Abstract
A 16-year-old boy with sickle cell anemia undergoes routine screening with transcranial Doppler ultrasonography to assess the risk of stroke. This examination shows an abnormally elevated blood-flow velocity in the middle cerebral artery. The hemoglobin level is 7.2 g per deciliter, the reticulocyte count is 12.5%, and the fetal hemoglobin level is 8.0%. Long-term treatment with red-cell transfusion is initiated to prevent stroke. A hematologist recommends prophylactic iron-chelating therapy.
THE CLINICAL PROBLEM
Long-term treatment with red-cell transfusion effectively prevents stroke and other complications of sickle cell anemia1 and can sustain patients with chronic congenital and acquired refractory anemia, including thalassemia major, Diamond–Blackfan anemia, myelodysplastic syndromes, myelofibrosis, aplastic anemia, and other disorders. In the United States, 10,000 to 20,000 patients with sickling disorders receive repeated transfusions. An estimated 4000 to 5000 patients with myelodysplastic syndromes and other forms of acquired refractory anemia require red-cell transfusions. The number of patients with transfusion-dependent thalassemia in the United States is smaller — probably less than 1000.2 However, globally, almost 100,000 patients with thalassemia syndromes undergo transfusions. The majority of these patients are in low- and middle-income countries.3
Because humans lack any effective means to excrete excess iron, long-term transfusion alone inexorably produces the clinical problem of iron overload. In patients with thalassemia who undergo transfusion from infancy, iron-induced liver disease and endocrine disorders develop during childhood and are almost inevitably followed in adolescence by death from iron-induced cardiomyopathy.4 In patients with sickle cell anemia, although iron-induced complications appear to develop later, eventually, liver disease with cirrhosis as well as cardiac and pancreatic iron deposition can develop.5,6 The annual per-patient costs of care for complications of iron overload are estimated at $15,000 to $20,000.7,8
PATHOPHYSIOLOGY AND EFFECT OF THERAPY
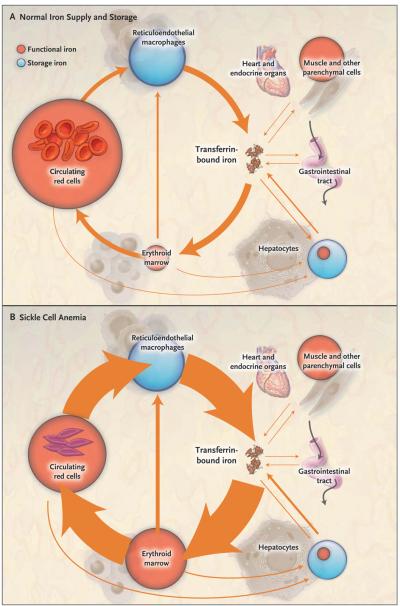
At the end of their life span, transfused red cells are phagocytosed by reticuloendothelial macrophages in the liver, bone marrow, and spleen (Fig. 1). Their hemoglobin is digested, and the iron is freed from heme and released into the cytosol. Early in the course of long-term transfusion, most of this additional iron can be stored within reticuloendothelial macrophages. Gradually, limits on the capacity of macrophages to retain iron result in the release of excess iron into plasma.9 Transferrin binds the released iron, with an increase in the plasma iron concentration and transferrin saturation. As the transferrin saturation increases, hepatocytes are recruited to serve as storage sites for the excess iron.
Figure 1. Iron Supply and Storage in Sickle Cell Anemia with Long-Term Red-Cell Transfusion and Iron-Chelating Therapy.
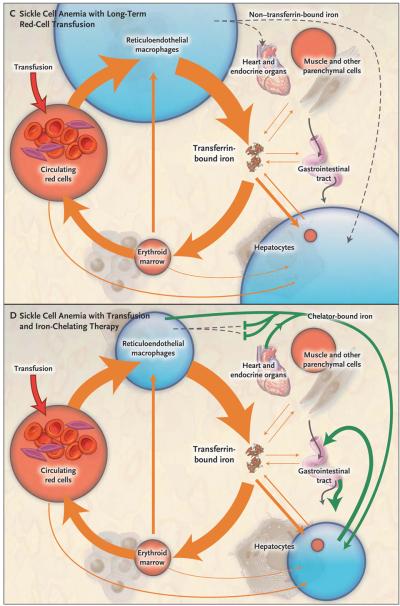
In all four panels, the area of the red and blue circles is roughly proportional to the amount of iron in each pool, and the width of the arrows is roughly proportional to the daily magnitude of the iron flux. Panel A shows normal iron supply and storage in a healthy person without sickle cell disease (i.e., with hemoglobin A). The major pathway of internal iron exchange is a unidirectional flow from plasma transferrin to the erythroid marrow to circulating red cells to reticuloendothelial macrophages and back to plasma transferrin (orange arrows). In the circulating plasma, virtually all iron is bound to transferrin. Panel B shows that in sickle cell anemia, hemolysis shortens the average life span of the red cell from about 4 months to 5 or 6 weeks, increasing red-cell catabolism by reticuloendothelial macrophages and increasing iron delivery to the erythroid marrow by 6 to 8 times the normal rate. There is little ineffective erythropoiesis, and iron absorption from the gastrointestinal tract is not increased. Panel C shows that long-term red-cell transfusion decreases erythroid marrow activity to 2 to 3 times the normal rate but results in accumulation of iron in reticuloendothelial macrophages and hepatocytes. Eventually, the capacity for safe storage is exceeded, with the appearance of plasma non–transferrin-bound iron (dashed arrow) and its progressive deposition in the heart and endocrine organs. In Panel D, the green arrows show that iron-chelating therapy with deferasirox can clear plasma non–transferrin-bound iron and remove excess iron from the liver, heart, and other organs, with subsequent excretion through the bile into the stool.
