Abstract
Gamma-aminobutyric acid (GABA) is a major inhibitory neurotransmitter in the brain. Understanding the GABA concentration, in vivo, is important to understand normal brain function. Using MEGA point resolved spectroscopy (MEGA-PRESS) sequence with interleaved water scans to detect subject motion, GABA level of sensorimotor cortex was measured using a voxel was identified from a functional MRI scan. The GABA level in a 20 × 20 × 20 mm3 voxel consisting of 37 ± 7% GM, 52 ± 12% WM, and 11 ± 8% CSF in the sensorimotor region was measured to be 1.43 ± 0.48 mM. In addition, using linear regression analysis, GABA concentrations within gray and white matter were calculated to be 2.87 ± 0.61 and 0.33 ± 0.11 mM, respectively.
Keywords: GABA, MRS, sensorimotor cortex, gray matter, white matter
1. Introduction
Spectroscopic measurement of GABA has drawn much attention in recent years due to its importance as a major inhibitory transmitter in human brain. Abnormalities in GABA level have been associated with several neuropsychiatric disorders [1]. However, due to the very low concentration of GABA in brain, its Nuclear Magnetic Resonance (NMR) signal-to-noise ratio is low. This makes in vivo GABA measurement very difficult in clinical situations. More importantly, it is difficult to separate GABA resonance peaks from overlapping peaks from other metabolites at the field strength of most clinical scanners. Thus, spectral editing is required for GABA measurement.
GABA is the primary inhibitory neurotransmitter and is critical to normal brain function. Accurate measurement of in vivo GABA levels in specific brain regions may provide unique, information about the underlying mechanisms of brain function in both healthy and diseased states. The present study focuses on GABA in the sensorimotor cortex, which is clinically relevant for several reasons. Reduction in GABA has been reported to facilitate long term potentiation-like activity in motor cortex [2,3]. GABAergic inhibition has been identified as one of the mechanisms operating in use-dependent plasticity in intact human motor cortex, suggesting similarities in the mechanisms underlying this form of plasticity and long-term potentiation [4]. Profound reorganization in somatosensory cortex has been associated with motor learning [5] and rapid modulation of GABA concentration in sensorimotor cortex during motor learning has been reported [6]. This reorganization can be suppressed with the GABA agonist lorazepam [5]. Decreased sensorimotor cortex GABA levels have been observed in focal dystonia [7], and rapid fluctuation of GABA level in sensorimotor cortex induced by acute deafferentation have been reported [8].
At clinical field strengths, J-difference spectral editing is widely used [9–12] in order to measure the GABA concentration, in-vivo. Since J-difference editing is a subtraction technique, subject motion can lead to misleading results [13]. Identification and exclusion of motion-corrupted data is extremely important in scans involving J-difference editing sequences. Failure to do so can lead to erroneous results [13].
Regional fluctuations as well as large differences in GABA concentration in cortical gray matter and sub-cortical white matter have been reported by several investigators [14–17]. For this reason it is important to separate out the cortical gray matter GABA concentration at sensorimotor cortex from the white matter GABA concentration in single voxel spectroscopic measurements where the voxel size is large enough to include different tissue types.
In the present study, using MEGA-PRESS sequence [12] with proper quality control for motion [13], we have measured in vivo GABA levels in the sensorimotor cortex in a manner suitable for use with patients (small voxel and short scan time). In addition, using linear regression analysis, we have obtained GABA concentration in cortical gray matter and white matter in the sensorimotor cortex (SMC). This constitutes the first single-voxel MR spectroscopy study to measure the sensorimotor cortex GABA level in a clinically relevant way, while controlling for subject motion and accounting for possible gray/white matter dependence of GABA.
