Abstract
Increased oxidative stress and inflammation are highly prevalent in chronic kidney disease (CKD), yet few studies have investigated whether oral antioxidant therapy can alter markers of inflammation or oxidative stress in CKD. The purpose of this study was to investigate whether a combination of mixed tocopherols and alpha lipoic acid (ALA) would alter biomarkers of oxidative stress and inflammation in subjects with Stage 3–4 CKD.
Methods
This was a prospective, randomized, double-blind, placebo-controlled pilot trial. 62 subjects were enrolled, and were randomly assigned to receive the combination of mixed tocopherols 666 IU/day plus ALA 600mg/day or their matching placebos for a total of 8 weeks. Plasma F2-isoprostane and protein thiol concentration were measured as biomarkers of oxidative stress, and C-reactive protein (CRP) and interleukin-6 (IL-6) concentration as biomarkers of systemic inflammation.
Results
There were no significant differences in demographics, diabetic status, or estimated glomerular filtration rate (eGFR) between study treatment and placebo groups at baseline. 58 of 62 randomized subjects (93%) completed the study protocol. After two months of treatment, there were no significant changes in F2-isoprostanes, protein thiols, CRP and IL-6 concentrations with mixed tocopherols and ALA treatment compared to matching placebos, whether analyzed as intention to treat or as treated. Diabetic status and baseline body mass index did not influence the results.
Conclusions
Combination oral mixed tocopherols and ALA treatment for 2 months does not influence biomarkers of oxidative stress and inflammation in Stage 3–4 CKD patients.
Introduction
Cardiovascular disease is the leading cause of morbidity and mortality in the United States, and the presence of chronic kidney disease (CKD) is now recognized to be associated with cardiovascular disease risk 1. Moderate to severe CKD has been shown to be independently associated with a graded increase in risk for hospitalizations, cardiovascular events, and risk of death, which are not fully explained by traditional Framingham risk factors 2. Consequently, pilot studies of novel therapies focusing on “non-traditional” mediators of cardiovascular disease in the CKD population are warranted.
Biomarkers of oxidative stress and inflammation are known to be markedly elevated in moderate -severe stages of CKD, suggesting that these may be target pathways for risk reduction in CKD. 3. Despite this knowledge, specific therapies attempting to mitigate the oxidative and inflammatory milieu in CKD have not been thoroughly investigated. Thus, it is not currently known whether antioxidant therapy can reduce the oxidative stress burden and potentially modulate the inflammatory response in CKD patients.
For this study, we hypothesized that a combination of oral antioxidants in subjects with moderate to severe CKD (Stage 3–4) could decrease biomarkers of oxidative stress and inflammation. In order to test this hypothesis, we performed a prospective, randomized, double-blind, placebo-controlled pilot trial. Sixty-two subjects with Stage 3 and 4 CKD were randomly assigned to receive either a combination of mixed tocopherols and alpha lipoic acid or their matching placebos for a total of 2 months. We examined the effects of this intervention on the plasma concentraion of F2-isoprostanes, protein thiols, C-reactive protein (CRP), and interleukin-6 (IL-6).
Methods
Study Design
This was a double blind, placebo-controlled, randomized pilot investigation examining 62 subjects with moderate to severe CKD. After informed consent was obtained, baseline enrollment data and blood work were obtained during a one week control period. Subjects were then assigned to one of two study groups by a block randomization strategy in a 1:1 ratio. Subjects were stratified according to the presence or absence of diabetes mellitus (DM). The study compared combination anti-oxidant therapy with mixed tocopherols (alpha, gamma, beta, and delta) approximately 666 IU daily plus alpha lipoic acid 600mg daily with matching placebo. The choice of antioxidants was based on the presumption that there would be synergy between the more hydrophilic, water soluble thiol containing antioxidant (alpha lipoic acid) and the more lipid soluble tocopherols. Our previous data in dialysis patients further suggested that a combination of mixed tocopherols might have more anti-inflammatory properties than pure alpha tocopherol. 4 Mixed tocopherols were provided as one capsule of 666 IU (Yasoo Health, Inc, Johnson City, TN, USA) and alpha lipoic acid was provided as two capsules of 300mg each (Jarrow Industries Inc, Santa Fe Springs, CA, USA). Identical matching capsules were provided to the placebo group prepared by the Vanderbilt University Medical Center (VUMC) Investigational Pharmacy Services. Subjects took a total of 3 capsules per day for a total of 8 weeks.
