Abstract
Peptides and proteins, evolved by nature to perform vital biological functions, would constitute ideal candidates for therapeutic intervention were it not for their generally poor pharmacokinetic profiles. Nonpeptide peptidomimetics have thus been pursued because they might overcome these limitations while maintaining both the potency and selectivity of the parent peptide or protein. Since the late 1980s, we have sought to design, synthesize, and evaluate a novel, proteolytically stable nonpeptide peptidomimetic scaffold consisting of a repeating structural unit amenable to iterative construction; a primary concern is maintaining both the appropriate peptide-like side-chains and requisite hydrogen bonding. In this Account, we detail how efforts in the Smith–Hirschmann laboratories culminated in the identification of the 3,5-linked polypyrrolinone scaffold.
We developed effective synthetic protocols, both in solution and on solid supports, for iterative construction of diverse polypyrrolinones that present functionalized peptide-like side-chains. As a result of the rigid nature of the pyrrolinone scaffold, control over the backbone conformation could be exerted by modulation of the stereogenicity of the constituent monomers and the network of intramolecular hydrogen bonding. The extended conformation of the homochiral 3,5-linked polypyrrolinone scaffold proved to be an excellent mimic for β-strands and β-sheets. Application to enzyme inhibitor design and synthesis led not only to modest inhibitors of the aspartic acid protease renin and the matrix metalloprotease class of enzymes, but importantly to bioavailable HIV-1 protease inhibitors with subnanomolar binding constants.
The design and synthesis of a competent peptide–pyrrolinone hybrid ligand for the class II major histocompatibility complex (MHC) antigen protein HLA-DR1 further demonstrated the utility of the 3,5-polypyrrolinone motif as a mimic for the extended polyproline type II peptide backbone. Equally important, we sought to define, by synthesis, the additional conformational space accessible to the polypyrrolinone structural motif, with the ultimate goal of accessing pyrrolinone-based turn and helix mimetics. Towards this end, a mono-N-methylated bispyrrolinone was found to adopt an extended helical array in the solid state. Subsequent synthesis of d,l-alternating (heterochiral) tetrapyrrolinones both validated the expected turn conformations in solution and led to a functionally active mimetic of a peptidal β-turn (similar to somatostatin). Finally, the design, synthesis, and structural evaluation of both acyclic and cyclic heterochiral (that is, d,l-alternating) hexapyrrolinones yielded nanotube-like assemblies in the solid state. Taken together, these results illustrate the remarkable potential of the 3,5-linked polypyrrolinone scaffold as β-strand, β-sheet, β-turn, and potentially helical peptidomimetics.
Keywords: Peptides to Peptidomimetics; Pyrrolinone Scaffold; Homochiral 3,5-Linked Polypyrrolinones; β–strand/β-sheet Mimetic; Conformationally Diverse Peptidomimetic – Pyrrolinone Based Turn and Helix Mimics
Introduction
In the late 1980’s Hirschmann, Nicolaou and Smith initiated collaborative research programs at the University of Pennsylvania to develop novel nonpeptide peptidomimetic scaffolds with improved pharmacokinetic properties.1 This collaboration was based on the Hirschmann hypothesis that secondary amide-bonds of peptides and proteins were primarily responsible for their poor pharmacokinetic properties.2 Two conceptually different programs were initiated, one directed at the design of nonpeptide peptidomimetic receptor angonists/antagonists,3 the second focused on the development of protease enzyme inhibitors.4 For the receptor angonists/antagonists program, the concept – innovative at the time – entailed peptide backbone replacement with a scaffold that would display the requisite peptide-like side-chains with trajectories similar to those found in turned peptide ligands, without regard for ligand backbone to receptor hydrogen bonding. For the protease inhibitor program, scaffolds were sought to mimic the well known extended β-strand conformation of native peptide substrates, including both side-chain trajectories and importantly substrate-enzyme hydrogen bonding. This review will focus on the evolution of pyrrolinone-based nonpeptide peptidomimetic program, highlighting the design, synthesis and biological validation of pyrrolinone based scaffolds.
Initial Design
Proteolytic enzymes (cf. aspartic acid and serine proteases) bind substrates in an extended β-strand conformation via an extensive array of backbone-to-backbone hydrogen bonds, in conjunction with side-chain interactions (Figure 1A),5,6 Thus, in contrast to the design of β-turn mimics that bind receptors, β-strand peptidomimetics for use as protease inhibitors require not only appropriate side-chain trajectories, but also optimal hydrogen-bond registration with the enzyme backbone.
Figure 1.

(A) Typical Aspartic Acid Protease Active Site, (B) Comparison of Amide. and Pyrrolinone Units, (C) Design of 3,5- and 2,5-Pyrrolinones, (D) Registrations of 3,5- and 2,5-Linked Pyrrolinone Scaffolds.
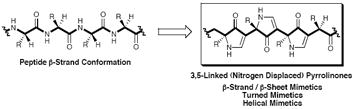
Early on, a decision was made to devise a β-strand mimetic that would incorporate a repeating structural unit, thus facilitating general application to a spectrum of problems by structural unit modification. The requirements of a repeating unit, that would appropriately project peptide-like side-chains, maintain the requisite hydrogen bonds, and prove proteolytically stable, led to the design of a vinylogous amide7 based repeating core, attractive for the following reasons: (1) the amide and vinylogous amide NH possess similar pKa values;8 (2) the nitrogen and carbonyl of amides and vinylogous amides display similar hydrogen bonding potential; (3) vinylogous amides are proteolytic stable; and (4) the vinylogous amide moiety provides backbone rigidity with an element of preorganization. To optimize side-chain and hydrogen bond registration, vis-à-vis a native peptide sequence, the vinylogous amide was incorporated into a five-membered pyrrolinone ring (Figure 1B). For translation of a peptide chain into a “nitrogen-displaced” polypyrrolinone mimic see Figure 1C. A conceptually similar exercise, involving displacement of the carbonyl groups, provides an alternate peptidomimetic backbone, termed 2,5-linked “carbonyl displaced” polypyrrolinones. Compared to a peptide β-strand, the pyrrolinone rings occupy somewhat different registrations relative to the pleates of β-strands (Figure 1D). Thus unique chemical, structural and biological characteristics for each scaffold could be envisioned (vide infra).
Monte Carlo conformational searches for model nitrogen and carbonyl displaced tetrapyrrolinones (1 and 2) were employed to determine the most favorable conformation. For the 3,5-linked nitrogen displaced tetrapyrrolinone (1), three low energy conformational classes were observed (Figure 2A). In contrast, only a single low energy class, possessing an extended backbone conformation (2a), was observed for the 2,5-linked carbonyl displaced tetrapyrrolinone 2 (Figure 2B). Importantly, both extended low energy conformations (1a and 2a) incorporate repeating intramolecular hydrogen bonds between the carbonyl of the pyrrolinone ring and the adjacent pyrrolinone NH hydrogen (Figure 2C), anticipated to stabilize the extended conformation of polypyrrolinone scaffolds. Somewhat worrisome; however, was the potential for decomposition of 2,5-linked polypyrrolinone scaffold upon attack of a nucleophile (Figure 2D). We thus decided to focus initially (and principally) on the 3,5-linked scaffold.9
Figure 2.
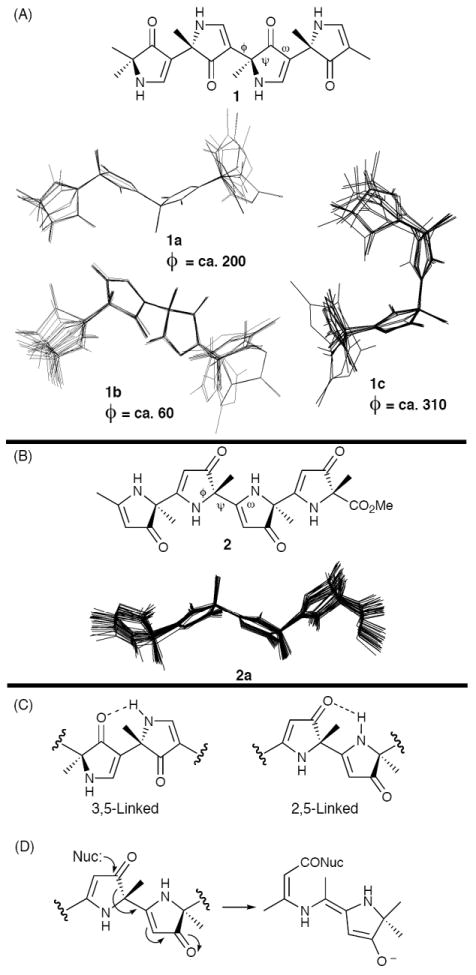
Monte Carlo Generated Backbone Conformations for Model Tetrapyrrolinones 1 (A) and 2 (B); (C) Predicted Intramolecular Hydrogen Bond Between Adjacent Pyrrolinone Rings; (D) Potential Susceptibility of the 2,5-linked Pyrrolinone Scaffold to Nucleophilic Decomposition.
Construction of 3,5-Linked Polypyrrolinones
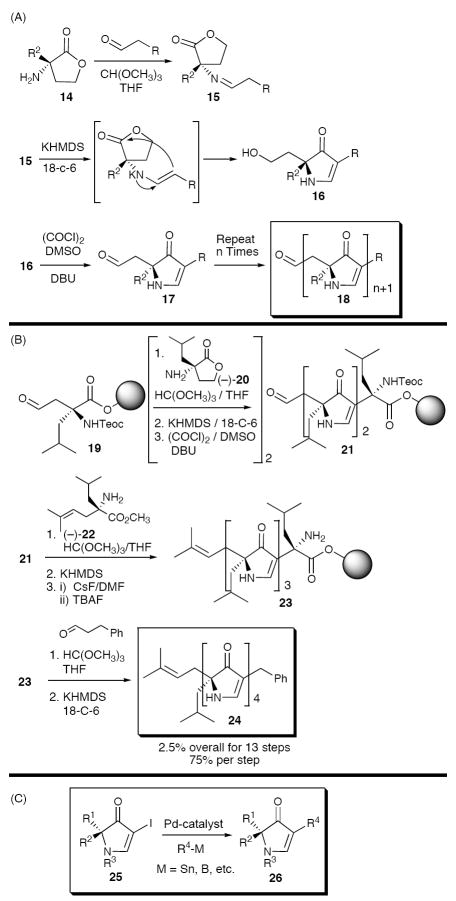
The Hiroi retron10 (Scheme 1A), involving condensation of aminoesters with preestablished α-stereogenicity and aldehyde building blocks, was selected as the foundation for our pyrrolinone synthetic program. Application and extension of this sequence to iterative construction of polypyrrolinones was substantially validated in our laboratory (Scheme 1B). 11
Scheme 1.