2. Materials and methods
Data acquisition
All of the procedures involved in obtaining these data were approved by the institutional review board (IRB) of Cleveland Clinic. MR scans were performed using a 3 tesla Siemens Trio scanner (Erlangen, Germany). A circularly polarized (CP) head coil was used. Nineteen healthy volunteers were scanned with a sequence based on the MEGA-PRESS sequence as designed by Mescher et al[12]. In this sequence the outer members of the 3.01 ppm GABA resonance triplet are refocused in one scan by a frequency selective pulse (ON resonance), and not refocused in the subsequent scan (OFF resonance), while the central member and the overlapping creatine resonance peaks are unaffected. Subtraction of the spectra from the two scans suppresses the creatine peak and the central member of the triplet and retains the outer members. In order to identify motion-corrupted data, we used water signal based interleaved navigator pulses [13], which have been shown to be an effective way to discard only the portion of the motion-corrupted data from a scan, and to reduce possible misinterpretation of the edited spectra.
The complete protocol consisted of the following scans: (i) localizer to get scout image, (ii) gradient echo field mapping, (iii) whole brain T1 weighted magnetization prepared rapid gradient echo scan (MPRAGE), (iv) functional MR scan for voxel localization, (v) single voxel MEGA-PRESS scan with interleaved navigator, (vi) MEGA-PRESS scan with interleaved navigator and with an inversion pulse for metabolite nulling, (vii) PRESS scan, (viii) PRESS without water suppression, (ix) repetition of the localizer scan.
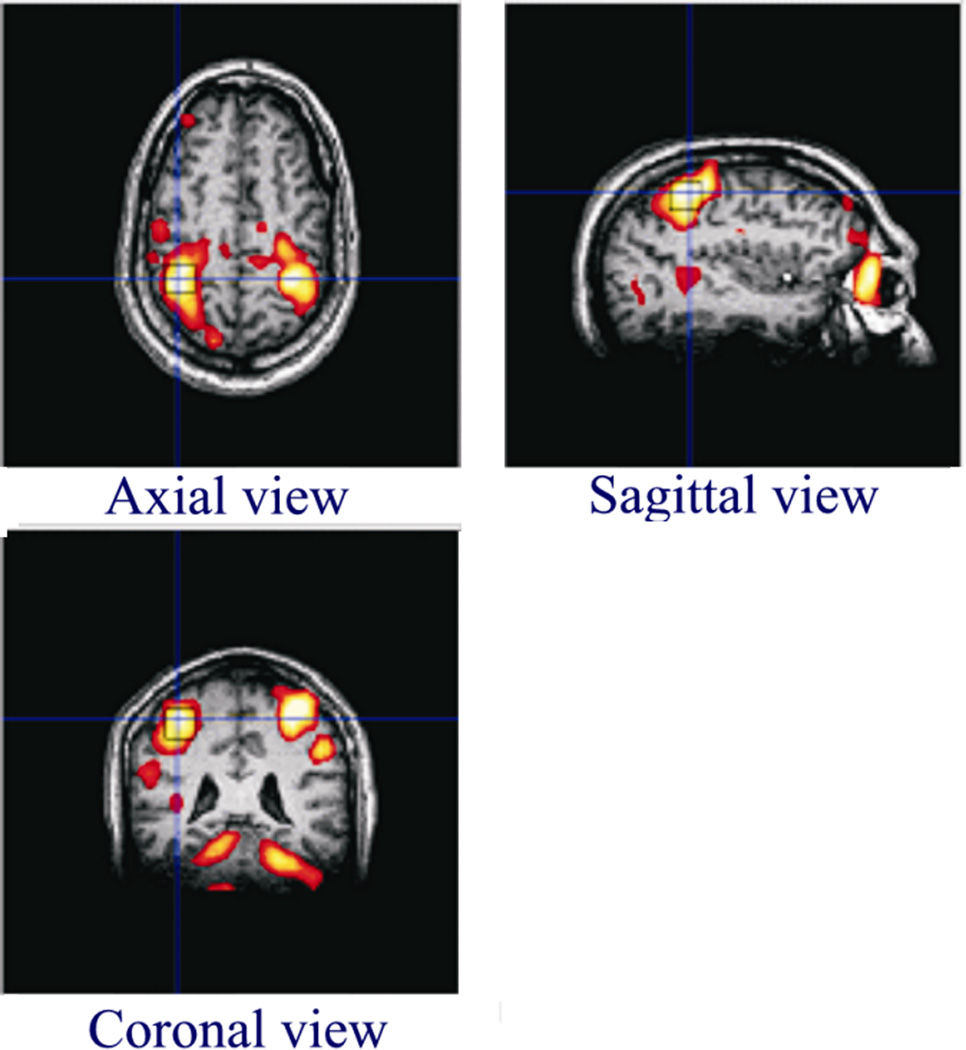
The motor cortex was identified prior to the spectroscopy scan from the activated regions in SMC determined from the fMRI scan (scan iv). In this scan, each subject performed self-paced bilateral finger tapping (index finger simultaneously in opposition to the thumb on each hand) in blocks of interleaved 32 second ON and 32 second OFF pattern. The gradient-echo echo-planar scan parameters were as follows: TR = 2000 ms, TE = 30 ms, flip angle = 90°, 32 transverse sections, slice thickness = 4 mm without any inter-slice gap, 24 cm×24-cm field of view, 64×64 matrix, 1954 Hz/pixel receiver bandwidth, 160 volume repetitions. The voxel selection was accomplished by utilizing real time fMRI Student’s t-statistic activation map generation program Neuro3D (Siemens Medical Solutions). A single voxel (2.0 cm × 2.0 cm × 2.0 cm) centered at the area of maximum activation in the right hemisphere pre-central gyrus, was selected for the spectroscopy scans, as shown in Fig. 1. The fMRI activation was thresholded at t ≥ 4.0 (p < 0.001) for this purpose.
Fig. 1.
Placement of a 20×20×20 mm3 voxel in the motor cortex. The voxel placement was done based upon area of maximum activation following a bilateral index finger tapping task.
