Abstract
The neuropeptide PACAP is an informational molecule released from stress-transducing neurons. It exerts post-synaptic effects required to complete hypothalamo-pituitary-adrenocortical (HPA) and hypothalamo-splanchnico-adrenomedullary (HSA) circuits activated by psychogenic and metabolic stressors. PACAP-responsive (in cell culture models) and PACAP-dependent (in vivo) transcriptomic responses in the adrenal gland, hypothalamus, and pituitary upon activation of these circuits have been identified. Gene products produced in response circuits during stress include additional neuropeptides and neurotransmitter biosynthetic enzymes and neuroprotective factors. Major portions of HPA and HSA stress responses are abolished in PACAP-deficient mice. This deficit occurs at the level of both the adrenal medulla (HSA axis) and the hypothalamus (HPA axis). PACAP-dependent transcriptional stress responses are conveyed through non-canonical cyclic AMP- and calcium-initiated signaling pathways within the HSA circuit. PACAP transcriptional regulation of the HPA axis, in the hypothalamus, is likely to be mediated via canonical cyclic AMP (cAMP) signaling through protein kinase A.
Keywords: PACAP, stress, HPA axis
The stress response may be defined operationally as the response of the neuroendocrine network to systemic or environmental perturbations outside of the normal physiological range. Stress responses in mammals are mediated through specific neural and neuroendocrine circuits. The propagation of signaling through these circuits is itself a cellular stressor. In other words, a ‘stressed neuron’ is one propagating an organismic stress response through a neuronal stress circuit. Thus, the cellular stress response is a point of entry to identifying targets for pharmacological modulation of organismic stress perception and processing.
Neuropeptides play a special role in the stress response. As informational molecules stored in large dense-core vesicles, neuropeptides are released preferentially at higher firing rates, as occur during neurotransmission activated by stress in both the central and peripheral nervous systems. Fast and slow transmission are the two major modes of intercellular communication that underlie nervous system function. Fast transmission by glutamate, GABA, and acetylcholine involves direct gating of plasma membrane ionic channels for point-to-point communication, and slow transmission, by dopamine, neuropeptides, adenosine, glutamate and acetylcholine1 at metabotropic receptors, involves the mobilization of second messengers like calcium and cAMP, producing the long-term changes underlying cellular plasticity that encode experience.
Bloom et al. made the acute observation that neuropeptides represent the language of stress because the enhanced neuronal firing required to message systemic or environmental stressors to the brain, and execute homeostatic signaling to the periphery, is characterized not only by increased release of classical neurotransmitters from small synaptic vesicles, but the selective release of informational molecules, among them neuropeptides, from large dense-core vesicles (LDCVs). Acting mainly through metabotropic, rather than ionotropic post-synaptic receptors, neuropeptides convey information for post-synaptic adaptation through long-term secretory as well as gene-encoded changes, rather than short-term electrical changes, in the post-synaptic cell [1, 2].
Can microarray analysis of neuropeptide signaling help us understand, in an unbiased fashion, the ‘emergency response’ role of neuropeptides such as PACAP during stress? This short review will examine this question and demonstrate how microarray analysis in combination with informed measurement of key proteins involved in transcription and hormone biosynthesis in the neuroendocrine stress axes have helped to uncover the role of PACAP as a key regulators of signaling in major stress transducing regions of the brain and in the periphery (hypothalamus and adrenal gland). The role of PACAP in mediating stress responses may also be connected to its emerging function in neuroprotection during episodes of hypoxia, ischemia, and traumatic injury, and this connection is also briefly explored here.
PACAP as an “emergency response” peptide
Pituitary adenylate cyclase-activating polypeptide (PACAP) was discovered in 1989 by Miyata, Arimura, Coy and co-workers in a screen for hypothalamic hypophysiotropic hormones elevating cAMP in perfused pituitary [3]. It is an ancient and well-conserved peptide that exists in a 38-amino acid and a 27-amino acid form processed from a prohormone precursor [4]. Processing of PACAP to its 27- and 38 forms (the latter predominates), occurs through the processing enzymes PC1 and 2 and the amidating enzyme PAM [5, 6]except in gonads where PC4 is used [7]. An analog of PACAP in Drosophila has been shown by Zhang, Feany and others to function both in the brain and at the neuromuscular junction through a dual signaling pathway involving both ion channel opening and cAMP [8, 9], and this dual signaling paradigm characterizes PACAP signaling in mammalian nervous system as well [4]. PACAP is a neurotrophic factor, promoting the survival of cortical neuronal progenitor cells [10], cortical neurons [11], dorsal root ganglion cells [12], cerebellar granule cells [13], and peripheral sympathetic neurons [14]. PACAP elicits both PC12 cell [15] and pluripotent stem cell differentiation [16]. It is also neuroprotective in ischemia in vivo and in response to oxidative stress in granule cell culture [17], and increases cell survival in response to multiple stressors including environmental toxins, hypoxia, and excitotoxins in various cell culture systems [13, 18, 19]. PACAP is also cardioprotective in response to cardiotoxin and ischemic insult [20]. Arimura remarked at a meeting of the Editorial Board of J. Mol. Neurosci. New Orleans several years ago that the 10-fold higher levels of PACAP in brains of the diving turtle [21], a species chronically exposed to brain ischemia, was his inspiration for the investigation of PACAP action as a neuroprotective agent in stroke (vide infra).
