Abstract
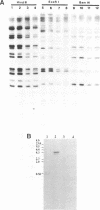
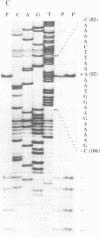
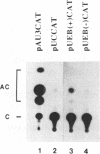
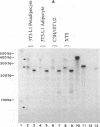
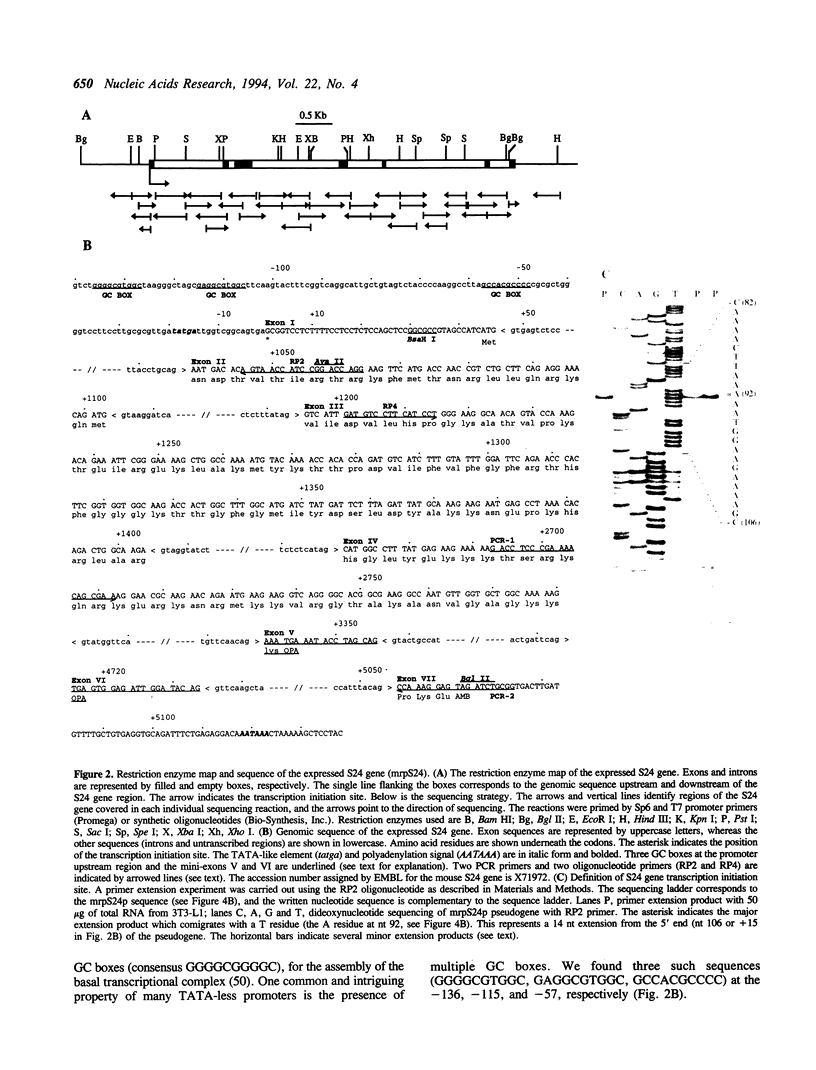
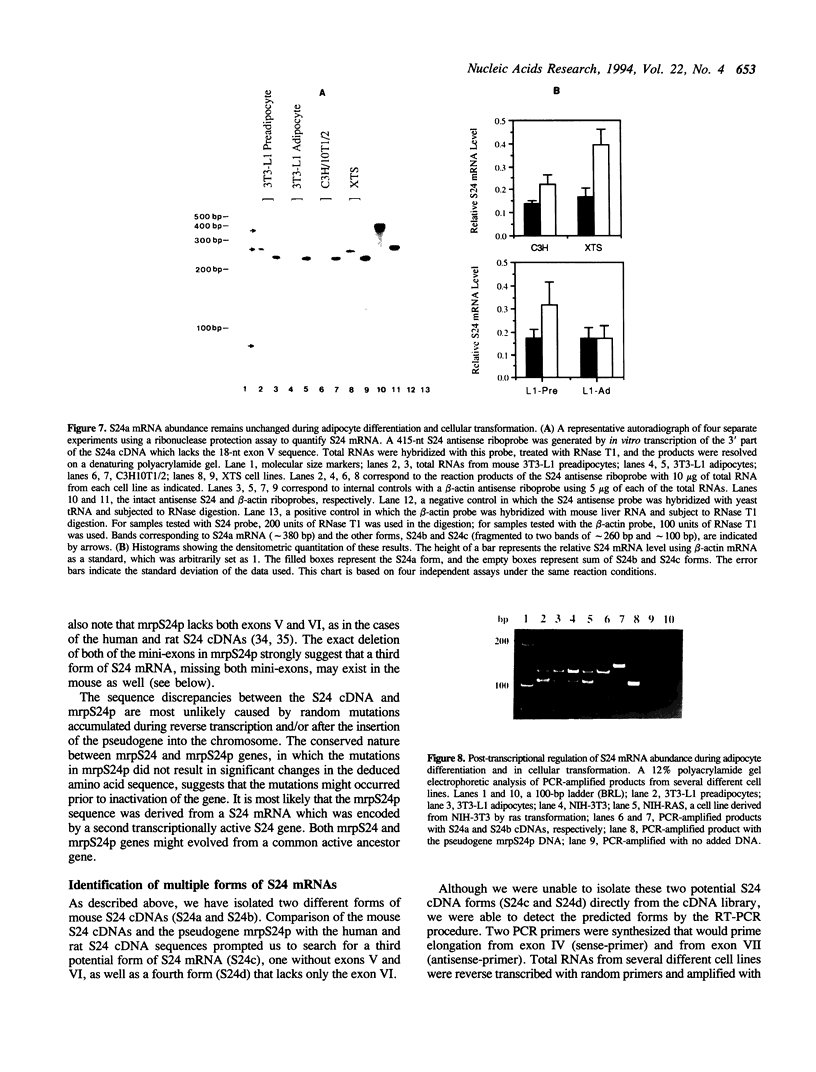
A family of 16 genes encoding the mouse ribosomal protein S24 was identified, and four members from this family were cloned. A single expressed intron-containing S24 gene (termed mrpS24) and one pseudogene (mrpS24p) were completely sequenced and characterized. The mrpS24 gene has seven exons and six introns spanning over 5.1 x 10(3) nucleotides (nt). The cap site of S24 was mapped to a G residue four nt upstream of a polypyrimidine tract and 15 nt downstream of a TATA-like (TATGA) element. The 5' region (-325 to +33) of the mrpS24 gene has a functional promoter that was able to express the fused chloramphenicol acetyltransferase (CAT) reporter gene. Two different forms of mouse S24 cDNA clones were previously isolated. Sequence analysis showed that one of these cDNA clones (termed S24a) lacks the entire exon V sequence (18 nt), and the deduced amino acid sequence is missing a C-terminal lysine residue encoded by the other cDNA (S24b). The pseudogene mrpS24p is flanked by an 11-bp direct repeat, and its sequence is almost identical to the S24 cDNA sequence, but it lacks two mini-exons, V and VI (20 nt), as in the cases of the human and rat S24 cDNAs. RT-PCR experiments demonstrated the existence of a third form (S24c) that similarly lacks both of the mini-exons, and suggested that different species of S24 mRNA might arise from alternative splicing of the mini-exons V and VI. Northern blot analysis showed that S24 expression is down- and up-regulated during adipocyte differentiation and in cellular transformation, respectively. RNase protection assays and RT-PCR experiments suggested that these cell-specific changes of S24 mRNA levels are mainly due to fluctuations in S24c mRNA level. Our results provide the first indication that a ribosomal protein gene is regulated by alternative usage of two mini-exons in a cell-specific manner.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaldi F., Beccari E., Bozzoni I., Luo Z. X., Pierandrei-Amaldi P. Nucleotide sequences of cloned cDNA fragments specific for six Xenopus laevis ribosomal proteins. Gene. 1982 Mar;17(3):311–316. doi: 10.1016/0378-1119(82)90147-0. [DOI] [PubMed] [Google Scholar]
- Atchison M. L., Meyuhas O., Perry R. P. Localization of transcriptional regulatory elements and nuclear factor binding sites in mouse ribosomal protein gene rpL32. Mol Cell Biol. 1989 May;9(5):2067–2074. doi: 10.1128/mcb.9.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard G. F., Staniunas R. J., Bao S., Mafune K., Steele G. D., Jr, Gollan J. L., Chen L. B. Increased expression of human ribosomal phosphoprotein P0 messenger RNA in hepatocellular carcinoma and colon carcinoma. Cancer Res. 1992 Jun 1;52(11):3067–3072. [PubMed] [Google Scholar]