The spectroscopy scans were performed on the selected voxel. The parameters for the GABA editing scan were: TR = 2700 ms, TE = 68 ms, water suppression bandwidth = 60 Hz, editing frequency-selective pulse bandwidth = the lowest allowable bandwidth of ~42 Hz, voxel size = 8 mL (2.0 cm × 2.0 cm × 2.0 cm), NEX = 96, total acquisition time = 8 min 38 sec. The frequency of the Gaussian editing pulse was set at the GABA C3H resonance frequency (1.90 ppm) for the ON resonance scan, while for the OFF resonance scan it was set at 1.50 ppm, which is symmetrical with respect to the 1.70 ppm macromolecule M4 resonance, in order to minimized macromolecule contamination [18]. For the metabolite nulling scan an inversion recovery time (TI) of 650 ms was used to identify any residual macromolecule contribution resulting from frequency drifts [19]. The TI of the scan was determined experimentally by scanning motor cortex of a control subject with different TIs and choosing the TI that minimize creatine peak in the spectrum. The parameters for the PRESS scans were: TR = 2700 ms, TE = 68 ms, NEX = 48 (NEX =1 for the PRESS scan without water suppression). Water suppression for the spectroscopy scans was done using WET (Water suppression Enhanced through T1 effects) technique [20].
Data analysis
MRS data were analyzed using jMRUI software package [21]. The first four measurements from each scan were ignored in order to ensure steady state magnetization. The un-suppressed navigator water signal amplitude for each measurement was determined using a Hankel Lanczos Squares Singular Value Decomposition (HLSVD) filter [22], and time-courses of water signal amplitude were obtained. As outlined in reference [13], datasets having more than 3% signal fluctuation were considered to be motion-corrupted, and only the data-points preceding the onset of motion were considered for analysis. In situations where motion happened towards the beginning of the scan, not enough good data-points could be recovered and the whole datasets were discarded.
The post-processing consisted of zero order phase correction and frequency shift correction of the individual subspectra using residual water as reference, summing up the individual phase- and frequency-corrected spectra, apodization by a 5 Hz Gaussian filter, and zero filling. The OFF-resonance spectrum was subtracted from the ON-resonance spectrum to obtain the final edited spectrum.
Next, the gray matter, white matter and CSF contribution to the voxel composition (fGM_vol, fWM_vol and fCSF_vol) was performed by using the FAST segmentation algorithm [23] of the FSL software library [24] with the anatomical 3D MPRAGE as the base image, and applying a mask at the voxel location. The percent volume of gray matter, white matter and CSF were used in order to perform absolute quantification of GABA level.
