natural compounds. Antioxid. Redox Signal. 2006;8:99–106. doi: 10.1089/ars.2006.8.99. [DOI] [PubMed] [Google Scholar]
- 90.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. U S A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, Harada T, Engel JD, Yamamoto M. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 92.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shibahara S, Muller RM, Taguchi H. Transcriptional control of rat heme oxygenase by heat shock. J. Biol. Chem. 1987;262:12889–12892. [PubMed] [Google Scholar]
- 94.Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AM. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J. Biol. Chem. 1997;272:5375–5381. [PubMed] [Google Scholar]
- 95.Takeda K, Ishizawa S, Sato M, Yoshida T, Shibahara S. Identification of a cis-acting element that is responsible for cadmium-mediated induction of the human heme oxygenase gene. J. Biol. Chem. 1994;269:22858–22867. [PubMed] [Google Scholar]
- 96.Kronke G, Bochkov VN, Huber J, Gruber F, Bluml S, Furnkranz A, Kadl A, Binder BR, Leitinger N. Oxidized phospholipids induce expression of human heme oxygenase-1 involving activation of cAMP-responsive element-binding protein. J. Biol. Chem. 2003;278:51006–51014. doi: 10.1074/jbc.M304103200. [DOI] [PubMed] [Google Scholar]
- 97.Yang G, Nguyen X, Ou J, Rekulapelli P, Stevenson DK, Dennery PA. Unique effects of zinc protoporphyrin on HO-1 induction and apoptosis. Blood. 2001;97:1306–1313. doi: 10.1182/blood.v97.5.1306. [DOI] [PubMed] [Google Scholar]
- 98.Sikorski EM, Hock T, Hill-Kapturczak N, Agarwal A. The story so far: Molecular regulation of the heme oxygenase-1 gene in renal injury. Am. J. Physiol. Renal Physiol. 2004;286:F425–F441. doi: 10.1152/ajprenal.00297.2003. [DOI] [PubMed] [Google Scholar]
- 99.Shibahara S, Sato M, Muller RM, Yoshida T. Structural organization of the human heme oxygenase gene and the function of its promoter. Eur. J. Biochem. 1989;179:557–563. doi: 10.1111/j.1432-1033.1989.tb14583.x. [DOI] [PubMed] [Google Scholar]
- 100.Tyrrell RM, Applegate LA, Tromvoukis Y. The proximal promoter region of the human heme oxygenase gene contains elements involved in stimulation of transcriptional activity by a variety of agents including oxidants. Carcinogenesis. 1993;14:761–765. doi: 10.1093/carcin/14.4.761. [DOI] [PubMed] [Google Scholar]
- 101.Muraosa Y, Shibahara S. Identification of a cis-regulatory element and putative trans-acting factors responsible for 12-O-tetradecanoylphorbol-13-acetate (TPA)-mediated induction of heme oxygenase expression in myelomonocytic cell lines. Mol. Cell Biol. 1993;13:7881–7891. doi: 10.1128/mcb.13.12.7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nascimento AL, Luscher P, Tyrrell RM. Ultraviolet A (320–380 nm) radiation causes an alteration in the binding of a specific protein/protein complex to a short region of the promoter of the human heme oxygenase 1 gene. Nucleic Acids Res. 1993;21:1103–1109. doi: 10.1093/nar/21.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deramaudt BM, Remy P, Abraham NG. Upregulation of human heme oxygenase gene expression by Ets-family proteins. J. Cell Biochem. 1999;72:311–321. [PubMed] [Google Scholar]
- 104.Lavrovsky Y, Schwartzman ML, Levere RD, Kappas A, Abraham NG. Identification of binding sites for transcription factors NF-kappa B and AP-2 in the promoter region of the human heme oxygenase 1 gene. Proc. Natl. Acad. Sci. USA. 1994;91:5987–5991. doi: 10.1073/pnas.91.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deramaudt TB, da Silva JL, Remy P, Kappas A, Abraham NG. Negative regulation of human heme oxygenase in microvessel endothelial cells by dexamethasone. Proc. Soc. Exp. Biol. Med. 1999;222:185–193. doi: 10.1046/j.1525-1373.1999.d01-130.x. [DOI] [PubMed] [Google Scholar]