With continued transfusion, macrophages and hepatocytes can no longer retain all the surplus iron. Iron then enters plasma in amounts that exceed the transport capacity of circulating transferrin. As a consequence, non–transferrin-bound iron appears in the plasma (Fig. 1) as a heterogeneous assortment of iron complexes that appear to be the major mediators of extrahepatic tissue damage in transfusional iron overload.10 Non–transferrin-bound plasma iron enters specific cells, particularly hepatocytes, cardiomyocytes, anterior pituitary cells, and pancreatic beta-cells. In these cells, iron accumulation leads to the generation of reactive oxygen species, resulting in damage to lipids, proteins, DNA, and subcellular organelles, including lysosomes and mito chondria. This injury may result in cellular dysfunction, apoptosis, and necrosis.
Therapy with chelating agents that form a complex with iron and promote its excretion can clear plasma non–transferrin-bound iron, remove excess iron from cells, and maintain or return body iron to safe levels (Fig. 1). (An interactive graphic depicting iron supply and storage in sickle cell anemia with long-term red-cell transfusion and iron-chelating therapy is available with the full text of this article at NEJM.org.) Two iron-chelating agents are approved for use in North America (Table 1): parenteral deferoxamine mesylate (Desferal, Novartis) and oral deferasirox (Exjade, Novartis).
Table 1.
Iron-Chelating Agents in Clinical Use.
| Variable | Deferoxamine | Deferasirox | Deferiprone |
|---|---|---|---|
| Chelator-iron complex | Hexadentate, 1:1 complex | Tridentate, 2:1 complex | Bidentate, 3:1 complex |
| Usual dose | 25–50 mg/kg/day | 20–40 mg/kg/day | 75–100 mg/kg/day |
| Administration | Subcutaneous or intravenous, 8–10 hr/day, 5–7 days/wk | Oral, once daily | Oral, three times daily |
| Plasma half-life | 20–30 min | 8–16 hr | 2–3 hr |
| Route of elimination | Biliary and urinary | Predominantly biliary | Predominantly urinary |
| Regulatory approval | Approved in United States, Canada, Europe, and other countries | Approved in United States, Canada, Europe, and other countries | Not approved in United States or Canada; approved in Europe11 and other countries |
| Indication | Transfusional iron overload | Transfusional iron overload | Transfusional iron overload in patients with thalassemia major, when deferoxamine therapy is contraindicated or inadequate |
| Adverse effects | Irritation at the infusion site, ocular and auditory disturbances, growth retardation and skeletal changes, allergy, respiratory distress syndrome with higher-than-recommended doses12 | Gastrointestinal disturbances, rash, increase in serum creatinine level; potentially fatal renal and hepatic impairment or failure, gastrointestinal hemorrhage13 | Agranulocytosis and neutropenia; gastrointestinal disturbances, arthropathy, increased liver-enzyme levels, low plasma zinc level, progression of hepatic fibrosis associated with increase in iron overload or hepatitis C11 |
Deferoxamine is a siderophore (an iron-binding compound) produced by the bacterium Streptomyces pilosus. It is poorly absorbed after oral administration and is rapidly cleared; consequently, subcutaneous or intravenous administration is necessary. One molecule of deferoxamine binds a single atom of iron, forming a feroxamine complex that is virtually inert metabolically. Plasma iron chelated with deferoxamine is eliminated predominantly by the kidneys. Hepatocytes efficiently take up deferoxamine, which then chelates hepatocellular iron, with the feroxamine excreted in the bile. Within cells, deferoxamine is localized to lysosomes, where it induces autophagy of cytosolic ferritin. Lysosomal degradation of cytosolic ferritin releases iron that is bound by deferoxamine, and the chelated iron is then cleared from the cell.14
In contrast to deferoxamine, the synthetic chelator deferasirox is well absorbed from the gastrointestinal tract and is cleared from the circulation slowly.13,15 Two molecules of deferasirox are needed to bind a single atom of iron. Like deferoxamine, deferasirox forms complexes with plasma iron, but deferasirox–iron complexes are eliminated predominantly through a hepatobiliary route. Hepatocytes readily take up deferasirox, which chelates hepatocellular iron. The deferasirox–iron complexes are then excreted in the bile.15 Within cells, deferasirox chelates cytosolic iron, leading to ferritin degradation by the proteasome.14
A third iron chelator, the synthetic oral agent deferiprone (Ferriprox, Apotex; Kelfer, Cipla), is not approved for use in the United States or Canada. In the European Union and some other countries, it is approved specifically for patients with thalassemia major when deferoxamine is contraindicated or inadequate (Table 1).