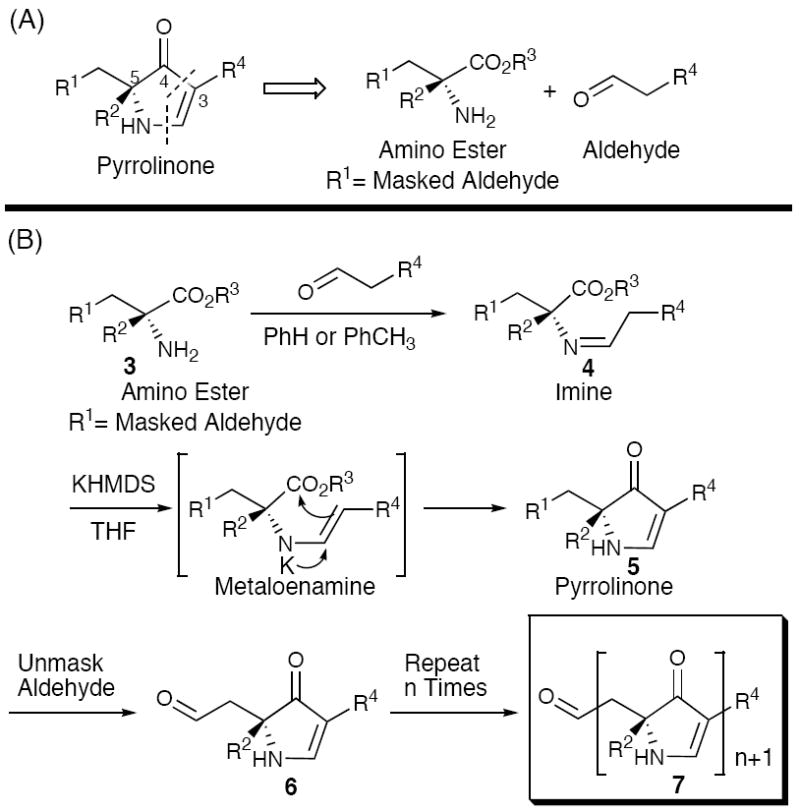
(A) Retrosynthetic Analysis of the 3,5-Pyrrolinone Unit. (B) Iterative 3,5-Pyrrolinone Synthesis via Metalloenamine Mediated Cyclization.
To construct the requisite amino acid ester building blocks, we adopted a modification of the Seebach12/Karady13 chemistry for the self-regeneration of stereogenic centers (Scheme 2A), initially exploiting a tert-butyl carbamate (Boc) protecting group for the amines and an olefin (i.e., prenyl group) for the masked aldehydes (cf. 12). To expedite iterative polypyrrolinone construction, a second protecting group strategy was introduced that employed a Cbz-carbamate and an acetal (cf. 13).14 Use of acetals both eliminated the need for oxidative cleavage of the olefin for subsequent iterations, a transformation that proved incompatible with some amino acid-like side chains, and provided flexibility vis-à-vis deprotection.15
Scheme 2.
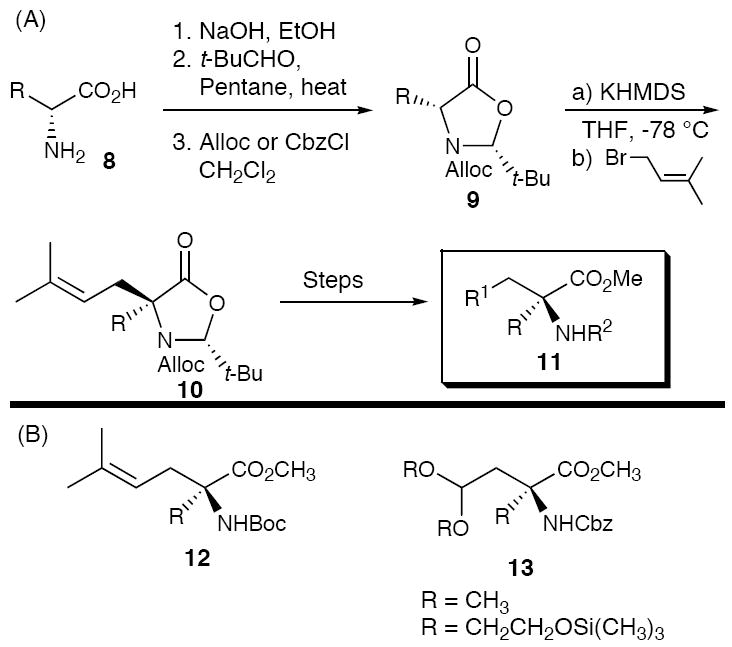
(A) Synthesis of Amino Ester and Aldehyde Building Blocks. (B) Amino Ester Building Blocks for Approaches A and B.
Validation of the polypyrrolinone scaffold as a β-strand mimetic (vide infra) prompted extension of the second-generation pyrrolinone synthetic protocol to solid support to permit construction of potential polypyrrolinone libraries. Here a third-generation strategy was required,16 employing amino lactones (cf. 14). The sequence retained the earlier two-step imine/metalloenamine cyclization (Scheme 1B), which when applied to the lactone, releases an alcohol requiring only mild oxidation to generate the aldehyde for iterative chain extension (Scheme 3A). Importantly, the requisite α-aminolactone building blocks proved readily available,17 thus facilitating the synthesis of polypyrrolinones on solid-support (Scheme 3B). Equally important for library construction, we developed a cross-coupling protocol to access diverse C-terminal peptidomimetics from a common precursor (Scheme 3C).18
Scheme 3.
(A) Third Generation Pyrrolinone Synthesis. (B) Synthesis of Polypyrrolinones on Solid Support. (C) Palladium-Catalyzed Functionalization of C-terminal Pyrrolinones.
Validation of the Extended 3,5-Polypyrrolinone Conformation as a β –Strand Mimic
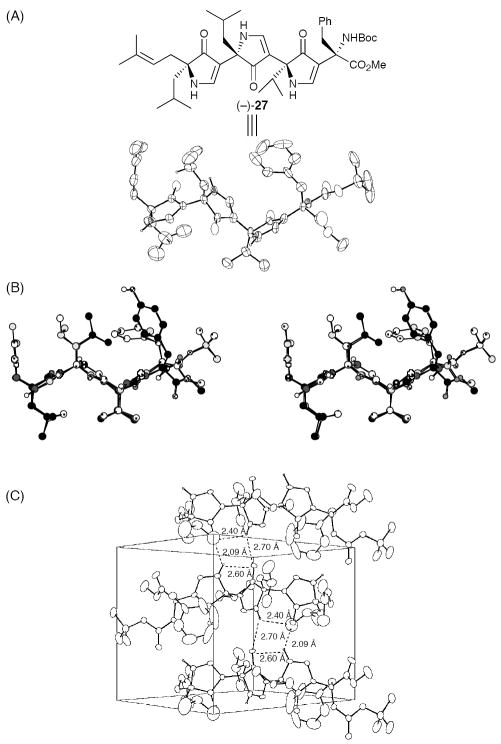
Prior to the development of prospective pyrrolinone-based enzyme inhibitors, experimental support for the proposed extended β-strand/β-sheet like conformation was sought. Trispyrrolinone (−)-27, a potential mimic of the Precigoux equinine tetrapeptide methyl ester, H-Leu-Leu-Val-Tyr-OMe,19 was designed and synthesized. Single crystal X-ray analysis revealed the anticipated extended β-strand-like conformation in the solid state (Figure 3A), with both the side-chain trajectories and carbonyl orientations overlaying remarkably well with the corresponding tetrapeptide (Figure 3B).4 Analysis of the unit cell further revealed interstrand hydrogen bonding with head-to-tail molecule stacking, similar to that found in antiparallel β-sheets (Figure 3C)
Figure 3.
(A) ORTEP Plot of Trispyrrolinone (−)-27; (B) Overlay with the Crystal Structure of the Corresponding Tetrapeptide (Stereoview); (C) Unit Cell Illustration Highlighting the Antiparallel Packing of (−)-27 (C).20
Single crystal X-ray analysis of the des-Boc trispyrrolinone [cf. amine (−)-28] also revealed an extended β-strand-like conformation; however, now stacking in the solid state in a parallel β-sheet like arrangement, as observed for the equinine tetrapeptide (Figure 4).4 That the nitrogen displaced pyrrolinone scaffold forms interstrand hydrogen bonds, stabilizing respectively antiparallel and parallel sheet formation was also evident in the crystallographic packing of 27 and 28.
Figure 4.
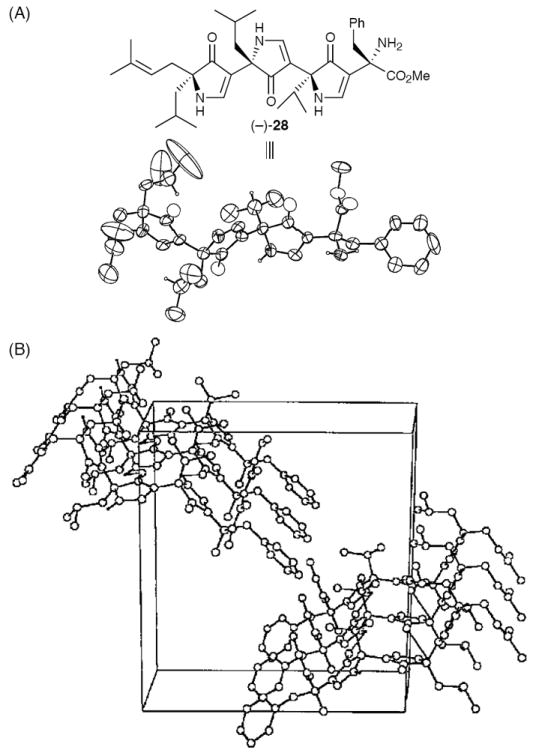
ORTEP Plot (A) and Unit Cell (B) for Trispyrrolinone Amine (−) 28.20
In similar fashion, solution FT-IR studies demonstrated that the NH and the carbonyl of adjacent pyrrolinone rings, as predicted, participate in a six-membered ring intramolecular hydrogen bond (Figure 5).21 Variable temperature 1H NMR studies were also informative. The N-terminal pyrrolinone NH proton in (−)-29, which cannot form an intramolecular H-bond, exhibits a large temperature chemical shift dependence, whereas the C-terminal pyrrolinone NH proton displays only a small chemical shift temperature dependence due to intramolecular H-bonding. From the outset however, we recognized that a more definitive test demonstrating the extended conformation would be the successful application of the polypyrroline structural motif to a relevant biological problem, or as Professor Hirschmann often stated: “Let the enzyme or receptor be the judge!”
