Abstract
We determined the temporal dynamic of cambial activity and xylem development of stone pine (Pinus cembra L.) throughout the treeline ecotone. Repeated micro-sampling of the developing tree ring was carried out during the growing seasons 2006 and 2007 at the timberline (1950 m a.s.l.), treeline (2110 m a.s.l.) and within the krummholz belt (2180 m a.s.l.) and the influence of climate variables on intra-annual wood formation was determined.
At the beginning of both growing seasons, highest numbers of cambial and enlarging cells were observed at the treeline. Soil temperatures at time of initiation of cambial activity were c. 1.5 °C higher at treeline (open canopy) compared to timberline (closed canopy), suggesting that a threshold root-zone temperature is involved in triggering onset of above ground stem growth.
The rate of xylem cell production determined in two weekly intervals during June through August 2006-2007 was significantly correlated with air temperature (temperature sums expressed as degree-days and mean daily maximum temperature) at the timberline only. Lack of significant relationships between tracheid production and temperature variables at the treeline and within the krummholz belt support past dendroclimatological studies that more extreme environmental conditions (e.g., wind exposure, frost desiccation, late frost) increasingly control tree growth above timberline.
Results of this study revealed that spatial and temporal (i.e. year-to-year) variability in timing and dynamic of wood formation of Pinus cembra is strongly influenced by local site factors within the treeline ecotone and the dynamics of seasonal temperature variation, respectively.
Keywords: Cambium, intra-annual growth, Pinus cembra, temperature, tracheid production
Introduction
There is extensive evidence that at high altitudes temperature has a direct effect on formation of new tissues and tree growth (see reviews by Körner 1998; Jobbagy and Jackson 2000; Grace et al. 2002). Since environmental factors (e.g., temperature, precipitation, solar radiation) change gradually with increasing altitude and the treelines of the world occur at seasonal mean air and ground temperatures between 5.5 and 7.5 °C (Körner 1998) and a global mean of 6.7 ±0.8 °C (Körner and Paulsen 2004), respectively, a threshold tissue temperature below which no growth processes occur is to be expected (cf. Hellmers et al. 1970; Grace et al. 1989). Recently, Rossi et al. (2007) linked wood formation in conifers at high altitudes with temperature and found evidence of the existence of thermal limits in xylogenesis.
Annual rings of trees are the most valuable and long-term climate proxy-data at an annual or even sub-annual resolution. Dendroclimatological methods are generally applied to identify the climatic factors most closely associated with variations in tree growth (Fritts 1976; Hughes 2002). These methods include the calculation of response functions, i.e. multiple regression techniques and/or Pearson-product-moment correlation analysis (Cook and Kairiukstis 1990), which reveal relationships between climate variables (e.g., monthly mean temperature and total precipitation for individual months of the year prior to growth and of the current year) and tree-ring series. Numerous dendroclimatological studies conducted in subalpine regions have shown that summer temperatures limit annual increment growth of trees (e.g., Eckstein and Aniol 1981; Oberhuber 2004; Büntgen et al. 2005; Frank and Esper 2005; Carrer et al. 2007). However, dendroclimatological studies, which analyse long-term data sets comprising several decades to centuries, are hampered by the fact that there is a lack of knowledge in the physiological basis of growth-climate relationships. On the other hand, ring width and cell number are highly correlated tree-ring parameters (Camarero et al. 1998; Vaganov et al. 2006). Therefore, it could be assumed that climate affects both parameters equally. That the determination of intra-annual dynamics of tracheid cell production and maturation are useful for understanding tree relationships with climate has been shown by e.g., Antonova and Stasova (1993), Mäkinen et al. (2003), Deslauriers and Morin (2005) and Rossi et al. (2006c, 2007).
Several studies on cellular phenology of annual ring formation at alpine timberline, where tree growth is strongly temperature-limited, have been published recently by Rossi et al. (2006b, 2006c, 2007, 2008). The aim of these studies, which were carried out in the eastern Italian Alps, was to assess timing and dynamics of wood formation in high-altitude conifer species, to define temperature thresholds for cambial activity and to determine age-dependent effects on xylogenesis.
Here, we focus on temporal dynamics of cambial activity and xylogenesis in Pinus cembra within the treeline ecotone in the Central Austrian Alps (Mt. Patscherkofel) during two growing seasons. Loris (1981) already in 1970s carried out a pioneer study on radial growth and cambial activity of Pinus cembra at timberline on Mt. Patscherkofel and found a close correlation between temperature sum and tracheid production. However, a comparison of the temporal dynamics of xylem development throughout the treeline ecotone in the Alps has not yet been published. Hence, study plots were selected along a southwest facing altitudinal transect including the timberline (upper limit of trees forming a closed canopy), treeline (isolated trees above the timberline ≥ 3 m height) and “krummholz” belt (outposts of short-stature individuals). We also hypothesized that open canopy above timberline, which causes advanced snow-melt and heating of root-zone at the beginning of the growing season compared to delayed soil warming under closed canopy at timberline (see Körner and Paulsen 2004), favours earlier onset of cambial activity of tree stems. That shoot growth and activity is tightly controlled by below ground signals has been reviewed by Tranquillini (1979) and Körner (1998). Since regional variability of climate-growth relationships of Pinus cembra has been reported recently by Carrer et al. (2007), this study also contributes to evaluation of response variability of xylogenesis to site related climate limiting factors.
Material and methods
Study area
The study area is situated at Mt. Patscherkofel (2246 m a.s.l.) near Innsbruck, in western Austria (47°12′N, 11°27′E). Mt. Patscherkofel is located in the Central Austrian Alps within an inner-alpine dry zone, where the local climate is strongly influenced by warm and dry southerly winds (Föhn), which most frequently occur in spring (March-May). This special situation is reflected in local climate. During the period 1967-2004 mean annual precipitation at the top of Mt. Patscherkofel was 890 mm with a maximum during summer (June-August: 358 mm) and minimum in winter (December-February: 147 mm). Although year-to-year variation in snow cover exists, south-facing slopes above timberline have only small patches of snow in April. Mean annual temperature at timberline during the same period was 2.5 °C and the coldest and warmest months were February (−4.3 °C) and July (10.0 °C), respectively.
Since the dominant conifer in the study area and at the timberline in the central part of the Eastern Alps is stone pine (Pinus cembra L.) - European larch (Larix decidua Mill.) and spruce (Picea abies (L.) Karst.) are scattered at some sites – we concentrated on the analysis of growth dynamics of this tree species. Study plots were selected on a southwest facing slope across the treeline ecotone, i.e. the transition zone from tall closed forest at timberline to the krummholz belt. Hence, the altitudinal gradient included the timberline (1950 m a.s.l.), treeline (2110 m a.s.l.) and krummholz limit (2180 m a.s.l.). Trees at the timberline and treeline reached a height of 10-14 m and 3-5m, respectively, whereas Pinus cembra krummholz was 50-100 cm tall. Krummholz-individuals, which showed no more than modest damage due to extreme environmental conditions, especially winter desiccation, were selected.
The geology of the Mt. Patscherkofel region (Tuxer Alpen as part of the Central Tyrolean Alps) is dominated by gneisses and schist. According to the World Base for Soil Resources (FAO 1998), the soil at the study site is classified as a haplic podzol, a soil type typical for the Central Austrian Alps (Neuwinger 1970).