CLINICAL EVIDENCE
The use of deferoxamine therapy antedates the common use of randomized, controlled trials to establish the efficacy of medical treatments. Only one small, randomized trial has compared chelation plus deferoxamine with no therapy; this trial enrolled 20 children with β-thalassemia. After a mean of 5.8 years of treatment with intramuscular deferoxamine, the mean hepatic iron concentration was 25.9 mg per gram of liver tissue (dry weight) in the deferoxamine group and 42.2 mg per gram in the control group.16 At 14 years, one death had occurred in the deferoxamine group and six deaths had occurred in the control group.17
In lieu of randomized trials, observational studies have investigated the effects of deferoxamine in the management of transfusion-related iron overload. One study involved 977 children with transfusion-dependent thalassemia major who survived beyond the first decade of life.18 Subsequent survival was examined according to 5-year birth cohorts beginning in 1960; deferoxamine was introduced in 1975. The survival rate increased progressively in each 5-year cohort (see Fig. 1 in the Supplementary Appendix, available at NEJM.org). The survival rate was significantly higher among children born after 1975 than among those in previous cohorts.
Deferasirox has been compared with deferoxamine in a few short-term trials sponsored by Novartis.19-23 In the largest of these trials, 586 children with β-thalassemia were randomly assigned to either agent, with dosing according to the baseline hepatic iron concentration.19 The primary end point was the percentage of subjects with either a maintained or reduced hepatic iron concentration at 1 year; this end point was reached in 52.9% of patients assigned to deferasirox and in 66.4% of patients assigned to deferoxamine. This result, which did not meet a prespecified noninferiority target, was attributed to the relative underdosing of deferasirox. The total hepatic iron concentration decreased by a mean of 2.4 mg per gram (dry weight) in the deferasirox group and by 2.9 mg per gram in the deferoxamine group. No trial has established the long-term effectiveness of deferasirox in preventing organ toxicity or improving survival.
Deferiprone has also been compared with deferoxamine in several small, randomized trials.24 As is the case with deferasirox, no long-term trials have been performed to evaluate the effect of deferiprone on organ function or survival.
CLINICAL USE
Iron-chelating therapy should be considered in all patients who require long-term red-cell transfusion. Such patients include those with sickle cell disease, myelodysplastic syndromes, thalassemia major, Diamond–Blackfan anemia, aplastic anemia, and other congenital and acquired forms of refractory anemia.
There are alternatives to chelation in some patients. Some of the underlying disorders requiring transfusion may be cured by hematopoietic stem-cell transplantation. In some patients with sickle cell disease, exchange transfusion may reduce or obviate the need for iron chelation. Infrequently, phlebotomy may be an option for the removal of excess iron in the event of cure or remission of a refractory anemia. Iron-chelating therapy itself may sometimes decrease or eliminate the need for transfusion in patients with myelodysplasia25 or myelofibrosis. Chelation therapy may not be needed in patients with myelodysplasia or other acquired refractory anemias who have an estimated survival of less than 1 year.26
With the exception of these groups, iron-chelating therapy is indicated in almost all patients requiring long-term red-cell transfusion. Iron chelation is contraindicated in patients who are hypersensitive to the chelating agent or excipients in the chelator formulation, and it requires specialized management in patients with several renal disease or anuria. Chelators should be avoided or used with great caution in patients who are pregnant or breast-feeding.
Ideally, iron-chelating therapy should be initiated prophylactically, before clinically significant iron accumulation has occurred. Treatment should begin when patients have received between 10 and 20 red-cell transfusions. Patients who have already undergone repeated transfusion without sufficient chelation can also be successfully treated, but they may require more intensive regimens (see below).
Evaluation of the patient before the initiation or adjustment of iron-chelating therapy includes a detailed characterization of the underlying disorder, with thorough documentation of the history of transfusion and chelation; determination of the body iron load by measurement of hepatic iron and serum ferritin concentrations; estimation of the rate of transfusional iron loading; and assessment of cardiac iron deposition. The techniques for assessing cardiac iron overload, transfusional iron loading, and body burden are described in the Supplementary Appendix. The extent of any existing iron-induced hepatic, cardiac, or endocrine dysfunction should be established, and in children and adolescents, growth and maturation should be assessed. Nutritional evaluation with correction of deficiencies is recommended.27
In the United States and Canada, the choice of an iron chelator for transfusional iron overload is either parenteral deferoxamine or oral deferasirox. The decision is best made with the patient and, if the patient is a child, with his or her parents. Despite the lack of data on long-term effectiveness, most patients now opt for deferasirox because of the ease of oral administration. Deferasirox is preferred for prophylactic or maintenance therapy. Deferoxamine, which has been proved to reverse iron-induced heart disease and increase long-term survival,28 may be indicated if deferasirox is ineffective in a particular patient, and it may be favored for severe iron overload, especially with cardiac involvement. Conversely, deferasirox may be the better choice in patients who are unable to tolerate subcutaneous infusions of deferoxamine. Deferasirox also may be substituted for deferoxamine after successful clearance of cardiac iron. Deferiprone is available in the United States on a compassionate-use basis, usually in combination with deferoxamine, in patients in whom iron-induced heart failure has developed or who are at high risk for the development of heart failure.29,30
Deferoxamine is administered subcutaneously or intravenously, usually with a portable pump, for 8 to 10 hours each day, 5 to 7 days per week. Subcutaneous administration is preferred except in patients with severe cardiac iron deposition, for whom continuous intravenous deferoxamine therapy is recommended.28,31 Deferasirox is administered orally once daily, and deferiprone is administered orally three times daily.