PACAP: a neurotransmitter at the adrenomedullary synapse
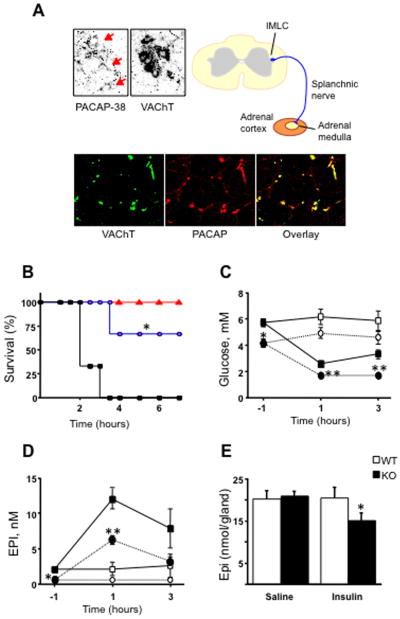
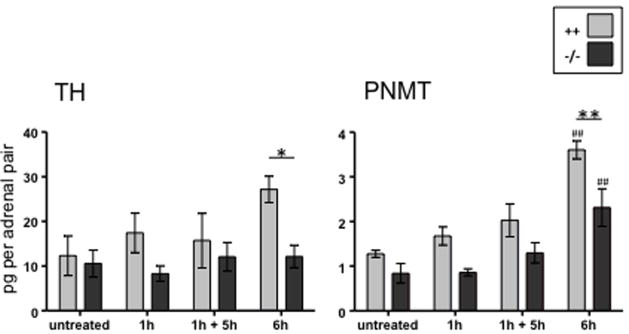
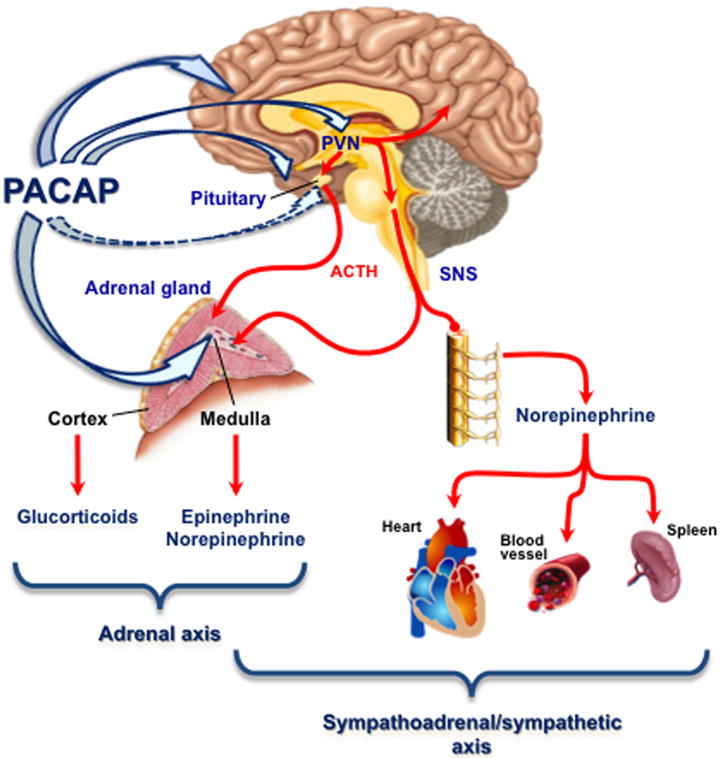
PACAP was shown to potently activate catecholamine secretion and biosynthesis in the adrenal medulla ([22], and references therein) and later to be present in neurons innervating the adrenal medulla [23, 24]. Hamelink et al. [25] demonstrated how PACAP acts as an emergency response peptide in paraphysiological situations. PACAP, present at the adrenomedullary synapse, is absolutely required for survival from prolonged hypoglycemia—mice without PACAP do not survive insulin administration, while wild-type mice become comatose but recover. This is because activation of tyrosine hydroxylase to provide enhanced catecholamine synthesis and release to allow gluconeogenesis to reestablish euglycemia (Figure 1) does not occur in PACAP-deficient mice. Induction of TH and PNMT mRNA after insulin is also PACAP-dependent (Stroth et al., in preparation), and PACAP-dependent induction of mRNAs encoding TH and PNMT also occurs in adrenal gland during restraint stress (Figure 2; from [26]). It is noteworthy that effects of stress on TH and PNMT are time-dependent—occurring only after six hours although catecholamine release begins much earlier, and is prolonged, during immobilization [27].