The GABA concentration was obtained in two steps:
- [GABA]/[Cr] ratio was obtained in the first step, from scans v and vi, using the following equation:
where G* is the area under the 3.01 ppm peak from the edited (subtracted) spectrum, M is the area under the macromolecule resonance peak at 2.99 ppm in the metabolite nulled spectrum after correcting the amplitude for T1 relaxation effects, ICr is the area under the 3.95 ppm methylene peak of creatine, and EE is the editing efficiency. The area under the peaks were measured using Advanced Method for Accurate, Robust, and Efficient Spectral fitting (AMARES) algorithm[25]. EE was obtained in a fashion similar to that by Terpstra et al [19] by comparing the unmodulated GABA signal relative to glycine from a PRESS scan (TR = 2700 ms, TE = 68 ms) with the edited GABA signal with respect to glycine from the same voxel of the same GABA-glycine phantom. In reference [19] T2 of Cr methyl and C4H GABA resonances were assumed to be identical. Mlynarik et al [26] reported T2 of Cr methyl and methylene resonances at the occipital lobe at 3T. Assuming similar T2 of the Cr resonances in occipital and motor areas, the difference in T2 relaxation effects between Cr methyl and methylene resonances was calculated to be negligible at TE=68ms. Hence, the T2 of the Cr methylene and of the C4H GABA resonance was assumed to be negligible in this study. Also, since the T1 of GABA is comparable with that of other metabolites [12], the T1 difference between GABA and Cr was neglected. We have used the methylene resonance of Cr instead of the widely used methyl resonance [9,19] in this calculation since, the use of methyl resonance can introduce a systematic error in the measurement of [GABA]/[Cr] ratio [27].[1] - Next, the creatine level, [Cr], was calculated from scans vii and viii, using the following equation as in [28]:
where, Icr is the area under the 3.93 ppm peak, fGM, fWM, and fCSF are fractions of gray matter, white matter and CSF water in the voxel, RH2O_GM, RH2O_WM, and RH2O_CSF are the relaxation attenuation factors for water in different tissue types, SH2O_obs is the area under the water peak in scan viii, RCr is the relaxation attenuation for creatine methylene resonance, #HCr (= 2) is the number of protons in Cr methylene, and [H2O] (= 55 M) is the concentration of pure water. fGM, fWM, and fCSF were calculated as in reference [28] using the volume fraction of GM, WM, and CSF in the voxel (fGM_vol, fWM_vol and fCSF_vol).[2]
The GABA concentration, [GABA] was next determined by taking the product of [1] and [2]. Finally, a statistical regression analysis was performed as in reference [29], and the gray matter and white matter GABA concentrations were calculated by plotting [GABA] vs fGM/(fGM+fWM); the GM and WM GABA concentration were estimated by extrapolating the regression line to fGM/(fGM+fWM) = 1 and 0 respectively.