The dose of an iron-chelating agent is determined by three principal factors: the presence or absence of cardiac iron overload, the rate of transfusional iron loading, and the body iron burden (see the Supplementary Appendix and Table 2). In brief, if cardiac iron overload is present, ridding the heart of the excess iron becomes the critical therapeutic goal. In the absence of cardiac iron overload, the long-term objective is to maintain the body iron at a level that permits safe storage while avoiding chelator toxicity. The greater the rate of transfusional iron loading, the greater the dose of an iron chelator that will be needed to control the accumulation of iron.
Table 2.
Usual Doses of Deferoxamine or Deferasirox for Transfusional Iron Overload.*
| Hepatic Iron Concentration |
No Cardiac Iron Overload (T2★, ≥20 msec) | Cardiac Iron Overload (T2★, <20 msec) | |||
|---|---|---|---|---|---|
| Daily Transfusional Iron Intake | Mild to Moderate | Severe | |||
| <0.3 mg/kg of body weight | 0.3 to 0.5 mg/kg of body weight | >0.5 mg/kg of body weight | T2★ 10 to <20 msec | T2★, <10 msec | |
| ≥15 mg/g, dry weight | Deferoxamine: 40–50 mg/kg/day, 8 to 10 hr/day, 6 or 7 days/wk, by subcutaneous infusion; deferasirox: oral dose of 30–40 mg/kg daily | Deferoxamine: 40–50 mg/kg/day, 8 to 10 hr/day, 6 or 7 days/wk, by subcutaneous infusion; deferasirox: oral dose of 30–40 mg/kg daily | Deferoxamine: 40–50 mg/kg/day, 8 to 10 hr/day, 6 or 7 days/wk, by subcutaneous infusion; deferasirox: oral dose of 30–40 mg/kg daily | Deferoxamine: 50 mg/kg/day by continuous intravenous infusion28; deferasirox: oral dose of 40 mg/kg daily, but uncertain efficacy in reducing cardiac iron | Deferoxamine: 50 mg/kg/day by continuous intravenous infusion28; deferasirox: oral dose of 40 mg/kg daily, but uncertain efficacy in reducing cardiac iron |
| 7 to <15 mg/g, dry weight | Deferoxamine: 30–40 mg/kg/day, 8 to 10 hr/day, 5 days/wk, by subcutaneous infusion; deferasirox: oral dose of 20–30 mg/kg daily | Deferoxamine: 40–50 mg/kg/day, 8 to 10 hr/day, 6 or 7 days/wk, by subcutaneous infusion; deferasirox: oral dose of 30–40 mg/kg daily | Deferoxamine: 40–50 mg/kg/day, 8 to 10 hr/day, 6 or 7 days/wk, by subcutaneous infusion; deferasirox: oral dose of 30–40 mg/kg daily | Deferoxamine: 40–50 mg/kg/day, 8 to 10 hr/day, 6 or 7 days/wk, by subcutaneous infusion; deferasirox: oral dose of 30–40 mg/kg daily | Deferoxamine: 40–50 mg/kg/day by continuous infusion28; deferasirox: oral dose of 30–40 mg/kg daily |
| 3 to <7 mg/g, dry weight | Deferoxamine: 30–40 mg/kg/day, 8 to 10 hr/day, 5 days/wk, by subcutaneous infusion; deferasirox: oral dose of 20–30 mg/kg daily | Deferoxamine: 30–40 mg/kg/day, 8 to 10 hr/day, 5 days/wk, by subcutaneous infusion; deferasirox: oral dose of 20–30 mg/kg daily | Deferoxamine: 30–40 mg/kg/day, 8 to 10 hr/day, 5 days/wk, by subcutaneous infusion; deferasirox: oral dose of 20–30 mg/kg daily | Deferoxamine: 40–50 mg/kg/day, 8 to 10 hr/day, 6 or 7 days/wk, by subcutaneous infusion; deferasirox: oral dose of 30–40 mg/kg daily | Deferoxamine: 40–50 mg/kg/day by continuous infusion28; deferasirox: oral dose of 30–40 mg/kg daily |
| <3 mg/g, dry weight | Deferoxamine: suspend therapy; deferasirox: suspend therapy | Deferoxamine: suspend therapy; deferasirox: suspend therapy | Deferoxamine: suspend therapy; deferasirox: suspend therapy | Deferoxamine: adjust intravenous or subcutaneous dose according to therapeutic index32; deferasirox: adjust oral dose, monitoring renal and hepatic function and blood count | Deferoxamine: adjust intravenous or subcutaneous dose according to therapeutic index32; deferasirox: adjust oral dose, monitoring renal and hepatic function and blood count |
To minimize interference with growth and skeletal development, the dose of deferoxamine in young children should not exceed 25 to 30 mg per kilogram of body weight. The dose should be adjusted according to the therapeutic index.32 The bioavailability of deferasirox may affect the response. T2★ denotes the cardiac effective transverse relaxation time on magnetic resonance imaging.
