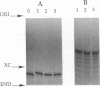
Abstract
The efficiency of oligodeoxynucleotide deprotection is greatly enhanced using a combination of: (a) ethanolamine, and especially a mixture of hydrazine, ethanolamine and methanol, in place of the usual aqueous ammonia; (b) tert-butylphenoxyacetyl amino protecting groups, and (c) oxalyl link between the first nucleotide and the polymeric support. The extent of base modification, particularly of C, is shown to be extremely low, and the quality of deprotected oligonucleotides is as high as in the case of ammonia deprotection. This method is also shown to be applicable to the preparation of phosphorothioate and methylphosphonate oligodeoxynucleotides and oligoribonucleotides.
Full text
PDF
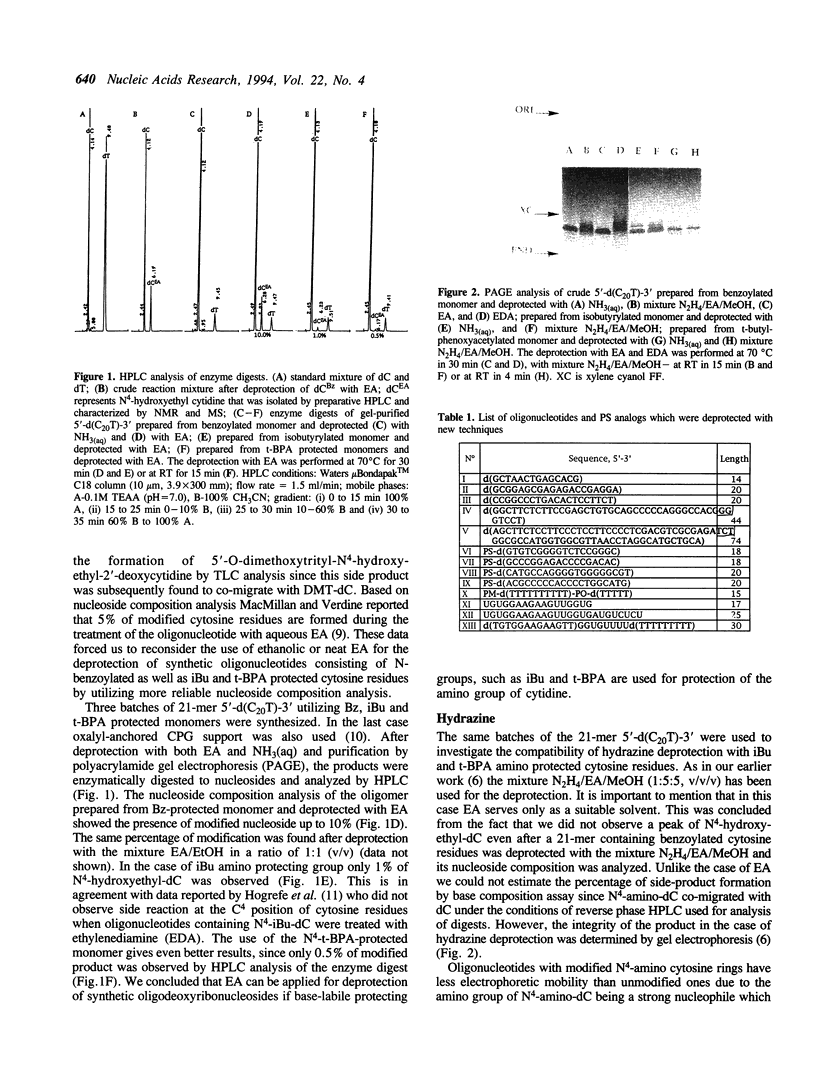
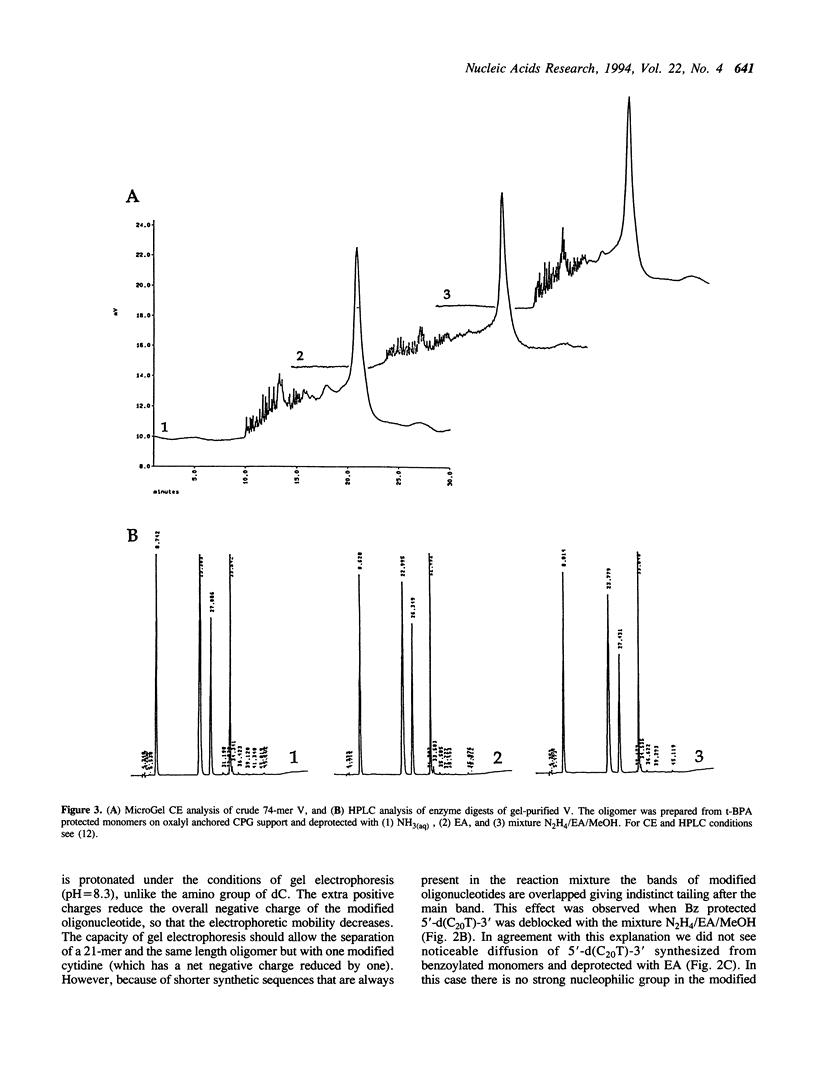



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alul R. H., Singman C. N., Zhang G. R., Letsinger R. L. Oxalyl-CPG: a labile support for synthesis of sensitive oligonucleotide derivatives. Nucleic Acids Res. 1991 Apr 11;19(7):1527–1532. doi: 10.1093/nar/19.7.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M. K., Ghosh K., Cohen J. S. Phosphorothioate-phosphodiester oligonucleotide co-polymers: assessment for antisense application. Anticancer Drug Des. 1993 Feb;8(1):15–32. [PubMed] [Google Scholar]
- Hogrefe R. I., Vaghefi M. M., Reynolds M. A., Young K. M., Arnold L. J., Jr Deprotection of methylphosphonate oligonucleotides using a novel one-pot procedure. Nucleic Acids Res. 1993 May 11;21(9):2031–2038. doi: 10.1093/nar/21.9.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Reddy M. P., Murakami A., Blake K. R., Lin S. B., Agris C. H. Solid-phase syntheses of oligodeoxyribonucleoside methylphosphonates. Biochemistry. 1986 Sep 9;25(18):5092–5097. doi: 10.1021/bi00366a017. [DOI] [PubMed] [Google Scholar]