Figure 1. PACAP a neurotransmitter at the mouse adrenomedullary synapse in vivo.
A. PACAP -38 and a marker for cholinergic neurons, VAChT, are co-localized in neuronal cell bodies the intermediolateral column (IMLC) of the spinal cord, from whence splanchnic sympathetic innervation of the adrenal gland originates, and the splanchnicoadrenomedullary nerve terminals innervating chromaffin cells of the adrenal medulla.
B. PACAP-deficient mice treated with insulin (2 units/kg) die within hours (black) unless rescued with i.p. PACAP (blue) or glucose (red). Wild-type mice invariably survive insulin challenge.
C. Following insulin administration, wild-type mice show decrease blood levels of glucose (filled squares) compared to saline injection (open squares) while PACAP-deficient mice show a more profound hypoglycemia (filled circles) than wild-type after insulin compared to saline (open circles).
D. Lack of compensatory glucose elevation in PACAP-deficient mice can be explained by a less pronounced elevation in plasma epinephrine after insulin administration in PACAP-deficient compared to wild-type mice (symbols mean the same as in C.). E. Decreased epinephrine levels in blood may be explained in part by lack of epinephrine release which is stimulated by PACAP, but also a failure of repletion of released epinephrine due to a failure of PACAP knock-out mice to up-regulate tyrosine hydroxlyase (not shown) resulting in adrenomedullary depletion of epinephrine in the stress PACAP-deficient, but not in the stressed wild-type, adrenal gland. Adapted from Hamelink et al., Proc. Natl. Acad. Sci. USA 99: 461-466, 2002.
Figure 2. PACAP-dependent induction of mRNAs encoding TH and PNMT in adrenal gland during restraint stress.
Restraint stress, as well as insulin shock (Stroth et al., in preparation), results in up-regulation of both tyrosine hydroxylase (TH) and phenylethanolamine methyltransferase (PNMT) mRNA in adrenal gland after 6 hours, and this up-regulation is absent in PACAP-deficient mice. Note that 1 hour of restraint stress is not sufficient for PACAP-dependent induction of TH and PNMT mRNA, either when measured immediately following the stressor (1h) or after 5 hours of non-restraint following on the 1 hours restraint stress (1h + 5h). As indicated in the key, data obtained from wild-type C57Bl/6 mice are shown in grey, while those obtained from PACAP-deficient mice of the same strain are shown in black. Adapted from Stroth and Eiden, Neuroscience 165: 1025-1030, 2010.
In order to determine if PACAP induces additional cellular plasticity associated with stress transduction at the adrenomedullary synapse, we performed microarray experiments in cultured chromaffin cells exposed to PACAP for six hours, corresponding to the period of prolonged stress in vivo causing up-regulation of TH and PNMT mRNA. We wished in particular to test the hypothesis that prolonged neuronal stress transduction, itself potentially stressful to neurons due to prolonged calcium elevation and metabolic demand during prolonged episodes of secretion, is accompanied by induction of calcium-neuroprotective proteins in the post-synaptic cell. A number of growth factors, cytokines and neuropeptides are induced by prolonged exposure of chromaffin cells to PACAP [28]. Among these is stanniocalcin-1 (Stc1). Stc1 is a 56 kD protein which interacts with a mitochondrial receptor to enhance mitochondrial calcium uptake and protect cardiomyocytes and neurons against toxicity associated with excessive calcium uptake [29-34].