During treatment, tests to monitor chelator-associated toxicity should be performed, depending on the potential adverse effects of the specific agent to be used (see Table 1 and the Adverse Effects section below). In patients who receive deferoxamine, these tests include annual assessments of auditory function and vision. In patients who receive deferasirox, serum creatinine, serum aminotransferases, and bilirubin levels and complete blood counts should be assessed monthly. In patients who receive deferiprone, weekly assessment of complete blood counts and monthly assessments of serum aminotransferases should be performed.
The effectiveness of iron-chelating therapy is best monitored by periodic measurements of cardiac iron concentrations by magnetic resonance imaging (MRI), of cardiac function, and of hepatic iron concentrations and by review of the actual rate of transfusional iron loading once or twice a year, depending on the severity of the iron overload (see the Supplementary Appendix). Dose adjustments can be made according to the guidelines in Table 2. Serum ferritin concentrations are usually measured at least quarterly. As at baseline, hepatic, cardiac, and endocrine function should be assessed periodically along with nutritional status, and, in children and adolescents, growth and maturation should be monitored.
In the United States, on the basis of 2006 wholesale acquisition prices, the annual costs of deferoxamine for iron-chelating therapy have been estimated to range from $6,824 to $29,209, plus $9,286 for infusion, and the estimated annual costs of deferasirox range from $24,404 to $53,095, with the actual cost depending on dose and body weight.7 Administration of a chelator will be needed as long as transfusion is continued and will be lifelong in most patients.
ADVERSE EFFECTS
Discomfort or pain at the site of subcutaneous infusion develops in almost all patients treated with subcutaneous deferoxamine, and induration or erythema develops in some patients. These symptoms can often be mitigated with topical anesthetic or glucocorticoid creams. Visual and auditory toxicity associated with deferoxamine has been reported, and in one series involving 89 patients treated with this agent, 13 presented with vision loss, deafness, or both.33 Subsequent studies suggest a much lower incidence of toxicity,34 and these risks can be minimized by not exceeding doses of 50 mg per kilogram of body weight in patients with iron overload and by decreasing the dose as the hepatic iron concentration approaches normal levels. Although treatment with deferoxamine may reduce endocrine complications of iron overload, such as a delay of puberty, the chelator itself can interfere with growth,35 apparently as a result of skeletal dysplasia.36 To minimize this effect, the dose of deferoxamine in young children should not exceed 25 to 30 mg per kilogram.37
In the registration trial of deferasirox described above,19 gastrointestinal disturbances occurred in approximately 15% of patients, rash in 11%, and increases in serum creatinine levels in 38%. Similar rates have been observed in subsequent trials.20-22 In January 2010, on the basis of postmarketing studies, the Food and Drug Administration required a change in the prescribing information for deferasirox. The new information states that the drug could cause potentially fatal renal and hepatic impairment or failure as well as gastrointestinal hemorrhage.13 These adverse effects were reported to occur more frequently in older patients and in patients with high-risk myelodysplastic syndromes, thrombocytopenia, or underlying renal or hepatic impairment.
The most common adverse effects of deferiprone are diarrhea and gastrointestinal effects, arthropathy (including severe arthritis with clinically significant disability), increased levels of serum liver enzymes, and progression of hepatic fibrosis associated with an increase in iron overload or hepatitis C.11 The most serious adverse effects are agranulocytosis (incidence, 1.1%) and neutropenia (incidence, 4.9%); weekly monitoring of the neutrophil count is recommended. Neurologic abnormalities have been reported with higher-than-recommended doses of deferiprone.38
AREAS OF UNCERTAINTY
Several areas of uncertainty exist with regard to the optimal approach to iron-chelating therapy. First, a variety of binary combinations of chelating agents are being examined in off-label uses, with either synchronous or sequential administration. The clinical usefulness of such combination therapies is unclear at present, in the absence of unequivocal evidence of the superiority of any specific combination over treatment with a single agent.24
Second, MRI performed to evaluate the iron content of the liver, heart, and other organs has become the method of choice for guiding iron-chelating therapy, but calibrated methods are not available for all patients. A 1-year study of deferasirox involving patients with various types of anemias used an alternative approach; the investigators based the initial dose on the rate of transfusional iron loading and subsequently adjusted the dose according to measurements of serum ferritin levels and safety markers.23 The long-term efficacy and safety of this strategy are uncertain.