- Beccari E., Mazzetti P., Mileo A., Bozzoni I., Pierandrei-Amaldi P., Amaldi F. Sequences coding for the ribosomal protein L14 in Xenopus laevis and Xenopus tropicalis; homologies in the 5' untranslated region are shared with other r-protein mRNAs. Nucleic Acids Res. 1986 Oct 10;14(19):7633–7646. doi: 10.1093/nar/14.19.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D. L. Does steric interference between splice sites block the splicing of a short c-src neuron-specific exon in non-neuronal cells? Genes Dev. 1991 Mar;5(3):389–402. doi: 10.1101/gad.5.3.389. [DOI] [PubMed] [Google Scholar]
- Bommer U. A., Lutsch G., Stahl J., Bielka H. Eukaryotic initiation factors eIF-2 and eIF-3: interactions, structure and localization in ribosomal initiation complexes. Biochimie. 1991 Jul-Aug;73(7-8):1007–1019. doi: 10.1016/0300-9084(91)90142-n. [DOI] [PubMed] [Google Scholar]
- Bommer U. A., Stahl J., Henske A., Lutsch G., Bielka H. Identification of proteins of the 40 S ribosomal subunit involved in interaction with initiation factor eIF-2 in the quaternary initiation complex by means of monospecific antibodies. FEBS Lett. 1988 Jun 6;233(1):114–118. doi: 10.1016/0014-5793(88)81366-8. [DOI] [PubMed] [Google Scholar]
- Brown S. J., Jewell A., Maki C. G., Roufa D. J. A cDNA encoding human ribosomal protein S24. Gene. 1990 Jul 16;91(2):293–296. doi: 10.1016/0378-1119(90)90103-x. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Paz V., Olvera J., Wool I. G. The primary structure of rat ribosomal protein S24. FEBS Lett. 1990 Mar 26;262(2):253–255. doi: 10.1016/0014-5793(90)80203-u. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Wool I. G. The structure of a gene containing introns and encoding rat ribosomal protein P2. Nucleic Acids Res. 1991 Sep 25;19(18):4895–4900. doi: 10.1093/nar/19.18.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. T., Roufa D. J. The transcriptionally active human ribosomal protein S17 gene. Gene. 1988 Oct 15;70(1):107–116. doi: 10.1016/0378-1119(88)90109-6. [DOI] [PubMed] [Google Scholar]
- Chester K. A., Robson L., Begent R. H., Talbot I. C., Pringle J. H., Primrose L., Macpherson A. J., Boxer G., Southall P., Malcolm A. D. Identification of a human ribosomal protein mRNA with increased expression in colorectal tumours. Biochim Biophys Acta. 1989 Dec 22;1009(3):297–300. doi: 10.1016/0167-4781(89)90119-x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chung S., Perry R. P. Importance of introns for expression of mouse ribosomal protein gene rpL32. Mol Cell Biol. 1989 May;9(5):2075–2082. doi: 10.1128/mcb.9.5.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo P., Yon J., Fried M. The organization and expression of the human L7a ribosomal protein gene. Biochim Biophys Acta. 1991 Dec 2;1129(1):93–95. doi: 10.1016/0167-4781(91)90218-b. [DOI] [PubMed] [Google Scholar]