3. Results and discussion
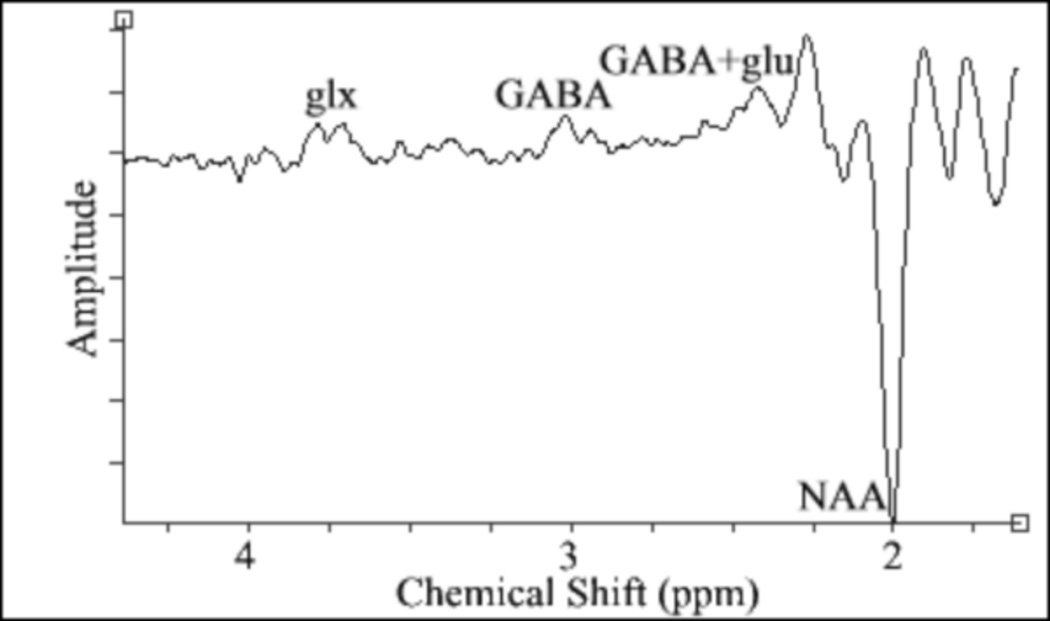
Eight of the nineteen acquired datasets were discarded because of unacceptable subject motion as determined by the criteria in our prior work [13]. Of the remaining eleven datasets, measurable GABA signal was observed in ten, while presence of GABA was inconclusive in one dataset. Sixteen out of a total 96 timepoints were discarded in one dataset because of motion, while all 96 timepoints were acceptable in the remaining nine datasets. A representative single subject GABA edited spectrum is shown in Fig. 2. GABA and glu peaks are clearly visible in the edited spectrum. Using MEGA-PRESS data from the ten subjects (age 38.4 ± 13.6 years) with measurable GABA signal, the [GABA]/[Cr] ratio of a 20 × 20 × 20 mm3 voxel in the sensorimotor region was determined to be 0.15 ± 0.05. The creatine concentration within the same voxel was determined to be 9.22 ± 0.81 mM, and the GABA concentration was 1.43 ± 0.48 mM. The mean voxel composition by volume was 37 ± 7% GM, 52 ± 12% WM, and 11 ± 8% CSF.
Fig. 2.
A representative single subject GABA edited spectrum. The 3.01 ppm GABA peak is clearly visible. The glutamate (glu) and glutamate+glutamine (glx) coedited resonances are also seen.
A plot of [GABA] vs fGM/(fGM+fWM) is shown in Fig. 3a. Following the regression analysis (R = 0.5077, P = 0.13, standard error= 0.29), the mean cortical gray matter and sub-cortical white matter GABA concentrations in the sensorimotor region were calculated to be 2.65 ± 0.57 and 0.36 ± 0.11 mM respectively. Individual measurement error was ~30%, and the datapoint with the maximum deviation from the linear fit (after considering the measurement error) was taken the to be an outlier. Upon discarding the single outlier, the GM and WM GABA concentrations were calculated to be 2.87 ± 0.61 and 0.33 ± 0.11 mM respectively. A plot of [GABA] vs fGM/(fGM+fWM) without the outlier is shown in Fig. 3b (R = 0.6415, P = 0.06, standard error = 0.23). The [GABA]/[Cr] ratio within the SMC voxel for these remaining nine subjects was 0.16 ± 0.04, and the GABA concentration was 1.51 ± 0.44 mM.
Fig. 3.
