Abstract
Background
Cocaine abuse and dependence continue to be widespread. Currently there are no pharmacotherapies shown to be effective in the treatment of cocaine dependence.
Methods
A 33-week outpatient clinical trial of fluoxetine (60 mg/day, p.o.) for cocaine dependence was conducted that incorporated abstinence-contingent voucher incentives. Participants (n=145) were both cocaine and opioid dependent and treated with methadone. A stratified randomization procedure assigned subjects to one of four conditions: fluoxetine plus voucher incentives (FV), placebo plus voucher incentives (PV), fluoxetine without vouchers (F), and placebo without vouchers (P). Dosing of fluoxetine/placebo was double blind. Primary outcomes were treatment retention and cocaine use based on thrice-weekly urine testing.
Results
The PV group had the longest treatment retention (mean of 165 days) and lowest probability of cocaine use. The adjusted predicted probabilities of cocaine use were: 65% in the P group, 60% in the F group, 56% in the FV group, and 31% in the PV group.
Conclusions
Fluoxetine was not efficacious in reducing cocaine use in patients dually dependent on cocaine and opioids.
Keywords: Cocaine, Contingency management, Fluoxetine, Methadone
1. Introduction
Numerous studies have examined the efficacy of a wide variety of medications for cocaine dependence treatment and none have consistently demonstrated positive outcomes (Vocci et al., 2005). Fluoxetine is a selective serotonin reuptake inhibitor (SSRI) that has FDA approval for a variety of psychiatric conditions including depression and panic disorder. Initial findings from preclinical studies or case reports showed promising outcomes for the treatment of cocaine dependence with fluoxetine (Pilla et al., 1999; Koobe & Caine 1999; Carroll et al., 1990; Batki et al., 1993). However, studies in humans have been equivocal (Kosten et al., 1992; Grabowski et al., 1995); some studies have shown significant decreases in cocaine use or effects with fluoxetine (Walsh et al., 1994; Schmitz et al., 1998), while others have found no such effect (Batki et al., 1996; Covi et al., 1995; Oliveto et al., 1995). Human laboratory data indicate that fluoxetine can significantly alter the effects of cocaine (Walsh et al., 1994) and limited data from clinical trials suggested efficacy for primary outcomes related to cocaine use (Grabowski et al., 1995; Schmitz et al., 1998). Similarly, clinical trials showed a decrease in cocaine positive urine samples for methadone maintained cocaine abusers who were treated with fluoxetine (Grabowski et al., 1995) and non-opioid dependent cocaine abusers who were treated with fluoxetine combined with an incentive intervention (Schmitz et al., 1998).
While there have been several reports on the use of SSRIs for the treatment of cocaine abuse/dependence, including some with newer SSRIs that show evidence of potential efficacy (e.g., Moeller et al., 2007), most papers report results from studies that enrolled small numbers of patients and/or were open label. Expectancy effects from open label studies could influence outcomes and studies with small sample sizes may be underpowered to detect treatment effects. The only double blind placebo controlled study that ensured fluoxetine medication ingestion (accomplished in the context of methadone maintenance treatment), found a trend for less cocaine use among participants who received active fluoxetine (Grabowski et al., 1995). The promising previous studies have generally found that higher doses of fluoxetine appear to be more effective than lower doses in the treatment of cocaine dependence (Walsh et al., 1994; Batki et al., 1996; Schmitz et al., 1998).
Voucher-based reinforcement therapy, a form of contingency management (CM), has been used as an adjunctive therapy to increase cocaine abstinence, treatment attendance, and retention in treatment (Higgins et al. 1991). CM is based on operant conditioning whereby vouchers are used as a positive reinforcement for recovery behaviors such as abstinence and achievement of treatment goals. Meta-analysis has established that CM has a moderate effect size (d=0.66) (Prendergast et al. 206). Schmitz and colleagues (1998) found that fluoxetine had a beneficial effect in the context of added incentives for being cocaine abstinent.
The purpose of this study was to assess whether fluoxetine could be an effective pharmacotherapy for the treatment of cocaine dependence. The study was designed to maximize the likelihood of detecting an effect. Thus, the subjects were also opioid dependent and maintained on methadone to ensure daily-supervised fluoxetine/placebo dose administration and to reduce dropout, which has been a serious problem in cocaine treatment clinical trials. Voucher incentives were used to enhance and to experimentally control the level of motivation, and semi-quantitative as well as qualitative urine results were obtained to increase the sensitivity to detect a statistically significant effect.