- De Benedetti A., Rhoads R. E. Overexpression of eukaryotic protein synthesis initiation factor 4E in HeLa cells results in aberrant growth and morphology. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8212–8216. doi: 10.1073/pnas.87.21.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudov K. P., Perry R. P. The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell. 1984 Jun;37(2):457–468. doi: 10.1016/0092-8674(84)90376-3. [DOI] [PubMed] [Google Scholar]
- Elvin P., Kerr I. B., McArdle C. S., Birnie G. D. Isolation and preliminary characterisation of cDNA clones representing mRNAs associated with tumour progression and metastasis in colorectal cancer. Br J Cancer. 1988 Jan;57(1):36–42. doi: 10.1038/bjc.1988.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Manfredini R., Tagliafico E., Rossi E., Donelli A., Torelli G., Torelli U. Noncoordinated expression of S6, S11, and S14 ribosomal protein genes in leukemic blast cells. Cancer Res. 1990 Sep 15;50(18):5825–5828. [PubMed] [Google Scholar]
- Fisher E. M., Beer-Romero P., Brown L. G., Ridley A., McNeil J. A., Lawrence J. B., Willard H. F., Bieber F. R., Page D. C. Homologous ribosomal protein genes on the human X and Y chromosomes: escape from X inactivation and possible implications for Turner syndrome. Cell. 1990 Dec 21;63(6):1205–1218. doi: 10.1016/0092-8674(90)90416-c. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan N., Kelley D. E., Perry R. P. Delta, a transcription factor that binds to downstream elements in several polymerase II promoters, is a functionally versatile zinc finger protein. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9799–9803. doi: 10.1073/pnas.88.21.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan N., Perry R. P. A characterization of the elements comprising the promoter of the mouse ribosomal protein gene RPS16. Nucleic Acids Res. 1989 Jul 11;17(13):5323–5337. doi: 10.1093/nar/17.13.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan N., Perry R. P. Functional dissection of a mouse ribosomal protein promoter: significance of the polypyrimidine initiator and an element in the TATA-box region. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1526–1530. doi: 10.1073/pnas.87.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. L., Coggin D. L., King C. R. High-level expression of the ribosomal protein L19 in human breast tumors that overexpress erbB-2. Cancer Res. 1993 Mar 15;53(6):1403–1408. [PubMed] [Google Scholar]
- Iwasaki K., Nagata S., Mizumoto K., Kaziro Y. The purification of low molecular weight form of polypeptide elongation factor 1 from pig liver. J Biol Chem. 1974 Aug 10;249(15):5008–5010. [PubMed] [Google Scholar]