Figure 5.
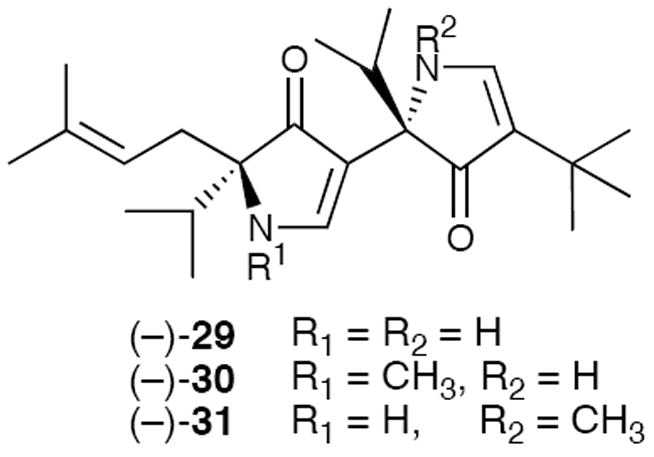
Pyrrolinones Used in Solution Phase Structural Studies.
Enzyme Inhibition Employing the 3,5-Linked Pyrrolinone Scaffold
As a first test of the 3,5-linked polypyrrolinone scaffold, we sought to design inhibitors of renin, a critical target in the late 1980s for intervention in the renin-angiotensinogen cascade regulating blood pressure.22 Elements of several known renin inhibitors23 were employed in our initial design. Ultimately, monopyrrolinone 32 and bispyrrolinone 33 were selected based on modeling studies, and constructed via our first generation synthetic protocol (Figure 6). Pleasingly in vitro assays of (+)-32 and (−)-33 revealed IC50 values of 18 μM and 0.6 μM, respectively.24 Observation of activity, albeit modest, was taken as the first evidence that the 3,5-pyrrolinonone scaffold in fact held promise as a β-strand mimic.
Figure 6.
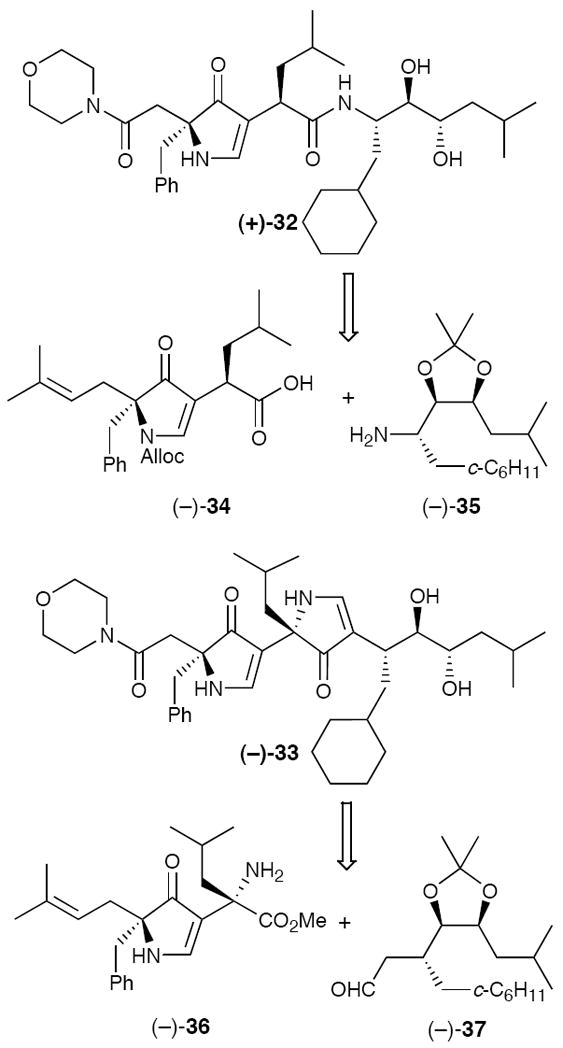
Design and Synthesis of Pyrrolinone-Based Renin Inhibitors.
Design, Synthesis and Biological Evaluation of HIV-1 Protease Inhibitors
In 1988, the identification that the HIV-1 protease was an aspartic acid protease proved seminal in the search for effective interventions in the HIV pandemic.25 Not surprisingly, we turned to the design and synthesis of pyrrolinone-based HIV-1 protease inhibitors. Initially, we employed the early Merck peptidal protease inhibitor L-682,679 (Figure 7)26 as a design template. Replacement of the P1’-P2’ dipeptide with a bispyrrolinone, possessing both the appropriate side-chains and the P2-P1 and P3’ units, led to a series of prospective bispyrrolinone HIV-1 protease inhibitors (cf. 38-40).24,27
Figure 7.
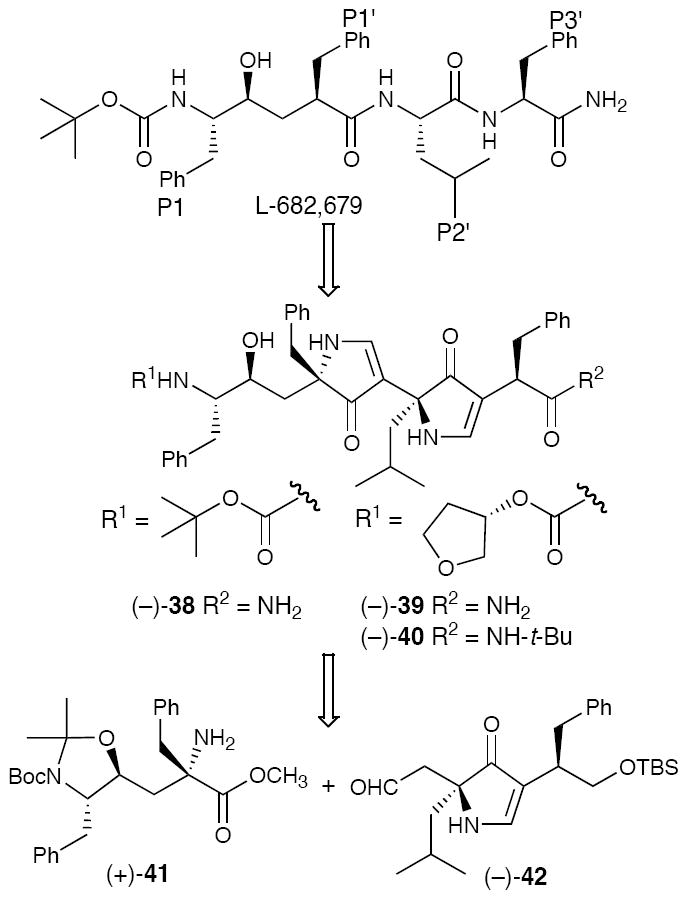
Design and Synthesis of Prospective Bispyrrolinone-Based HIV-1 Protease Inhibitors.
When tested for activity both in enzyme inhibitory (IC50) and cellular (CIC95) assays, furanyl carbamates (−)-39 and (−)-40 proved potent at the low nanomolar range (Table 1). That in purified enzyme assays (IC50), (−)-39 and (−)-40 proved less active than L-682,679, while in cellular assays (CIC95) more active [i.e., lower CIC95 to IC50 (C/I) ratios], suggested that the inhibitors were more cell-permeable than the analogous peptides.28 The improved transport properties were attributed to the presence of the intramolecular hydrogen bonds between the adjacent pyrrolinone rings, that decreased the desolvation energy required for passage from the extracellular aqueous phase into and through the cellular membrane.
Table 1.
Biological Activity of Bispyrrolinone HIV-1 Protease Inhibitors. 50% Inhibitory Concentration (IC50); Cellular 95% Inhibitory Concentration (CIC95)
| Inhibitor | N-Terminus | C-Terminus | IC50 (nM) | CIC95 (nM) | C/I |
|---|---|---|---|---|---|
| L-682,679 | Boc | NH2 | 0.6 | 6,000 | 10,000 |
| (−)-38 | Boc | NH2 | 10 | 1,500 | 150 |
| (−)-39 | Furanyl Carbamate | NH2 | 1.3 | 800 | 615 |
| (−)-40 | Furanyl Carbamate | NH-t-Bu | 3.3 | 800 | 242 |
Monopyrrolinone HIV-1 Protease Inhibitors
While translation of a peptidyl protease inhibitor into bispyrrolinone congeners proved successful, the bispyrrolone inhibitors were not orally bioavailable in dogs, presumably due, at least in part, to their high molecular weight (ca. 730). A series of lower molecular weight monopyrrolinone inhibitors typified by (−)-43 (MW = 583), based on L-685,807 and Indinavir,29 were therefore designed and synthesized (Figure 8).30
Figure 8.