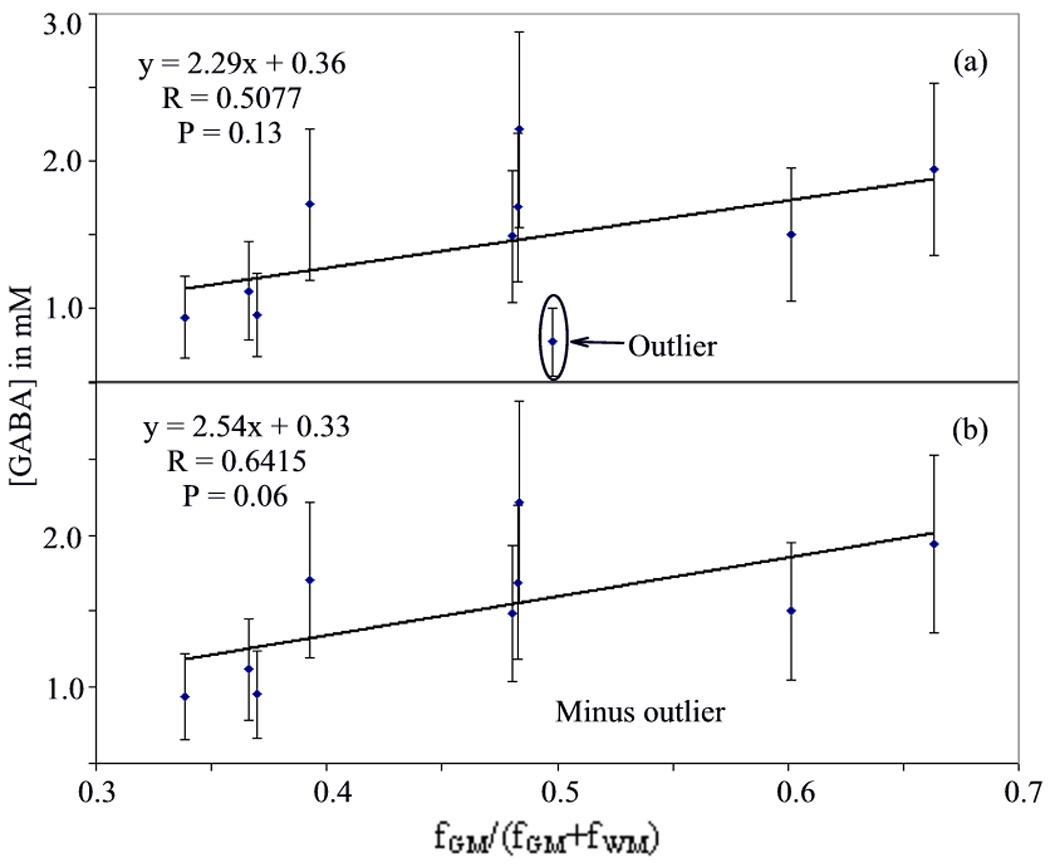
Linear fitting to determine the GM and WM GABA concentrations with (a) data from 10 subjects, and (b) data after removing the outlier.
The mean GABA level obtained from this study is in agreement with 1.49 ± 0.28 mM as obtained by Floyer-Lea et al. [6] (36 subjects, mean age: 25 years; range: 20–31 years). Although both studies were performed in sensorimotor cortex, Floyer-Lea et al. [6] did not account for varying GABA concentrations from the different tissue-types within the selected voxel. [GABA]/[Cr] level obtained from the current study is different from 0.20 ± 0.06 obtained by Levy et al. [7] (17 subjects). However, the voxel size used in that study [7] was 25 × 25 × 20 mm3, which is larger than that used in the current study. Also, similar to the case with the Floyer-Lea study [6], the voxel composition was not reported in reference [7], which could have contribution in the difference in the measured ratio. Using four subjects, Kalra et al. [30] reported a motor cortex GABA concentration of 0.72 ± 0.11 mM, which is different from our finding. However, the voxel size in reference [30] was 25 × 25 × 50 mm3, which is much larger than 20 × 20 × 20 mm3 used in the current study. The average CSF content in the voxels was significantly (100%) higher in the work of Kalra et el, which could contribute to the difference in the measured GABA level. In addition to the differences in voxel size and tissue composition, none of these prior studies accounted for possible subject motion, despite the long scan time required for the studies.