- Jagus R., Anderson W. F., Safer B. The regulation of initiation of mammalian protein synthesis. Prog Nucleic Acid Res Mol Biol. 1981;25:127–185. doi: 10.1016/s0079-6603(08)60484-5. [DOI] [PubMed] [Google Scholar]
- Kawai S., Nishizawa M. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 1984 Jun;4(6):1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller E. B., Noon W. A. Intron splicing: a conserved internal signal in introns of animal pre-mRNAs. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7417–7420. doi: 10.1073/pnas.81.23.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A., Meyuhas O. A multigene family of intron lacking and containing genes, encoding for mouse ribosomal protein L7. Nucleic Acids Res. 1984 May 11;12(9):3763–3776. doi: 10.1093/nar/12.9.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaris-Karatzas A., Montine K. S., Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5' cap. Nature. 1990 Jun 7;345(6275):544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- Lazaris-Karatzas A., Sonenberg N. The mRNA 5' cap-binding protein, eIF-4E, cooperates with v-myc or E1A in the transformation of primary rodent fibroblasts. Mol Cell Biol. 1992 Mar;12(3):1234–1238. doi: 10.1128/mcb.12.3.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafune K., Wong J. M., Staniunas R. J., Lu M. L., Ravikumar T. S., Chen L. B., Steele G. D., Jr Ubiquitin hybrid protein gene expression during human colon cancer progression. Arch Surg. 1991 Apr;126(4):462–466. doi: 10.1001/archsurg.1991.01410280064009. [DOI] [PubMed] [Google Scholar]
- Mager W. H. Control of ribosomal protein gene expression. Biochim Biophys Acta. 1988 Jan 25;949(1):1–15. doi: 10.1016/0167-4781(88)90048-6. [DOI] [PubMed] [Google Scholar]
- Mariottini P., Amaldi F. The 5' untranslated region of mRNA for ribosomal protein S19 is involved in its translational regulation during Xenopus development. Mol Cell Biol. 1990 Feb;10(2):816–822. doi: 10.1128/mcb.10.2.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyuhas O., Thompson E. A., Jr, Perry R. P. Glucocorticoids selectively inhibit translation of ribosomal protein mRNAs in P1798 lymphosarcoma cells. Mol Cell Biol. 1987 Aug;7(8):2691–2699. doi: 10.1128/mcb.7.8.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M., Pettersson I., Hinterberger M., Karmas A., Steitz J. A. The U1 small nuclear RNA-protein complex selectively binds a 5' splice site in vitro. Cell. 1983 Jun;33(2):509–518. doi: 10.1016/0092-8674(83)90432-4. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Yen T. S., Wang Y. F., Kam W. K., Rutter W. J. Cloning and characterization of a human ribosomal protein gene with enhanced expression in fetal and neoplastic cells. Nucleic Acids Res. 1987 Nov 11;15(21):8919–8934. doi: 10.1093/nar/15.21.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue-Geile K., Geiser J. R., Shu M., Miller C., Wool I. G., Meisler A. I., Pipas J. M. Ribosomal protein genes are overexpressed in colorectal cancer: isolation of a cDNA clone encoding the human S3 ribosomal protein. Mol Cell Biol. 1991 Aug;11(8):3842–3849. doi: 10.1128/mcb.11.8.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh B. F., Tjian R. Transcription from a TATA-less promoter requires a multisubunit TFIID complex. Genes Dev. 1991 Nov;5(11):1935–1945. doi: 10.1101/gad.5.11.1935. [DOI] [PubMed] [Google Scholar]