- 106.Takahashi S, Matsuura N, Kurokawa T, Takahashi Y, Miura T. Cooperation of the transcription factor hepatocyte nuclear factor-4 with Sp1 or Sp3 leads to transcriptional activation of the human haem oxygenase-1 gene promoter in a hepatoma cell line. Biochem. J. 2002;367:641–652. doi: 10.1042/BJ20020819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takahashi S, Takahashi Y, Ito K, Nagano T, Shibahara S, Miura T. Positive and negative regulation of the human heme oxygenase-1 gene expression in cultured cells. Biochim. Biophys. Acta. 1999;1447:231–235. doi: 10.1016/s0167-4781(99)00156-6. [DOI] [PubMed] [Google Scholar]
- 108.Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am. J. Hum. Genet. 2000;66:187–195. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fu WP, Sun C, Dai LM, Yang LF, Zhang YP. Relationship between COPD and polymorphisms of HOX-1 and mEPH in a Chinese population. Oncol. Rep. 2007;17:483–488. [PubMed] [Google Scholar]
- 110.Fu WP, Zhao ZH, Fang LZ, Sun C, Liu L, Zhang JQ, Zhang YP, Dai LM. Heme oxygenase-1 polymorphism associated with severity of chronic obstructive pulmonary disease. Chin. Med. J. (Engl) 2007;120:12–16. [PubMed] [Google Scholar]
- 111.Nakayama K, Kikuchi A, Yasuda H, Ebihara S, Sasaki T, Ebihara T, Yamaya M. Heme oxygenase-1 gene promoter polymorphism and decline in lung function in Japanese men. Thorax. 2006;61:921. doi: 10.1136/thx.2006.066423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guenegou A, Leynaert B, Benessiano J, Pin I, Demoly P, Neukirch F, Boczkowski J, Aubier M. Association of lung function decline with the heme oxygenase-1 gene promoter microsatellite polymorphism in a general population sample. Results from the European Community Respiratory Health Survey (ECRHS), France. J. Med. Genet. 2006;43:e43. doi: 10.1136/jmg.2005.039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Siedlinski M, van Diemen CC, Postma DS, Boezen HM. Heme oxygenase 1 variations and lung function decline in smokers: proof of replication. J. Med. Genet. 2008;45:400. doi: 10.1136/jmg.2008.058123. [DOI] [PubMed] [Google Scholar]
- 114.Alexeeff SE, Litonjua AA, Wright RO, Baccarelli A, Suh H, Sparrow D, Vokonas PS, Schwartz J. Ozone exposure, antioxidant genes, and lung function in an elderly cohort: VA normative aging study. Occup. Environ. Med. 2008;65:736–742. doi: 10.1136/oem.2007.035253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.He JQ, Ruan J, Connett JE, Anthonisen NR, Pare PD, Sandford AJ. Antioxidant gene polymorphisms and susceptibility to a rapid decline in lung function in smokers. Am. J. Respir. Crit. Care Med. 2002;166:323–328. doi: 10.1164/rccm.2111059. [DOI] [PubMed] [Google Scholar]
- 116.Hersh CP, Demeo DL, Lange C, Litonjua AA, Reilly JJ, Kwiatkowski D, Laird N, Sylvia JS, Sparrow D, Speizer FE, Weiss ST, Silverman EK. Attempted replication of reported chronic obstructive pulmonary disease candidate gene associations. Am. J. Respir. Cell Mol. Biol. 2005;33:71–78. doi: 10.1165/rcmb.2005-0073OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hirai H, Kubo H, Yamaya M, Nakayama K, Numasaki M, Kobayashi S, Suzuki S, Shibahara S, Sasaki H. Microsatellite polymorphism in heme oxygenase-1 gene promoter is associated with susceptibility to oxidant-induced apoptosis in lymphoblastoid cell lines. Blood. 2003;102:1619–1621. doi: 10.1182/blood-2002-12-3733. [DOI] [PubMed] [Google Scholar]
- 118.Petrache I, Otterbein LE, Alam J, Wiegand GW, Choi AM. Heme oxygenase-1 inhibits TNF-alpha-induced apoptosis in cultured fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;278:L312–L319. doi: 10.1152/ajplung.2000.278.2.L312. [DOI] [PubMed] [Google Scholar]