Third, in patients with myelodysplastic syndromes who have undergone long-term transfusion and for whom prolonged survival is anticipated, iron-chelating therapy may be appropriate. In individual patients, the benefit of such treatment may vary, given the morbidity associated with chelation, the variable prognosis for the underlying disorder, and the latency period between the onset of the transfusion and the development of clinical manifestations of iron overload. Data are lacking from prospective, randomized trials examining the clinical circumstances in which morbidity and mortality improve with iron-chelating therapy in these patients39; one such trial is currently recruiting patients.40
Fourth, there is evidence to suggest that iron overload is associated with lower rates of cardiomyopathy, endocrinopathy, and other conditions among patients with sickle cell disease than among patients with thalassemia.41 The effects of the systemic inflammatory state on iron handling in patients with sickle cell disease,42 as well as differences in the rate and duration of transfusion and in the age of the patient at the initiation of long-term transfusion, the extent of ineffective erythropoiesis, and gastrointestinal iron absorption, may be involved. It is unclear whether these data provide sufficient grounds to recommend a higher body iron threshold for chelation therapy in patients with sickle cell disease, especially given the potential risks of iron-induced liver disease.
Finally, there is a lack of certainty with respect to the optimal hepatic iron concentrations (Table 2) for minimizing the risk that hepatic fibrosis will progress to cirrhosis and its ultimate complication, hepatocellular carcinoma.43,44
GUIDELINES
Guidelines and consensus statements on the management of sickle cell disease,45,46 thalassemia,32,47 Diamond–Blackfan anemia,48 aplastic anemia,49 and myelodysplastic syndromes26,50 all include recommendations for iron-chelating therapy for transfusional iron overload. All these guidelines are generally consistent with the approach outlined in this review, although there are variations in the individual recommendations, depending in part on the year of publication and the specific underlying disorder. For example, the guidelines for iron chelation in thalassemia32,47 generally endorse measurement of serum ferritin levels as a useful way to monitor iron overload, whereas the guidelines for the management of sickle cell disease45,46 emphasize that ferritin levels can be altered by liver disease and inflammation. The guidelines on the management of thalassemia by the Italian Society of Hematology47 endorse deferoxamine as first-line therapy over the oral chelators, whereas most of the other guidelines do not state an explicit preference in this regard.
RECOMMENDATIONS
After discussing the need for iron-chelating therapy with the patient and his family, I would describe the advantages and disadvantages of subcutaneous deferoxamine and oral deferasirox so that a genuinely informed decision can be made. At present, the most frequent choice is oral deferasirox. Before the initiation of treatment, I would obtain information about the number of previous transfusions and the rate of ongoing transfusion and would arrange for cardiac T2* and hepatic transverse relaxation rate (R2) measurements, an MRI evaluation of cardiac function, and echocardiographic and electrocardiographic studies. Auditory and ophthalmic testing, including slit-lamp examination and dilated-fundus examination, should be performed. Laboratory tests should include a complete blood count with a differential count and measurement of serum creatinine, serum aminotransferases, bilirubin levels, and iron indexes. After transfusion of a total of 10 to 20 units of blood, or with the hepatic iron concentration between 3 and 7 mg per gram, I would administer once-daily oral therapy with deferasirox at a dose of 20 mg per kilogram. With good support and careful monitoring, this regimen, adjusted as needed, should provide long-term protection against the complications of transfusional iron overload in this patient.
Supplementary Material
Acknowledgments
Supported by grants from the St. Giles Foundation, the Food and Drug Administration (R01 FD003702), and the National Institutes of Health (R37 DK049108; R01 DK066251).
Footnotes
Dr. Brittenham reports that his institution filed a patent application in April 2010 for a rapid MRI method. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the author are available with the full text of this article at NEJM.org.
References
- 1.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 2.Vichinsky EP, MacKlin EA, Waye JS, Lorey F, Olivieri NF. Changes in the epidemiology of thalassemia in North America: a new minority disease. Pediatrics. 2005;116(6):e818–e825. doi: 10.1542/peds.2005-0843. [DOI] [PubMed] [Google Scholar]
- 3.Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood. 2010;115:4331–6. doi: 10.1182/blood-2010-01-251348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weatherall DJ, Clegg JB. The thalassemia syndromes. 4th ed. Blackwell Science; Oxford, England: 2001. [Google Scholar]
- 5.Darbari DS, Kple-Faget P, Kwagyan J, Rana S, Gordeuk VR, Castro O. Circumstances of death in adult sickle cell disease patients. Am J Hematol. 2006;81:858–63. doi: 10.1002/ajh.20685. [DOI] [PubMed] [Google Scholar]
- 6.Finch CA, Lee MY, Leonard JM. Continuous RBC transfusions in a patient with sickle cell disease. Arch Intern Med. 1982;142:279–82. [PubMed] [Google Scholar]
- 7.Delea TE, Sofrygin O, Thomas SK, Baladi JF, Phatak PD, Coates TD. Cost effectiveness of once-daily oral chelation therapy with deferasirox versus infusional deferoxamine in transfusion-dependent thalassaemia patients: US healthcare system perspective. Pharmacoeconomics. 2007;25:329–42. doi: 10.2165/00019053-200725040-00005. [DOI] [PubMed] [Google Scholar]
- 8.Delea TE, Edelsberg J, Sofrygin O, et al. Consequences and costs of noncompliance with iron chelation therapy in patients with transfusion-dependent thalassemia: a literature review. Transfusion. 2007;47:1919–29. doi: 10.1111/j.1537-2995.2007.01416.x. [DOI] [PubMed] [Google Scholar]
- 9.Fillet G, Beguin Y, Baldelli L. Model of reticuloendothelial iron metabolism in humans: abnormal behavior in idiopathic hemochromatosis and in inflammation. Blood. 1989;74:844–51. [PubMed] [Google Scholar]
- 10.Breuer W, Hershko C, Cabantchik ZI. The importance of non-transferrin bound iron in disorders of iron metabolism. Transfus Sci. 2000;23:185–92. doi: 10.1016/s0955-3886(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 11.European Medicines Agency Ferriprox (deferiprone): summary of product characteristics. European public assessment report 2009. (rev. ed. 14) ( http://www.ema.europa.eu/humandocs/Humans/EPAR/ferriprox/ferriprox.htm.)