2. Methods
2.1. Study Design
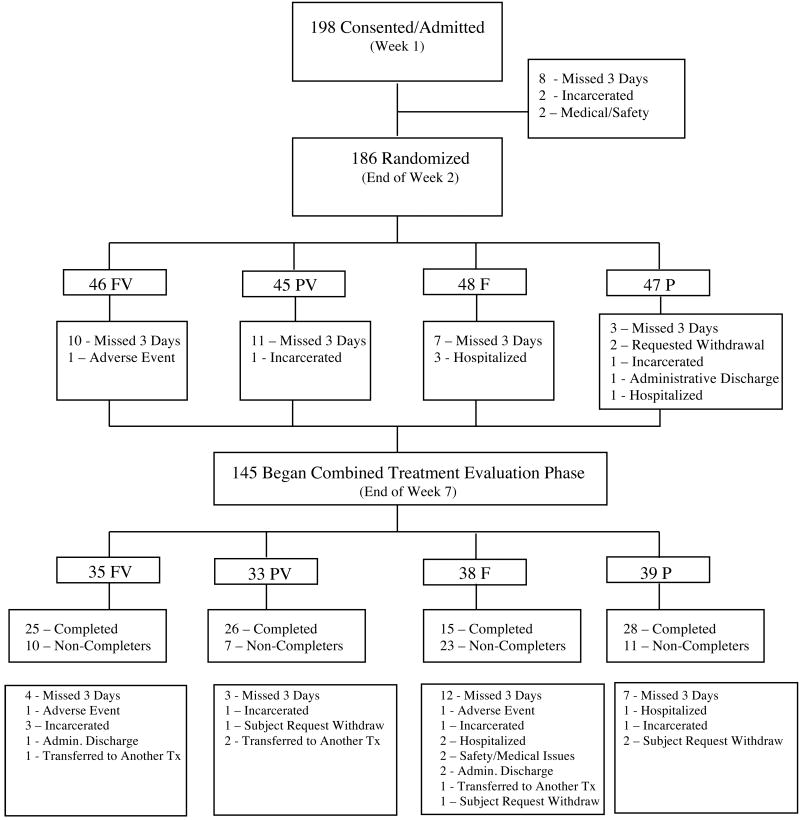
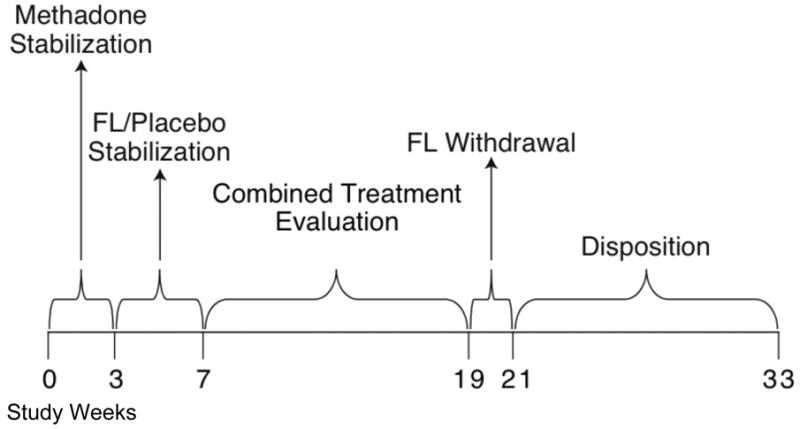
This study was a 33-week outpatient double blind clinical trial of fluoxetine for cocaine dependence that was conducted from 2001 to 2005. Study participants were both cocaine and opioid dependent and a stratified randomized procedure was used to assign subjects to one of the following conditions: fluoxetine plus vouchers (FV), placebo plus vouchers (PV), fluoxetine without vouchers (F) and placebo without vouchers (P). After an initial intake evaluation to determine protocol eligibility, participants were admitted to the study; there were five study phases (Figure 1). For the first three weeks of the study (Phase 1) subjects were stabilized on methadone and received placebo capsules. During study week 3, subjects were randomly assigned, and stratified by sex, age (split at <38 vs. >/= 38 years old), depressive symptoms (based upon week 2 Beck Depression Inventory scores spilt at <15 vs. >/=15) and baseline semi-quantitative cocaine urinalysis (benzoylecgonine levels split at 40,000ng/ml). During the second phase of the study subjects were stabilized on fluoxetine (60 mg/day) or placebo capsules (weeks 4-7). The third phase of the study (weeks 8-19) was the combined treatment evaluation period. At the start of this phase (week 8) subjects were notified of their voucher condition and were maintained on active fluoxetine or placebo; this was the period of primary study interest. During the fourth phase of the study (weeks 20-21) subjects were withdrawn from fluoxetine (or placebo). During the final study phase (weeks 22-33), treatment disposition occurred. Subjects were able to either continue methadone therapy on-site or were referred to another treatment facility in the area based on their treatment preferences. This study was approved by the Johns Hopkins University human subjects Institutional Review Board (IRB).
Figure 1. Study Phases.
Throughout the trial, data were collected from a variety of sources. Trained clinical staff administered the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1995) at admission, and the Addiction Severity Index (ASI) (McLellan et al., 1980) at admission and weeks 7, 12 and 17 of the study. Nursing staff monitored vital signs, adverse effects and concomitant medications as well as collected breathalyzers at intake, admission and thereafter every four weeks. Counseling staff recorded data on the number of counseling sessions and a modified global assessment of functioning. Self-reported data were collected weekly in a computerized assessment center monitored by a research assistant and included the Beck Depression Inventory (BDI) (Beck et al., 1961), State Trait Anxiety Inventory (STAI) (Spielberger 1983), past week drug use questionnaire, visual analog scale ratings of cocaine effects, assessments of dose adequacy for methadone and fluoxetine, a symptom checklist of potential side effects, a medication identification questionnaire, and a treatment retention form.
2.2. Subjects
Study subject inclusion criteria included: 1) DSM-IV diagnosis of current opioid and cocaine dependence (based upon evaluation with the SCID), 2) eligible to receive methadone maintenance therapy per federal guidelines, 3) between 18-60 years of age, 4) no significant chronic medical illness, and 5) no serious psychiatric illness (e.g., schizophrenia). Exclusion criteria included: 1) urine sample positive for methadone on the day of admission, 2) symptomatic HIV infection, 3) laboratory tests results found to be unacceptable for participation in the study as determined by medical staff not involved in the study as investigators, and 4) a positive pregnancy test for females. The primary mode of study recruitment was through the methadone clinic where the study was conducted. Additional recruitment strategies included posting flyers in clinical care settings and advertising in local print newspapers for patients interested in seeking treatment for their use of opiates and cocaine.