- 119.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase-1 suppresses endothelial cell apoptosis. J. Exp. Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brouard S, Berberat PO, Tobiasch E, Seldon MP, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappa B to protect endothelial cells from tumor necrosis factor-alpha-mediated apoptosis. J. Biol. Chem. 2002;277:17950–17961. doi: 10.1074/jbc.M108317200. [DOI] [PubMed] [Google Scholar]
- 121.Kim HP, Wang X, Suh GY, Zhang J, Ryter SW, Choi AMK. Heat shock factor-1 mediates the cytoprotective effect of carbon monoxide. J. Immunol. 2005;175:2622–2629. doi: 10.4049/jimmunol.175.4.2622. [DOI] [PubMed] [Google Scholar]
- 122.Liu XM, Chapman GB, Peyton KJ, Schafer AI, Durante W. Antiapoptotic action of carbon monoxide on cultured vascular smooth muscle cells. Exp. Biol. Med. (Maywood) 2003;228:572–575. doi: 10.1177/15353702-0322805-30. [DOI] [PubMed] [Google Scholar]
- 123.Liu XM, Chapman GB, Peyton KJ, Schafer AI, Durante W. Carbon monoxide inhibits apoptosis in vascular smooth muscle cells. Cardiovasc. Res. 2002;55:396–405. doi: 10.1016/s0008-6363(02)00410-8. [DOI] [PubMed] [Google Scholar]
- 124.Thom SR, Fisher D, Xu YA, Notarfrancesco K, Ischiropoulos H. Adaptive responses and apoptosis in endothelial cells exposed to carbon monoxide. Proc. Natl. Acad. Sci. U S A. 2000;97:1305–1310. doi: 10.1073/pnas.97.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu XM, Chapman GB, Wang H, Durante W. Adenovirus-mediated heme oxygenase-1 gene expression stimulates apoptosis in vascular smooth muscle cells. Circulation. 2002;105:9–84. doi: 10.1161/hc0102.101369. [DOI] [PubMed] [Google Scholar]
- 126.Fogg S, Agarwal A, Nick HS, Visner GA. Iron regulates hyperoxia-dependent human heme oxygenase 1 gene expression in pulmonary endothelial cells. Am. J. Respir. Cell Mol. Biol. 1999;20:797–804. doi: 10.1165/ajrcmb.20.4.3477. [DOI] [PubMed] [Google Scholar]
- 127.Lee PJ, Alam J, Sylvester SL, Inamdar N, Otterbein L, Choi AM. Regulation of heme oxygenase-1 expression in vivo and in vitro in hyperoxic lung injury. Am. J. Respir. Cell Mol. Biol. 1996;14:556–568. doi: 10.1165/ajrcmb.14.6.8652184. [DOI] [PubMed] [Google Scholar]
- 128.Lee PJ, Alam J, Wiegand GW, Choi AM. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc. Natl. Acad. Sci. U S A. 1996;93:10393–10398. doi: 10.1073/pnas.93.19.10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Otterbein LE, Otterbein SL, Ifedigbo E, Liu F, Morse DE, Fearns C, Ulevitch RJ, Knicklebein R, Flavell RA, Choi AM. MKK3 mitogen activated protein kinase pathway mediates carbon monoxide-induced protection against oxidant induced lung injury. Am. J. Pathol. 2003;163:2555–2563. doi: 10.1016/S0002-9440(10)63610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang X, Shan P, Jiang G, Zhang SS, Otterbein LE, Fu XY, Lee PJ. Endothelial STAT3 is essential for the protective effects of HO-1 in oxidant-induced lung injury. FASEB J. 2006;20:2156–2158. doi: 10.1096/fj.06-5668fje. [DOI] [PubMed] [Google Scholar]
- 131.Wang X, Wang Y, Kim HP, Nakahira K, Ryter SW, Choi AM. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J. Biol. Chem. 2007;282:1718–1726. doi: 10.1074/jbc.M607610200. [DOI] [PubMed] [Google Scholar]
- 132.Zhang X, Shan P, Alam J, Davis RJ, Flavell RA, Lee PJ. Carbon monoxide modulates Fas/Fas ligand, caspases, and Bcl-2 family proteins via the p38alpha mitogen-activated protein kinase pathway during ischemia-reperfusion lung injury. J. Biol. Chem. 2003;278:22061–22070. doi: 10.1074/jbc.M301858200. [DOI] [PubMed] [Google Scholar]