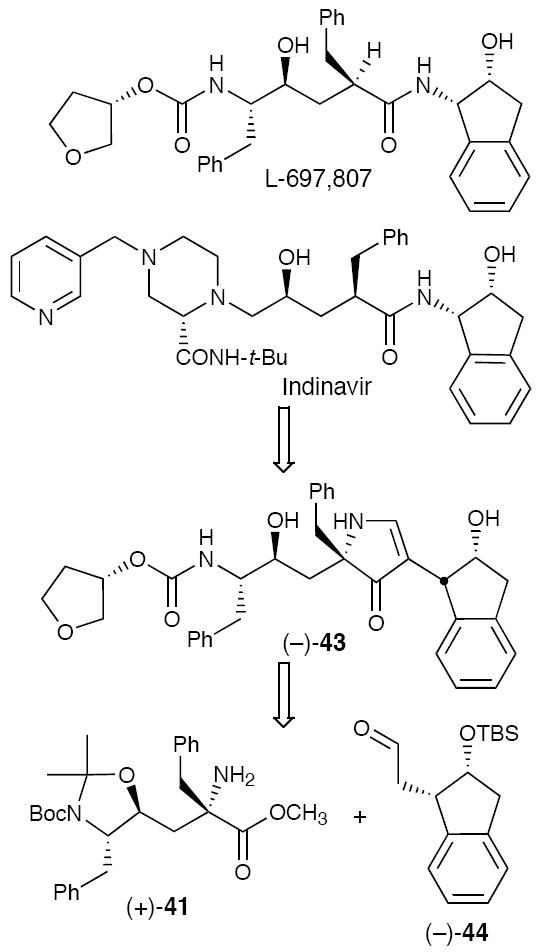
Design of Monopyrrolinone HIV-1 Protease Inhibitors.
Biological evaluation of (−)-43 and related (P2/P1’) congeners revealed that the inhibitors exploiting the monopyrrolinone scaffold were indeed quite potent against the wild-type HIV-1 protease (Table 2), displaying lower C/I ratios than either the peptide or bispyrrolinones based inhibitors, and thus were anticipated to have improved cell membrane transport properties. Administration of (−)-43 in two dogs revealed oral bioavailability of ca. 13%.
Table 2.
HIV-1 Protease Bioassay Data for Monopyrrolinone and Related Amide-based Inhibitors. 50% Inhibitory Concentration (IC50); Cellular 95% Inhibitory Concentration (CIC95)
| Inhibitor | IC50 (nM) | CIC95 (nM) | C/I |
|---|---|---|---|
| Indinavir | 0.36 | 25-100 | 69-277 |
| L-682,679 | 0.6 | 6 000 | 10 000 |
| (−)-39 | 1.3 | 800 | 615 |
| L-697,807 | 0.03 | 3 | 100 |
| (−)-43 | 2.0 | 100-250 | 50-125 |
Equally important, X-ray analysis of (−)-43 co-crystallized with the HIV-1 protease30a provided the foundation for an extended Penn/Merck program to design additional modified monopyrrolinone-based inhibitors with improved binding affinity. From 1994-2005, repeated rounds of design and synthesis, employing molecular modeling and X-ray analysis of co-crystal structures, culminated in the discovery of (−)-48,31 the most potent (in vitro) monopyrrolinone-based HIV-1 protease inhibitor prepared to date in our laboratory (Figure 9). Thus our early hypothesis that the pyrrolinone scaffold holds considerable potential as a β-strand mimetic had been validated.
Figure 9.
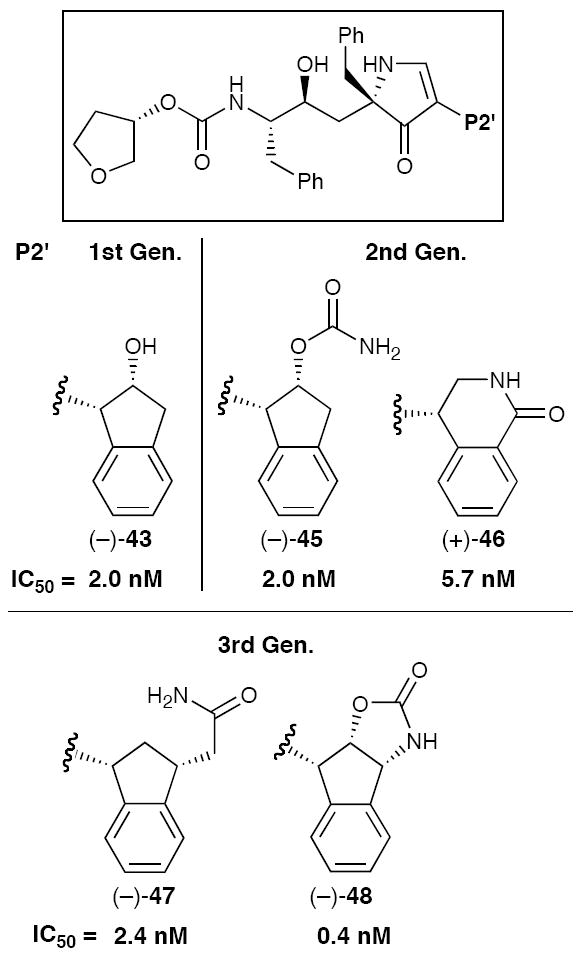
Development of Monopyrrolinone-Based HIV-1 Protease Inhibitors.
Major Histocompatibility Complex Hybrid Ligands and Matrix Metalloproteases Inhibitors
Building on the success of the aspartic acid protease program, we turned to other proteins and enzymes of biomedical significance, known to prefer binding extended β-strand conformations. The class II major histocompatibility complex (MHC) comprises a series of extracellular membrane-bound proteins found on specialized antigen-presenting T-cells, with the MHC protein HLA-DR1 specifically linked to increased susceptibility towards rheumatoid arthritis.32 In 1994 the late Don Wiley and colleagues, established that class II MHC molecules bind antigenic peptides in an extended, polyproline type II conformation.33 In collaboration with Olsen at Hoffmann La Roche, we initiated a program to design a pyrrolinone-peptide hybrid ligand for HLA-DR1.34 The design was based on an analog of the potent peptide HA 306-318 (Figure 10).35 We sought to mimic the peptide with pyrrolinone-peptide hybrid 50 (Figure 10). The bispyrrolinone segment was constructed via our second-generation protocol to provide Fmoc-protected bispyrrolinone amino acid (−)-51, that was incorporated into peptide 50 via Fmoc-based solid-phase synthesis.
Figure 10.

A Pyrrolinone-Peptide Hybrid Ligand for the Class II MHC HLA-DR1.
Affinity-binding experiments revealed that the pyrrolinone-peptide hybrid (50) was a competent ligand for HLA-DR1 (IC50 137 nM), compared both to HA 306-318 (89 nM) and the control peptide 49 (176 nM).34 Equally exciting, the X-ray structure of co-crystallized 50 and HLA-DR1 obtained by the Wiley group34b mimicked closely the polyproline type II conformation of peptide HA-306-318, a result that demonstrates that the pyrrolinone scaffold can serve as a direct replacement of an amino acid sequence in a bioactive peptide, involving an extended conformation.
We also explored the design of inhibitors for matrix metalloproteases (MMPs), a family of zinc-containing enzymes known to bind substrates in extended conformations, which have been implicated in a variety of disease states.36 Employing peptidyl inhibitor Ro-31-4724 (IC50 = 9 nM for MMP-1)37 as the design template, bispyrrolinones (−)-55 and (−)-56 were synthesized (Figure 11).38 Although the bispyrrolinone MMP inhibitors displayed only modest activity (ca. low μM), conformation of the pyrrolinone scaffold as β-strand mimics had again been achieved.
Figure 11.
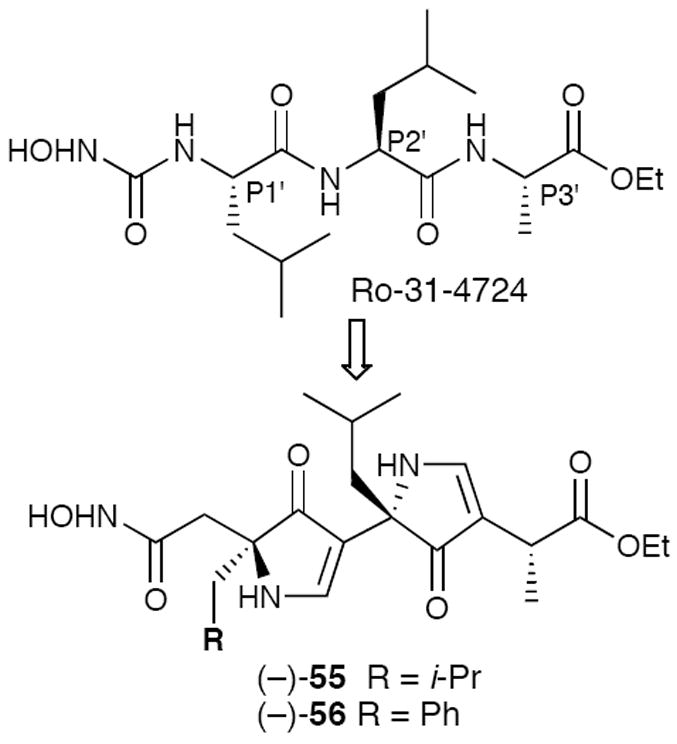
Pyrrolinone-Based MMP Inhibitors.
Alternative Conformations for the 3,5-Linked Polypyrrolinone Scaffold
An evolving interest of the Smith/Hirschmann collaboration was the accessibility of alternative conformational space for 3,5-linked polypyrrolinones. Towards this end, early molecular modeling calculations (Figure 1a-e) had suggested that the 3,5-linked backbone could not only adopt the extended β-strand conformation, stabilized by an intramolecular hydrogen bond, but also turn and twisted conformations similar to the other secondary conformations of peptides and proteins (Figure 2A).4 Tactics to access the broader range of polypyrroline conformational space were envisioned to include modulation of the α-stereogenicity, the side-chain structure, and/or the presence of intramolecular hydrogen bonding.
N-Methylated 3,5-Linked Pyrrolinones: Disruption of Intramolecular Hydrogen Bonding
To explore the hypothesis that disrupting the intramolecular hydrogen bond between the pyrrolinone units would lead to additional backbone conformations, structural analysis of a series of model N-methylated bispyrrolinones such as (−)-31 (Figure 5) was undertaken.39 Crystallographic analysis revealed that (−)-31 had a ϕ angle of 177°, and that the individual molecules assembled to form a beautiful helical array in the solid-state (Figure 12).39 Although attempts to enforce a helical array by covalent linking the bispyrrolinone units proved unrewarding,39b observation of a helical array in the solid state of (−)-31 provided the first evidence of the wider range of conformational diversity available to the 3,5-linked polypyrrolinone structural motif.
Figure 12.