- Reed R., Maniatis T. Intron sequences involved in lariat formation during pre-mRNA splicing. Cell. 1985 May;41(1):95–105. doi: 10.1016/0092-8674(85)90064-9. [DOI] [PubMed] [Google Scholar]
- Reed R., Maniatis T. The role of the mammalian branchpoint sequence in pre-mRNA splicing. Genes Dev. 1988 Oct;2(10):1268–1276. doi: 10.1101/gad.2.10.1268. [DOI] [PubMed] [Google Scholar]
- Reed R. The organization of 3' splice-site sequences in mammalian introns. Genes Dev. 1989 Dec;3(12B):2113–2123. doi: 10.1101/gad.3.12b.2113. [DOI] [PubMed] [Google Scholar]
- Rhoads D. D., Dixit A., Roufa D. J. Primary structure of human ribosomal protein S14 and the gene that encodes it. Mol Cell Biol. 1986 Aug;6(8):2774–2783. doi: 10.1128/mcb.6.8.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro H. S., Roncari D. A. The C/EBP-binding region and adjacent sites regulate expression of the adipose P2 gene in human preadipocytes. Mol Cell Biol. 1991 Apr;11(4):2303–2306. doi: 10.1128/mcb.11.4.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro H. S., Xu L. Nucleotide sequences of a cDNA clone encoding mouse ribosomal protein S24. Nucleic Acids Res. 1991 Dec 11;19(23):6647–6647. doi: 10.1093/nar/19.23.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. H. The origin and evolution of retroposons. Int Rev Cytol. 1985;93:187–279. doi: 10.1016/s0074-7696(08)61375-3. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Green M. R. Role of the 3' splice site consensus sequence in mammalian pre-mRNA splicing. Nature. 1985 Oct 24;317(6039):732–734. doi: 10.1038/317732a0. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Krainer A. R., Maniatis T., Green M. R. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984 Aug;38(1):317–331. doi: 10.1016/0092-8674(84)90553-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp M. G., Adams S. M., Elvin P., Walker R. A., Brammar W. J., Varley J. M. A sequence previously identified as metastasis-related encodes an acidic ribosomal phosphoprotein, P2. Br J Cancer. 1990 Jan;61(1):83–88. doi: 10.1038/bjc.1990.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. RNA splicing and genes. JAMA. 1988 Nov 25;260(20):3035–3041. [PubMed] [Google Scholar]
- Siliciano P. G., Guthrie C. 5' splice site selection in yeast: genetic alterations in base-pairing with U1 reveal additional requirements. Genes Dev. 1988 Oct;2(10):1258–1267. doi: 10.1101/gad.2.10.1258. [DOI] [PubMed] [Google Scholar]