Signaling pathways for PACAP-regulated stress-responsive genes
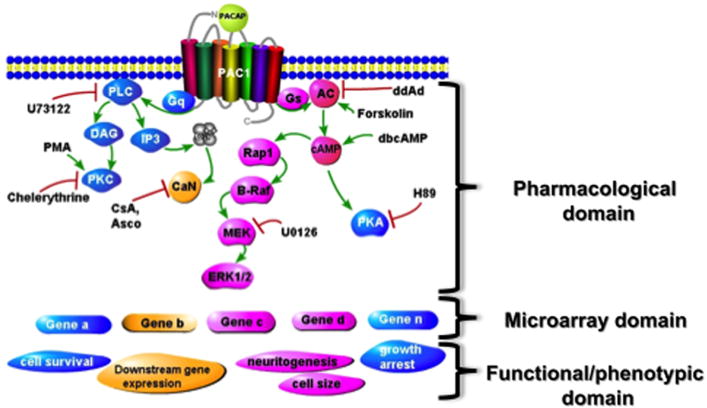
PC12 cells have been a convenient model for gathering sufficient numbers of a pure population of cells to perform combined pharmacological, biochemical, and microarray analysis of signaling pathways underlying PACAP action, and tie together PACAP-initiated cellular signaling pathways, inducible genes, target genes, and cellular outcomes (see Figure 3). In particular, this is a model system in which the transitional transcription (gene expression that occurs during the transition from state A to state B), often difficult to define in vivo, can be systematically analyzed. Thus, in previous studies, we determined that PACAP expression leading to a full neuritogenic response of PC12 cells can be obtained by PACAP exposure for 6 hours [35], and chose this time point for initial work, while also performing time courses to establish the temporal order of gene transcriptional changes occurring during the process of differentiation [36]. The pharmacology of neuritogenesis was defined using inhibitors of PKA, ERK, and other putative nodes in PACAP signaling pathways (Figure 3). This in turn to allow categorization of induced genes based on their pharmacological profile, as potential actors in PACAP-initiated processes including proliferation, neurite extension, cell size, growth arrest, survival following serum withdrawal, as well as further alterations in the downstream transcriptome of the cell itself, including those encoding late or end-stage differentiation-associated proteins, both pan-neuronal and specific to particular sublineages such as noradrenergic, cholinergic, etc. This strategy allowed the characterization of Egr1 as a PACAP-dependent gene required for neuritogenesis [37].
Figure 3. Analysis of scheme used to identify signaling pathways to activation of key genes controlling various facets of cellular function in PC12 cells.
PACAP signaling through the PAC1 receptor activates Gq- and Gs-dependent signaling that results in gene activation. Protein products of induced genes are required for various cellular functions including increased cell size, growth arrest, neuritogenesis, activation of specialized genes for chemical coding of neurotransmission (TH, NPY, sodium and calcium channels, etc), and protection from cell death. By correlating the effects of specific inhibitors on the total PC12 cel transcriptome using microarray, with their effects on cellular phenotype (neuritogenesis, growth arrest, etc), linkages between signaling pathways, gene induction, and cellular function can be established, and then tested with siRNA treatment directed at candidate genes underlying function. Examples of this approach are discussed in text, and see Ravni et al., Mol. Pharmacol. 73: 1688-1708, 2008.
The signaling pathway through which Egr1 gene induction occurs in response to PAC1 receptor activation is a unique cAMP-initiated, but protein kinase A-independent one, first characterized in bovine chromaffin cells [38]. Egr1 transcription is also up-regulated in the adrenal gland after restraint stress [26]. We have identified additional genes, including Stc1 and galanin (GAL), that are elevated in bovine chromaffin cells and in stress and also regulated by the non-canonical cAMP pathway as determined by resistance to H89 inhibition and blocked by U0126 (Stroth et al., in preparation). In contrast, available evidence suggests that PACAP regulation of CRH biosynthesis at the level of the hypothalamus involves canonical (PKA-dependent) cAMP signaling [39].
PACAP and neuroprotection: linkage to stress-related signaling?