Xylem sampling and determination of wood formation
Seasonal wood formation dynamics were monitored during the growing seasons 2006 and 2007 by taking small punched cores from 5 trees/site of the outermost tree rings (micro-cores) with a diameter and length of 2.5 mm and c. 2 cm, respectively (Rossi et al. 2006a). To determine the variability in intra-annual wood formation between trees at each plot (i.e., timberline, treeline and krummholz site), individual trees were randomly selected. Samples were taken on the slope-parallel side of the stem following a spiral trajectory up the stem and at timberline from c. 15 cm below breast height (1.3 m) to c. 15 cm above. At treeline and within the krummholz belt, samples were taken above the ground at c. 50 (± 15 cm) and c. 15 cm (± 5 cm), respectively. A distance of c. 2 cm in tangential and longitudinal direction was kept to avoid lateral influence of wound reactions on adjacent sampling positions.
Based on previous dendroclimatological studies carried out within the study area (Oberhuber 2004; Pfeifer et al. 2005; Oberhuber et al. 2008) xylem formation was expected to primarily occur in June-July. Therefore, micro-cores were taken at all study plots during June through October 2006 in weekly intervals to include the whole dynamic of xylem formation. Based on results of cambial activity in the first year sampling in 2007 was carried out from late April through October in about 10-day intervals. Because of small diameter of tree stems at the krummholz site, sampling at this plot was reduced to about once every two weeks in both growing seasons.
Collected core samples were prepared for light microscopy. Immediately after extraction cores were fixed in a solution of 70 % ethanol, propionic acid and 40 % formaldehyde (mixing ratio: 90/5/5), subsequently embedded in glycolmethacrylate (Technovit 7100) and polymerized after adding an accelerator. Transverse sections of c. 12 μm were cut with a microtome, stained with a water solution of 0.05 % cresyl fast violet and observed under a light microscope with polarised light to differentiate the development of xylem cells, i.e. the discrimination between tracheids in enlarging and cell-wall thickening phase (Antonova and Stasova 1993; Deslauriers et al. 2003; Rossi et al. 2006b). The number of cambial cells (i.e., fusiform cells lacking radial enlargement), radial enlarging cells, cells undergoing secondary wall thickening and mature xylem cells were counted on all sampled cores in three radial rows. Xylem formation was considered to have begun and to be complete, when one horizontal row of cells was detected in the enlarging phase and cell wall thickening and lignification was completed, respectively. Total xylem cell number was determined by adding the number of cells in radial enlargement and in cell wall thickening and the number of mature xylem cells (Deslauriers et al. 2003; Rossi et al. 2006c). Values, i.e. the number of cells in different zones of 5 cores (trees) per date and for each site, were averaged.
Standardization of cell number and fitting of xylem growth
Because cell number varies within the tree circumference and hence among different samples, standardization is required (Rossi et al. 2003). The total cell number of the previous tree ring was recorded in every sample and used for a cell number correction for each tree. Cell number in each j-sample (i.e., micro-cores taken throughout growing seasons 2006 and 2007) and by each i-phase (i.e., enlarging, wall thickening and mature tracheids) was corrected as follows:
where:
ncij = corrected cell number
nij = counted cell number
nm= mean cell number of previous ring of all j-samples
ns = cell number of previous ring for each j-sample
Short-term variation in the measured time series (number of tracheids) were modelled with a Gompertz function using the nonlinear regression procedure included in the Origin software package (OriginLab Corporation, Northampton, MA, USA). The Gompertz equation proved its versatility to describe growth limiting processes and to assess cell dynamics of tree-ring growth (Zeide 1993; Camarero et al. 1998; Deslauriers and Morin 2005; Rossi et al. 2003, 2006c).
Microclimate records and cell production/climate relationships
Air and soil temperatures were collected automatically at all sites. Soil temperatures were recorded at 5 cm soil depths with type-T thermocouples, whereby at the timberline the sensor was placed in a manner that a complete shading by the forest canopy was ensured. On the other hand, the temperature sensor was not shaded at the krummholz site, whereas the sensor was shaded transiently at the treeline as justified by larger tree crowns. Statistical significance of differences of monthly and seasonal mean values were tested with Student’s t-test at p <0.05.
Daily precipitation was recorded at a meteorological station on top of Mt. Patscherkofel (2246 m a.s.l.). Soil water potential in the top 10 cm soil layer was monitored at all sites with equitensiometers (Model EQ3, Ecomatik, Dachau-Munich, Germany). Measuring intervals for all sensors were 30 min. Mean daily temperature and soil water potential were calculated by averaging all measurements (48 values/day).
Degree days (d.d.), an index which represents a measure of accumulated heat, were calculated according to Baskerville and Emin (1969) as the integral of degrees above 5 °C times the number of days, integrated over sampling intervals. In particular, a curve was fitted to the recorded daily maximum and minimum air temperatures starting on January 1st and the area of the curve above the base temperature was calculated. The base temperature, i.e. the lower threshold temperature for cambial growth of 5 °C was chosen based on results by Loris (1981), who found that radial increments of Pinus cembra within the study area ceased whenever air temperatures fell below c. 5 °C. Degree days at time of initiation of cambial activity were calculated for the timberline, where continuous air temperature records, i.e. daily maxima and minima, were available throughout the year.
Relationships between cell production and climate variables during the growing seasons 2006 and 2007 were determined for the period June through August. Xylem cell number increases (including cells in enlarging and wall thickening phase and mature cells) were calculated for two weekly intervals based on developed Gompertz models. For the same intervals mean daily maximum air and soil temperatures, degree days and precipitation sums were calculated. Pearson product-moment correlation analysis (r) was applied to identify the climatic factors most closely associated with tracheid production.
Results
Microclimate during growing seasons 2006 and 2007
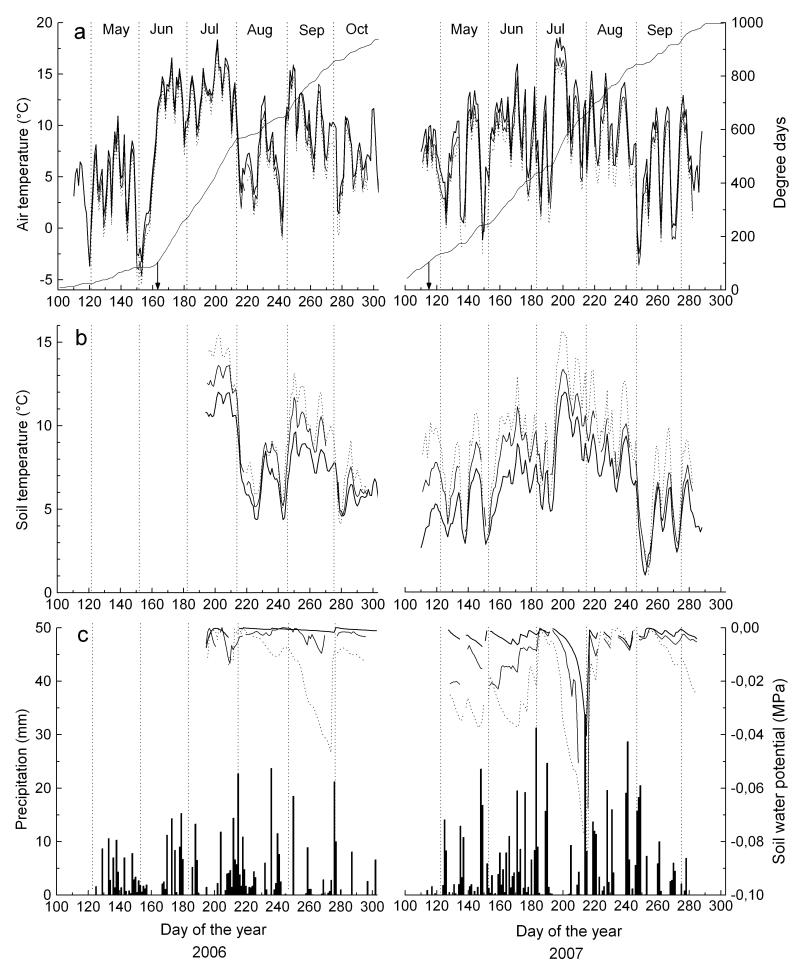
Climate in 2006 was characterized by a prolonged late frost event occurring between 30 May and 8 June (minimum air temperature at timberline fell to −4.9 °C), a warm June and July with mean air temperature exceeding the long-term mean (LTM; 1967-2004) in July by 3.8 °C, followed by a sharp temperature drop in August (August mean air temperature was 3.2 °C below LTM). September and October were also warmer than LTM (Fig. 1, Table 1). In 2007 the growing period was extended due to occurrence of exceptionally mild temperatures in spring (3.4 °C above LTM). Cumulated heat sums in spring (March - May) 2006 and 2007 reached 81 and 231 d.d., respectively. Summer (June - August) in 2007 was slightly warmer than in 2006 and exceeded LTM by 1.3 °C. In both growing seasons highest mean air temperatures were recorded in July. Precipitation in June-July 2006 and 2007 was c. 35 % and c. 5 % below LTM, respectively. Soil water potential did not decrease below −0.08 MPa throughout the treeline ecotone during a dry period lasting from 10 July to 1 August 2007 (Fig. 1).