Using image segmentation and non-linear least square fitting in a CSI study, Choi et al. [17] reported GM and WM GABA concentration of 1.30 ± 0.36 mM and 0.16 ± 0.16 mM respectively in frontal and parietal lobes (N = 13). Using an approach of separating GM and WM GABA level by two-voxel data analysis, Choi et al. [31] reported GM and WM concentration of 1.1 ± 0.1 mM and 0.4 ± 0.1 mM respectively in medial prefrontal and left frontal lobes (N = 3). The GM GABA concentration obtained from this study is higher than those obtained by IY Choi et al [17] and C Choi et al [31]. On the other hand the WM GABA concentration obtained from this study is higher than that reported by IY Choi et al [17], but is statistically not different from that reported by C Choi et al [31]. The GM to WM GABA ratio, however, is in agreement with IY Choi et al [17]. The higher GM GABA concentration reported in this study compared with those in [17] and [31] may be reflective of the highly heterogeneous GABA distribution observed in biopsy study [14], which can be validated in future studies by placing voxels in different regions. It should be noted that the region of interest of the current study was functionally localized using fMRI. Further, the well-characterized anatomy of the underlying brain structure augments the fMRI data to ensure consistent localization within a given region brain parenchyma.
4. Conclusion
Using a MEGA-PRESS sequence combined with a navigator echo approach that permits excellent control of subject-motion related corruption of data, we have measured cortical GABA level in the sensorimotor cortex. Using linear regression analysis, we measured of gray matter and white matter GABA concentration from single voxel spectroscopy study localized with fMRI to the sensorimotor region. This approach of functionally defining the region of interest using fMRI can be used in other cortical regions to obtain cortical and subcortical GABA concentrations in different brain regions.
Acknowledgements
This work was supported by grants from National Institutes of Health and National Multiple Sclerosis Society. We greatly acknowledge Dr. Mark Brown for his generous support with pulse sequence development, and Ms. Jian Lin and Ms. Blessy Mathew for assisting in data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chang L, Cloak CC, Ernst T. Magnetic resonance spectroscopy studies of GABA in neuropsychiatric disorders. J Clin Psychiatry. 2003;64 Suppl 3:7–14. [PubMed] [Google Scholar]
- 2.Castro-Alamancos MA, Donoghue JP, Connors BW. Different forms of synaptic plasticity in somatosensory and motor areas of the neocortex. J Neurosci. 1995;15(7 Pt 2):5324–5333. doi: 10.1523/JNEUROSCI.15-07-05324.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castro-Alamancos MA, Connors BW. Short-term synaptic enhancement and long-term potentiation in neocortex. Proc Natl Acad Sci U S A. 1996;93(3):1335–1339. doi: 10.1073/pnas.93.3.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci U S A. 2000;97(7):3661–3665. doi: 10.1073/pnas.050350297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pleger B, Schwenkreis P, Dinse HR, Ragert P, Hoffken O, Malin JP, Tegenthoff M. Pharmacological suppression of plastic changes in human primary somatosensory cortex after motor learning. Exp Brain Res. 2003;148(4):525–532. doi: 10.1007/s00221-002-1324-1. [DOI] [PubMed] [Google Scholar]
- 6.Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J Neurophysiol. 2006;95(3):1639–1644. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- 7.Levy LM, Hallett M. Impaired brain GABA in focal dystonia. Ann Neurol. 2002;51(1):93–101. [PubMed] [Google Scholar]
- 8.Levy LM, Ziemann U, Chen R, Cohen LG. Rapid modulation of GABA in sensorimotor cortex induced by acute deafferentation. Ann Neurol. 2002;52(6):755–761. doi: 10.1002/ana.10372. [DOI] [PubMed] [Google Scholar]
- 9.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90(12):5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keltner JR, Wald LL, Christensen JD, Maas LC, Moore CM, Cohen BM, Renshaw PF. A technique for detecting GABA in the human brain with PRESS localization and optimized refocusing spectral editing radiofrequency pulses. Magn Reson Med. 1996;36(3):458–461. doi: 10.1002/mrm.1910360319. [DOI] [PubMed] [Google Scholar]