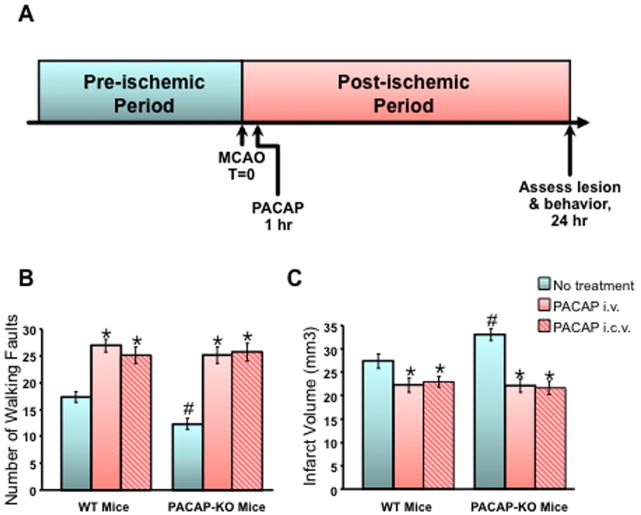
PACAP-deficient mice show aberrant locomotor activity and lack of adaptation to novelty in exploratory behavior, and PAC1 receptor-deficient mice show impaired social investigation reminiscent of autistic behaviors in humans [40, 41]. We have also shown that PACAP is neuroprotective in ischemic insult ([42, 43]; Figure 4) in mice, as previously demonstrated by Reglodi et al. in the rat [44]. Furthermore, lack of PACAP expression in cerebral cortex increases the size of the ischemic lesion after middle cerebral artery occlusion (MCAO), a mouse model for stroke, and enhances the neurological damage caused by MCAO, while treatment with PACAP (even when administered an hour after the ischemic event) significantly decreases lesion size and improves neurological outcome [43]. Ohta et al. have also determined that lack of endogenous PACAP exacerbates tissue damage after stroke, and further, that the protective effects of PACAP seem to require patent regulation of IL-6 gene expression, suggesting that induction of IL-6 by PACAP may contribute to its neuroprotective actions [45]. Because PACAP does not induce IL-6 in cortical neuronal cultures (Holighaus, unpublished observations), it is most likely that PACAP’s effects on IL-6 induction are mediated through astrocytes or perhaps even microglial cells in the brain in situ. However, PACAP does induce neuroprotective genes, including BDNF [11], and the neuro- and cardioprotective protein Stc1 (Holighaus unpublished observations), directly in cultured cortical neurons, as first observed in PC12 cells expressing physiological levels of the PAC1hop receptor [28]. Indeed PACAP regulation of Stc1 occurs in cerebrocortical neuronal cultures, and appears to use the non-canonical cAMP pathway identified in bovine chromaffin cells for regulation of Stc1, GAL and other genes (Holighaus, unpublished observations).
Figure 4. PACAP an endogenous neuroprotectant in stroke in the mouse.
A. The effects of middle cerebral artery occlusion (MCAO) on ischemic lesion size and neurological deficits were compared in wild-type and PACAP-deficient mice in which PACAP or saline was administered one hour after MCAO, and lesion size and neurological function assessed 24 hours later.
B. Effects of PACAP administered either intravenously (i.v., red bars) or intracerebroventricularly (i.c.v., red hatched bars) one hour after MCAO on neurological outcome (change in number of walking faults-higher number equals greater recovery/less damage) assessed at 24 hours post-MCAO.
C. Effects of PACAP administered either intravenously or intracerebroventricularly (symbols as in B.) one hour after MCAO on neurological outcome (larger infarct volume size reflects greater extent of brain damage) assessed at 24 hours post-MCAO
Adapted from Chen et al., Regul Pept. 137: 4-19, 2006 and see this reference for additional details.
PACAP and the HPA and HSA stress axes
The importance of PACAP at the splanchnicoadrenomedullary has been documented [25], as summarized in Figure 1. At the same time, PACAP deficiency does not affect elevation of CORT levels 2 hours after induction of hypoglycemia by administration of insulin (i.e. conditions under which PACAP is absolutely required for full epinephrine secretion, maintenance of blood glucose, and survival) [25]. It was thus initially surprising to us to find that following restraint stress in mice, induction of immediate early genes in hypothalamus; elevation of ACTH secretion; induction of steroidogenic enzyme mRNA in adrenal cortex; and elevation of plasma CORT were all (Figure 5) significantly attenuated in PACAP-deficient mice ([26]; Stroth et al., in preparation).
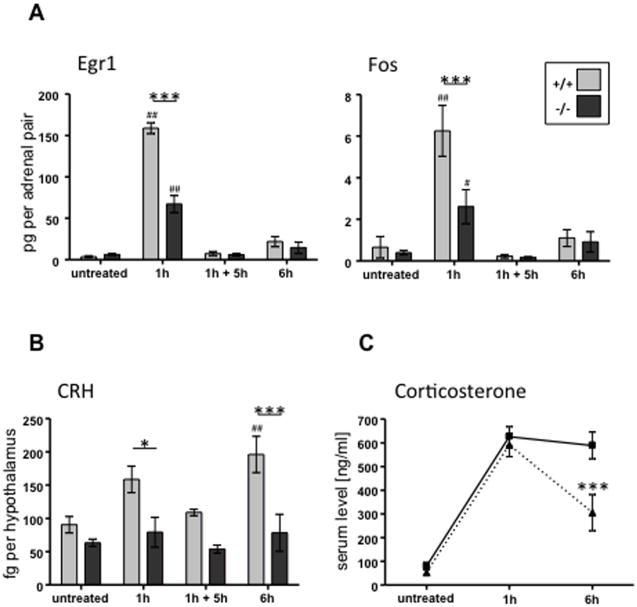
Figure 5. Induction of immediate early genes in adrenal gland, CRH mRNA in hypothalamus, and corticosterone in blood after restraint stress is PACAP-dependent.