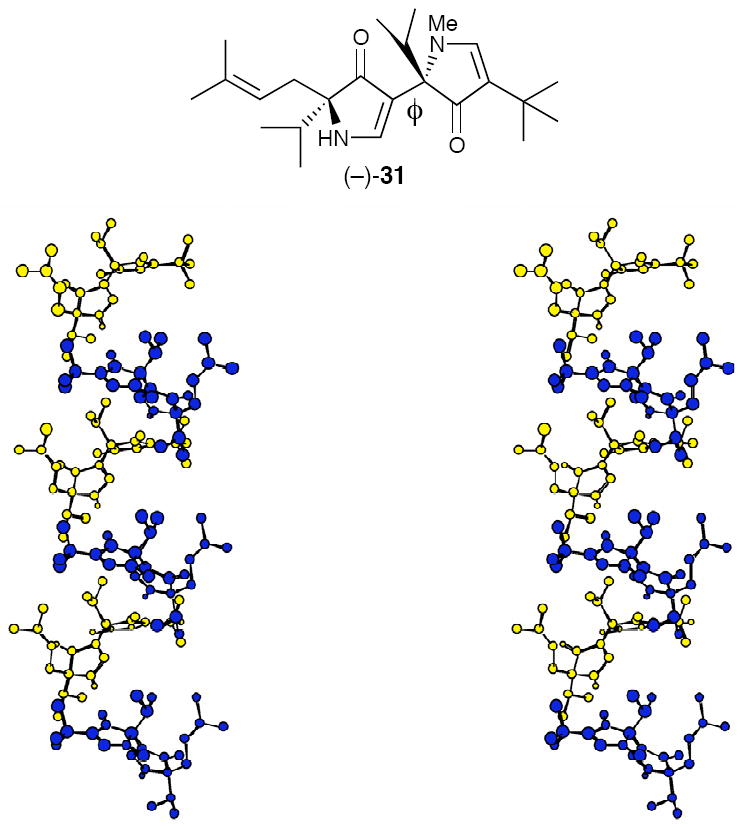
Stereoview of the X-ray Structure of (−)-31.40
D,L-Alternating Polypyrrolinones: Computational Analysis, Synthesis and Structural Evaluation
In addition to disrupting intramolecular hydrogen bonding, we targeted modulation of the stereogenicity α to the pyrrolinone carbonyl to expand the 3,5-polypyrrolinone conformational space. Based on the established ability of D-amino acids to stabilize β-turns,41 as well as the demonstrated turn conformations observed with peptides containing D- and L-amino acids,42 we reasoned that a sequence of alternating D,L-linked pyrrolinones might preferentially adopt a turn structure.
Computational analysis of heterochiral D,L-alternating 3,5-linked pyrrolinones revealed that the low energy conformations not only adopt turn conformations (Figure 13),14 but importantly predicted that the family of turn conformations would again accommodate intramolecular hydrogen bonding between the adjacent pyrrolinone rings. Moreover, the intramolecular hydrogen bonding would enforce the β-turn-like conformation. With this as background, the synthesis of an initial D,L-alternating tetrapyrrolinone (−)-58 was achieved exploiting the second generation protocol. A series of variable concentration NMR, 2D-NMR, and FT-IR experiments revealed that intramolecular hydrogen bonding within tetrapyrrolinone (−)-58 did in fact lead to a turned conformation in solution (Figure 14).14
Figure 13.
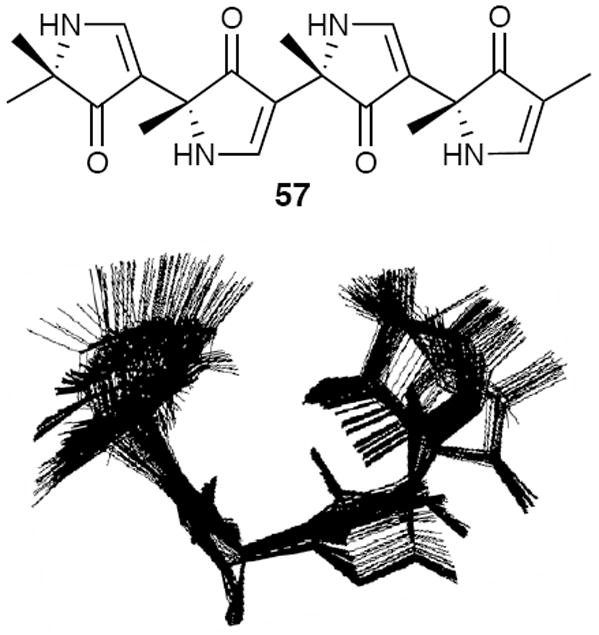
D,L,D,L-Tetrapyrrolinone 57 and the Low Energy Structures from a Monte Carlo Conformational Search.
Figure 14.
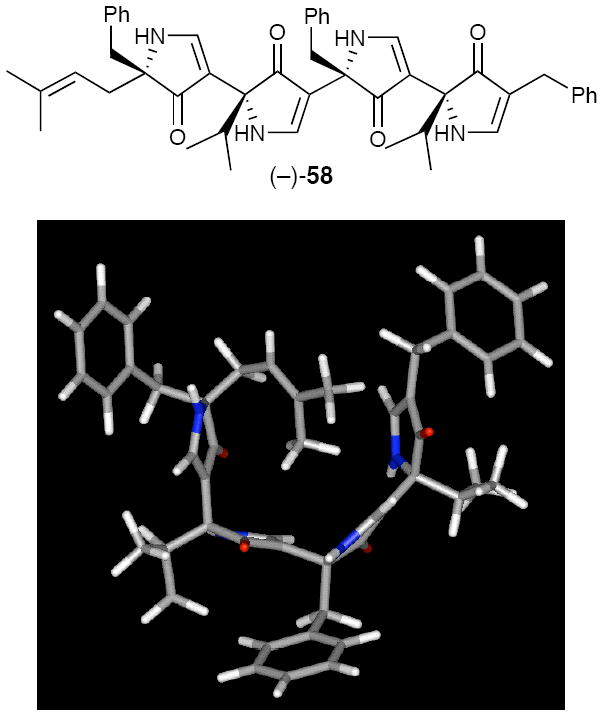
The Solution Structure of (−)-58.
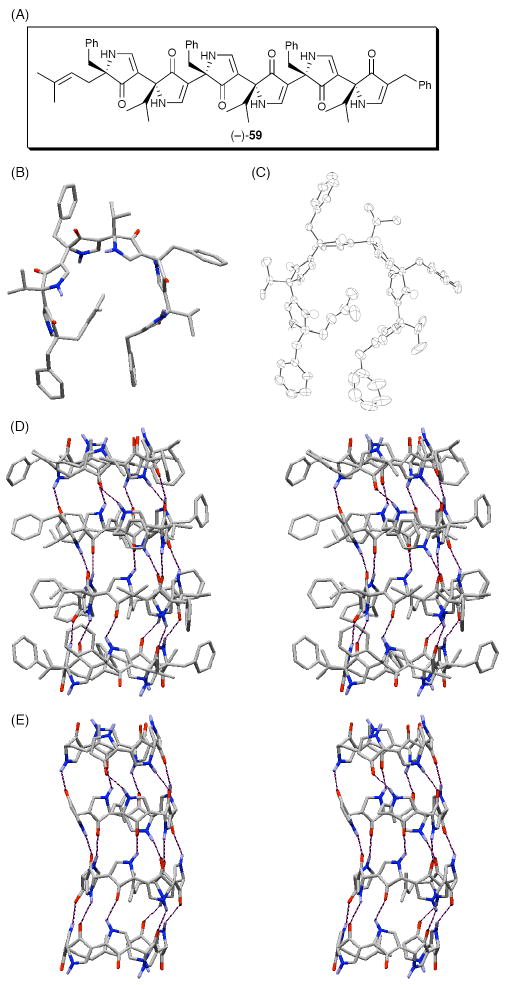
Having demonstrated by rational design that a tetrapyrrolinone scaffold can adopted a β-turn like conformation, we next constructed a D,L-alternating hexapyrrolinone (59, Figure 15A).43 A series of 2D-NMR experiments again revealed a flat, G-shaped turn conformation of (−)-59 in CDCl3 (Figure 15B). Pleasingly, X-ray analysis of crystalline (−)-59 confirmed the flat G-shaped structure (Figure 15C), similar to the low energy conformation observed in solution. Of equal interest, the unit cell revealed that (−)-59 self-assembles into a nanotube-like quaternary structure (Figure 15D and E), with the monomers arrayed in an antiparallel fashion.
Figure 15.
(A) D,L-alternating Hexapyrrolinone (−)-59; (B) The Predicted Solution Structure of (−)-59; (C) ORTEP Diagram of (−)-59; Stereoviews Illustrating a Nanotube-Like Assembly of (−)-59 in the Solid State; (D) Side View with Intermolecular Hydrogen Bonds Illustrated; and (E) With Side-Chains Removed.
A Biologically Relevant β-Turn Peptidomimetic Based on the Polypyrrolinone Structural Motif
To validate a heterochiral (D,L-alternating) polypyrrolinone turn mimic in a biologically relevant system, recall the “Hirschmann motto”, we turned to somatostatin (Somatotropin Release Inhibiting Factor, SRIF-14), the endogenous, cyclic tetradecapeptide hormone that regulates endocrine and exocrine secretion. Somatostatin was of course well known to Hirschmann and colleagues, having demonstrated at Merck that a β-turn is both necessary and sufficient for somatostatin receptor binding and signal transduction.44 A series of D,L-mixed tetrapyrrolinones, incorporating the turn side-chain sequence of L-363,301 (cf, Phe7, Trp8, Lys9, Thr10)44 were envisioned as prospective pyrrolinone-based SRIF mimetics (Figure 16).
Figure 16.

Prospective Pyrrolinone-Based SRIF Mimetics.
Although the synthesis of tetrapyrrolinone 60 possessing an i+1 indole side-chain mimic proved elusive, three D,L-alternating tetrapyrrolinone SRIF mimetics (−)-61, (+)-62 and (+)-63 displaying aromatic indole surrogates were constructed.45 Binding affinities were determined at two somatostatin receptors (hsst 4 and 5, Table 3). Despite the modest affinities relative to SRIF, the potential utility of the pyrrolinone scaffold as a β-turn peptidomimetic had been validated.
Table 3.