Figure 1.
Climate variables recorded during the growing seasons 2006 and 2007 within the treeline ecotone. a Mean daily air temperature and degree days (d.d.). Arrows indicate day of the year, when 100 d.d. were reached at timberline. For improved clarity d.d. are shown only for the timberline. b Mean daily soil temperature. c Daily precipitation sum and soil water potential in 2007. In a-c study sites are denoted by thick and thin solid lines for timberline and treeline, respectively, and dotted lines for the krummholz site.
Table 1.
Seasonal (spring = March-May; summer = June-August) and monthly mean air temperature, degree days and precipitation sum during 2006 and 2007 growing seasons. Long-term mean values ± standard deviation of air temperature were available from timberline (LTM-Pk; 1967-2004). Accordingly, seasonal and monthly mean air temperature and degree days during study years are shown for the timberline plot. Anom2006 and Anom2007 are air temperature anomalies relative to the 1901-2000 average from high-elevation meteorological stations in the Alps in 2006 and 2007, respectively (Auer et al. 2007). Precipitation records are from top of Mt. Patscherkofel (LTM-Pk; 1967-2004).
| Air temperature (°C) | Degree days | Precipitation (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LTM-Pk | 2006 | 2007 | Anom2006 | Anom2007 | 2006 | 2007 | LTM-Pk | 2006 | 2007 | |
| Spring | 0.6 ± 1.4 | 0.4 | 4.0 | 0.6 | 3.1 | 81 | 231 | 201 ± 55 | 174 | 154 |
| Summer | 9.3 ± 1.4 | 9.9 | 10.6 | 1.4 | 1.4 | 562 | 586 | 358 ± 63 | 278 | 409 |
| Jun | 7.8 ± 1.9 | 9.2 | 10.1 | 2.1 | 2.4 | 187 | 188 | 109 ± 35 | 81 | 120 |
| Jul | 10.0 ± 1.9 | 13.8 | 11.3 | 4.3 | 1.1 | 298 | 227 | 127 ± 39 | 70 | 108 |
| Aug | 10.1 ± 1.9 | 6.9 | 10.6 | − 2.3 | 0.7 | 90 | 188 | 121 ± 50 | 126 | 182 |
| Sep | 7.1 ± 2.0 | 11.0 | 5.5 | 3.5 | − 1.6 | 198 | 88 | 78 ± 29 | 34 | 101 |
| Oct | 4.1 ± 2.2 | 7.2 | 3.9 | 4.0 | 0.2 | 98 | 80 | 48 ± 29 | 53 | 24 |
Underneath the closed tree canopy at the timberline, soil temperatures during May through August 2007 were about 1.6 and 2.6 °C lower compared to soil temperatures recorded at treeline and within the krummholz belt, respectively (Table 2). In May 2007, when cambial activity was resumed at all sites, mean daily soil temperature reached 4.9, 6.4 and 7.5 °C at the timberline, treeline and krummholz site, respectively. Lower soil temperatures at the treeline (2110 m a.s.l.) compared to the krummholz site (2180 m a.s.l.) were due to partial shading of the soil surface throughout the day by larger tree crowns (Table 3), whereas at the krummholz site shading of soil surface by crown of small trees (tree height 0.5 – 1 m) was minimal. Highest mean monthly soil temperatures were recorded in July (Fig. 1).
Table 2.
Monthly and summer (June-August) mean daily soil temperatures (°C) during the growing season 2007 throughout the treeline ecotone. Statistical significant differences of mean values at p ≤ 0.05 within the treeline ecotone are indicated by different letters (SD = standard deviation).
| Soil temperature (°C) mean ± SD |
|||
|---|---|---|---|
| May | June-August | September | |
| Timberline | 4.9a ± 1.4 | 7.6a ± 1.9 | 4.1a ± 1.8 |
| Treeline | 6.4b ± 1.6 | 9.3b ± 1.8 | 4.5ab ± 1.8 |
| Krummholz | 7.5b ± 2.1 | 10.3c ± 2.5 | 5.9b ± 2.7 |
Table 3.
Characteristics of Pinus cembra trees selected for sampling micro-cores (n = 5 trees/site) throughout the treeline ecotone (SD = standard deviation, RW = ring width).
| Site | Altitudey (m a.s.l.) |
Tree height (m) |
Crown diameter1 (m) |
Stem diameter2 (cm) |
Age3 (yr) mean5 ± SD |
RW4 (mm) mean5 ± SD |
|---|---|---|---|---|---|---|
| Timberline | 1950 | 10 - 14 | 4 - 5 | 30-50 | 80a ± 11 | 2.10a ± 0.66 |
| Treeline | 2110 | 3 - 5 | 2.5 - 3.0 | 15-20 | 70a ± 15 | 2.32a ± 0.68 |
| Krummholz | 2180 | 0.5 - 1.0 | 0.35 - 0.60 | 5-7 | 22b ± 5 | 1.92a ± 0.78 |
Range of maximum crown diameter.
Range of tree diameter measured at sampling height (see Material and Methods).
Approximate age of selected trees was calculated by adding 15, 10 and 5 yr to mean number of measured tree rings of increment cores taken at c.1.3 m (timberline), c. 50 cm (treeline) and c. 15 cm stem height (krummholz site), respectively.
Mean values during the period 2003-2005.
Mean tree age and ring width of selected trees throughout the treeline ecotone labelled by different letters are different at p ≤ 0.05 (Student’s t-test).