Subjects
Subjects were recruited from the outpatient nephrology clinics at Maine Medical Center (MMC) in Portland, Maine and VUMC in Nashville, Tennessee. Recruitment began in December, 2005 and continued until December, 2006. Criteria for study participation included patients with CKD of any etiology followed in nephrology clinics, the presence of stage III-IV CKD (as defined by an eGFR 30–59 mL/min for stage 3, and eGFR 15–29 mL/min for stage 4) measured by the Modification of Diet in Renal Disease (MDRD) formula 5, age > 18 and < 75 years, and those who could provide informed consent for study participation. Exclusion criteria included: subjects with acute inflammatory illnesses; history of heart failure; hospitalization within 6 weeks prior to study initiation; severe co-morbid complications; previous or anticipated kidney transplantation; subjects on chronic anti-inflammatory therapy or vitamin supplementation; hypersensitivity to mixed tocopherols or alpha lipoic acid; on experimental drug protocols; pregnant women and other vulnerable populations. Subjects underwent study visits at baseline (prior to initiation of study medication), month 1, and month 2 (conclusion). Demographics, medical history, and blood for routine chemistries and nutritional biomarkers were collected at the baseline visit. Additional blood was collected for biomarkers of inflammation and oxidative stress at baseline, month 1 and 2 visits. Compliance and adverse event assessments were performed at months 1 and 2.
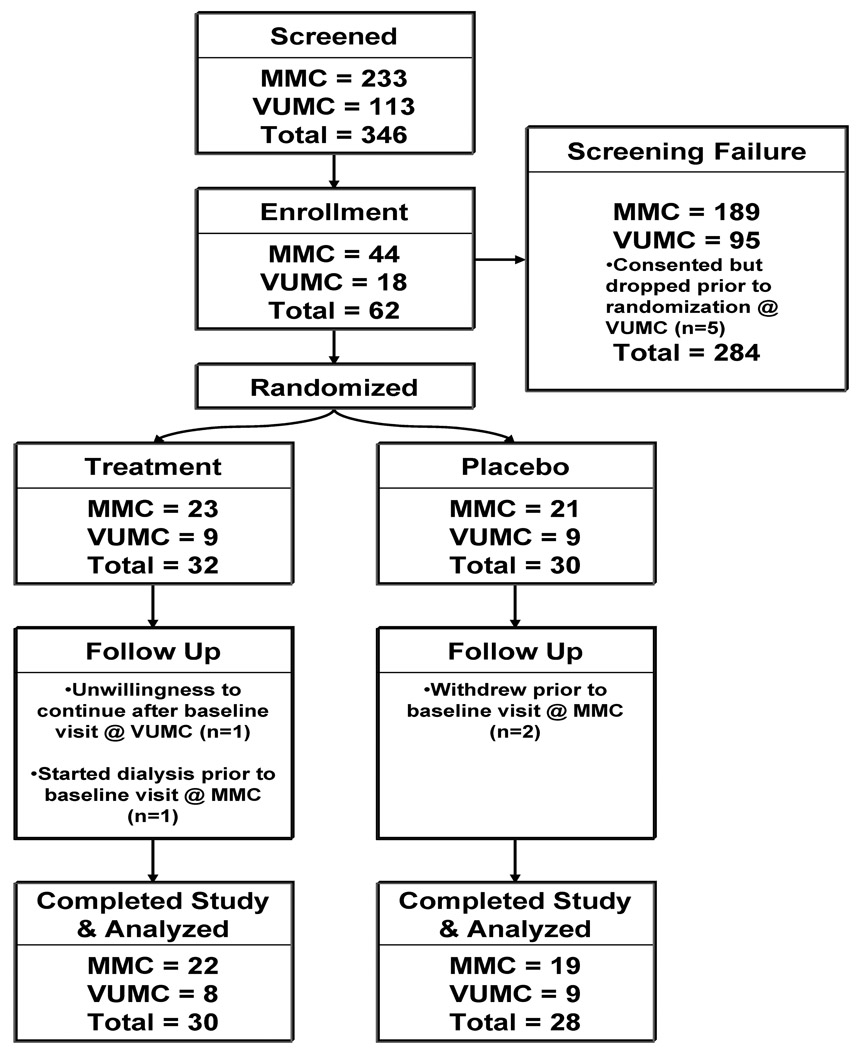
A total of 346 (MMC = 233 and VUMC = 113) subjects were screened for eligibility using the inclusion and exclusion criteria described above (Figure 1). There were 284 screen failures (MMC = 189 and VUMC = 95). The primary reason for exclusion was refusal to participate. There were no particular criteria that dominated the reason for exclusion. Overall, the patient population was representative of Stage 3 CKD since it was comparable to other publications in similar cohorts. 6–7 A total of 5 subjects at VUMC consented, but voluntarily decided not to participate in the study prior to randomization.