A. PACAP-dependent regulation of immediate early genes in hypothalamus following restraint stress.
B. Regulation of CRH mRNA in hypothalamus during restraint is abolished in PACAP-deficient mice.
C. Sustained corticosterone secretion induced by restraint stress requires PACAP.
Adapted from Stroth and Eiden, Neuroscience 165: 1025-1030, 2010.
Induction of CRH mRNA in hypothalamus by restraint stress, as shown in Figure 5, is completely dependent on the presence of PACAP. It is not yet known whether PACAP release from neurons directly innervating CRH neurons of the paraventricular nucleus controls CRH gene transcription in stress, although several anatomical lines of evidence suggest this is the case [46-48]. In addition, reports of PACAP expression in the parvocellular neurons themselves, suggest that PACAP in addition to regulating CRH biosynthesis, could be co-released with CRH to regulate ACTH secretion directly [49, 50]. It will be of particular interest to elucidate the roles of PACAP in promoting HPA axis activation in acute and chronic (prolonged) stress. Our initial observations suggest that initial (acute) HPA responses are relatively independent of PACAP, while prolonged stress responses are sustained by PACAP-dependent mechanisms (see Figure 5C), possibly reflecting adequacy of pre-stored CRH for acute responses, and a need for stimulus-secretion-synthesis coupling to sustain HPA axis activation in response to stress.
Conclusions and future prospects
Several conclusions about PACAP involvement in stress transduction and neuroprotection during stress can be inferred from the data obtained so far, both from our own laboratory and that of others. However, several questions remain to be addressed, as enumerated below, before the role of PACAP in these functions emerges with sufficient clarity to exploit therapeutically in traumatic brain injury, post-traumatic stress disorder and other anxiety disorders.
First, PACAP regulates stress hormone biosynthesis in adrenal gland via up-regulation of at least two enzymes involved in epinephrine biosynthesis, TH and PNMT. Second, PACAP acts at the level of the hypothalamus to control induction, though not basal levels, of CRH mRNA. Third, PACAP control of stress hormone biosynthesis appears to be specialized for prolonged versus acute stress axis activation. Fourth, at least in its effects on the chromaffin cell of the adrenal medulla, and possibility also in cerebrocortical neurons, PACAP utilizes a novel ERK-mediated cAMP-dependent/PKA-dependent signaling pathway to activate a ‘stress response transcriptome’ that includes other neuropeptides, neuroprotective factors, and prohormone convertases. Finally, it is noteworthy that PACAP modulation of stress response in the brain may extend even beyond the HPA and HSA axes. May and colleagues have characterized PACAP induction of a cohort of neuropeptides in cultured post-ganglionic sympathetic neurons in culture [14, 51]. These results imply an action of PACAP to mediate stress responses specific to the sympathetic nervous system (SNS) although these have not yet been investigated in vivo despite the critical role of SNS activation in acute and chronic homeostatic and allostatic responses to a wide range of stressful stimuli [27, 52-54]. Hammack and colleagues have observed that PACAP expression in the bed nucleus of the stria terminalis is greatly enhanced by prolonged stress, and have postulated a potential anxiogenic role for PACAP in the limbic system [55, 56]. The effects of PACAP at these different levels of stress response are summarized in Figure 6.
Figure 6. PACAP is a master regulator of stress circuits in brain and periphery.
Depiction of the levels of the central and peripheral nervous system at which PACAP acts to modulate the efferent limb of systemic and processive stress responses at the levels of the HPA and HAS axes, and afferent (or processing nodes) within the limbic system that integrate processive stress responses, such as anxiogenesis. The involvement of PACAP in the output of the sympathetic nervous system (independently of the HSA axis) is not yet clear (see text).