Binding Affinities of Pyrrolinone-Based SRIF Mimetics.
| Ligand | IC50 hsst 4 | IC50 hsst 5 |
|---|---|---|
| (−)-61 | 2.14 μM | 2.44 μM |
| (+)-62 | 4.04 μM | 1.27 μM |
| (+)-63 | 2.05 μM | 38% at 10 μM |
| SRIF-14 | 0.111 nM | 0.362 nM |
Macrocyclic D,L-Alternating Hexapyrrolinones: Design, Synthesis and Structure Evaluation
The nanotube-like architecture of (−)-59 in the solid-state, suggested ring closure to achieve macrocyclic hexapyrrolinones 64 and 65. Importantly, Monte Carlo conformational searches for 64 predicted a flat conformation for the monomers with the potential for an antiparallel stacking arrangement, in agreement with the observed stacking in the crystalline open-chain hexapyrrolinone (−)-59.43
Macrocyclic hexapyrrolinone 64 was subsequently prepared,46 although the yield for the macrocyclization step proved quite modest (ca. 12%). Importantly, the propensity of macrocycle (+)-64 to self-assemble in solution was demonstrated via a series of 1H NMR studies. Unfortunately, crystals of (+)-64 suitable for X-ray analysis were not forthcoming. Lacking a crystal structure of (+)-64, an alternate hexapyrrolinone 65 was designed and synthesized (Scheme 4), with the expectation that the reduced flexibility of the isopropyl side-chains would facilitate crystal growth.
Scheme 4.
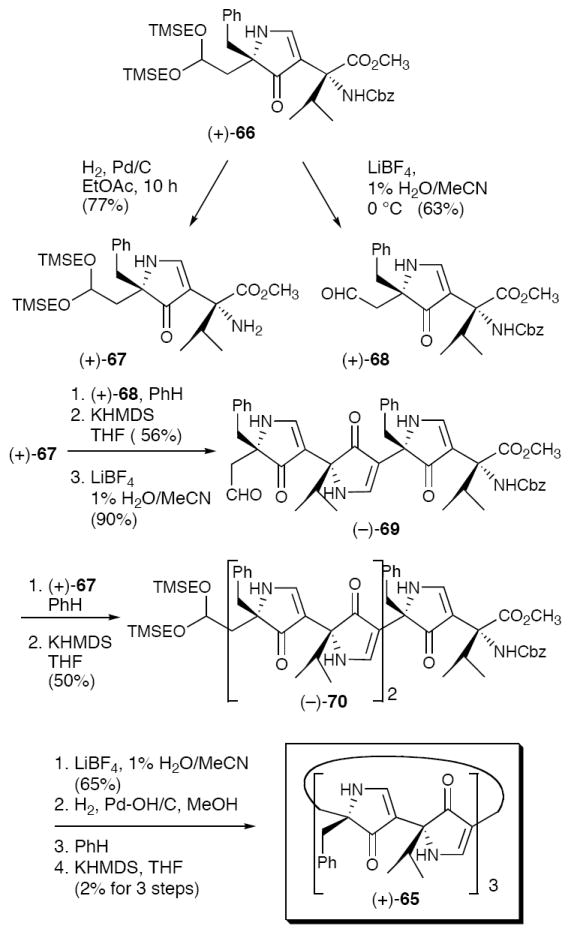
Convergent Synthesis of Macrocyclic Hexapyrrolinone (+)-65.
Pleasingly, crystals suitable for X-ray analysis of (+)-65 were obtained (Figure 18);46 however, unlike the open chain hexapyrrolinones (−)-59, (+)-65 was found to assemble into an infinite, staggered, nanotube-like array, with four pyrrolinone rings participating in intermolecular hydrogen bonding. The first steps toward the design, synthesis and structural characterization of novel pyrrolinone-based nanostructures had thus been achieved with the synthesis and structural characterization of (+)-64 and (+)-65.
Figure 18.
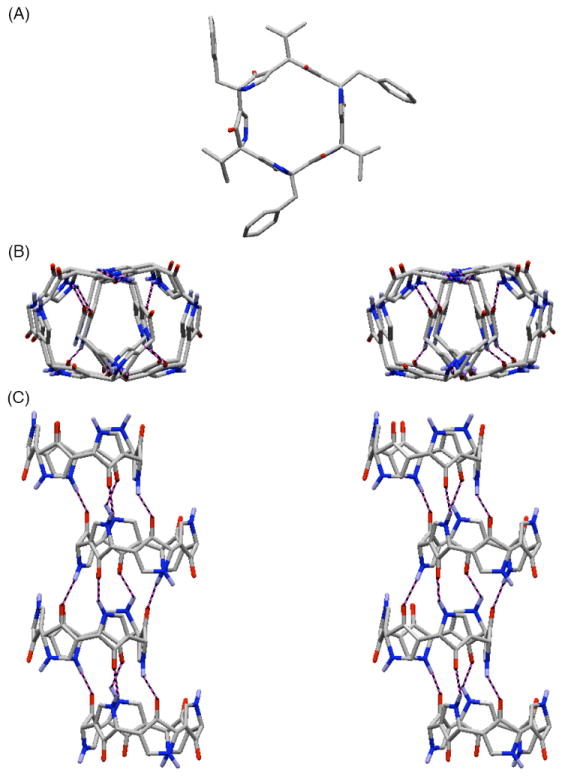
(A) X-ray Structure of (+)-65; The Nanotube-like Assembly of (+)-65 in the Solid-State (Side Chains Omitted); Stereoview from the Top (B) and Side View (C).
Summary
The polypyrrolinone scaffold, designed as a non-peptide peptidomimetic incorporating both hydrogen bond capacity and side-chain diversity, lead to the development of a robust metalloenamine-based synthesis, followed by expansion to include diverse amino-acid-like side-chains, flexible protecting-group strategies, and importantly translation to solid-support for the eventual construction of polypyrroline libraries. Homochiral 3,5-linked polypyrrolinones adopt extended β-strand/sheet-like conformations, while heterochiral (D,L-alternating) polypyrrolinones lead to turned structures. Importantly homochiral mono and bispolypyrrolinones were validated as inhibitors of renin, matrix metalloproteases and potent bioavailable HIV-1 protease inhibitors, as well as a competent peptide-pyrrolinone hybrid ligand for the class II MHC HLA-DR1. Finally, the turned conformations of heterochiral pyrrolinones were shown to be both biologically relevant as β-turn mimics and capable of producing novel nanotube-like structures. Taken together, the results of the Hirschmann-Smith pyrrolinone program illustrate the remarkable potential of the pyrrolinone structural motif as a privileged scaffold for molecular mimicry, capable of generating β-strand/β-sheet, β-turn and potential helical peptidomimetics.
Figure 17.
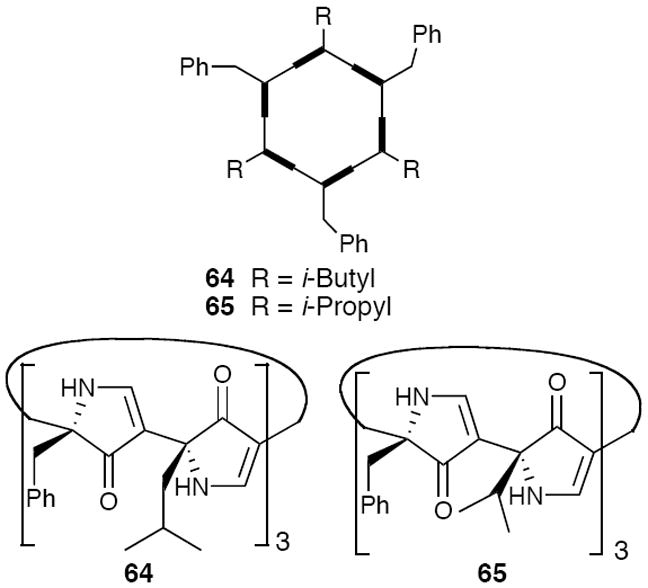
Designed Macrocyclic Hexapyrrolinones.
Acknowledgments
ABS dedicates this review to the memory of Ralph Hirschmann, friend, colleague and mentor. We thank our many co-workers and collaborators who have contributed greatly to this research program, and whose names appear in the references cited. Financial support was provided by the NIH, through Grant GM-41821, and by the Merck Research Laboratories and Hoffmann La Roche.
Biographies
Amos B. Smith, III was born in Lewisburg, PA, in 1944 and in 1966 completed Bucknell University’s inaugural B.S.-M.S. degree in chemistry with Professor H. W. Heine. After a year of medical school at the University of Pennsylvania, he entered The Rockefeller University, completing his Ph.D. degree in 1972 and spending a year as a Research Associate, both with Professor W. C. Agosta. He then joined the University of Pennsylvania, where he is currently the Rhodes-Thompson Professor of Chemistry and a Full Member of the Monell Chemical Senses Center. From 1988 to 1996, he served as Chair of the Department of Chemistry. In addition, he holds Honorary Memberships in the Kitasato Institute and the Pharmaceutical Society of Japan, and serves as the inaugural Editor-in-Chief of Organic Letters. In 2009, he was awarded a D.Sc. (honoris causa) from Queens University Belfast (Northern Ireland) for his contributions to Organic Chemistry.
Adam K. Charnley was born in 1978 and spent his early years in Lapeer Michigan. He obtained a B.S. degree from the University of Notre Dame in 2000 as a combined chemistry-business major. In 2005 he completed his Ph.D. in Chemistry at the University of Pennsylvania under the guidance of Professor Amos B. Smith, III. During that time, he was awarded a Bristol-Myers Squibb Graduate Fellowship in Synthetic Organic Chemistry. From 2006 to 2008, he worked in the laboratory of Professor Erik J. Sorensen at Princeton University as an NIH NRSA Postdoctoral Fellow. In 2008, he joined GlaxoSmithKline, where he is currently a Principal Scientist in the Pattern Recognition Receptor DPU of the Immuno-Inflammation Center of Excellence for Drug Discovery.
Ralph F. Hirschmann (1922-2009) was born in Bavaria, Germany, and came to the U.S. in his teens. He graduated from Oberlin College and then served in the U.S. Army in the Pacific Theater during World War II. He resumed his education at the University of Wisconsin, Madison, where he was the Sterling Winthrop Fellow. In 1950 he completed his Ph.D. studies under the guidance of W. S. Johnson and joined Merck & Co., Inc. In 1987, at age 65, he retired from Merck, where he was Senior Vice President for Basic Research, and joined the faculty at the University of Pennsylvania as the Makineni Professor of Bioorganic Chemistry. At Merck, his team discovered Mevacor, Vasotec, Prinivil, Primaxin, Proscar, and Ivermectin. In 1969, Robert G. Denkewalter, Hirschmann, and their collaborators reported simultaneously with the Merrifield group (Rockefeller University) the first total synthesis of an enzyme in solution.