Figure 1.
Study enrollment flowchart
A total of 62 subjects (MMC = 44 and VUMC = 18) enrolled and were randomized into treatment (MMC = 23 and VUMC = 9) and placebo (MMC = 21 and VUMC = 9) groups, respectively. Of the total randomized, 58 subjects (93%) completed the study for data analysis (Treatment Arm: MMC = 22 and VUMC = 8; Placebo Arm: MMC = 19 and VUMC = 9). Four subjects failed to complete the study protocol; one subject started dialysis; while three subjects withdrew. Three subjects reported an adverse event which resolved with no intervention (n = 1 had nausea; n =2 had diarrhea). One of these subjects also reported hypotension caused by diarrhea, which resolved without intervention or hospitalization. The study was approved by each center’s respective Institutional Review Board, and all patients provided written informed consent prior to study enrollment.
Outcomes
The primary outcome assessed was a change in plasma F2-isosprostane concentration, a lipid peroxidation endproduct as a biomarker of oxidative stress. The secondary outcomes were changes in plasma protein thiols (a biomarker indicating endogenous anti-oxidant capacity) and plasma IL-6 and serum CRP as biomarkers of inflammation.
We hypothesized that the intervention would lower F2-isoprostane levels by 40% with no change in the placebo arm. While we did not have any preliminary data to base our sample size, we speculated that this would be a clinically significant decrease. It was also anticipated that based on a standard deviation of 48.8 pg/ml, 30 subjects in each arm would have given us a detectable range of 35.91 pg/ml between intervention and placebo arms with probability (power) 0.8 and a two sided type I error rate of 0.0.” 8 This change was considered to be clinically significant. In our previously published data, the mean plasma F2-isoprostane level was 96.2 pg/ml with a standard deviation of 48.8 pg/ml.9 If the true difference in the mean response of matched pairs were to be 38, we needed to study 19 pairs of subjects to be able to reject the null hypothesis that this response difference is zero with probability (power) 0.9. The Type I error probability associated with this test of the null hypothesis is 0.05. Under these circumstances, a sample size of 30 in the experimental group (assuming a drop-out rate of up to 50%) would have at least 90% power.
Analytical Procedures
All blood sampling were performed at the General Clinical Research Center (VUMC) or Research Core Laboratory (Maine Medical Center Research Institute) of the participating institutions. Blood sample measurements included routine chemistries, nutritional markers (serum albumin, lipid panel, glucose, HgbA1c), serum CRP, plasma IL-6, F2-isoprostanes, and protein thiols. Venous blood was drawn into Vacutainer® (Becton-Dickinson, Franklin Lakes, NJ, USA) tubes containing ethyldiaminetetraacetic acid (EDTA) supplemented with 1000U/mL catalase and serum separator tubes containing clot activator for plasma and serum separation, respectively. Samples for plasma collection were transported on ice and immediately centrifuged at 20°C at 1700g for 15 minutes, while the samples for serum collection were allowed to clot at room temperature prior to centrifugation. Supernatants were stored in aliquots at −70° C until further use.
Plasma F2-isoprostane Concentration
Plasma F2-isoprostane concentration were measured by gas chromatography/negative-ion chemical ionization mass spectrometry as described by Morrow et al 10. The precision of the assay is ± 6%, with an accuracy of 96%. Data are expressed in nanograms per milliliter. The previously published normal value for F2-isoprostane is 0.050 ng/mL 9.