- 133.Zhang X, Shan P, Otterbein LE, Alam J, Flavell RA, Davis RJ, Choi AM, Lee PJ. Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 mitogen-activated protein kinase and involves caspase-3. J. Biol. Chem. 2003;278:1248–1258. doi: 10.1074/jbc.M208419200. [DOI] [PubMed] [Google Scholar]
- 134.Zhang X, Shan P, Alam J, Fu XY, Lee PJ. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J. Biol. Chem. 2005;280:8714–8721. doi: 10.1074/jbc.M408092200. [DOI] [PubMed] [Google Scholar]
- 135.Rahman I, MacNee W. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radic. Biol. Med. 1996;21:669–681. doi: 10.1016/0891-5849(96)00155-4. [DOI] [PubMed] [Google Scholar]
- 136.Rennard SI. Cigarette smoke in research. Am. J. Respir. Cell Mol.Biol. 2004;31:479–480. doi: 10.1165/rcmb.F284. [DOI] [PubMed] [Google Scholar]
- 137.Liu X, Conner H, Kobayashi T, Kim H, Wen F, Abe S, Fang Q, Wang X, Hashimoto M, Bitterman P, Rennard SI. Cigarette smoke extract induces DNA damage but not apoptosis in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2005;33:121–129. doi: 10.1165/rcmb.2003-0341OC. [DOI] [PubMed] [Google Scholar]
- 138.Hoshino Y, Mio T, Nagai S, Miki H, Ito I, Izumi T. Cytotoxic effects of cigarette smoke extract on an alveolar type II cell-derived cell line. Am. J. Physiol. 2001;281:L509–L516. doi: 10.1152/ajplung.2001.281.2.L509. [DOI] [PubMed] [Google Scholar]
- 139.Park JW, Kim HP, Lee S-J, Wang X, Wang Y, Ifedigbo E, Watkins SC, Ohba M, Ryter SW, Vyas YM, Choi AM. Protein kinase Cα and ζ differentially regulate death-inducing signaling complex formation in cigarette smoke extract-induced apoptosis. J. Immunol. 2008;180:4668–4678. doi: 10.4049/jimmunol.180.7.4668. [DOI] [PubMed] [Google Scholar]
- 140.Fukano Y, Yoshimura H, Yoshida T. Heme oxygenase-1 gene expression in human alveolar epithelial cells (A549) following exposure to whole cigarette smoke on a direct in vitro exposure system. Exp. Toxicol. Pathol. 2006;57:411–418. doi: 10.1016/j.etp.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 141.Knorr-Wittmann C, Hengstermann A, Gebel S, Alam J, Muller T. Characterization of Nrf2 activation and heme oxygenase-1 expression in NIH3T3 cells exposed to aqueous extracts of cigarette smoke. Free Radic. Biol. Med. 2005;39:1438–1448. doi: 10.1016/j.freeradbiomed.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 142.Palozza P, Serini S, Curro D, Calviello G, Igarashi K, Mancuso C. beta-Carotene and cigarette smoke condensate regulate heme oxygenase-1 and its repressor factor Bach1: relationship with cell growth. Antioxid. Redox Signal. 2006;8:1069–1080. doi: 10.1089/ars.2006.8.1069. [DOI] [PubMed] [Google Scholar]
- 143.Reiter TA, Pang B, Dedon P, Demple B. Resistance to nitric oxide-induced necrosis in heme oxygenase-1 overexpressing pulmonary epithelial cells associated with decreased lipid peroxidation. J. Biol. Chem. 2006;281:36603–36612. doi: 10.1074/jbc.M602634200. [DOI] [PubMed] [Google Scholar]
- 144.Kim HP, Wang X, Chen Z-H, Lee S-J, Huang M-H, Wang Y, Ryter SW, Choi AMK. Autophagic proteins regulate cigarette smoke induced apoptosis: protective role of heme oxygenase-1. Autophagy. 2008;4:887–895. doi: 10.4161/auto.6767. [DOI] [PubMed] [Google Scholar]
- 145.Jimbo A, Fujita E, Kouroko Y, Ohnishi J, Inohara N, Kuida K, Sakamaki K, Yonehara S, Momoi T. ER stress induces caspase-8 activation, stimulating cytochrome c release and caspase-9 activation. Exp. Cell Res. 2003;283:156–166. doi: 10.1016/s0014-4827(02)00033-2. [DOI] [PubMed] [Google Scholar]
- 146.Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, del Rio G, Bredesen DE, Ellerby HM. Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J. Biol. Chem. 2002;277:21836–21842. doi: 10.1074/jbc.M202726200. [DOI] [PubMed] [Google Scholar]