Dynamics of tree- ring growth
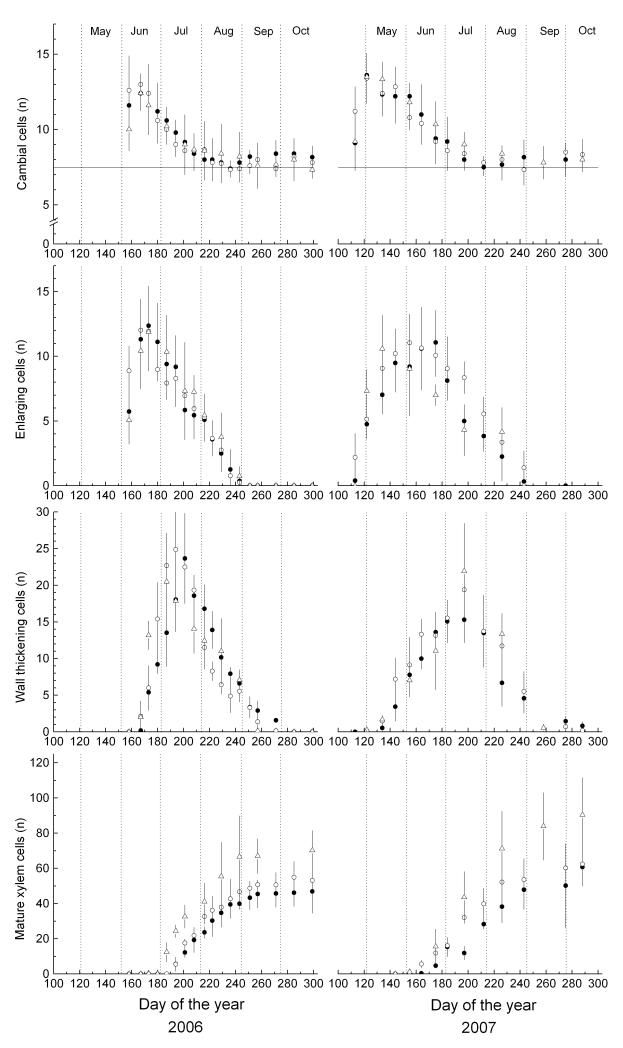
The dormant cambium consisted of 7 to 8 cells, when there was no cambial activity from September 2006 through April 2007. Although annual dynamics of cambial activity were similar within the treeline ecotone in 2006 and 2007, earlier onset of cell division at the treeline can be deduced from higher numbers of cambial cells determined at first sampling dates (Fig. 2). First samplings in 2006 began in early June (7 June), when trees from all sites already showed wide cambial zones, thereby preventing a precise determination of onset of cambial activity. In 2007 sampling started on 23 April and in early May, the number of cells in the cambial zone rapidly increased to about 13, whereby the greatest increase in numbers of cambial cells was observed at the treeline (Fig. 2). Cambial activity started at timberline, when temperature sum had reached c. 95 d.d. In 2006 a corresponding heat-sum was reached on 11 June, i.e. a few days after first determination of cambial activity, when the number of cells in the cambial zone had already increased to 10-13, which might indicate that the amount of warmth necessary for initiation of cambial activity varies among years. Cambial cells reached their maximum at all plots on 16 June 2006 and 2 May 2007, respectively. Termination of cambial activity within the ecotone occurred about end of July. High variability in number of cambial cells between trees precludes a more precise determination and a differentiation between sites, although a delayed cessation of cell division by about 2 wk at the krummholz site might be deduced from less steep decreasing trend in number of cambial cells. Cambial activity in 2007 throughout the treeline ecotone lasted from end of April to end of July, i.e. cambial cells divided throughout c. 90 d.
Figure 2.
Number of cells in the cambial zone, in radial enlargement, in secondary wall thickening and lignification and mature xylem cells during 2006 and 2007 within the treeline ecotone. Bars represent standard deviations and horizontal thin lines indicate number of dormant cambial cells. Study sites are denoted by filled and open circles for timberline and treeline, respectively, and open triangles for the krummholz site.
Delayed bell-shaped curves (enlarging and wall thickening cells) and a growing S-shaped curve (mature cells) characterize the dynamics of cell differentiation (Fig. 2). In 2006 and 2007 higher numbers of enlarging cells were recorded early in the growing season at the treeline. The onset of radial enlargement in 2007 in some individuals occurred on 23 April at the timberline and treeline and on 2 May at the krummholz site. In 2006 and 2007 enlarging cells were detected throughout the treeline ecotone until end of August (243 d).
Corresponding to cambial activity and number of cells in the enlargement phase at the beginning of the growing season in 2006 and 2007 at treeline, higher numbers of wall thickening cells were detected early in the growing season at the treeline and krummholz site compared to the timberline. In 2006 and 2007 first tracheids were undergoing wall thickening around 16 June and 2 May, respectively. On the other hand, wall thickening and lignification was completed about 4 wk earlier at higher elevation (mid to end of September and mid to end of October at krummholz site and timberline, respectively). This developmental phase lasted about 14 days longer in 2007 compared to 2006 (Table 4). Whereas in 2006 first mature tracheids were detected on 13 July 2006 at the timberline and treeline, in 2007 maturation of some tracheids was already completed around 9 June (160 d) at the treeline and around 20 June at the timberline (Fig. 2). Within the krummholz belt first mature tracheids were detected on 6 July 2006 and 16 June 2007, respectively. Hence, mature xylem cells were found about 4 wk earlier in 2007 compared to 2006. In 2007 duration of wood formation including all developmental phases from cell enlargement to the end of wall thickening and lignification lasted for about 5 and 6 months at the krummholz site and timberline, respectively (Table 4).
Table 4.
Onset, end and overall duration of wood formation of Pinus cembra along the treeline ecotone in 2007, number of mature xylem cells and ring width in 2006 and 2007 (n = 5 trees/site). Onset and hence duration of wood formation in 2006 could not be determined precisely and were therefore omitted (cf. text). Timing of wood formation is given in days of the year. All values are means ± standard deviation.