Plasma Protein Thiol Concentration
Plasma protein reduced thiol group content was assayed according to the method of Ellman11 as modified by Hu 12 as we have previously described 13. Briefly, 1 mL of buffer containing 0.1 mol/L Tris, 10 mmol/L EDTA, pH 8.2, and 50 µl plasma were added to cuvettes, followed by 50 µl 10 mmol/L 5’5’dithio-bis (2-nitrobenzoic acid) (DTNB) in methanol. Blanks were run for each sample, prepared as described previously, with the exception that there was no DTNB in the methanol. Following incubation for 15 minutes at room temperature, sample absorbance was read at 412 nm on a Lambda 2 spectrophotometer (Perkins Elmer, Norwalk, CT, USA). Sample and reagent blanks were also subtracted. The concentration of sulfhydryl group was determined using the 5-thio-2-nitrobenzoic acid (TNB) molar extinction coefficient of 14,100 M−1 cm−1. The coefficient of variation for this assay was 2.67%. Data are reported as micromoles per liter. The previously published normal value for protein reduced thiol content is 328 umol/L 14.
Serum C-Reactive Protein Concentration
Plasma CRP levels were measured by ELISA using high sensitivity kits from Diagnostic Systems Laboratories (Webster, Texas) and expressed in milligrams per liter. Normal levels for hsCRP is < 3 mg/L
Plasma Interleukin-6 Concentration
Plasma IL-6 cytokine concentrations were determined by ELISA with kits from BioSource International (Camarillo, CA, USA). Data are expressed in picograms per milliliter. Normal value for IL-6 is < 5 pg/mL 15.
Statistical Analyses
Patient baseline characteristics were compared by using the chi-square test for categorical variables, and by using the Mann-Whitney U test for continuous variables. Data are presented as proportions for categorical variables and mean ± standard deviation (SD) for continuous variables. Plasma concentration of F2-isosprostanes, protein thiols, IL-6 and CRP, between combination anti-oxidant therapy and placebo groups were compared at baseline, 1 and 2 months separately by using Mann-Whitney U tests. The effect of the combination anti-oxidant therapy was assessed by comparing the change in outcome variables at 30 days or 60 days from baseline between the treatment arms using general linear models (GLM) with bootstrap covariance accounting for correlation among repeated measures within a patient. The difference in change from baseline was assessed in the bootstrap-GLM by including an interaction term between treatment and time. Baseline value of outcome variable was adjusted as a model covariate as well as other baseline covariates including age, body mass index (BMI), gender, race, presence or absence of DM and eGFR. The effect of treatment at each of the two time points was assessed only when the global test was rejected. Residuals were assessed graphically for normality and transformation on the dependent variable was done to correct non-normal residuals if needed. All analyses were performed with R-software version 2.7.2 (www.r-project.org) and a 2-sided P-value <0.05 was required to reject the null hypothesis.
Results
Table 1 shows the baseline characteristics of both treatment groups and the combined cohort. There was a total of 58 CKD subjects completing the protocol who were randomized to either the study (N= 30) or placebo (N= 28) groups. The overall mean eGFR of the combined cohort was 38.2 ± 11 mL/min. There were site specific differences except race such that all subjects at MMC were Caucasians whereas 4 out of 17 subjects were African American at VUMC. The mean eGFR was not significantly different between study and placebo groups. The mean age of the placebo group was slightly higher compared to the treatment group (64.5 ± 8.8 years vs. 58.6 ± 12.0 years, p = 0.047). Gender, race, presence of diabetes mellitus, anthropometric measurements, and smoking status were not significantly different between the groups. The primary and secondary outcome variables at baseline are summarized in Table 2. Plasma concentrations of F2-isoprostanes, IL-6, CRP and protein thiols were similar to previous reports in subjects with CKD 14. There were no differences in measured biomarkers between the study and the placebo groups.
Table 1.