2.3. Vouchers
The behavioral intervention consisted of an escalating system of voucher payments for evidence of sustained abstinence from cocaine use as determined by qualitative urine toxicology testing. Subjects received vouchers for goods and services equivalent to the monetary value earned for no cocaine use. Subjects received a voucher worth $2.50 for their first cocaine negative urine sample starting in week 8. Vouchers for subsequent consecutive cocaine negative urines increased in value by increments of $1.50. In addition, subjects received a $10 bonus for every three consecutive cocaine negative urine samples. A cocaine positive urine sample or missing a scheduled urine test during the 12-week period resulted in a re-setting of the payment schedule back to the initial value for a cocaine negative urine ($2.50). The maximum possible earnings (i.e., 12 weeks of continuous abstinence producing 36 consecutive cocaine negative urines) under this schedule was $1155 (an average of $96.25 per week for the 12 weeks). This voucher schedule is similar to that previously shown to be effective (Higgins et al., 1991).
2.4. Urine Samples
Urine samples were collected, thrice weekly, under supervision and tested on-site for cocaine (benzoylecgonine). Urine samples were tested using a Syva 30R Chemical Batch Analyzer. Serial dilutions of qualitative positive tests, using a standard cut-off of 300 ng/ml, were conducted in order to derive quantitative benzoylecgonine concentrations. Qualitative cocaine urine results were collected for the entire duration of the study, whereas quantitative cocaine urine results were only collected for weeks 2-19.
2.5. Standard Care
Counseling occurred in conjunction with methadone maintenance therapy. Participants received psychosocial counseling (individual and group) based on a manualized staged-levels of treatment. Subjects could be administratively discharged for violating the clinic rules (i.e., threatening staff or other patients, dealing drugs) and participants who missed three consecutive days of methadone medication were discharged from the study and clinic.
2.6. Medications
All medications were prepared onsite and were directly administered by research nursing staff under the supervision of the pharmacy staff. No take home doses of medication were permitted during any phase of the study. An automated computer-controlled dispensing system was used for methadone dosing and subjects were titrated to 100 mg per day of oral methadone during study weeks 1-3. Participants who could not tolerate 100 mg per day of methadone had their dose induction halted at a tolerable dose. All methadone was administered in liquid form as a constant volume, mixed with cherry syrup to mask taste and ensure palatability.
All capsules were identical in weight and appearance and were dispensed in blister packages. Fluoxetine was prepared in capsules containing either 20 mg or 40 mg of active drug. There were two forms of placebo capsules. The first type contained lactose and the second type contained lactose and 10 mg of diphenhydramine. Diphenhydramine has been effectively used as a placebo control in previous clinical trials testing cocaine pharmacotherapies (Covi et al., 1995). Two lactose only placebo capsules were administered to all participants for the first three weeks of the study, prior to randomization. Starting in week four, subjects that were randomized to placebo received one diphenhydramine capsule and one placebo capsule each day. Subjects randomized to the fluoxetine condition received 20 mg per day for 3 days, then had 20 mg dose increases every 3-4 days until they were stabilized on 60 mg per day. During the fourth phase of the study (weeks 20-21), subjects were withdrawn from fluoxetine/placebo over a 2-week period. Patients and medical staff were blinded to both the fluoxetine/placebo and methadone doses as only the pharmacy staff (who did not have subject contact) were aware of random assignment and dosage.
2.7. Outcome Measures
The primary outcome measures were: 1) cocaine use, and 2) treatment retention. Cocaine use was operationalized as a dichotomous variable of use versus no use based on either positive urine toxicology testing (obtained thrice weekly) or subjects' self-report of cocaine use (based on once weekly self-reports of cocaine use). Treatment retention was operationalized as the number of days between study admission and discharge.
Secondary outcome measures included: side effects, opioid use based on urine toxicology screening, opioid use based on self-reports, any self-reported intravenous drug use, ASI composite scores, depression symptoms, anxiety symptoms, self-reports of cocaine craving, and clinicians ratings of modified global assessment of cocaine severity.
2.8. Analyses
The statistical analyses were conducted using Stata SE Version 10.2 (College Station, TX). All of the analysis used an intent-to-treat (ITT) sample, which included all subjects still enrolled at the beginning of week 8 (n=145), the start of the combined treatment evaluation phase of the study. Chi-square, Fishers Exact and ANOVA were used to determine statistical differences between the different treatment conditions on baseline characteristics. Statistical significance was defined as a p≤0.05. Survival analysis was used to assess treatment retention, and failure was defined as dropping out before completing the combined evaluation phase (week 19). Longitudinal data analyses (LDA) were used to model cocaine use using a population average model with maximum likelihood estimates. Longitudinal data analysis is appropriate because it accounts for the correlation of repeated measures on the same subjects (Fitzmauice, Laird, & Ware 2004), and robust estimation of standard errors was used because it is a more conservative estimation if the underlying correlation structure was not accurately identified. Given that the primary outcome variable (cocaine use) is dichotomous, the coefficients are presented as odds ratios for ease of interpretation. The correlation structure chosen allowed for unbalanced data and therefore all available data points are used in the estimation procedure.
The frequency and number of urine toxicology tests varied per subjects due to missed clinic days and holidays. Therefore the time variable for cocaine use based on urine toxicology screening was modeled as urine test number ranging from 1-36 (3 times weekly for 12 weeks). This procedure was used to ensure that each subject had no more than 3 urine test results per week and a maximum total of 36 test results during the 12 weeks. For self-reported cocaine use, time was modeled as study week.