| Onset | End | Duration | Xylem cells (n) | Ring width (mm) | ||||
|---|---|---|---|---|---|---|---|---|
| 2007 | 2006 | 2007 | 2007 | 2006 | 2007 | 2006 | 2007 | |
| Timberline | 117 ± 5 | 285 ± 0 | 301 ± 7 | 184 ± 7 | 47 ± 13 | 61 ± 11 | 1.94 ± 0.69 | 1.84 ± 0.72 |
| Treeline | 113 ± 0 | 273 ± 6 | 283 ± 7 | 170 ± 7 | 53 ± 7 | 62 ± 10 | 1.90 ± 0.83 | 2.07 ± 0.92 |
| Krummholz | 124 ± 5 | 260 ± 6 | 276 ± 16 | 152 ± 15 | 72 ± 32 | 90 ± 21 | 2.20 ± 0.95 | 2.17 ± 0.63 |
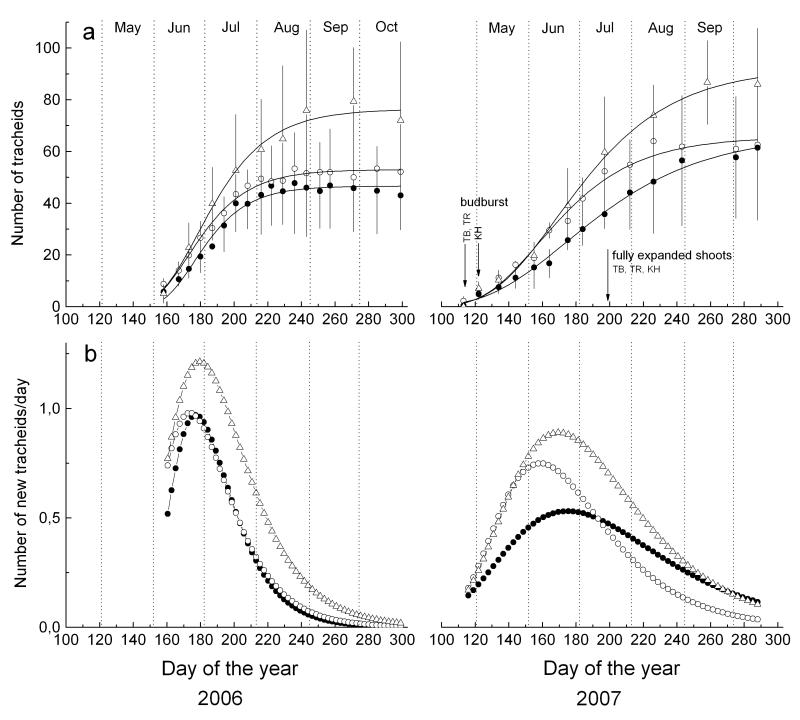
Increase in total number of tracheids (including cells in enlarging and wall thickening phase and mature cells) during the growing seasons 2006 and 2007 are depicted in Fig. 3. Maximum daily growth rates and total number of new tracheids produced were highest at the krummholz site. Higher cell production rate in 2006 did not lead to a higher tree-ring cell number compared to 2007 (Table 4). Based on modelled number of xylem cell number increase by applying the Gompertz function, the maximum cell production rate (tracheids d−1) in 2006 and 2007 occurred around summer solstice (172 d of the year) except for the treeline in 2007 (Table 5). Thereafter, daily xylem growth rates decreased, although highest mean daily air temperatures were recorded in July (Fig. 1, Table 1). At the timberline and treeline in 2007 budburst occurred by the end of April and about one week later at the krummholz site. On 2 May krummholz individuals showed swelled and partly opened buds, whereas new shoots 0.5 - 1 cm in length were already detectable at the treeline and timberline. Throughout the treeline ecotone current year shoots in 2007 were fully expanded in mid July.
Figure 3.
a Dynamics of xylem growth (including enlarging, wall thickening and mature xylem cells) within the treeline ecotone modelled by applying the Gompertz function and phenological events in 2007 (TB = timberline, TR = treeline, KH = krummholz site). b Daily xylem growth rates calculated on basis of modelled growth during 2006 and 2007. Symbols as in Fig. 2.
Table 5.
Parameters of the Gompertz function for xylem cell dynamic (see Fig. 3) and R2 of the model (A = upper asymptote; Ip = inflection point; κ = rate of change parameter; mean values ± standard deviation).
| Site | Year | A (n cells) |
Ip (day of the year) |
κ | R2 |
|---|---|---|---|---|---|
| Timberline | 2006 | 47 ± 1.0 | 176 ± 1.3 | 0.057 ± 0.006 | 0.976 |
| 2007 | 67 ± 2.3 | 174 ± 2.3 | 0.022 ± 0.002 | 0.995 | |
| Treeline | 2006 | 53 ± 0.7 | 172 ± 0.9 | 0.050 ± 0.003 | 0.989 |
| 2007 | 66 ± 2.2 | 157 ± 2.2 | 0.031 ± 0.003 | 0.986 | |
| Krummholz | 2006 | 76 ± 2.5 | 178 ± 2.0 | 0.043 ± 0.005 | 0.986 |
| 2007 | 92 ± 3.4 | 168 ±2.7 | 0.026 ± 0.003 | 0.995 |
Overall, xylogenesis in 2007 compared to 2006 was characterized by (i) a considerably earlier development of tracheids (4 to 5 weeks difference in emergence of first tracheids undergoing wall thickening and reaching complete maturation) and (ii) a lower production rate, i.e. number of new tracheids developed per day (Fig. 3, Table 5).
Relationship between xylogenesis and climate
Mean maximum air temperature during summer significantly correlated with number of tracheids produced in two weekly periods in 2006 and 2007 at the timberline only (Fig. 4). Correlation coefficients using mean and minimum air temperature were lower at all plots and are therefore not shown. On the other hand, d.d. showed a closer relationship with tracheid production throughout the treeline ecotone, although correlations were statistically significant at the timberline only. No significant correlations were found between wood formation and soil temperature or precipitation.
Figure 4.
Pearson product-moment correlations between mean number of tracheids produced (n = 5 trees/site) in two weekly intervals during June through August 2006-2007 and climate parameters (mean daily maximum temperature, d.d., precipitation sum) at the timberline (a-d), treeline (e-h) and krummholz site (i-l). ** p ≤ 0.01; * p ≤ 0.05.
Discussion
For 2007, we approximate the duration of wood formation at the timberline to have been from 27 April (first cells in enlarging phase) to 28 October (complete maturation of tracheids). Loris (1981) previously determined the period of wood formation of Pinus cembra within the study area at timberline in 1979 by simply counting developed tracheids and dated onset and end of tracheid production in mid May and end of September, respectively. Considering that (i) a differentiation of tracheid cell types (enlarging, wall-thickening, mature cells) might have extended the determined duration of tracheid production especially in autumn and (ii) spring, summer and autumn mean temperatures in 1979 were 4.3, 2.9 and 0.7 °C below recorded temperatures in 2007, both results are quite comparable.
Hence, Pinus cembra produce tracheids for about 6 months, which is substantially longer compared to about 4 months (c. 130 d) reported for this species at timberline (2080 m a.s.l.) in the eastern Italian Alps (Rossi et al. 2008). This discrepancy can be explained by lower elevation of our timberline site (130 m altitudinal difference) and an extraordinary warm spring in 2007, which caused early onset of cambial activity. Additionally, because (i) Mt. Patscherkofel is situated within an inner-alpine dry zone, where annual precipitation is about 20 % lower than at the study site in the eastern Italian Alps (see Rossi et al. 2008), and (ii) the local climate is also strongly influenced by warm and dry southerly winds in spring (Föhn), more advanced snow-melt and heating of root-zone at the beginning of the growing season is reasonable to suppose within the study area.
Although early initiation of above ground stem growth will make trees more vulnerable to late frost events (cf. Cannel and Smith 1986), which can adversely affect shoot development and flowering, an extended growing season not only causes a surplus in the carbon balance of trees, but also favours maturation of needles, shoots and buds against detrimental effects of winter stress, especially frost desiccation (Baig and Tranquillini 1980). Hence, lengthening and warming of the growing season, which has been reported on a global and regional scale (e.g., Menzel and Fabian 1999, Wieser 2004) is expected to result in increased tree growth and upward shift of the alpine treeline (cf. Dullinger et al. 2004, Gehrig-Fasel et al. 2007).
The amount of warmth necessary for initiation of cambial activity at timberline in 2007 was 96 d.d. and possibly slightly lower in 2006, which agrees with the temperature-sum threshold of 85 to 90 d.d. reported for onset of wood formation in Pinus sylvestris at the northern treeline (Schmitt et al. 2004). However, statistically significant variation among years in d.d. before onset of cambial activity was reported by Seo et al. (2008), indicating that other or additional factors are involved in initiation of cambial activity of Pinus sylvestris (see Savidge 1996).