Baseline demographic characteristics of the study patients. Data presented as Mean ± SD. No statistically significant differences were observed between groups except placebo group being slightly older. BMI: Body Mass Index, MI: History of myocardial infarction, CVA: history of Cerebrovascular event, PVD: history of peripheral vascular disease
| N | Placebo N = 28 |
Drug N = 30 |
Combined N = 58 |
P-value | ||
|---|---|---|---|---|---|---|
| Age (years) | 58 | (64.5 ± 8.8) | (58.6 ± 12.0) | (61.4 ± 10.9) | 0.0471 | |
| BMI | 58 | (32.2 ± 7.5) | (32.9 ± 8.7) | (32.6 ± 8.0) | 0.971 | |
| Gender | Male | 58 | 57% (16) | 53% (16) | 55% (32) | 0.772 |
| Female | 43% (12) | 47% (14) | 45% (26) | |||
| Race | White | 58 | 93% (26) | 93% (28) | 93% (54) | 0.942 |
| Black | 7% (2) | 7% (2) | 7% (4) | |||
| Diabetic status | Non-Diabetic | 58 | 43% (12) | 43% (13) | 43% (25) | 0.972 |
| Diabetic | 57% (16) | 57% (17) | 57% (33) | |||
| Weight (kg) | 58 | (93 ± 22) | (98 ± 29) | (95 ± 26) | 0.781 | |
| Blood | Systolic | 58 | 135 ± 17 | 140 ± 14 | 138 ± 16 | 0.331 |
| Pressure | Diastolic | 58 | 71 ± 11 | 76 ± 10 | 73 ± 11 | 0.131 |
| MI | 58 | 29% | 10% | 19% | 0.12 | |
| CVA | 58 | 11% | 10% | 10% | 0.92 | |
| PVD | 58 | 11% | 10% | 10% | 0.92 |
Tests used:
Wilcoxon test;
Pearson test
Table 2.
Study variables at baseline and visits 1 and 2 per study interventions. Data presented as Median and ranges. No statistically significant differences were observed between groups.
| Baseline | Month 1 | Month 2 | ||||
|---|---|---|---|---|---|---|
| Placebo | Intervention | Placebo | Intervention | Placebo | Intervention | |
| F2.iso (ng/mL) | 0.067 (0.023, 0.197) | 0.060 (0.024, 0.126) | 0.052 (0.014, 0.183) | 0.056 (0.020, 0.094) | 0.061 (0.024, 0.205) | 0.067 (0.030, 0.168) |
| CRP (mg/L) | 7.7 (1.3, 87.2) | 7.4 (0.1, 119.0) | 10.7 (0.7, 48.8) | 7.9 (0.3, 127.0) | 9.4 (0.8, 75.5) | 7.5 (0.1, 53.9) |
| IL-6 (pg/mL) | 5.8 (2.0, 27.9) | 4.7 (2.0, 31.8) | 6.8 (2.0, 63.0) | 6.0 (2.0, 59.0) | 5.5 (2.0, 47.0) | 5.6 (2.0, 24.0) |
| Thiols (umol/L) | 315 (254, 459) | 299 (229, 441) | 333 (245, 376) | 300 (166, 427) | 322 (225, 360) | 300 (247, 427) |
| BMI ( | 32 (20, 46) | 32 (21, 60) | 32.5 (20, 46) | 32 (21, 60) | 31.5 (20, 46) | 32 (21, 46) |
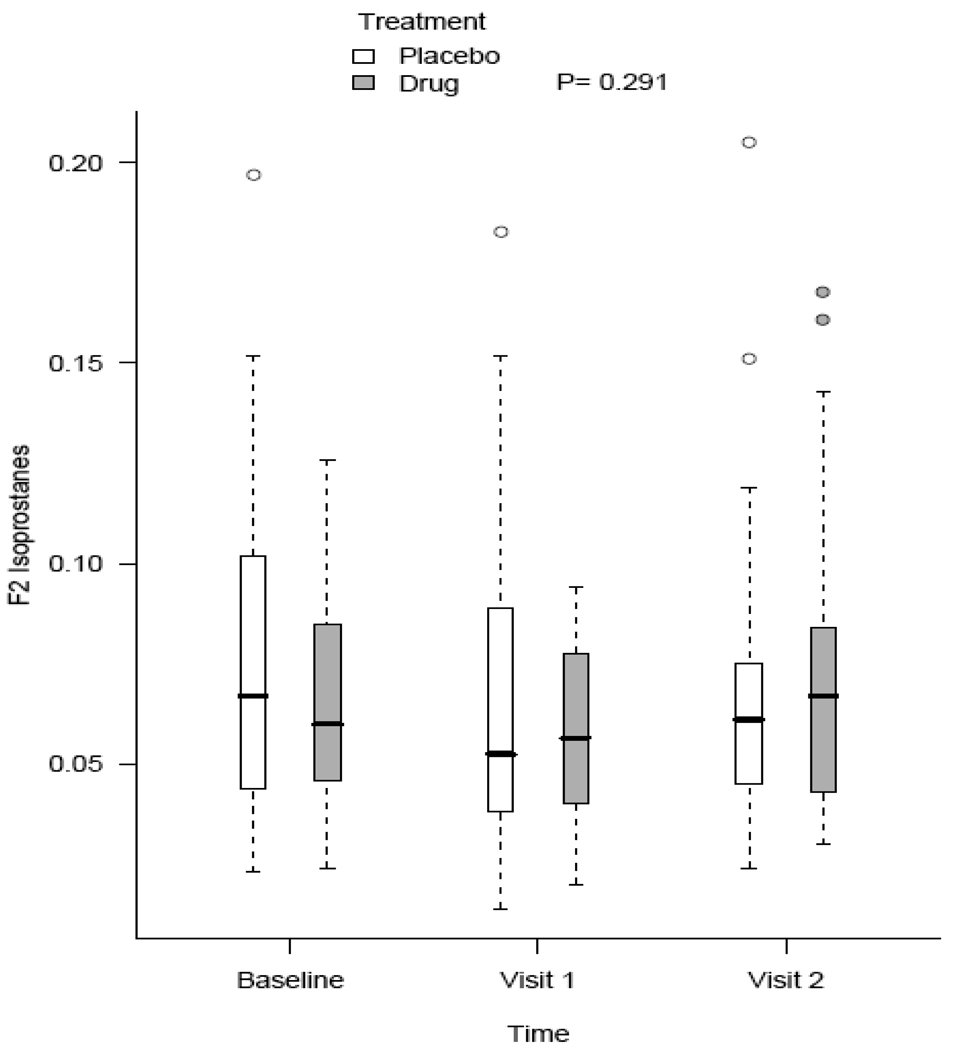