- 147.Liu XM, Peyton KJ, Ensenat D, Wang H, Schafer AI, Alam J, Durante W. Endoplasmic reticulum stress stimulates heme oxygenase-1 gene expression in vascular smooth muscle. Role in cell survival. J. Biol. Chem. 2005;280:872–877. doi: 10.1074/jbc.M410413200. [DOI] [PubMed] [Google Scholar]
- 148.Kim KM, Pae HO, Zheng M, Park R, Kim YM, Chung HT. Carbon monoxide induces heme oxygenase-1 via activation of protein kinase R-like endoplasmic reticulum kinase and inhibits endothelial cell apoptosis triggered by endoplasmic reticulum stress. Circ. Res. 2007;101:919–927. doi: 10.1161/CIRCRESAHA.107.154781. [DOI] [PubMed] [Google Scholar]
- 149.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 150.Foresti R, Green CJ, Motterlini R. Generation of bile pigments by haem oxygenase: a refined cellular strategy in response to stressful insults. Biochem. Soc. Symp. 2004;71:177–192. doi: 10.1042/bss0710177. [DOI] [PubMed] [Google Scholar]
- 151.Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc. Natl. Acad. Sci. USA. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tang LM, Wang YP, Wang K, Pu LY, Zhang F, Li XC, Kong LB, Sun BC, Li GQ, Wang XH. Exogenous biliverdin ameliorates ischemia-reperfusion injury in small-for-size rat liver grafts. Transplant Proc. 2007;39:1338–1344. doi: 10.1016/j.transproceed.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 153.Nakao A, Murase N, Ho C, Toyokawa H, Billiar TR, Kanno S. Biliverdin administration prevents the formation of intimal hyperplasia induced by vascular injury. Circulation. 2005;112:587–591. doi: 10.1161/CIRCULATIONAHA.104.509778. [DOI] [PubMed] [Google Scholar]
- 154.Ollinger R, Bilban M, Erat A, Froio A, McDaid J, Tyagi S, Csizmadia E, Graca-Souza AV, Liloia A, Soares MP, Otterbein LE, Usheva A, Yamashita K, Bach FH. Bilirubin: a natural inhibitor of vascular smooth muscle cell proliferation. Circulation. 2005;112:1030–1039. doi: 10.1161/CIRCULATIONAHA.104.528802. [DOI] [PubMed] [Google Scholar]
- 155.Maines MD. New insights into biliverdin reductase functions: linking heme metabolism to cell signaling. Physiology (Bethesda) 2005;20:382–389. doi: 10.1152/physiol.00029.2005. [DOI] [PubMed] [Google Scholar]
- 156.Miralem T, Hu Z, Torno MD, Lelli KM, Maines MD. siRNA-mediated gene silencing of human biliverdin reductase, but not that of heme oxygenase-1, attenuates arsenite-mediated induction of the oxygenase and increases apoptosis in 293A kidney cells. J. Biol. Chem. 2005;280:17084–17092. doi: 10.1074/jbc.M413121200. [DOI] [PubMed] [Google Scholar]
- 157.Pachori AS, Smith A, McDonald P, Zhang L, Dzau VJ, Melo LG. Heme-oxygenase-1-induced protection against hypoxia/reoxygenation is dependent on biliverdin reductase and its interaction with PI3K/Akt pathway. J. Mol. Cell Cardiol. 2007;43:580–592. doi: 10.1016/j.yjmcc.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Balla G, Vercellotti GM, Eaton JW, Jacob HS. Iron loading of endothelial cells augments oxidant damage. J. Lab. Clin. Med. 1990;116:546–554. [PubMed] [Google Scholar]
- 159.Greene BT, Thorburn J, Willingham MC, Thorburn A, Planalp RP, Brechbiel MW, Jennings-Gee J, Wilkinson J, Torti FM, Torti SV. Activation of caspase pathways during iron chelator-mediated apoptosis. J. Biol. Chem. 2002;277:25568–25575. doi: 10.1074/jbc.M110345200. [DOI] [PubMed] [Google Scholar]
- 160.Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 1999;13:1800–1809. doi: 10.1096/fasebj.13.13.1800. [DOI] [PubMed] [Google Scholar]
- 161.Suttner DM, Sridhar K, Lee CS, Tomura T, Hansen TN, Dennery PA. Protective effects of transient HO-1 overexpression on susceptibility to oxygen toxicity in lung cells. Am. J. Physiol. 1999;276:L443–L451. doi: 10.1152/ajplung.1999.276.3.L443. [DOI] [PubMed] [Google Scholar]