On the other hand, wood formation in 2007 lasted for about 5 months at the krummholz site, indicating that favourable climate conditions for tree growth prevailed even at 2180 m a.s.l. This finding might indicate that excluding anthropogenic influence and deleterious effects due to climate extremes (strong wind exposure within the krummholz belt causes shoot damage due to winter desiccation especially in years with low snow depth; Tranquillini 1979), Pinus cembra could prosper within the study area at higher altitudes. This is supported by (i) occurrence of a treeline site on a steep rocky outcrop at 2200 m a.s.l. nearby, where Pinus cembra reaches a height of c. 8 m, and (ii) recent establishment of Pinus cembra beyond present treeline into abandoned alpine grasslands (Oberhuber, unpublished observations). It has also to be noted that due to strong climate warming of the recent three decades in the Alps (Auer et al. 2007), the limiting effect of temperature on tree growth at the elevation of actual treeline ecotone is reduced.
Impact of root zone temperature on initiation of cambial activity
At the beginning of the growing seasons 2006 and 2007 higher numbers of cells in the cambial zone and in differentiation process were found at sites with open canopy, i.e. at treeline and krummholz site, where root-zone temperatures in May exceeded those at timberline by 1.5 and 2.6 °C, respectively. Hence, our results might indicate that soil temperature plays an important role for triggering cambial activity and cell differentiation. Roots of small tree groups and isolated trees benefit from stand fragmentation above the timberline due to earlier snow melt and increased soil warming under open sparse canopy (cf. Körner and Paulsen 2004). Dependence of initiation of cambial activity at the northern treeline on date of snow melt was reported by Kirdyanov et al. (2003). That low root-zone temperatures in spring may delay the establishment of planted conifer seedlings by impairing shoot growth was found by Lopushinsky and Kaufmann (1984) and Vapaavuori et al. (1992). Several authors also report direct influences of soil temperature on above-ground metabolism (e.g., Hellmers et al. 1970; Havranek 1972; DeLucia 1986; Scott et al. 1987; Day et al. 1989). Simultaneous bud burst at timberline and treeline may also indicate an influence of below ground temperature on above ground shoot growth at the beginning of the growing season. However, experimental studies dealing with the effect of soil temperature on bud phenology of conifer seedlings yielded ambiguous results (e.g., Lopushinsky and Max 1990; Domisch et al. 2001) indicating that species-specific responses may exist.
Delayed bud opening and wood formation within the krummholz belt might be explained by increased environmental stress at higher elevations, especially occurrence of frost desiccation (Tranquillini 1979). Additionally, delayed bud burst and initiation of cambial activity can be regarded as an adaptation to prevent late frost injuries to current-year needles and to the developing xylem.
Effect of microclimate on wood formation
Throughout June to August, air temperature (mean maximum air temperature and d.d.) positively influenced tracheid production at timberline, which is consistent with previous ecophysiological and dendroclimatological studies of Pinus cembra within the treeline ecotone on Mt. Patscherkofel (Loris 1981; Oberhuber 2004) and studies from other authors (e.g., Nicolussi 1994; Frank and Esper 2005; Carrer et al. 2007). At higher altitudes, i.e. at treeline and within the krummholz-belt, more extreme environmental conditions (wind exposure) might override influence of temperature on shoot growth.
Maximum daily growth rates within the treeline ecotone peaked around summer solstice and not during the warmest period of the study years, which supports the hypothesis suggested by Rossi et al. (2006c) that the photoperiodic growth constraint is an adaptation allowing tracheid differentiation to be completed before winter. Gričar et al. (2007) reported that experimental heating of Norway spruce stems (Picea abies (L.) Karst.) did not prolong cambial activity at the end of the growing period, whereas cambial activity increased at higher temperatures in the first part of the growing season. Antonova and Stasova (1993, 1997) and Kirdyanov et al. (2003) found that primarily warm temperatures early in the growing season affect current cambial activity and tracheid production of Siberian conifers.
That additional intrinsic and/or extrinsic factors are involved in tracheid production is indicated by (i) different growth trends developed in 2006 and 2007 after maximum growth rates were reached and (ii) higher daily cell production rates in 2006, which did not lead to a higher tree-ring cell number compared to 2007 (cf. Savidge 1996; Plomion et al. 2001). This is concordant with Deslauriers and Morin (2005), who suggested that the dynamic of seasonal temperature variation might influence the shape of the Gompertz sigmoid function (slope at the inflection point, daily maximum production rate) but not the total number of tracheids formed.
Because krummholz-trees are coupled to the shelter and warmth associated with the ground (Smith et al. 2003) they profit from their small stature (0.5 – 1 m tall), which results in higher tissue temperatures (Wilson et al. 1987; Grace and Norton 1990). This size effect might explain higher daily growth rates of krummholz individuals compared to trees > 3 m tall at lower altitude, which experience convective cooling through tight atmospheric coupling (Grace 1988; Körner 2003). On the other hand, age-dependent decrease in tree-ring width is typical for coniferous trees and is related to increasing tree size (Fritts 1976; Bräker 1981). Hence, higher daily production of new tracheids and comparable ring widths found within the krummholz belt compared to trees at timberline and treeline during the study period might have been caused by considerably lower tree age. Furthermore, smaller tracheid radial diameters determined at the krummholz-limit than at lower altitude (data not shown) necessarily result in higher daily cell production, when similar annual increments are observed. That more stressful growth conditions cause a decrease in tracheid radial diameter was also reported by Camarero et al. (1998).
Though warm conditions in spring 2007 lengthened the growing season, annual increments throughout the treeline ecotone in 2007 were not significantly higher compared to previous years. Higher xylem cell numbers found in 2007 compared to 2006 can be explained by higher percentage of radially flattened latewood cells in 2007. Recently, Oberhuber et al. (2008) also report a weak growth response of Pinus cembra on Mt. Patscherkofel to the 2003 summer heat-wave. Hence, there is supporting evidence that besides summer temperature other climate variables, like temperature in previous fall and during early spring (March), winter precipitation, and weather extremes (late frost), which have been determined by evaluating growth-climate relationships (e.g., Frenzel and Maisch 1981; Oberhuber 2004; Pfeifer et al. 2005; Carrer et al. 2007) are influencing total tracheid production (i.e. radial growth) of Pinus cembra. That ring width and the number of tracheids are highly correlated, i.e. tracheids of wide rings are not larger than those of narrow rings, was reported for several coniferous species from low and high altitudes (Camarero et al. 1998, Wang et al. 2002, Mäkinen et al. 2003).
No significant relations were found between precipitation and tracheid production throughout the treeline ecotone. These results are not surprising, as soil water potential during a dry period in July 2007 was > −0.08 MPa (corresponds to a soil water content of 30 % (vol.); Guggenberger 1980) even at the krummholz site, where coarse textured shallow soils prevail (< 5 cm humus layer). Occurrence of drought stress in 2007 within the treeline ecotone is therefore unlikely. That soil moisture effects at the timberline on Mt. Patscherkofel are regarded as being of minor importance for tree growth has also been reported by Tranquillini (1979) and Wieser (2004).
Conclusion
Based on our present results, initiation of cambial activity in late spring is possibly influenced by a threshold root-zone temperature, which is reached earlier under open canopy at the treeline and within the krummholz belt than under closed canopy at timberline. On the other hand, wood formation during June-August was not significantly correlated with soil temperature throughout the treeline ecotone, which supports the view of Rossi et al. (2007) that soil temperature is not the main limiting factor affecting tracheid production at high-altitude.