References
- 1.Hirschmann RF, Nicolaou KC, Angeles AR, Chen JS, Smith AB., III The β-D-Glucose Scaffold as a β-Turn mimetic. Accts Chem Res. 2009;42:1511–1520. doi: 10.1021/ar900020x.; also see Accts Chem Res. 2008;41(10).
- 2.Smith AB, III, Keenan TP, Holcomb RC, Sprengeler PA, Guzman MC, Wood JL, Carroll PJ, Hirschmann R. Design, Synthesis, and Crystal Structure of a Pyrrolinone-Based Peptidomimetic Possessing the Conformation of a β-Strand: Potential Application to the Design of Novel Inhibitors of Proteolytic Enzymes. J Am Chem Soc. 1992;114:10672–10674. [Google Scholar]
- 3.(a) Nicolaou KC, Salvino JM, Raynor K, Pietranico S, Reisine T, Freidinger RM, Hirschmann R. In: Design and Synthesis of a Peptidomimetic Employing β-D-Glucose for Scaffolding. Rivier JE, Marshall GR, editors. ESCOM; Leiden: 1990. pp. 881–884. [Google Scholar]; (b) Hirschmann R, Nicolaou KC, Pietranico S, Salvino J, Leahy EM, Sprengeler PA, Furst G, Smith AB, III, Strader CD, Cascieri MA, Candelore MR, Donaldson C, Vale W, Maechler L. Nonpeptidal Peptidomimetics with β-D-Glucose Scaffolding. A Partial Somatostatin Agonist Bearing a Close Structural Relationship to a Potent, Selective Substance P Antagonist. J Am Chem Soc. 1992;114:9217–9218. [Google Scholar]
- 4.Smith AB, III, Guzman MC, Sprengeler PA, Keenan TP, Holcomb RC, Wood JL, Carroll PJ, Hirschmann R. De Novo Design, Synthesis, and X-Ray Crystal Structures of Pyrrolinone-Based β-Strand Peptidomimetics. J Am Chem Soc. 1994;116:9947–9962. [Google Scholar]
- 5.For a review see: Tyndall JDA, Nall T, Fairlie DP. Proteases Universally Recognize Beta Strands In Their Active Sites. Chem Rev. 2005;105:973–999. doi: 10.1021/cr040669e..
- 6.Rich DH. In: Comprehensive Medicinal Chemistry. Sammes PG, Taylor JB, editors. Vol. 4. Pergamon; New York: 1990. pp. 391–441. [Google Scholar]
- 7.Greenhill JV. Enaminones. Chem Soc Rev. 1977;6:277–294. [Google Scholar]
- 8.(a) Huisgen R, Brade H, Huisgen R. Die Basizitätskonstanten Offenkettiger Carbonsäure-Amide. Chem Ber. 1957;90:1432–1436. [Google Scholar]; (b) Greenhill JV. Enaminones. Chem Soc Rev. 1977;6:277–294. [Google Scholar]
- 9.For the synthesis and structural analysis of a 2,5-trispyrrolinone see: Smith AB, III, Knight SD, Sprengeler PA, Hirschmann R. The Design and Synthesis of 2,5-Linked Pyrrolinones. A Potential Non-Peptide Peptidomimetic Scaffold. Bioorg Med Chem. 1996;4:1021–1034. doi: 10.1016/0968-0896(96)00094-6..
- 10.Hiroi K, Achiwa K, Yamada S-I. Stereochemical Studies. IX. Asymmetric Synthesis of 2-Alkylcyclo-hexanones with Enamine Alkylation. Chem Pharm Bull. 1972;20:246–257. [Google Scholar]
- 11.Smith AB, III, Holcomb RC, Guzman MC, Keenan TP, Sprengeler PA, Hirschmann R. An Effective Synthesis of Scalemic 3,5,5-Trisubstituted Pyrrolin-4-Ones. Tetrahedron Lett. 1993;34:63–66. [Google Scholar]
- 12.(a) Seebach D, Naef R. Enantioselective Generation and Diastereoselective Reactions of Chiral Enolates Derived from α-Heterosubstituted Carboxylic Acids. Preliminary Communication. Helv Chim Acta. 1981;64:2704–2708. [Google Scholar]; (b) Seebach D, Fadel A. N,O-Acetals from Pivalaldehyde and Amino Acids for the α-Alkylation with Self-Reproduction of the Center of Chirality. Enolates of 3-Benzoyl-2-(tert-butyl)-1,3-oxazolidin-5-ones. Helv Chim Acta. 1985;68:1243–1250. [Google Scholar]; For a review see: Seebach D, Sting AR, Hoffmann M. Self-Regeneration of Stereocenters (SRS) – Applications, Limitations, and Abandonment of a Synthetic Principle. Angew Chem Int Ed. 1996;35:2708–2748..
- 13.Karady S, Amato JS, Weinstock LM. Enantioretentive Alkylation of Acyclic Amino Acids. Tetrahedron Lett. 1984;25:4337–4340. [Google Scholar]; (b) Amato JS, Weinstock LM, Karady S. US Patent. 4:508–921.
- 14.Smith AB, III, Wang W, Sprengeler PA, Hirschmann R. Design, Synthesis, and Solution Structure of a Pyrrolinone-Based β-Turn Peptidomimetic. J Am Chem Soc. 2000;122:11037–11038. [Google Scholar]
- 15.Smith AB, III, Benowitz AB, Favor DA, Sprengeler PA, Hirschmann R. A Second-Generation Synthesis of Scalemic 3,5,5-Trisubstituted Pyrrolin-4-Ones: Incorporation of Functionalized Amino Acid Side-Chains. Tetrahedron Lett. 1997;38:3809–3812. [Google Scholar]
- 16.Smith AB, III, Liu H, Hirschmann R. A Second-Generation Synthesis of Polypyrrolinone Nonpeptidomimetics: Prelude to the Synthesis of Polypyrrolinones on Solid Support. Org Lett. 2000;2:2037–2040. doi: 10.1021/ol0059293. [DOI] [PubMed] [Google Scholar]
- 17.Smith AB, III, Liu H, Okumura H, Favor DA, Hirschmann R. Synthesis of Polypyrrolinones on Solid Support. Org Lett. 2000;2:2041–2044. doi: 10.1021/ol005931u. [DOI] [PubMed] [Google Scholar]
- 18.Charnley AK. Ph. D. Thesis, University of Pennsylvania. 2005. [Google Scholar]
- 19.Precigoux G, Courseille C, Geoffre S, Leroy F. Crystal Sructure of the 10-13 Sequence of Angiotensinogen L-leucyl-L-leucyl-L-valyl-L-tyrosyl Methyl Ester. J Am Chem Soc. 1987;109:7463–7465. [Google Scholar]
- 20.Adapted with permission from Smith AB, III, Guzman MC, Sprengeler PA, Keenan TP, Holcomb RC, Wood JL, Carroll PJ, Hirschmann R. De Novo Design, Synthesis, and X-Ray Crystal Structures of Pyrrolinone-Based β-Strand Peptidomimetics. J Am Chem Soc. 1994;116:9947–9962.. Copyright 1994 American Chemical Society.
- 21.Guzman MC. Ph.D. Thesis, University of Pennsylvania. 1994. [Google Scholar]
- 22.Brewster UC, Setaro JF, Perazella MA. The Renin-Angiotensin-Aldosterone System: Cardiorenal Effects and Implications for Renal and Cardiovascular Disease States. Am J Med Sci. 2003;326:15–24. doi: 10.1097/00000441-200307000-00003.. and references cited therein.