- 12.Novartis Pharmaceuticals Desferal (deferoxamine mesylate) prescribing information. 2010 ( http://www.desferal.com.)
- 13.Novartis Pharmaceuticals Exjade (deferasirox) prescribing information. 2010 ( http://www.exjade.com.)
- 14.De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood. 2009;114:4546–51. doi: 10.1182/blood-2009-05-224188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waldmeier F, Bruin GJ, Glaenzel U, et al. Pharmacokinetics, metabolism, and disposition of deferasirox in beta-thalassemic patients with transfusion-dependent iron overload who are at pharmacokinetic steady state. Drug Metab Dispos. 2010;38:808–16. doi: 10.1124/dmd.109.030833. [DOI] [PubMed] [Google Scholar]
- 16.Barry M, Flynn DM, Letsky EA, Risdon RA. Long-term chelation therapy in thalassaemia major: effect on liver iron concentration, liver histology, and clinical progress. BMJ. 1974;2:16–20. doi: 10.1136/bmj.2.5909.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Modell B, Letsky EA, Flynn DM, Peto R, Weatherall DJ. Survival and desferrioxamine in thalassaemia major. Br Med J (Clin Res Ed) 1982;284:1081–4. doi: 10.1136/bmj.284.6322.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borgna-Pignatti C, Rugolotto S, De Stefano P, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–93. [PubMed] [Google Scholar]
- 19.Cappellini MD, Cohen A, Piga A, et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006;107:3455–62. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- 20.Piga A, Galanello R, Forni GL, et al. Randomized phase II trial of deferasirox (Exjade, ICL670), a once-daily, orally-administered iron chelator, in comparison to deferoxamine in thalassemia patients with transfusional iron overload. Haematologica. 2006;91:873–80. [PubMed] [Google Scholar]
- 21.Porter J, Galanello R, Saglio G, et al. Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study. Eur J Haematol. 2008;80:168–76. doi: 10.1111/j.1600-0609.2007.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vichinsky E, Onyekwere O, Porter J, et al. A randomised comparison of deferasirox versus deferoxamine for the treatment of transfusional iron overload in sickle cell disease. Br J Haematol. 2007;136:501–8. doi: 10.1111/j.1365-2141.2006.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cappellini MD, Porter J, El-Beshlawy A, et al. Tailoring iron chelation by iron intake and serum ferritin: the prospective EPIC study of deferasirox in 1744 patients with transfusion-dependent anemias. Haematologica. 2010;95:557–66. doi: 10.3324/haematol.2009.014696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts DJ, Brunskill SJ, Doree C, Williams S, Howard J, Hyde CJ. Oral deferiprone for iron chelation in people with thalassaemia. Cochrane Database Syst Rev. 2007;3:CD004839. doi: 10.1002/14651858.CD004839.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Jensen PD, Heickendorff L, Pedersen B, et al. The effect of iron chelation on haemopoiesis in MDS patients with transfusional iron overload. Br J Haematol. 1996;94:288–99. doi: 10.1046/j.1365-2141.1996.d01-1795.x. [DOI] [PubMed] [Google Scholar]
- 26.Gattermann N. Overview of guidelines on iron chelation therapy in patients with myelodysplastic syndromes and transfusional iron overload. Int J Hematol. 2008;88:24–9. doi: 10.1007/s12185-008-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Claster S, Wood JC, Noetzli L, et al. Nutritional deficiencies in iron overloaded patients with hemoglobinopathies. Am J Hematol. 2009;84:344–8. doi: 10.1002/ajh.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis BA, Porter JB. Long-term outcome of continuous 24-hour deferoxamine infusion via indwelling intravenous catheters in high-risk beta-thalassemia. Blood. 2000;95:1229–36. [PubMed] [Google Scholar]
- 29.Cohen AR. Compassionate use of deferiprone for patients with thalassemia and iron-induced heart disease. (ClinicalTrials.gov identifier no. NCT00293098.) ( http://www.clinicaltrials.gov/ct2/show/NCT00293098?term=deferiprone+thalassemia&rank=2.)