- 11.Hetherington HP, Newcomer BR, Pan JW. Measurements of human cerebral GABA at 4.1 T using numerically optimized editing pulses. Magn Reson Med. 1998;39(1):6–10. doi: 10.1002/mrm.1910390103. [DOI] [PubMed] [Google Scholar]
- 12.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11(6):266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharyya PK, Lowe MJ, Phillips MD. Spectral quality control in motion-corrupted single-voxel J-difference editing scans: an interleaved navigator approach. Magn Reson Med. 2007;58(4):808–812. doi: 10.1002/mrm.21337. [DOI] [PubMed] [Google Scholar]
- 14.Banay-Schwartz M, Palkovits M, Lajtha A. Heterogeneous distribution of functionally important amino acids in brain areas of adult and aging humans. Neurochem Res. 1993;18(4):417–423. doi: 10.1007/BF00967245. [DOI] [PubMed] [Google Scholar]
- 15.Perry TL, Hansen S, Berry K, Mok C, Lesk D. Free amino acids and related compounds in biopsies of human brain. J Neurochem. 1971;18(3):521–528. doi: 10.1111/j.1471-4159.1971.tb11980.x. [DOI] [PubMed] [Google Scholar]
- 16.Jensen EJ, Deb Frederick B, Renshaw PF. Grey and white matter GABA level differences in the human brain using two-dimensional, J-resolved spectroscopic imaging. NMR Biomed. 2005;18:570–576. doi: 10.1002/nbm.994. [DOI] [PubMed] [Google Scholar]
- 17.Choi IY, Lee SP, Merkle H, Shen J. In vivo detection of gray and white matter differences in GABA concentration in the human brain. Neuroimage. 2006;33(1):85–93. doi: 10.1016/j.neuroimage.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Henry PG, Dautry C, Hantraye P, Bloch G. Brain GABA editing without macromolecule contamination. Magn Reson Med. 2001;45(3):517–520. doi: 10.1002/1522-2594(200103)45:3<517::aid-mrm1068>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Terpstra M, Ugurbil K, Gruetter R. Direct in vivo measurement of human cerebral GABA concentration using MEGA-editing at 7 Tesla. Magn Reson Med. 2002;47(5):1009–1012. doi: 10.1002/mrm.10146. [DOI] [PubMed] [Google Scholar]
- 20.Ogg RJ, Kingsley PB, Taylor JS. WET, a T1- and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. J Magn Reson B. 1994;104(1):1–10. doi: 10.1006/jmrb.1994.1048. [DOI] [PubMed] [Google Scholar]
- 21.Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. Magma. 2001;12(2–3):141–152. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- 22.Pijnappel WWF, et al. SVD-based quantification of magnetic resonance signals. J. Magn. Reson. 1992;97:122–124. [Google Scholar]
- 23.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 24.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 25.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129(1):35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- 26.Mlynarik V, Gruber S, Moser E. Proton T (1) and T (2) relaxation times of human brain metabolites at 3 Tesla. NMR Biomed. 2001;14(5):325–331. doi: 10.1002/nbm.713. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharyya PK. ISMRM Workshop on MR Spectroscopy and Neurotransmitter Function in Neuropsychiatric Disorders. Quebec City, PQ, Canada: 2008. Systematic Error in the Measurement of [GABA]/[Cr] Ratio Using Methyl Resonance of Creatine. [Google Scholar]
- 28.Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med. 2006;55(6):1219–1226. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- 29.Hetherington HP, Pan JW, Mason GF, Adams D, Vaughn MJ, Twieg DB, Pohost GM. Quantitative 1H spectroscopic imaging of human brain at 4.1 T using image segmentation. Magn Reson Med. 1996;36(1):21–29. doi: 10.1002/mrm.1910360106. [DOI] [PubMed] [Google Scholar]
- 30.Kalra S, Choi C, Allen PS. Motor Cortex GABA Concentration as measured by Double-Quantum Filtering. Proc. Intl. Soc. Mag. Reson. Med. 2006;14:3062. [Google Scholar]
- 31.Choi C, Bhardwaj PP, Kalra S, Casault CA, Yasmin US, Allen PS, Coupland NJ. Measurement of GABA and contaminants in gray and white matter in human brain in vivo. Magn Reson Med. 2007;58(1):27–33. doi: 10.1002/mrm.21275. [DOI] [PubMed] [Google Scholar]