The final analyses of cocaine use are presented as two models with use based on urine toxicology screening and self-reports. Model one includes separate bi-variable (unadjusted odds ratios) equations for each of the independent variables and model two is a single multi-variable equation (adjusted odds ratios). For ease of interpretation the adjusted odds ratios were transformed into predicted probabilities of cocaine use. The Optimal Design Software (Spybrook et al., 2008) was used to conduct a post-hoc test of power.
The bi-variable models include cocaine use as the dependent variable and separate equations for the following independent variables: treatment group and demographic characteristics (age, education level, race, gender, legal problems and employment status). The demographic characteristics included in the model were factors known to be associated with treatment response. Younger age, employment and not having legal problems are associated with better treatment outcomes (Grella et al. 1999; Hser et al. 2006). Race and gender have been differentially associated with treatment outcomes (Tuchman 2010; Brower & Carey 2003). The multi-variable model includes all of the independent variables that were statistically significant in the bi-variable equations (p≤0.05), as well as a treatment group by time interaction term. The models for the primary and secondary outcomes were similar with two exceptions. The primary outcome variables were based on data from the combined evaluation phase of the study, whereas the secondary outcome variables used data from the entire duration of the study. Opioid use and intravenous (IV) drug use were modeled as dichotomous variables, whereas the other secondary outcome measures were modeled as continuous variables.
3. Results
3.1. Baseline demographic characteristics
The CONSORT diagram (see Figure 2) displays the attrition of subjects throughout the 33 weeks of the study. Twelve subjects were excluded from the study prior to randomization and 22% (n=41) of those randomized discontinued the study prior to week 8. Among those 41 subjects who were randomized but did not begin the combined evaluation phase of the study: 31 were discharged because they missed three consecutive clinic days, 4 were hospitalized, 2 were incarcerated, 2 requested withdrawal, 1 had an adverse event and 1 was administratively discharged. The intent-to-treat analysis was restricted to those subjects (n=145) who were still enrolled at the start of the combined evaluation of the study (week 8) because: 1) the behavioral intervention began at this time, and 2) subjects may not have been stabilized on fluoxetine prior to this time.
Figure 2. Study Profile.
The randomized groups were balanced in terms of demographic characteristics and baseline BDI scores, with the exception of baseline legal involvement (see Table 1). A higher proportion of subjects in the FV group were involved with the criminal justice system at baseline. The average age of the 145 participants was 39 years old, 45.5% (n=66) were female, 48.3% (n=70) were Black and on average the subjects had 11.5 years of education. The majority of subjects did not have any legal issues (72.4%, n=105) and were unemployed (75.9%, n=110) at baseline. Furthermore, there were no statistically significant differences at baseline on any of the ASI composite scores (data not shown). The mean voucher earnings for participants in the FV group (n=35) was $302.87 and $553.44 in the PV group (n=33).
Table 1. Demographic & Psychosocial Characteristics Reported at Baseline by Treatment Group in ITT Sample (n=145).
| Baseline Characteristics | FV (N=35) |
PV (N=33) |
F (N=38) |
P (N=39) |
p-value |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean | 37.1 | 40.4 | 38.6 | 39.1 | NS |
| SD | 8.0 | 7.1 | 7.2 | 8.6 | |
| Gender (%) | |||||
| Male (79) | 54.3 | 57.6 | 57.9 | 48.7 | NS |