Mixed effect models were used to compare the anti-oxidant effect of mixed tocopherols and alpha lipoic acid compared to matching placebo for changes in measured biomarkers at months 1 and 2. Regarding the primary outcome, we adjusted for baseline F2-isoprostanes in addition to age, race, gender, baseline BMI, and estimated GFR. There were no statistically different changes in F2-isoprostanes over time between study and placebo groups (Figure 2). Additionally, there were no changes in protein thiols, IL-6, and CRP using similar mixed effect models. Adjusting for diabetic status did not have any significant effect on measured outcomes.
Figure 2.
Changes in F2-isosprostane concentrations (ng/mL) in response to placebo and study intervention. No statistically significant differences were observed between the groups.
Discussion
In this study, we evaluated whether a combination of mixed tocopherols and alpha-lipoic acid could alter biomarkers of oxidative stress and inflammation in subjects with stage 3 and 4 CKD. We found that this combination did not significantly change the primary (F2-isosprostanes) or secondary outcomes of oxidative stress biomarkers when compared to matching placebo after therapy in subjects with moderate to severe CKD. Similarly, no effect was observed in markers of inflammation (IL-6 and CRP). Furthermore, no changes in measured biomarkers were seen between groups when adjusting for BMI or diabetic status, variables known to influence oxidative stress and inflammation.7
Although a logical rationale exists for the administration of anti-oxidant therapy to mitigate the oxidative stress burden and inflammatory process in human disease, numerous randomized clinical trials with these agents have failed to improve cardiovascular and survival outcomes. Notably, the Heart Protection Study, the Group for the Study of Streptokinase in Myocardial Infarction (GISSI), the Heart Outcomes Prevention Evaluation (HOPE) Study, and the Study to Evaluate Carotid Ultrasound Changes in Patients Treated with Ramipril and Vitamin E (SECURE) all failed to show efficacy of oral anti-oxidants on clinical endpoints in the general population 16–19. Indirect evidence in kidney disease also suggested similar lack of significant effect. In sub-group analyses of the HOPE Study, 4.5 years of alpha tocopherol administration in middle-aged to elderly people with diabetes and cardiovascular disease did not have any effect on the incidence of diabetic nephropathy 20. In the same study, further analyses involving 993 people with mild to moderate kidney insufficiency (serum creatinine 1.4 to 2.3 mg/dL) revealed that Vitamin E supplementation did not have any effect on primary or secondary cardiovascular outcomes 21.