- 162.Kvam E, Hejmadi V, Ryter S, Pourzand C, Tyrrell RM. Heme oxygenase activity causes transient hypersensitivity to oxidative ultraviolet A radiation that depends on release of iron from heme. Free Radic. Biol. Med. 2000;28:1191–1196. doi: 10.1016/s0891-5849(00)00205-7. [DOI] [PubMed] [Google Scholar]
- 163.Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Baranano DE, Dore S, Poss KD, Snyder SH. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat. Cell Biol. 1999;1:152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- 164.Vile GF, Basu-Modak S, Waltner C, Tyrrell RM. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc. Natl. Acad. Sci. USA. 1994;91:2607–2610. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lin F, Girotti AW. Hemin-enhanced resistance of human leukemia cells to oxidative killing: antisense determination of ferritin involvement. Arch. Biochem. Biophys. 1998;352:51–58. doi: 10.1006/abbi.1998.0588. [DOI] [PubMed] [Google Scholar]
- 166.Berberat PO, Katori M, Kaczmarek E, Anselmo D, Lassman C, Ke B, Shen X, Busuttil RW, Yamashita K, Csizmadia E, Tyagi S, Otterbein LE, Brouard S, Tobiasch E, Bach FH, Kupiec-Weglinski JW, Soares MP. Heavy chain ferritin acts as an antiapoptotic gene that protects livers from ischemia reperfusion injury. FASEB J. 2003;17:1724–1726. doi: 10.1096/fj.03-0229fje. [DOI] [PubMed] [Google Scholar]
- 167.Baranano DE, Wolosker H, Bae BI, Barrow RK, Snyder SH, Ferris CD. A mammalian iron ATPase induced by iron. J. Biol. Chem. 2000;275:15166–15173. doi: 10.1074/jbc.275.20.15166. [DOI] [PubMed] [Google Scholar]
- 168.Choi BM, Pae HO, Jeong YR, Oh GS, Jun CD, Kim BR, Kim YM, Chung HT. Overexpression of heme oxygenase (HO)-1 renders Jurkat T cells resistant to fas-mediated apoptosis: involvement of iron released by HO-1. Free Radic. Biol. Med. 2004;36:858–871. doi: 10.1016/j.freeradbiomed.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 169.Mishra S, Fujita T, Lama VN, Nam D, Liao H, Okada M, Minamoto K, Yoshikawa Y, Harada H, Pinsky DJ. Carbon monoxide rescues ischemic lungs by interrupting MAPK-driven expression of early growth response 1 gene and its downstream target genes. Proc. Natl. Acad. Sci. USA. 2006;13:5191–5196. doi: 10.1073/pnas.0600241103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang X, Shan P, Jiang D, Noble PW, Abraham NG, Kappas A, Lee PJ. Small interfering RNA targeting heme oxygenase-1 enhances ischemia-reperfusion-induced lung apoptosis. J. Biol. Chem. 2004;279:10677–10684. doi: 10.1074/jbc.M312941200. [DOI] [PubMed] [Google Scholar]
- 171.Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am. J. Physiol. 1986;250:G749–G753. doi: 10.1152/ajpgi.1986.250.6.G749. [DOI] [PubMed] [Google Scholar]
- 172.Katori M, Buelow R, Ke B, Ma J, Coito AJ, Iyer S, Southard D, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 overexpression protects rat hearts from cold ischemia/reperfusion injury via an anti-apoptotic pathway. Transplantation. 2002;73:287–292. doi: 10.1097/00007890-200201270-00023. [DOI] [PubMed] [Google Scholar]
- 173.Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR, Kolls JK, Alam J, Ritter T, Volk HD, Farmer DG, Ghobrial RM, Busuttil RW, Kupiec-Weglinski JW. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J. Clin. Invest. 1999;104:1631–1639. doi: 10.1172/JCI7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Blydt-Hansen T, Katori M, Lassman C, Ke B, Coito AJ, Iyer S, Buelow R, Ettenger R, Busuttil RW, Kupiec-Weglinski JW. Gene transfer-induced local heme oxygenase-1 overexpression protects rat kidneys from ischemia/reperfusion injury. J. Am. Soc. Nephrol. 2002;14:745–754. doi: 10.1097/01.asn.0000050760.87113.25. [DOI] [PubMed] [Google Scholar]