| Female (66) | 45.7 | 42.4 | 42.1 | 51.3 | |
| Race (%) | |||||
| White (72) | 45.7 | 39.4 | 52.6 | 59.0 | NS |
| Black (70) | 51.4 | 57.6 | 44.7 | 41.0 | |
| Other (3) | 2.9 | 3.0 | 2.6 | 0 | |
| Education (years) | |||||
| Mean | 11.7 | 11.4 | 11.5 | 11.3 | NS |
| SD | 1.6 | 1.2 | 1.7 | 1.7 | |
| Legal Involvement (%) | |||||
| No (105) | 51.4 | 84.9 | 81.6 | 71.8 | <0.00 |
| Yes (40) | 48.6 | 15.1 | 18.4 | 28.2 | |
| Employed (%) | |||||
| No (110) | 71.4 | 72.7 | 81.6 | 76.9 | NS |
| Yes (35) | 28.6 | 27.3 | 18.4 | 23.1 | |
| BDI Total Score | |||||
| Mean | 12.1 | 15.6 | 13.5 | 13.0 | NS |
| SD | 9.6 | 11.4 | 8.7 | 9.4 | |
| STAI State Score | |||||
| Mean | 34.6 | 34.9 | 37.2 | 36.5 | NS |
| SD | 5.6 | 5.2 | 7.6 | 6.3 | |
| STAI Trait Score | |||||
| Mean | 39.5 | 40.3 | 40.0 | 40.4 | NS |
| SD | 6.1 | 4.7 | 6.6 | 5.3 | |
| Cocaine Cravinga | |||||
| Mean | 39.7 | 33.8 | 38.6 | 37.8 | NS |
| SD | 27.9 | 28.0 | 26.1 | 27.6 | |
| Heroin Cravinga | |||||
| Mean | 37.0 | 45.2 | 38.8 | 35.4 | NS |
| SD | 33.0 | 32.9 | 32.0 | 28.8 |
NOTE: NS=not significant, SD=standard deviation, FV=fluoxetine plus vouchers, PV=placebo plus vouchers, F=fluoxetine without vouchers, P=placebo without vouchers;
Cocaine and heroin craving was self-reported on a visual analog scale of 0-100 at study week 2
3.2. Retention in treatment
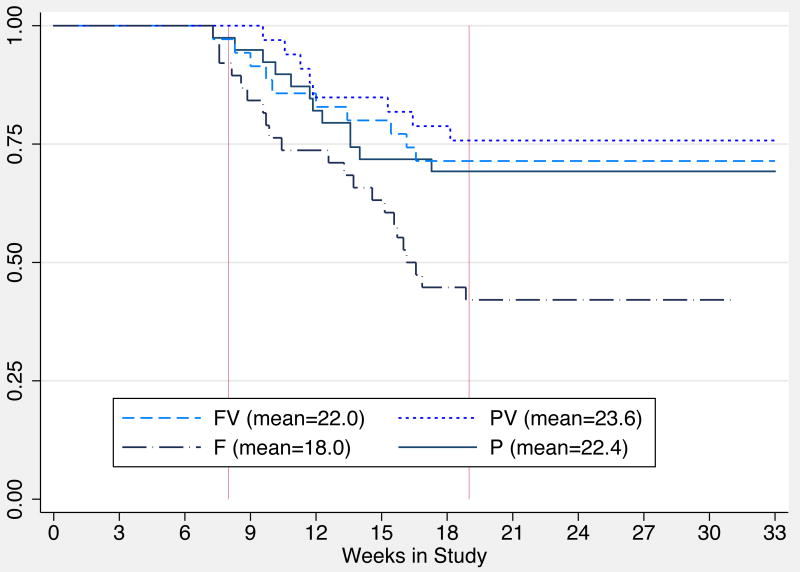
The average retention in weeks was: 22.0 (SD=7.5) in the FV group; 23.6 (SD=6.9) in the PV group; 18.0 (SD=7.5) in the F group; and 22.4 (SD=8.2) in the P group. The PV group was significantly more likely to stay in treatment longer compared to the F group (an average of 5.7 weeks longer; HSD-test 4.49). The survival analysis indicates a statistically significant difference in time to study drop out by group (χ=11.17, p=0.01). Kaplan Meier survival estimates are displayed in Figure 3. Thirty five percent of the sample (n=51) dropped out before completing the combined treatment evaluation phase of the study and the reasons for non-completion are displayed at the bottom of Figure 2.
Figure 3. Kaplan-Meier Survival Estimates of Treatment Retention by Treatment Group in the ITT Sample (n=145).
Note: Survival was defined as remaining in treatment through the end of week 18.
3.3. Cocaine Use
Model 1 for cocaine use (Table 2), presents the bi-variable logistic regression equations that are not adjusted to account for the effect of treatment condition over time nor other potentially important covariates. Based on the urine toxicological testing for cocaine use (first column, Table 2), subjects in the PV group were significantly less likely to use cocaine (76%) compared to the P group. Subjects in either F or FV groups were less likely to use cocaine (18%, p=0.66; 33%, p=0.40 respectively) compared to the P group, however this difference was not statistically significant. The subjects' age, years of education, race, legal status or employment status were not significantly related to cocaine use based on toxicology testing, but females had an elevated odds of cocaine use based on urine toxicology in this model (OR=2.44, p<0.00). Model 2 incorporates all of the variables that were statistically significant at the bi-variable level. Based on the urine toxicology results for cocaine use (second column, Table 2), there were no between group differences for the four study conditions nor group X time effects. The adjusted predicted probabilities of cocaine use based on urine testing across the 4 treatment groups were: 31% in the PV group, 56% in the FV group, 60% in the F group and 65% in the P group. The study was underpowered to detect differences in probabilities of cocaine use less than 0.25 (power=0.80) and therefore there is a high probability of making a type II error when comparing the fluoxetine and placebo groups.
Table 2. Longitudinal Logistic Regression Models of Cocaine Use (N=145).