- Ogilvie K. K., Usman N., Nicoghosian K., Cedergren R. J. Total chemical synthesis of a 77-nucleotide-long RNA sequence having methionine-acceptance activity. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5764–5768. doi: 10.1073/pnas.85.16.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polushin N. N., Pashkova I. N., Chakhmakhcheva O. G., Efimov V. A. Primenenie gidrazina dlia bystrogo deblokirovaniia sintetitcheskikh oligonukleotidov. Bioorg Khim. 1993 Mar;19(3):318–326. [PubMed] [Google Scholar]
- Polushin N. N., Pashkova I. N., Efimov V. A. Bystryi metod polnogo deblokirovaniia sinteticheskikh oligonukleotidov. Bioorg Khim. 1991 Aug;17(8):1145–1148. [PubMed] [Google Scholar]
- Polushin N. N., Pashkova I. N., Efimov V. A. Rapid deprotection procedures for synthetic oligonucleotides. Nucleic Acids Symp Ser. 1991;(24):49–50. [PubMed] [Google Scholar]
- Scaringe S. A., Francklyn C., Usman N. Chemical synthesis of biologically active oligoribonucleotides using beta-cyanoethyl protected ribonucleoside phosphoramidites. Nucleic Acids Res. 1990 Sep 25;18(18):5433–5441. doi: 10.1093/nar/18.18.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulhof J. C., Molko D., Teoule R. The final deprotection step in oligonucleotide synthesis is reduced to a mild and rapid ammonia treatment by using labile base-protecting groups. Nucleic Acids Res. 1987 Jan 26;15(2):397–416. doi: 10.1093/nar/15.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N. D., Davis P., Usman N., Pérez J., Hodge R., Kremsky J., Casale R. Labile exocyclic amine protection of nucleosides in DNA, RNA and oligonucleotide analog synthesis facilitating N-deacylation, minimizing depurination and chain degradation. Biochimie. 1993;75(1-2):13–23. doi: 10.1016/0300-9084(93)90019-o. [DOI] [PubMed] [Google Scholar]
- Uznanski B., Grajkowski A., Wilk A. The isopropoxyacetic group for convenient base protection during solid-support synthesis of oligodeoxyribonucleotides and their triester analogs. Nucleic Acids Res. 1989 Jun 26;17(12):4863–4871. doi: 10.1093/nar/17.12.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Ogilvie K. K., Pon R. T. Prevention of chain cleavage in the chemical synthesis of 2'-silylated oligoribonucleotides. Nucleic Acids Res. 1989 May 11;17(9):3501–3517. doi: 10.1093/nar/17.9.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]