- 175.Squiers E, Bruch D, Buelow R, Tice D. Pretreatment of small bowel isograft donors with cobalt-protoporphyrin decreases preservation injury. Transplant Proc. 1999;31:585–586. doi: 10.1016/s0041-1345(98)01566-8. [DOI] [PubMed] [Google Scholar]
- 176.Pileggi A, Molano R, Berney T, Cattan P, Vizzardelli C, Oliver R, Fraker C, Ricordi C, Pastori RL, Bach FH, Inverardi L. Heme oxygenase-1 induction in islet cells results in protection from apoptosis and improved in vivo function after transplantation. Diabetes. 2001;50:1983–1991. doi: 10.2337/diabetes.50.9.1983. [DOI] [PubMed] [Google Scholar]
- 177.Rucker M, Schafer T, Roesken F, Spitzer W, Bauer M, Menger M. Local heat-shock priming-induced improvement in microvascular perfusion in osteomyocutaneous flaps is mediated by heat-shock protein 32. Br. J. Surg. 2001;88:450–457. doi: 10.1046/j.1365-2168.2001.01682.x. [DOI] [PubMed] [Google Scholar]
- 178.Ozaki KS, Marques GM, Nogueira E, Feitoza RQ, Cenedeze MA, Franco MF, Mazzali M, Soares MP, Pacheco-Silva A, Câmara NO. Improved renal function after kidney transplantation is associated with heme oxygenase-1 polymorphism. Clin. Transplant. 2008;22:609–616. doi: 10.1111/j.1399-0012.2008.00832.x. [DOI] [PubMed] [Google Scholar]
- 179.Nath K, Balla G, Vercellotti G, Balla J, Jacob H, Levitt M. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J. Clin. Invest. 1992;90:267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Brune B, Ulrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of granulate cyclase. Mol. Pharmacol. 1987;32:497–504. [PubMed] [Google Scholar]
- 181.Sato K, Balla J, Otterbein L, Smith RN, Brouard S, Lin Y, Csizmadia E, Sevigny J, Robson SC, Vercellotti G, Choi AM, Bach FH, Soares MP. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J. Immunol. 2001;166:4185–4194. doi: 10.4049/jimmunol.166.6.4185. [DOI] [PubMed] [Google Scholar]
- 182.Otterbein LE, Zuckerbraun BS, Haga M, Liu F, Song R, Usheva A, Stachulak C, Bodyak N, Smith RN, Csizmadia E, Tyagi S, Akamatsu Y, Flavell RJ, Billiar TR, Tzeng E, Bach FH, Choi AM, Soares MP. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat. Med. 2003;9:183–190. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]
- 183.Nakao A, Kimizuka K, Stolz DB, Seda Neto J, Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS, Bauer AJ, Nalesnik MA, Otterbein LE, Geller DA, Murase N. Protective effect of carbon monoxide inhalation for cold-preserved small intestinal grafts. Surgery. 2003;134:285–292. doi: 10.1067/msy.2003.238. [DOI] [PubMed] [Google Scholar]
- 184.Faleo G, Neto JS, Kohmoto J, Tomiyama K, Shimizu H, Takahashi T, Wang Y, Sugimoto R, Choi AM, Stolz DB, Carrieri G, McCurry KR, Murase N, Nakao A. Carbon monoxide ameliorates renal cold ischemia-reperfusion injury with an upregulation of vascular endothelial growth factor by activation of hypoxia-inducible factor. Transplantation. 2008;85:1833–1840. doi: 10.1097/TP.0b013e31817c6f63. [DOI] [PubMed] [Google Scholar]
- 185.Kaizu T, Nakao A, Tsung A, Toyokawa H, Sahai R, Geller DA, Murase N. Carbon monoxide inhalation ameliorates cold ischemia/reperfusion injury after rat liver transplantation. Surgery. 2005;138:229–235. doi: 10.1016/j.surg.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 186.Song R, Kubo M, Morse D, Zhou Z, Zhang X, Dauber JH, Fabisiak J, Alber SM, Watkins SC, Zuckerbraun BS, Otterbein LE, Ning W, Oury TD, Lee PJ, McCurry KR, Choi AM. Carbon monoxide induces cytoprotection in rat orthotopic lung transplantation via anti-inflammatory and anti-apoptotic effects. Am. J. Pathol. 2003;163:231–242. doi: 10.1016/S0002-9440(10)63646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nakao A, Toyokawa H, Tsung A, Nalesnik MA, Stolz DB, Kohmoto J, Ikeda A, Tomiyama K, Harada T, Takahashi T, Yang R, Fink MP, Morita K, Choi AM, Murase N. Ex vivo application of carbon monoxide in University of Wisconsin solution to prevent intestinal cold ischemia/reperfusion injury. Am. J. Transplant. 2006;6:2243–2255. doi: 10.1111/j.1600-6143.2006.01465.x. [DOI] [PubMed] [Google Scholar]