Furthermore, results of this study confirmed the necessity that a combination of dendroclimatological and intra-annual wood anatomical studies are required to assess long- and short-term climatic influences on radial tree growth (cf. Gartner et al. 2002).
Acknowledgements
This work was supported by the Austrian Science Fund (Project No. FWF P18819-B03 “Temperature dependence of Pinus cembra (L.) stem growth and respiration along an altitudinal transect”). Precipitation data were provided by Zentralanstalt für Meteorologie und Geodynamik, Innsbruck, which is greatly acknowledged. We also thank anonymous reviewers for valuable suggestions and comments on improving the manuscript.
References
- Antonova GF, Stasova VV. Effects of environmental factors on wood formation in Scots pine stems. Trees. 1993;7:214–219. [Google Scholar]
- Antonova GF, Stasova VV. Effects of environmental factors on wood formation in larch. Trees. 1997;11:462–468. [Google Scholar]
- Auer I, Böhm R, Jurkovic A, Lipa W, Orlik A, Potzmann R, Schöner W, Ungersbök M, Matulla C, Briffa K, Jones P, Efthymiadis D, Brunetti M, Nanni T, Maugeri M, Mercalli L, Mestre O, Moisselin J-M, Begert M, Müller-Westermeier G, Kveton V, Bochnicek O, Stastny P, Lapin M, Szalai S, Szentimrey T, Cegnar T, Dolinar M, Gajic-Capka M, Zaninovic K, Majstorovic Z, Nieplova E. HISTALP – historical instrumental climatological surface time series of the Greater Alpine Region. Int J Climatol. 2007;27:17–46. [Google Scholar]
- Baig MN, Tranquillini W. The effects of wind and temperature on cuticular transpiration of Picea abies and Pinus cembra and their significance in desiccation damage at the alpine tree line. Oecologia. 1980;47:252–256. doi: 10.1007/BF00346828. [DOI] [PubMed] [Google Scholar]
- Baskerville GL, Emin P. Rapid estimation of heat accumulation from maximum and minimum temperatures. Ecology. 1969;50:514–517. [Google Scholar]
- Bräker OU. Der Alterstrend bei Jahrringdichten und Jahrringbreiten von Nadelhölzern und sein Ausgleich. Mitt Forstl Bundesvers Wien. 1981;142:75–102. [Google Scholar]
- Büntgen U, Esper J, Frank DC, Nicolussi K, Schmidhalter M. A 1052-year tree-ring proxy for Alpine summer temperature. Clim Dyn. 2005;25(2-3):141–153. [Google Scholar]
- Camarero JJ, Guerrero-Campo J, Gutiérrez E. Tree-ring growth and structure of Pinus uncinata and Pinus sylvestris in the Central Spanish Pyrenees. Arct Alp Res. 1998;30(1):1–10. [Google Scholar]
- Cannell MGR, Smith RI. Climatic warming, spring budburst and frost damage on trees. J Appl Ecol. 1986;23:177–191. [Google Scholar]
- Carrer M, Nola P, Eduards JL, Motta R, Urbinati C. Regional variability of climate-growth relationships in Pinus cembra high elevation forests in the Alps. J Ecol. 2007;95:1072–1083. [Google Scholar]
- Cook ER, Kairiukstis LA, editors. Methods of Dendrochronology. Applications in the environmental sciences. Kluwer; Dordrecht: 1990. [Google Scholar]
- Day TA, DeLucia EH, Smith WK. Influence of cold soil and snowcover on photosynthesis and leaf conductance in two Rocky Mountain conifers. Oecologia. 1989;80:546–552. doi: 10.1007/BF00380080. [DOI] [PubMed] [Google Scholar]
- DeLucia EH. Effect of low root temperature on net photosynthesis, stomatal conductance and carbohydrate concentration in Engelmann spruce (Picea engelmannii Parry ex Engelm.) seedlings. Tree Phys. 1986;2:143–154. doi: 10.1093/treephys/2.1-2-3.143. [DOI] [PubMed] [Google Scholar]
- Deslauriers A, Morin H, Begin Y. Cellular phenology of annual ring formation of Abies balsamea in the Quebec boreal forest (Canada) Can J For Res. 2003;33:190–200. [Google Scholar]
- Deslauriers A, Morin H. Intra-annual tracheid production in balsam fir stems and the effect of meteorological variables. Trees. 2005;19:402–408. [Google Scholar]
- Domisch T, Finér L, Lehto T. Effects of soil temperature on biomass and carbohydrate allocation in Scots pine (Pinus sylvestris) seedlings at the beginning of the growing season. Tree Phys. 2001;21:465–472. doi: 10.1093/treephys/21.7.465. [DOI] [PubMed] [Google Scholar]
- Dullinger S, Dirnbök T, Grabherr G. Modelling climate change-driven treeline shifts: relative effects of temperature increase, dispersal and invisibility. J Ecol. 2004;92:241–252. [Google Scholar]
- Eckstein D, Aniol RW. Dendroclimatological reconstruction of the summer temperatures for an alpine region. Mitt Forstl Bundesvers Wien. 1981;142:391–398. [Google Scholar]
- Frenzel B, Maisch I. Klimatische Analyse der Jahrringbreitenschwankungen an der alpinen Waldgrenze. Mitt Forstl Bundesvers Wien. 1981;142:399–416. [Google Scholar]
- Frank D, Esper J. Characterization and climate response patterns of a high-elevation, multi-species tree-ring network in the European Alps. Dendrochronologia. 2005;22:107–121. [Google Scholar]
- Fritts HC. Tree rings and climate. Academic Press; London: 1976. [Google Scholar]
- Gartner BL, Aloni R, Funada R, Lichtfuss-Gautier AN, Roig FA. Clues for dendrochronology from studies of wood structure and function. Dendrochronologia. 2002;20(1-2):53–61. [Google Scholar]
- Gehrig-Fasel J, Guisan A, Zimmermann NE. Tree line shifts in the Swiss Alps: Climate change or land abandonment? J Veg Sci. 2007;18:571–582. [Google Scholar]
- Grace J. The functional significance of short stature in montane vegetation. In: Werger MJA, Van der Aart PJM, During HJ, Verhoeven JTA, editors. Plant form and vegetation structure. SPB Academic Publishers; The Hague: 1988. pp. 201–209. [Google Scholar]
- Grace J, Norton DA. Climate and growth of Pinus sylvestris at its upper altitudinal limit in Scotland: evidence from tree growth-rings. J Ecol. 1990;78:601–610. [Google Scholar]
- Grace J, Allen SJ, Wilson C. Climate and the meristem temperatures of plant communities near the tree-line. Oecologia. 1989;79:198–204. doi: 10.1007/BF00388479. [DOI] [PubMed] [Google Scholar]
- Grace J, Berninger F, Nagy L. Impacts of climate change on the tree line. Ann Bot. 2002;90:537–544. doi: 10.1093/aob/mcf222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gričar J, Zupančič M, Čufar K, Primož O. Regular cambial activity and xylem and phloem formation in locally heated and cooled stem portions of Norway spruce. Wood Sci Techn. 2007;41(6):463–475. [Google Scholar]
- Guggenberger H. PhD-thesis. University of Innsbruck; 1980. Untersuchungen zum Wasserhaushalt der alpinen Zwergstrauchheide Patscherkofel. [Google Scholar]
- Havranek WM. Über die Bedeutung der Bodentemperatur für die Photosynthese und Transpiration junger Forstpflanzen und für die Stoffproduktion an der Waldgrenze. Angew Bot. 1972;46:101–116. [Google Scholar]