Despite the negative results from multiple trials in the general population, two pilot studies in stage 5 CKD patients on hemodialysis have suggested that antioxidants might have a beneficial effect. The Secondary Prevention with Antioxidants of Cardiovascular Disease in End Stage Renal Disease (SPACE) study was a randomized placebo-controlled trial of 196 subjects on chronic hemodialysis who were treated with 800 IU/day of alpha tocopherol or matching placebo over 1.5 years. Those randomized to the active arm showed a reduction in the composite cardiovascular disease endpoints of myocardial infarction, ischemic stroke, peripheral vascular disease, and unstable angina 22. However, no significant reductions in cardiovascular and total mortality were observed in the study. Tepel and colleagues conducted a prospective, randomized placebo-controlled trial in 134 ESRD subjects on chronic hemodialysis 23. Subjects were randomized to either acetylcysteine 600mg po bid or placebo for a median follow-up duration of 14 months. Similar to the SPACE Trial, acetylcysteine reduced the composite of cardiovascular event endpoints, but failed to reduce total mortality 23. Despite these encouraging pilot results, neither of these studies prospectively measured surrogate markers of oxidative stress or inflammation; thus, it is currently unknown whether the observed improvements in cardiovascular event rates with oral antioxidant therapy in the ESRD population observed in these two studies were accompanied by concomitant reduction in the inflammatory and oxidative burden in this population. Moreover, at this time, there is not a clear rationale for choice of antioxidants likely to have maximum biological effect in this population.
We reasoned that prior to embarking on larger event-driven clinical trials of antioxidant therapies, it is important to first assess the safety, tolerability and efficacy in lowering biomarkers of oxidative stress and/or inflammation in relevant populations.
The results obtained in our study demonstrated adequate safety and tolerability, but did not demonstrate efficacy in improving biomarkers of oxidative stress and inflammation. There are several potential explanations for this result. First, it is possible that either the dose or composition of antioxidants may be biologically ineffective in modulating the oxidative stress burden in this particular patient population. Additionally, given the relatively small sample size it is possible that we failed to observe a true effect on the primary outcome. However, it should be noted that, based on our results, in order to have sufficient power to observe a true effect (i.e. within 10% change in primary outcome) it would require over 300 subjects to be randomized to each arm. It is also possible that the study duration could have been too short to have an impact on measured biomarkers. Recent data in subjects with hypercholesterolemia suggested that statistically significant effects of Vitamin E supplementation on F2-isoprostane levels are only observed after 4 months 24. Due to these two major limitations, our data should be considered with caution. Finally, it is also possible that a negative effect on surrogate markers would not obviate a potentially beneficial effect on more clinically relevant cardiovascular outcomes including cardiovascular events.
In conclusion, combination oral mixed tocopherols and alpha lipoic acid treatment for 2 months does not decrease biomarkers of oxidative stress and inflammation in Stage 3 and 4 CKD patients. Future similar investigations should focus on either a longer duration of treatment, higher doses, or alternate composition of oral antioxidants or the use of other novel approaches that may decrease the oxidative and inflammatory burden in this population.
Acknowledgements
This study was supported in part by HL-070937 from National Heart, Lung and Blood Institute, K24 DK62849 from the National Institute of Diabetes, Digestive and Kidney Diseases and Clinical Translational Science Award 1UL-1RR024975 from the National Center for Research Resources. Luis F. Ramos was partially supported by the Vanderbilt Clinical and Translational Research Scholar Program 5KL2 RR024977.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov Identification Number NCT00308971
TAI and JH contributed equally in this work in designing the experiment, collecting and analyzing the data, and writing the manuscript. LFR contributed in collecting and analyzing the data, and writing the manuscript. AS and PW contributed to data analyses. JK contributed to data collection. EM and PL contributed to data analyses. All authors declared no conflict of interest with the work presented.