- 30.Borgna-Pignatti C, Cappellini MD, De Stefano P, et al. Cardiac morbidity and mortality in deferoxamine- or deferiprone-treated patients with thalassemia major. Blood. 2006;107:3733–7. doi: 10.1182/blood-2005-07-2933. [DOI] [PubMed] [Google Scholar]
- 31.Anderson LJ, Westwood MA, Holden S, et al. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127:348–55. doi: 10.1111/j.1365-2141.2004.05202.x. [DOI] [PubMed] [Google Scholar]
- 32.Cappellini MD, Cohen A, Eleftheriou A, Piga A, Porter J, Taher A. Guidelines for the clinical management of thalassemia. 2nd ed. rev. Thalassaemia International Federation; Nicosia, Cyprus: Nov, 2008. Iron overload; pp. 33–63. [PubMed] [Google Scholar]
- 33.Olivieri NF, Buncic JR, Chew E, et al. Visual and auditory neurotoxicity in patients receiving subcutaneous deferoxamine infusions. N Engl J Med. 1986;314:869–73. doi: 10.1056/NEJM198604033141402. [DOI] [PubMed] [Google Scholar]
- 34.Cohen A, Martin M, Mizanin J, Konkle DF, Schwartz E. Vision and hearing during deferoxamine therapy. J Pediatr. 1990;117:326–30. doi: 10.1016/s0022-3476(05)80556-6. [DOI] [PubMed] [Google Scholar]
- 35.De Sanctis V, Roos M, Gasser T, et al. Impact of long-term iron chelation therapy on growth and endocrine functions in thalassemia. J Pediatr Endocrinol Metab. 2006;19:471–80. [PubMed] [Google Scholar]
- 36.Chan YL, Pang LM, Chik KW, et al. Patterns of bone diseases in transfusion-dependent homozygous thalassemia major: predominance of osteoporosis and desferrioxamine-induced bone dysplasia. Pediatr Radiol. 2002;32:492–7. doi: 10.1007/s00247-002-0664-0. [DOI] [PubMed] [Google Scholar]
- 37.Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997;89:739–61. [Erratum, Blood 1997;89:2621.] [PubMed] [Google Scholar]
- 38.Beau-Salinas F, Guitteny MA, Donadieu J, Jonville-Bera AP, Autret-Leca E. High doses of deferiprone may be associated with cerebellar syndrome. BMJ. 2009;338:a2319. doi: 10.1136/bmj.a2319. [DOI] [PubMed] [Google Scholar]
- 39.Leitch HA. Controversies surrounding iron chelation therapy for MDS. Blood Rev. 2010 October 26; doi: 10.1016/j.blre.2010.09.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 40.Novartis Pharmaceuticals Myelodysplastic syndromes (MDS) event free survival with iron chelation therapy study (TELESTO). (ClinicalTrials.gov identifier no. NCT00940602.) ( http://www.clinicaltrials.gov/ct2/show/NCT00940602?term=telesto&rank=1.)
- 41.Vichinsky E, Butensky E, Fung E, et al. Comparison of organ dysfunction in transfused patients with SCD or beta thalassemia. Am J Hematol. 2005;80:70–4. doi: 10.1002/ajh.20402. [DOI] [PubMed] [Google Scholar]
- 42.Walter PB, Fung EB, Killilea DW, et al. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. Br J Haematol. 2006;135:254–63. doi: 10.1111/j.1365-2141.2006.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borgna-Pignatti C, Vergine G, Lombardo T, et al. Hepatocellular carcinoma in the thalassaemia syndromes. Br J Haematol. 2004;124:114–7. doi: 10.1046/j.1365-2141.2003.04732.x. [DOI] [PubMed] [Google Scholar]
- 44.Ko C, Siddaiah N, Berger J, et al. Prevalence of hepatic iron overload and association with hepatocellular cancer in end-stage liver disease: results from the National Hemochromatosis Transplant Registry. Liver Int. 2007;27:1394–401. doi: 10.1111/j.1478-3231.2007.01596.x. [DOI] [PubMed] [Google Scholar]
- 45.Division of Blood Diseases and Resources NHLBI . The management of sickle cell disease. National Heart, Lung, and Blood Institute; Bethesda, MD: 2002. ( http://www.nhlbi.nih.gov/health/prof/blood/sickle/sc_mngt.pdf.) [Google Scholar]
- 46.Standards for the clinical care of adults with sickle cell disease in the UK. Sickle Cell Society; London: 2008. ( http://www.sicklecellsociety.org/pdf/CareBook.pdf.) [Google Scholar]
- 47.Angelucci E, Barosi G, Camaschella C, et al. Italian Society of Hematology practice guidelines for the management of iron overload in thalassemia major and related disorders. Haematologica. 2008;93:741–52. doi: 10.3324/haematol.12413. [DOI] [PubMed] [Google Scholar]
- 48.Vlachos A, Ball S, Dahl N, et al. Diagnosing and treating Diamond Blackfan anaemia: results of an international clinical consensus conference. Br J Haematol. 2008;142:859–76. doi: 10.1111/j.1365-2141.2008.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marsh JC, Ball SE, Cavenagh J, et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147:43–70. doi: 10.1111/j.1365-2141.2009.07842.x. [DOI] [PubMed] [Google Scholar]
- 50.Bennett JM. Consensus statement on iron overload in myelodysplastic syndromes. Am J Hematol. 2008;83:858–61. doi: 10.1002/ajh.21269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.