| Cocaine Use based on Qualitative Urine Toxicology | Cocaine Use based on Self-Reports | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 (Bi-variable) | Model 2 (Multi-variable) | Model 1 (Bi-variable) | Model 2 (Multi-variable) | |||||||||
| OR | SE | p | AOR | SE | p | OR | SE | p | AOR | SE | p | |
| Treatment Groups | ||||||||||||
| P (control, reference) | ||||||||||||
| FV | 0.67 | 0.32 | 0.40 | 0.46 | 0.30 | 0.23 | 0.91 | 0.40 | 0.83 | 0.26 | 0.20 | 0.08 |
| PV | 0.24 | 0.11 | <0.00 | 0.43 | 0.27 | 0.17 | 0.43 | 0.20 | 0.06 | 0.15 | 0.12 | 0.02 |
| F | 0.82 | 0.38 | 0.66 | 0.84 | 0.48 | 0.75 | 1.43 | 0.60 | 0.39 | 0.36 | 0.26 | 0.16 |
| Treatment Group X Time | ||||||||||||
| P (control, reference) | ||||||||||||
| FV | 0.99 | 0.01 | 0.64 | 1.11 | 0.06 | 0.07 | ||||||
| PV | 0.98 | 0.02 | 0.34 | 1.10 | 0.07 | 0.16 | ||||||
| F | 1.02 | 0.01 | 0.15 | 1.14 | 0.06 | 0.02 | ||||||
| Age (years) | 1.03 | 0.02 | 0.18 | 1.01 | 0.02 | 0.47 | ||||||
| Education (years) | 1.06 | 0.11 | 0.59 | 0.95 | 0.09 | 0.63 | ||||||
| Race | ||||||||||||
| White (reference) | ||||||||||||
| Black | 0.67 | 0.21 | 0.19 | 1.23 | 0.38 | 0.52 | 1.06 | 0.34 | 0.86 | |||
| Other | 0.43 | 0.50 | 0.46 | 0.07 | 0.07 | 0.01 | 0.06 | 0.03 | <0.00 | |||
| Gender | ||||||||||||
| Male (reference) | ||||||||||||
| Female | 2.44 | 0.78 | <0.00 | 2.46 | 0.81 | <0.00 | 3.43 | 1.07 | <0.00 | 3.64 | 1.15 | <0.00 |
| Legal Problems | ||||||||||||
| No (reference) | ||||||||||||
| Yes | 1.35 | 0.51 | 0.42 | 1.10 | 0.38 | 0.79 | ||||||
| Employed | ||||||||||||
| No (reference) | ||||||||||||
| Yes | 0.53 | 0.19 | 0.08 | 0.50 | 0.19 | 0.07 | ||||||
NOTE: OR=Odds ratio, SE=Standard error, AOR=Adjusted odds ratio
Using the outcome of self-reported cocaine use, model 1 showed that no treatment group was significantly associated with use (third column, Table 2). The PV group had less cocaine use (57%) relative to the P (control) group, however this difference did not achieve statistical significance (p=0.06). In addition, in this model subjects in the other race category (versus White) and being female were significantly associated with an increased odds of self-reported cocaine use.
In contrast to the toxicological findings, at the initiation of the combined treatment phase of the study self-reported cocaine use for the PV group (fourth column, Table 2) had a lower odds of cocaine use (OR=0.15, p=0.02) and the F group had an increased odds of cocaine use during the combined treatment phase (OR=1.14, p=0.02). Females were again more likely to use cocaine in this model based on self-reports, even after controlling for treatment group.
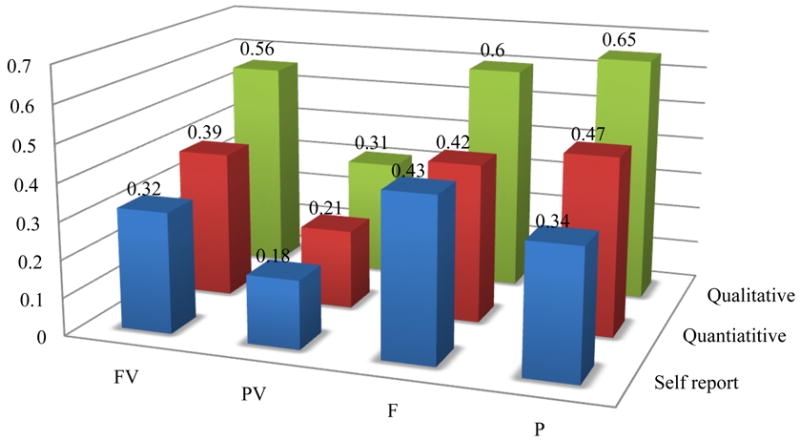
Sensitivity analyses were conducted to explore potential reasons why cocaine use based on urine testing in the PV group was significantly lower at the onset of the 12-week combined evaluation period and yet did not continue to decline during this study phase. The final multi-variable model, based on urine toxicology screen of cocaine use, was refitted using a spline at week 3, whereby a spline allows the model to have 2 separate slopes before and after week 3. The results suggested that there was a statistically significant 12% reduced odds of cocaine use in the PV group prior to week 3, but not thereafter. Additionally, the model was re-fit using the quantitative urine results. The quantitative urine results were first recoded into the following categories: no cocaine use, no new cocaine use, and cocaine use whereby no new cocaine use was calculated based on a 50% reduction in benzoylecgonine level from the previous urine test (Preston et al., 1997). The quantitative urine results were then recoded into no cocaine use/no new cocaine use versus cocaine use. The results of this analysis produced nearly the same results as those for the self-reports of cocaine use, however the probability of cocaine use across the groups was notably lower. Using the transformation of the quantitative urine results, the probability of a cocaine positive urine samples was 21% in the PV group, 39% in the FV group, 42% in the F group, and 47% in the P group. Figure 4 displays the comparison of the predicated probabilities of cocaine use based on the qualitative urine toxiocology results, the quantitative toxicology results (recoded using the Preston Rule), and the self-reports of cocaine use. There were no statistical differences between the predicated probabilities of cocaine use based on self-report compared to the quantitative urine toxicology, whereas there was a statistically significant greater proportion of cocaine use reported in the qualitative urine toxicology compared to both the quantitative urine toxicology and self-reported cocaine use in the FV, F and P groups. There were no statistical differences in the probability of cocaine use in the PV group.
Figure 4. Comparison of Predicated Probabilities of Cocaine Use Comparing Qualitative Urine Toxicology, Quantitative Urine Toxicology, and Self-Reports by Treatment Group.
3.4. Side Effects
There were no differences in the total number of side effects reported between subjects on fluoxetine compared to subjects who received placebo, however there were differences with respect to the presence/absence of specific symptoms. Subjects who received fluoxetine were more likely to report: dreaming more (OR=1.06, p=0.01, 95% CI: 1.01, 1.11), dry mouth (OR=1.07, p=0.01, 95% CI: 1.02, 1.12), tremors (OR=1.09, p=0.02, 95% CI: 1.01, 1.16), and nervousness (OR=1.08, p=0.03, 95% CI: 1.02, 1.14). Finally, there were no statistically significant differences in the total number of side effects reported at each study week between subjects who did and subjects who did not complete the combined evaluation phase of the study.