References
- 1.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney international. 2004;65:1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 4.Himmelfarb J, Kane J, McMonagle E, Zaltas E, Bobzin S, Boddupalli S, Phinney S, Miller G. Alpha and gamma tocopherol metabolism in healthy subjects and patients with end-stage renal disease. Kidney Int. 2003;64:978–991. doi: 10.1046/j.1523-1755.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 6.Ramos LF, Shintani A, Himmelfarb J, Ikizler TA. Determinants of plasma adiponectin levels in nondiabetic subjects with moderate to severe chronic kidney disease. J Ren Nutr. 2009;19:197–203. doi: 10.1053/j.jrn.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 7.Ramos LF, Shintani A, Ikizler TA, Himmelfarb J. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol. 2008;19:593–599. doi: 10.1681/ASN.2007030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupont WD, Plummer WD., Jr Power and sample size calculations for studies involving linear regression. Control Clin Trials. 1998;19:589–601. doi: 10.1016/s0197-2456(98)00037-3. [DOI] [PubMed] [Google Scholar]
- 9.Ikizler TA, Morrow JD, Roberts LJ, Evanson JA, Becker B, Hakim RM, Shyr Y, Himmelfarb J. Plasma F2-isoprostane levels are elevated in chronic hemodialysis patients. Clin Nephrol. 2002;58:190–197. doi: 10.5414/cnp58190. [DOI] [PubMed] [Google Scholar]
- 10.Morrow JD. The isoprostanes: their quantification as an index of oxidant stress status in vivo. Drug Metab Rev. 2000;32:377–385. doi: 10.1081/dmr-100102340. [DOI] [PubMed] [Google Scholar]
- 11.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 12.Hu ML, Louie S, Cross CE, Motchnik P, Halliwell B. Antioxidant protection against hypochlorous acid in human plasma. J Lab Clin Med. 1993;121:257–262. [PubMed] [Google Scholar]
- 13.Himmelfarb J, McMonagle E, McMenamin E. Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney international. 2000;58:2571–2578. doi: 10.1046/j.1523-1755.2000.00443.x. [DOI] [PubMed] [Google Scholar]
- 14.Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 15.Pupim LB, Himmelfarb J, McMonagle E, Shyr Y, Ikizler TA. Influence of Initiation of Maintenance Hemodialysis on Biomarkers of Inflammation and Oxidative Stress. Kidney Int. 2004;65:2371–2379. doi: 10.1111/j.1523-1755.2004.00656.x. [DOI] [PubMed] [Google Scholar]
- 16.Sharafuddin MJ, Stolpen AH, Dixon BS, Andresen KJ, Sun S, Lawton WJ. Value of MR angiography before percutaneous transluminal renal artery angioplasty and stent placement. J Vasc Interv Radiol. 2002;13:901–908. doi: 10.1016/s1051-0443(07)61773-4. [DOI] [PubMed] [Google Scholar]
- 17.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 18.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. The New England journal of medicine. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 19.Lonn E, Yusuf S, Dzavik V, Doris C, Yi Q, Smith S, Moore-Cox A, Bosch J, Riley W, Teo K. Effects of ramipril and vitamin E on atherosclerosis: the study to evaluate carotid ultrasound changes in patients treated with ramipril and vitamin E (SECURE) Circulation. 2001;103:919–925. doi: 10.1161/01.cir.103.7.919. [DOI] [PubMed] [Google Scholar]
- 20.Lonn E, Yusuf S, Hoogwerf B, Pogue J, Yi Q, Zinman B, Bosch J, Dagenais G, Mann JF, Gerstein HC. Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: results of the HOPE study and MICRO-HOPE substudy. Diabetes Care. 2002;25:1919–1927. doi: 10.2337/diacare.25.11.1919. [DOI] [PubMed] [Google Scholar]
- 21.Mann JF, Lonn EM, Yi Q, Gerstein HC, Hoogwerf BJ, Pogue J, Bosch J, Dagenais GR, Yusuf S. Effects of vitamin E on cardiovascular outcomes in people with mild-to-moderate renal insufficiency: results of the HOPE study. Kidney international. 2004;65:1375–1380. doi: 10.1111/j.1523-1755.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 22.Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, Knecht A, Weissgarten Y, Brunner D, Fainaru M, Green MS. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet. 2000;356:1213–1218. doi: 10.1016/s0140-6736(00)02783-5. [DOI] [PubMed] [Google Scholar]
- 23.Tepel M, van der Giet M, Statz M, Jankowski J, Zidek W. The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure: a randomized, controlled trial. Circulation. 2003;107:992–995. doi: 10.1161/01.cir.0000050628.11305.30. [DOI] [PubMed] [Google Scholar]
- 24.Roberts LJ, II, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD, Shyr Y, Morrow JD. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radical Biology and Medicine. 2007;43:1388–1393. doi: 10.1016/j.freeradbiomed.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]