- Hellmers H, Genthe MK, Ronco F. Temperature affects growth and development of Engelmann spruce. For Sci. 1970;16:447–452. [Google Scholar]
- Hughes MK. Dendrochronology in climatology – the state of the art. Dendrochronologia. 2002;20(1-2):95–116. [Google Scholar]
- Jobbagy EG, Jackson RB. Global controls of timberline elevation in the northern and southern hemispheres. Glob Ecol Biogeogr. 2000;9:253–268. [Google Scholar]
- Kirdyanov A, Hughes M, Vaganov E, Schweingruber F, Silkin P. The importance of early summer temperature and date of snow melt for tree growth in the Siberian Subarctic. Trees. 2003;17:61–69. [Google Scholar]
- Körner C. A re-assessment of high elevation treeline positions and their explanation. Oecologia. 1998;115:445–459. doi: 10.1007/s004420050540. [DOI] [PubMed] [Google Scholar]
- Körner C. Alpine plant life: functional plant ecology of high mountain ecosystems. 2nd edn Springer; Berlin Heidelberg New York: 2003. [Google Scholar]
- Körner C, Paulsen J. A world-wide study of high altitude treeline temperatures. J Biogeogr. 2004;31:713–732. [Google Scholar]
- Lopushinsky W, Kaufmann MR. Effects of cold soil on water relations and spring growth of Douglas-fir seedlings. For Sci. 1984;30:628–634. [Google Scholar]
- Lopushinsky W, Max TA. Effect of soil temperature on root and shoot growth and on budburst timing in conifer seedling transplants. New For. 1990;4:107–124. [Google Scholar]
- Loris K. Dickenwachstum von Zirbe, Fichte und Lärche an der alpinen Waldgrenze/Patscherkofel. Mitt Forstl Bundesvers Wien. 1981;142:417–441. [Google Scholar]
- Mäkinen H, Nöjd P, Saranpää P. Seasonal changes in stem radius and production of new tracheids in Norway spruce. Tree Phys. 2003;23:959–968. doi: 10.1093/treephys/23.14.959. [DOI] [PubMed] [Google Scholar]
- Menzel A, Fabian P. Growing season extended in Europe. Nature. 1999;397:659. [Google Scholar]
- Neuwinger I. Böden der subalpinen und alpinen Stufe in den Tiroler Alpen. Mitt Ostalpin-Dinar Ges Vegetationskde. 1970;11:135–150. [Google Scholar]
- Nicolussi K. Jahrringe und Massenbilanz. Dendroklimatologische Rekonstruktion der Massenbilanzreihe des Hintereisferners bis zum Jahr 1400 mittels Pinus cembra-Reihen aus den Ötztaler Alpen, Tirol. Z Gletscherk Glazialgeol. 1994;30:11–52. [Google Scholar]
- Oberhuber W. Influence of climate on radial growth of Pinus cembra within the alpine timberline ecotone. Tree Phys. 2004;24:291–301. doi: 10.1093/treephys/24.3.291. [DOI] [PubMed] [Google Scholar]
- Oberhuber W, Kofler W, Pfeifer K, Seeber A, Gruber A, Wieser G. Long-term changes in tree-ring–climate relationships at Mt. Patscherkofel (Tyrol, Austria) since the mid-1980s. Trees. 2008;22:31–40. doi: 10.1007/s00468-007-0166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer K, Kofler W, Oberhuber W. Climate related causes of distinct radial growth reductions in Pinus cembra during the last 200 yr. Veg Hist Archaeobot. 2005;14:211–220. [Google Scholar]
- Plomion C, Leprovost G, Stokes A. Wood formation in trees. Plant Phys. 2001;127:1513–1523. [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Deslauriers A, Morin H. Application of the Gompertz equation for the study of xylem cell development. Dendrochronologia. 2003;21:33–39. [Google Scholar]
- Rossi S, Anfodillo T, Menardi R. Trephor: a new tool for sampling microcores from tree stems. IAWA J. 2006a;27:89–97. [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T. Assessment of cambial activity and xylogenesis by microsampling tree species: an example at the Alpine timberline. IAWA J. 2006b;27:383–394. [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T, Morin H, Saracino A, Motta R, Borghetti M. Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol. 2006c;170(2):301–310. doi: 10.1111/j.1469-8137.2006.01660.x. [DOI] [PubMed] [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia. 2007;152(1):1–12. doi: 10.1007/s00442-006-0625-7. [DOI] [PubMed] [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T, Carrer M. Age-dependent xylogenesis in timberline conifers. New Phytol. 2008;177(1):199–208. doi: 10.1111/j.1469-8137.2007.02235.x. [DOI] [PubMed] [Google Scholar]
- Savidge RA. Xylogenesis, genetic and environmental regulation, a review. IAWA J. 1996;17:269–310. [Google Scholar]
- Schmitt U, Jalkanen R, Eckstein D. Cambium dynamics of Pinus sylvestris and Betula spp. in the northern boreal forest in Finland. Silv Fenn. 2004;38(2):167–178. [Google Scholar]
- Scott PA, Bentley CV, Fayle DCF, Hansell RIC. Crown forms and shoot elongation of white spruce at the treeline, Churchill, Manitoba, Canada. Arct Alp Res. 1987;19:175–186. [Google Scholar]
- Seo JW, Eckstein D, Jalkanen R, Rickebusch S, Schmitt U. Estimating the onset of cambial activity in Scots pine in northern Finland by means of the heat-sum approach. Tree Phys. 2008;28:105–112. doi: 10.1093/treephys/28.1.105. [DOI] [PubMed] [Google Scholar]
- Smith WK, Germino MJ, Hancock TE, Johnson DM. Another perspective on altitudinal limits of alpine timberlines. Tree Phys. 2003;23:1101–1112. doi: 10.1093/treephys/23.16.1101. [DOI] [PubMed] [Google Scholar]
- Tranquillini W. Ecological Studies. Vol. 31. Springer; Berlin: 1979. Physiological ecology of alpine timberline. Tree existence at high altitudes with special references to the European Alps. [Google Scholar]
- Vaganov EA, Hughes MK, Shashkin AV. Ecological Studies. Vol. 183. Springer; Berlin: 2006. Growth dynamics of conifer tree rings. Images of past and future environments. [Google Scholar]
- Vapaavuori EM, Rikala R, Ryyppö A. Effects of root temperature on growth and photosynthesis of conifer seedlings during shoot elongation. Tree Phys. 1992;10:217–230. doi: 10.1093/treephys/10.3.217. [DOI] [PubMed] [Google Scholar]
- Wang L, Payette S, Bégin Y. Relationship between anatomical and densitometric characteristics of black spruce and summer temperature at tree line in northern Quebec. Can J For Res. 2002;32:477–486. [Google Scholar]
- Wieser G. Seasonal variation of soil respiration in a Pinus cembra forest at the timberline in the Central Austrian Alps. Tree Phys. 2004;24:475–480. doi: 10.1093/treephys/24.4.475. [DOI] [PubMed] [Google Scholar]
- Wilson C, Grace J, Allen S, Slack F. Temperature and stature: a study of temperatures in montane vegetation. Funct Ecol. 1987;1:405–413. [Google Scholar]
- Zeide B. Analysis of growth equations. For Sci. 1993;39:594–616. [Google Scholar]