- 23.(a) Luly JR, BaMaung N, Soderquist J, Fung AKL, Stein H, Kleinert HD, Marcotte PA, Egan DA, Bopp B, Merits I, Bolis G, Greer J, Perun TJ, Plattner JJ. Renin Inhibitors. Dipeptide Analogues of Angiotensinogen Utilizing a Dihydroxyethylene Transition-State Mimic at the Scissile Bond To Impart Greater Inhibitory Potency. J Med Chem. 1988;31:2264–2276. doi: 10.1021/jm00120a005. [DOI] [PubMed] [Google Scholar]; (b) Izuka K, Kamijo T, Kubota T, Akahane K, Umeyama H, Kiso Y. New Human Renin Inhibitors Containing an Unnatural Amino Acid, Norstatine. J Med Chem. 1988;31:701–704. doi: 10.1021/jm00399a001. [DOI] [PubMed] [Google Scholar]
- 24.Smith AB, III, Hirschmann R, Pasternak A, Akaishi R, Guzman MC, Jones DR, Keenan TP, Sprengeler PA, Darke PL, Emini EA, Holloway MK, Schleif WA. Design and Synthesis of Peptidomimetic Inhibitors of HIV-1 Protease and Renin. Evidence for Improved Transport. J Med Chem. 1994;37:215–218. doi: 10.1021/jm00028a001. [DOI] [PubMed] [Google Scholar]
- 25.Brik A, Wong C-H. HIV-1 Protease: Mechanism and Drug Discovery. Org Biomol Chem. 2003;1:5–14. doi: 10.1039/b208248a. [DOI] [PubMed] [Google Scholar]
- 26.Vacca JP, Guare JP, deSolms SJ, Sanders WM, Giuliani EA, Young SD, Darke PL, Zugay J, Sigal IS, Schleif WA, Quintero JC, Emini EA, Anderson PS, Huff JR. L-687,908, a Potent Hydroxyethylene Containing HIV Protease Inhibitor. J Med Chem. 1991;34:1225–1228. doi: 10.1021/jm00107a050. [DOI] [PubMed] [Google Scholar]
- 27.Smith AB, III, Hirschmann R, Pasternak A, Guzman MC, Yokoyama A, Sprengeler PA, Darke PL, Emini EA, Schleif WA. Pyrrolinone-Based HIV Protease Inhibitors. Design, Synthesis, and Antiviral Activity: Evidence for Improved Transport. J Am Chem Soc. 1995;117:11113–11123. [Google Scholar]
- 28.Thompson WJ, Fitzgerald PMD, Holloway MK, Emini EA, Darke PL, McKeever BM, Schleif WA, Quintero JC, Zugay JA, Tucker TJ, Schwering JE, Homnick CF, Nunberg J, Springer JP, Huff JR. Synthesis and Antiviral Activity of a Series of HIV-1 Protease Inhibitors with Functionality Tethered to the P1 or P1’ Phenyl Design. J Med Chem. 1992;35:1685–1701. doi: 10.1021/jm00088a003. [DOI] [PubMed] [Google Scholar]
- 29.(a) Lyle TA, Wiscount CM, Guare JP, Thompson WJ, Anderson PS, Darke PL, Zugay JA, Emini EA, Schleif WA, Quintero JC, Dixon RAF, Sigal IS, Huff JR. Benzocycloalkyl Amines as Novel C-termini for HIV Protease Inhibitors. J Med Chem. 1991;34:1228–1230. doi: 10.1021/jm00107a051. [DOI] [PubMed] [Google Scholar]; (b) Vacca JP, Dorsey BD, Schleif WA, Levin RB, McDaniel SL, Darke PL, Zugay J, Quintero JC, Blahy OM, Roth E, Sardana VV, Schlabach AJ, Graham PI, Condra JH, Gotlib L, Holloway MK, Lin J, Chen I-W, Vastag K, Ostovic D, Anderson PS, Emini EA, Huff JR. L-735,524: An Orally Bioavailable Human Immunodeficiency Virus Type 1 Protease Inhibitor. Proc Natl Acad Sci U S A. 1994;91:4096–4100. doi: 10.1073/pnas.91.9.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.(a) Smith AB, III, Hirschmann R, Pasternak A, Yao W, Sprengeler PA, Holloway MK, Kuo LC, Chen Z, Darke PL, Schleif WA. An Orally Bioavailable Pyrrolinone Inhibitor of HIV-1 Protease: Computational Analysis and X-ray Crystal Structure of the Enzyme Complex. J Med Chem. 1997;40:2440–2444. doi: 10.1021/jm970195u. [DOI] [PubMed] [Google Scholar]; (b) Smith AB, III, Cantin L-D, Pasternak A, Guise-Zawacki L, Yao W, Charnley AK, Barbosa J, Sprengeler PA, Hirschmann R, Munshi S, Olen DB, Schleif WA, Kuo LC. Design, Synthesis, and Biological Evaluation of Monopyrrolinone-Based HIV-1 Protease Inhibitors. J Med Chem. 2003;46:1831–1844. doi: 10.1021/jm0204587. [DOI] [PubMed] [Google Scholar]
- 31.Smith AB, III, Charnley AK, Harada H, Beiger JJ, Cantin L-D, Kenesky CS, Hirschmann R, Munshi S, Olsen DB, Stahlhut MW, Schleif WA, Kuo LC. Design, Synthesis and Biological Evaluation of Monopyrrolinone-Based HIV-1 Protease Inhibitors Possessing Augmented P2 Side-Chains. Bioorg Med Chem Lett. 2006;16:859–863. doi: 10.1016/j.bmcl.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Todd JA, Acha-Orbea H, Bell JI, Chao N, Fronek Z, Jacob CO, McDermott M, Sinha AA, Timmerman L, Steinman L, McDevitt HO. A Molecular Basis for MHC Class II-associated Autoimmunity. Science. 1988;240:1003–1009. doi: 10.1126/science.3368786. [DOI] [PubMed] [Google Scholar]
- 33.Stern LJ, Brown JH, Jardetzky TS, Gorga JC, Urban RG, Strominger JL, Wiley DC. Crystal Structure of the Human Class II MHC Protein HLA-DR1 Complexed with an Influenza Virus Peptide. Nature. 1994;368:215–221. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]
- 34.(a) Smith AB, III, Benowitz AB, Sprengeler P, Barbosa P, Guzman MC, Hirschmann R, Scweiger EJ, Bolin DR, Nagy Z, Campbell RM, Cox DC, Olsen GL. Design and Synthesis of a Competent Pyrrolinone–Peptide Hybrid Ligand for the Class II Major Histocompatibility Complex Protein HLA-DR1. J Am Chem Soc. 1999;121:9286–9298. [Google Scholar]; (b) Lee KH, Olsen GL, Bolin DR, Benowitz AB, Sprengeler PA, Smith AB, III, Hirschmann R, Wiley DC. The Crystal Structure of a Pyrrolinone–Peptide Hybrid Ligand Bound to the Human Class II MHC Protein HLA-DR1. J Am Chem Soc. 2000;122:8370–8375. [Google Scholar]
- 35.(a) Hammer J, Belunis C, Bolin D, Papadopoulos J, Walsky R, Higelin J, Danho W, Sinigaglia F, Nagy ZA. High-Affinity Binding of Short Peptides to Major Histocompatibility Complex Class II Molecules by Anchor Combinations. Proc Natl Acad Sci U S A. 1994;91:4456–4460. doi: 10.1073/pnas.91.10.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bolin D, Campbell R. Hoffmann-La Roche Inc.; unpublished results. [Google Scholar]
- 36.For recent reviews of matrix metalloproteases see: Matrix Metalloproteinases. Biochim Biophys Acta. 2010;1803:1–150. doi: 10.1016/j.bbamcr.2010.01.016..
- 37.Johnson WH, Roberts NA, Borkakoti N. Collagenase Inhibitors: Their Design and Potential Therapeutic Use. J Enzyme Inhib. 1987;2:1–22. doi: 10.3109/14756368709030352. [DOI] [PubMed] [Google Scholar]
- 38.Smith AB, Nittoli T, Sprengeler PA, Duan JJ-W, Liu R-Q, Hirschmann RF. Design, Synthesis, and Evaluation of a Pyrrolinone-Based Matrix Metalloprotease Inhibitor. Org Lett. 2000;2:3809–3812. doi: 10.1021/ol000254p. [DOI] [PubMed] [Google Scholar]
- 39.(a) Smith AB, III, Favor DA, Sprengeler PA, Guzman MC, Carroll PJ, Furst GT, Hirschmann R. Molecular Modeling, Synthesis, and Structures of N-Methylated 3,5-Linked Pyrrolin-4-Ones Toward the Creation of a Privileged Nonpeptide Scaffold. Bioorg Med Chem. 1999;7:9–22. doi: 10.1016/s0968-0896(98)00234-x. [DOI] [PubMed] [Google Scholar]; (b) Favor DA. Ph.D. Thesis, University of Pennsylvania. 1998. [Google Scholar]
- 40.Reprinted from Bioorganic and Medicinal Chemistry, 7, Smith AB, III, Favor DA, Sprengeler PA, Guzman MC, Carroll PJ, Furst GT, Hirschmann R. Molecular Modeling, Synthesis, and Structures of N-Methylated 3,5-Linked Pyrrolin-4-Ones Toward the Creation of a Privileged Nonpeptide Scaffold, 9-22, Copyright 1999, with permission from Elsevier. doi: 10.1016/s0968-0896(98)00234-x..
- 41.Nutt RF, Veber DF, Saperstein R, Hirschmann R. Somatostatin Analogs Which Define the Role of the Lysine-9 Amino Group. Int J Peptide Protein Res. 1983;21:66–73. doi: 10.1111/j.1399-3011.1983.tb03079.x. [DOI] [PubMed] [Google Scholar]
- 42.(a) Karle IL, Handa BK, Hassall CH. The Conformation of the Cyclic Tetrapeptide L-Ser(O-t-Bu)-β-Ala-Gly-L-β-Asp(OMe) Containing a 14-Membered Ring. Acta Cryst. 1975;B31:555–560. [Google Scholar]; (b) De Santis P, Morosetti S, Rizzo R.Conformational Analysis of Regular Enantiomeric Sequences Macromolecules 1974752–58.4837982 [Google Scholar]; (c) Tomasic L, Lorenzi GP. Some Cyclic Oligopeptide with S2n Symmetry. Helv Chim Acta. 1987;70:1012–1016. [Google Scholar]; Clark TD, Buriak JM, Kobayashi K, Isler MP, McRee DE, Ghadiri MR. Cylindrical β-Sheet Peptide Assemblies. J Am Chem Soc. 1998;120:8949–8962.. and references cited therein.; (e) Hanessian S, Vinci V, Fettis K, Maris T, Viet MTP. Self-Assembly of Noncyclic Bis-d- and l-tripeptides into Higher Order Tubular Constructs: Design, Synthesis, and X-ray Crystal Superstructure. J Org Chem. 2008;73:1181–1191. doi: 10.1021/jo701621c. [DOI] [PubMed] [Google Scholar]
- 43.Smith AB, III, Wang W, Charnley AK, Carroll PJ, Kenesky CS, Hirschmann R. Design, Synthesis and Structural Analysis of D,L-Mixed Polypyrrolinones 1: From Nonpeptide Peptidomimetics to Nanotubes. Org Lett. 2010;12:2990–2993. doi: 10.1021/ol101007n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veber DF, Holly FW, Paleveda WJ, Nutt RF, Bergstrand SJ, Torchiana M, Glitzer MS, Saperstein R, Hirschmann R. Conformationally Restricted Bicyclic Analogs of Somatostatin. Proc Nat Acad Sci. 1978:2636–2640. doi: 10.1073/pnas.75.6.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith AB, III, Charnley AK, Mesaros EF, Kikuchi O, Wang W, Benowitz A, Chu C-L, Feng J-J, Chen K-H, Lin A, Cheng F-C, Taylor L, Hirschmann R. Design, Synthesis, and Binding Affinities of Pyrrolinone-Based Somatostatin Mimetics. Org Lett. 2005;7:399–402. doi: 10.1021/ol0476974. [DOI] [PubMed] [Google Scholar]
- 46.Smith AB, III, Xiong H, Charnley AK, Brenner M, Mesaros EF, Kenesky CS, Di Costanzo L, Christianson DW, Hirschmann R. Design, Synthesis, and Structural Analysis of D,L-Mixed Polypyrrolinones. 2. Macrocyclic Hexapyrrolinones. Org Lett. 2010;12:2994–2997. doi: 10.1021/ol101008y. [DOI] [PMC free article] [PubMed] [Google Scholar]