3.5. Secondary Outcome Measures
There were no between group differences in depression symptoms, anxiety symptoms, opioid use (based on either urine toxicology or self-reports), or self-reported IV drug use during the study. Irrespective of group assignment; the ASI drug and legal composite scores declined during the study. The PV group had a slightly higher ASI alcohol composite score and no other ASI scores differences were noted. Subjects in the PV group were overall less likely to report cocaine craving, but there were no statistically significant between group differences over time for craving scores. Subjects in the PV group had higher ratings, suggesting improvement, on the clinician-reported modified global assessment of cocaine severity.
Fluoxetine was not effective in reducing either depression or anxiety symptoms during the combined evaluation phase of the study, although there was a statistically significant reduction in total BDI scores (β.=-0.20, p<0.00) when all study weeks were included. In a sub-analysis of subjects with persistent clinically meaningful depressive symptoms at study week 4 (n=36) (Winstanley et al., 2008), fluoxetine was not effective in reducing depressive symptoms.
4. Discussion
The results of this study suggest that fluoxetine may not be efficacious in reducing cocaine use in patients that are dually dependent on cocaine and opioids. Subjects in the PV group had the best outcomes, with the longest duration of treatment retention (165 days), the lowest probability of cocaine use (69% cocaine abstinent) and the greatest improvement in their cocaine problem according to clinical staff ratings. The adjusted predicted probabilities of a cocaine positive urine sample across the 4 treatment groups were: 31% in the PV group, 56% in the FV group, 60% in the F group and 65% in the P group. The between group differences in cocaine use based on qualitative urine toxicology screens were not statistically significant, whereas based on the self-report of cocaine use the PV group had a lower odds of use and the F group had an increased odds of use. It is possible that the reduced probability of cocaine use in the PV group at the onset of the combined evaluation period was an expectancy effect reflecting the anticipation of receiving vouchers for cocaine abstinence. Overall, the BDI scores across all groups were clinically low at baseline and this may partially explain why a robust antidepressant effect for fluoxetine was not detectable.
A recent meta-analytic review of psychosocial interventions for substance use disorders found the largest effect size estimates were for contingency management (Dutra et al., 2008), and consistent with this finding, the present study found the best outcomes were observed in the behavioral (contingency management) intervention group (PV).
There were several strengths of the study design including ensured and observed dose ingestion, intensive urine testing, and the use of incentives to enhance the level of motivation. The reduction of cocaine use observed in the study suggests that subjects were able to decrease their use, however they were not responsive to the pharmacological intervention. The predicted probabilities of cocaine use (Figure 4) were highest using the qualitative toxicology reports and lowest using the self-report of cocaine use. This is consistent with studies that have found research participants may under-report use, and qualitative toxicology results may over-report use (Somoza et al. 2008), because participants may continue to test positive for several days after discontinuing cocaine use. It is interesting to note that there were no statistical differences in cocaine use in the PV group or between the self-report and the quantitative toxicology predicated probabilities of use (recoded using the Preston Rule). Most notably, the PV group had much lower probabilities of cocaine use across the different measures of cocaine use suggesting that this finding is robust.
There are five study limitations worth noting. First, the sample size was not large enough to detect a modest treatment effect. Second, there was a statistically significant between treatment group difference in legal involvement at baseline. Nearly 50% of subjects in the FV group had legal problems at baseline and subjects in the FV group were significantly more likely to report legal problems as a reason why they terminated early from treatment (OR=28.0, p=0.03). These results suggest that legal involvement may be an important consideration when stratifying randomization, particularly if treatment retention is a primary outcome variable. Third, subjects who terminated treatment early were more likely to report that either getting a job or keeping a job was a reason for early termination (OR=3.0, p=0.04) when compared to those who completed the study. This study was conducted in the context of methadone treatment and the numerous weekly assessments, in addition to daily supervised dosing, may have been challenging for subjects that obtained jobs during normal business hours. Fourth, the combined evaluation phase of the study did not begin until study week seven and this may contribute to the number of subjects who dropped out. Finally, this study was conducted within the context of methadone maintenance therapy and therefore subjects may not have been seeking treatment for their cocaine dependence.
5. Conclusion
In summary, this outpatient clinical trial was designed to maximize the detection of a positive anti-cocaine effect with fluoxetine: supervised daily doses were administered, a higher dose of this medication was used compared to several previous studies, and an array of outcome measures were collected to detect a signal for therapeutic effect. The results from the study showed that the enrolled population and the study methods were sensitive to detecting and responding to an effective therapeutic intervention, as evidenced by the significant decrease in cocaine use in the PV group. However, fluoxetine did not demonstrate efficacy and this finding is consistent with the conclusion of a meta-analysis of pharmacological treatments of cocaine dependence (de Lima et al., 2002).
The results suggest that vouchers may not have the anticipated efficacy when patients are taking fluoxetine, and that fluoxetine may actually attenuate the efficacy of vouchers. Anecdotally, clinicians report that fluoxetine may increase apathy (Hoehn-Saric et al., 1990), which in turn may minimize the perceived value of vouchers. In terms of treatment retention, the only statistically significant difference was between the PV and F groups which is interesting given that the study design did not include a non-contingent voucher group. While a non-contingent voucher condition may have improved treatment retention in the P and F groups, it may have obscured the finding that efficacy of vouchers may be decreased when patients are taking fluoxetine. Additional research on the relationship between fluoxetine and apathy maybe warranted.