- Smith M. R., Jaramillo M., Liu Y. L., Dever T. E., Merrick W. C., Kung H. F., Sonenberg N. Translation initiation factors induce DNA synthesis and transform NIH 3T3 cells. New Biol. 1990 Jul;2(7):648–654. [PubMed] [Google Scholar]
- Sterner D. A., Berget S. M. In vivo recognition of a vertebrate mini-exon as an exon-intron-exon unit. Mol Cell Biol. 1993 May;13(5):2677–2687. doi: 10.1128/mcb.13.5.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolan D. R., Hershey J. W., Traut R. T. Crosslinking of eukaryotic initiation factor eIF3 to the 40S ribosomal subunit from rabbit reticulocytes. Biochimie. 1983 Jul;65(7):427–436. doi: 10.1016/s0300-9084(83)80062-5. [DOI] [PubMed] [Google Scholar]
- Uchiumi T., Ogata K. Cross-linking study on localization of the binding site for elongation factor 1 alpha on rat liver ribosomes. J Biol Chem. 1986 Jul 25;261(21):9668–9671. [PubMed] [Google Scholar]
- Wagner M., Perry R. P. Characterization of the multigene family encoding the mouse S16 ribosomal protein: strategy for distinguishing an expressed gene from its processed pseudogene counterparts by an analysis of total genomic DNA. Mol Cell Biol. 1985 Dec;5(12):3560–3576. doi: 10.1128/mcb.5.12.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K. L., Konrad K. D., Woods D. F., Bryant P. J. Drosophila homolog of the human S6 ribosomal protein is required for tumor suppression in the hematopoietic system. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11302–11306. doi: 10.1073/pnas.89.23.11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann P., Nygård O. The spatial arrangement of the complex between eukaryotic initiation factor eIF-3 and 40 S ribosomal subunit. Cross-linking between factor and ribosomal proteins. Biochim Biophys Acta. 1983 Oct 13;741(1):103–108. doi: 10.1016/0167-4781(83)90015-5. [DOI] [PubMed] [Google Scholar]
- Wiedemann L. M., Perry R. P. Characterization of the expressed gene and several processed pseudogenes for the mouse ribosomal protein L30 gene family. Mol Cell Biol. 1984 Nov;4(11):2518–2528. doi: 10.1128/mcb.4.11.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wool I. G. The structure and function of eukaryotic ribosomes. Annu Rev Biochem. 1979;48:719–754. doi: 10.1146/annurev.bi.48.070179.003443. [DOI] [PubMed] [Google Scholar]
- Wu J., Manley J. L. Mammalian pre-mRNA branch site selection by U2 snRNP involves base pairing. Genes Dev. 1989 Oct;3(10):1553–1561. doi: 10.1101/gad.3.10.1553. [DOI] [PubMed] [Google Scholar]
- Zhuang Y., Weiner A. M. A compensatory base change in U1 snRNA suppresses a 5' splice site mutation. Cell. 1986 Sep 12;46(6):827–835. doi: 10.1016/0092-8674(86)90064-4. [DOI] [PubMed] [Google Scholar]
- Zhuang Y., Weiner A. M. A compensatory base change in human U2 snRNA can suppress a branch site mutation. Genes Dev. 1989 Oct;3(10):1545–1552. doi: 10.1101/gad.3.10.1545. [DOI] [PubMed] [Google Scholar]