- 188.Musameh MD, Green CJ, Mann BE, Fuller BJ, Motterlini R. Improved myocardial function after cold storage with preservation solution supplemented with a carbon monoxide-releasing molecule (CORM-3) J. Heart Lung Transplant. 2007;26:1192–1198. doi: 10.1016/j.healun.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 189.Gunther L, Berberat PO, Haga M, Brouard S, Smith RN, Soares MP, Bach FH, Tobiasch E. Carbon monoxide protects pancreatic β-cells from apoptosis and improves islet function/survival after transplantation. Diabetes. 2002;51:994–999. doi: 10.2337/diabetes.51.4.994. [DOI] [PubMed] [Google Scholar]
- 190.Nakao A, Kimizuka K, Stolz DB, Neto JS, Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS, Nalesnik MA, Otterbein LE, Murase N. Carbon monoxide inhalation protects rat intestinal grafts from ischemia/reperfusion injury. Am. J. Pathol. 2003;163:1587–1598. doi: 10.1016/S0002-9440(10)63515-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graca-Souza AV, Ollinger R, Czismadia E, May GA, Ifedigbo E, Otterbein LE, Bach FH, Soares MP. Heme oxygenase-1–derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. FASEB J. 2004;18:771–772. doi: 10.1096/fj.03-0921fje. [DOI] [PubMed] [Google Scholar]
- 192.Pannen BHJ, Köhler N, Hole B, Bauer M, Clemens MG, Geiger KK. Protective role of endogenous carbon monoxide in hepatic microcirculatory dysfunction after hemorrhagic shock in rats. J. Clin. Invest. 1998;102:1220–1228. doi: 10.1172/JCI3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Amersi F, Shen XD, Anselmo D, Melinek J, Iyer S, Southard DJ, Katori M, Volk HD, Busuttil RW, Buelow R, Kupiec-Weglinski JW. Ex vivo exposure to carbon monoxide prevents hepatic ischemia/reperfusion injury through p38 MAP kinase pathway. Hepatology. 2002;35:815–823. doi: 10.1053/jhep.2002.32467. [DOI] [PubMed] [Google Scholar]
- 194.Fondevila C, Shen XD, Tsuchiyashi S, Yamashita K, Csizmadia E, Lassman C, Busuttil RW, Kupiec-Weglinski JW, Bach FH. Biliverdin therapy protects rat livers from ischemia and reperfusion injury. Hepatology. 2004;40:1333–1341. doi: 10.1002/hep.20480. [DOI] [PubMed] [Google Scholar]
- 195.Adin CA, Croker BP, Agarwal A. Protective effects of exogenous bilirubin on ischemia–reperfusion injury in the isolated, perfused rat kidney. Am. J. Physiol. Renal Physiol. 2005;288:F778–F784. doi: 10.1152/ajprenal.00215.2004. [DOI] [PubMed] [Google Scholar]
- 196.Yamashita K, McDaid J, Ollinger R, Tsui TY, Berberat PO, Usheva A, Csizmadia E, Smith RN, Soares MP, Bach FH. Biliverdin, a natural product of heme catabolism, induces tolerance to cardiac allografts. FASEB J. 2004;18:765–767. doi: 10.1096/fj.03-0839fje. [DOI] [PubMed] [Google Scholar]
- 197.Nakao A, Neto JS, Kanno S, Stolz DB, Kimizuka K, Liu F, Bach FH, Billiar TR, Choi AM, Otterbein LE, Murase N. Protection against ischemia/reperfusion injury in cardiac and renal transplantation with carbon monoxide, biliverdin and both. Am. J. Transplant. 2005;5:282–291. doi: 10.1111/j.1600-6143.2004.00695.x. [DOI] [PubMed] [Google Scholar]
- 198.Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am. J. Physiol. 1999;276:L688–L694. doi: 10.1152/ajplung.1999.276.4.L688. [DOI] [PubMed] [Google Scholar]
- 199.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 200.Motterlini R, Mann BE, Foresti R. Therapeutic applications of carbon monoxide–releasing molecules. Expert. Opin. Investig. Drugs. 2005;14:1305–1318. doi: 10.1517/13543784.14.11.1305. [DOI] [PubMed] [Google Scholar]