Two important methodological considerations regarding study retention can be taken from this study and may be relevant to future studies. First, stratification on legal involvement may be necessary to ensure equitable study attrition between groups. Second, early study termination was associated with gaining employment. Studies with numerous assessments during standard business hours may negatively impact study attrition even though gaining employment is a positive outcome of treatment. This has implications for assumptions about missing data, as well as the design of studies.
The sample size may have precluded finding a small treatment effect and in subpopulations of patients that are treatment-resistant even modest progression towards abstinence may be clinically meaningful. Consistent with prior studies the present data support the conclusion that the incentive-based behavioral approaches remain at present the most effective treatments available for cocaine dependence.
Acknowledgments
Preliminary results of this study were presented at the College on the Problems of Drug Dependence in 2006 and 2008. The original study was supported by grants from the National Institute on Drug Abuse (DA10754 and DA00332) and further analyses were also supported by NIDA grants T32 DA07209 and DA023186.
Footnotes
Conflicts of Interest: The authors report no conflicts of interest relevant to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Batki SL, Manfredit LB, Jacob P, Jones RT. Fluoxetine for cocaine dependence in methadone maintenance: Quantitative plasma and urine cocaine/benzoylecgonine concentrations. J Clin Psychopharmacol. 1993;13:243–250. [PubMed] [Google Scholar]
- Batki SL, Washburn AW, Delucchi K, Jones RT. A controlled trial of fluoxetine in crack cocaine dependence. Drug Alc Depend. 1996;41:137–142. doi: 10.1016/0376-8716(96)01233-1. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Brower KK, Carey TL. Racially related health disparities and alcoholism treatment outcomes. Alcohol Clin Exp Res. 2003;27(8):1365–7. doi: 10.1097/01.ALC.0000080165.72243.03. [DOI] [PubMed] [Google Scholar]
- Durta L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York, NY: [Google Scholar]
- Fitzmauice GM, Laird NM, Ware JH. Applied Longitudinal Data Analysis. John Wiley & Sons, Inc.; Hoboken, NJ: 2004. [Google Scholar]
- Grabowski J, Rhoades H, Elk R, Schmitz J, David C, Creson D, Kirby K. Fluoxetine is ineffective for treatment of cocaine dependence or concurrent opiate and cocaine dependence: Two placebo-controlled, double-blind trials. J Clin Psychopharmacol. 1995;15:163–74. doi: 10.1097/00004714-199506000-00004. [DOI] [PubMed] [Google Scholar]
- Grella CE, Hser Y, Joshi V, Anglin MD. Patient histories, retention, and outcome models for younger and older adults in DATOS. Drug Alc Depend. 1999;57:151–66. doi: 10.1016/s0376-8716(99)00082-4. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991;148:1218–24. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, Lipsey JR, McLeod DR. Apathy and indifference in patients on fluvoxamine and fluoxetine. J Clin Psychopharmacol. 1990;10:343–5. [PubMed] [Google Scholar]
- Hser Y, Stark ME, Paredes A, Huang D, Anglin MD, Rawson R. A 12-year follow-up of a treated cocaine-dependent sample. J Subst Abuse Treat. 2006;30:219–26. doi: 10.1016/j.jsat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation for substance abuse patients: The Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Schmitz JM, Steinberg JL, Green CM, Reist C, Lai LY, Swann AC, Grabowski J. Citalopram combined with behavioral therapy reduces cocaine use: a double-blind, placebo-controlled trial. Am J Drug Alcohol Abuse. 2007;33:367–78. doi: 10.1080/00952990701313686. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwall L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101:1546–60. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Schuster CR, Cone EJ. Assessment of cocaine use with quantitative urinanalysis and estimation of new uses. Addiction. 1997;27:717–27. [PubMed] [Google Scholar]
- Schmitz JM, Rhoades HM, Elk R, Creson D, Hussein I, Grabowski J. Medication take-home doses and contingency management. Exp Clin Psychopharmacol. 1998;6:162–8. doi: 10.1037//1064-1297.6.2.162. [DOI] [PubMed] [Google Scholar]
- Somoza E, Somoza P, Lewis D, Li SH, Winhusen T, Chiang N, Vocci F, Horn P, Eklashef A. The SRPHK1 outcomes measure for cocaine-dependence trials combines self-report, urine benzoylecgonine levels, and the concordance between the two to determine a cocaine-use status for each study day. Drug Alc Depend. 2008;93:132–40. doi: 10.1016/j.drugalcdep.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Inventory for Adults. Mind Garden; Redwood City, VA: 1983. [Google Scholar]
- Spybrook J, Raudenbush SW, Liu X, Congdon R, Martinez A. Optimal Design for Longitudinal and Multilevel Research: Documentation for the “Optimal Design” Software 2008 [Google Scholar]
- Tuchman E. Women and addiction: The importance of gender issues in substance abuse research. J Addict Dis. 2010;29(2):127–38. doi: 10.1080/10550881003684582. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Sullivan JT, Fromme R, Bigelow GE. Fluoxetine alters the effects of intravenous cocaine in humans. J Clin Psychopharmacol. 1994;14:396–407. [PubMed] [Google Scholar]
- Winstanley EL, Strain EC, Bigelow GE. Lack of efficacy of fluoxetine in depressed cocaine dependent subjects. College on the Problems of Drug Dependence; 2008. [Google Scholar]