As a master regulator of stress responses throughout the neuroaxis, PACAP may represent only a ‘jack of all trades’ in mediating neuroprotective, neurotransmission, and gating functions in hypothalamic, hippocampal, amygdalar, and peripheral nervous transduction of homeostatic and allostatic stress responses. GAL, CRH, adenosine, and other neuropeptides may play more specialized roles at particular neuroanatomical locations and under specific stress conditions. PACAP does, however, deserve further attention as a major multi-level stress regulator whose modulation could affect deleterious sustained stress leaving intact the acute (fight or flight) stress response helpful for survival.
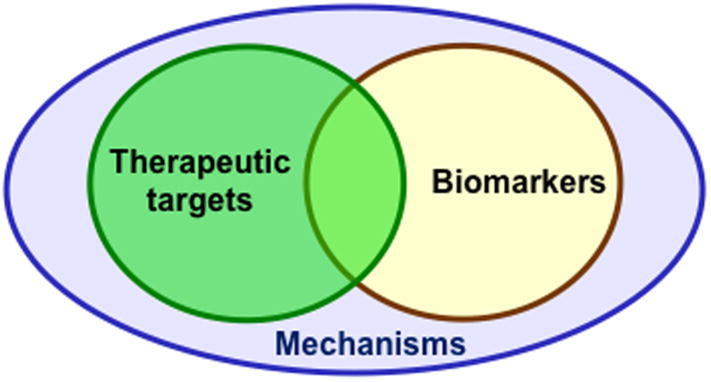
In summary, PACAP is paradigmatic as a neuropeptide regulator in stress, and some more general conclusions can also be drawn about microarray analysis of neuropeptide-regulated genes from the accumulating information about PACAP signaling. Microarray analysis reveals--in an unbiased way—gene targets related to mechanisms, biomarkers, and potential drug targets for stress-related disease. These may overlap, and may also function to reinforce each other in translational and reverse-translational approaches to understanding and treatment of stress disorders (Figure 7). Signaling pathways activated in stress may be dissected pharmacologically to allow manipulation of individual components, e.g. neuroprotection, stress hormone biosynthesis, neurotrophin production. Finally, deciding when neuropeptide agonists or antagonists should be sought as stress management therapeutics remains a key question, and one that can be addressed in part by microarray analysis of neuropeptide signaling to the nucleus in a variety of physiological contexts.
Figure 7. Gene expression analysis reveals mechanism, biomarkers, and therapeutic targets in stress.
Acknowledgments
Work described in this review was carried out under NIMH-IRP Projects Z01- MH002386-21, Z01- MH002386-22, Z01- MH002386-23, and Z01- MH002386-24. We thank our colleagues Abdel Elkahloun (NHGRI-NIMH-NINDS Microarray Core), Eberhard Weihe (Philipps University, Marburg) and members of the Section on Molecular Neuroscience (Tomris Mustafa) for critical reading of the manuscript.
Footnotes
This contribution summarizes the State-of-the-Art Lecture “Signaling in Stress: The ‘emergency response’ transcriptome” presented by L.E.E. at The 7th International Congress of Neuroendocrinology, July 11-15, 2010, Rouen, France.
Glutamate and acetylcholine are released from SSVs and act as both fast and slow transmitters depending on receptor interaction; DA is released from both SSVs and LDCVs and acts as a slow transmitter in both cases. Neuropeptides are uniquely released from LDCVs, in part because their prohormones and processing enzymes do not traffic to SSVs.
References
- 1.Eiden LE. Signaling During Exocytosis. In: Bradshaw R, Dennis E, editors. Handbook of Cell Signaling. Vol. 3. Academic Press; New York: 2003. pp. 375–392. [Google Scholar]
- 2.Hökfelt T, Bartfai T, Bloom F. Neuropeptides:opportunities for drug discovery. Lancet Neurology. 2003;2:463–472. doi: 10.1016/s1474-4422(03)00482-4. [DOI] [PubMed] [Google Scholar]
- 3.Miyata A, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- 4.Mustafa T, Eiden LE. The Secretin Superfamily: PACAP, VIP and Related Peptides. In: Lim R, editor. Handbook of Neurochemistry and Molecular Neurobiology: XIII. Neuroactive Peptides and Proteins. XIII. Springer Heidelberg; 2006. pp. 1–36. [Google Scholar]
- 5.Hansel DE, et al. Pituitary adenylyl cyclase-activating peptides and alpha-amidation in olfactory neurogenesis and neuronal survival in vitro. J Neurosci. 2001;21:4625–4636. doi: 10.1523/JNEUROSCI.21-13-04625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seidah NG, et al. The activation and physiological functions of the proprotein convertases. Int J Biochem Cell Biol. 2008;40:1111–1125. doi: 10.1016/j.biocel.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Li M, Mbikay M, Arimura A. Pituitary adenylate cyclase-activating polypeptide precursor is processed solely by prohormone convertase 4 in the gonads. Endocrinology. 2000;141:3723–3730. doi: 10.1210/endo.141.10.7717. [DOI] [PubMed] [Google Scholar]
- 8.Feany MB, Quinn WG. A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science. 1995;268:869–873. doi: 10.1126/science.7754370. [DOI] [PubMed] [Google Scholar]
- 9.Zhong Y. Mediation of PACAP-like neuropeptide transmission by coactivation of Ras/Raf and cAMP signal transduction pathways in Drosophila. Nature. 1995;375:588–592. doi: 10.1038/375588a0. [DOI] [PubMed] [Google Scholar]
- 10.DiCicco-Bloom E, et al. The PACAP ligand/receptor system regulates cerebral cortical neurogenesis. Ann N Y Acad Sci. 1998;865:274–289. doi: 10.1111/j.1749-6632.1998.tb11188.x. [DOI] [PubMed] [Google Scholar]
- 11.Frechilla D, et al. BDNF mediates the neuroprotective effect of PACAP-38 on rat cortical neurons. Neuroreport. 2001;12:919–923. doi: 10.1097/00001756-200104170-00011. [DOI] [PubMed] [Google Scholar]
- 12.Hokfelt T, Zhang X, Wiesenfeld-Hallin Z. Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci. 1994;17:22–30. doi: 10.1016/0166-2236(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 13.Vaudry D, et al. Pituitary adenylate cyclase-activating polypeptide stimulates both c-Fos gene expression and cell survival in rat cerebellar granule neurons through activation of the protein kinase A pathway. Neuroscience. 1998;84:801–812. doi: 10.1016/s0306-4522(97)00545-9. [DOI] [PubMed] [Google Scholar]
- 14.May V, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP)/PAC1HOP1 receptor activation coordinates multiple neurotrophic signaling pathways: Akt activation through phosphatidylinositol 3-kinase gamma and vesicle endocytosis for neuronal survival. J Biol Chem. 2010;285:9749–9761. doi: 10.1074/jbc.M109.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deutsch PJ, Sun Y. The 38-amino acid form of pituitary adenylate cyclase-activating polypeptide stimulates dual signaling cascades in PC12 cells and promotes neurite outgrowth. J Biol Chem. 1992;267:5108–5113. [PubMed] [Google Scholar]
- 16.Falluel-Morel A, et al. The neuropeptide pituitary adenylate cyclase-activating polypeptide exerts anti-apoptotic and differentiating effects during neurogenesis: focus on cerebellar granule neurones and embryonic stem cells. J Neuroendocrinol. 2007;19:321–327. doi: 10.1111/j.1365-2826.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- 17.Vaudry D, et al. Endogenous PACAP acts as a stress response peptide to protect cerebellar neurons from ethanol or oxidative insult. Peptides. 2005;26:2518–2524. doi: 10.1016/j.peptides.2005.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka J, et al. Neuronal protection from apoptosis by pituitary adenylate cyclase-activating polypeptide. Regul Peptides. 1997;72:1–8. doi: 10.1016/s0167-0115(97)01038-0. [DOI] [PubMed] [Google Scholar]
- 19.Waschek JA. VIP and PACAP receptor-mediated actions on cell proliferation and survival. Ann N Y Acad Sci. 1996;805:290–300. doi: 10.1111/j.1749-6632.1996.tb17491.x. [DOI] [PubMed] [Google Scholar]
- 20.Mori H, et al. Cardioprotective effect of endogenous pituitary adenylate cyclase-activating polypeptide on Doxorubicin-induced cardiomyopathy in mice. Circ J. 2010;74:1183–1190. doi: 10.1253/circj.cj-09-1024. [DOI] [PubMed] [Google Scholar]
- 21.Reglodi D, et al. Pituitary adenylate cyclase activating polypeptide is highly abundant in the nervous system of anoxia-tolerant turtle, Pseudemys scripta elegans. Peptides. 2001;22:873–878. doi: 10.1016/s0196-9781(01)00412-0. [DOI] [PubMed] [Google Scholar]
- 22.Hamelink C, Weihe E, Eiden LE. PACAP: an ‘emergency response’ co-transmitter in the adrenal medulla. In: Vaudry H, Arimura A, editors. Pituitary Adenylate Cyclase-Activating Polypeptide. Kluwer-Academic Press; Norwell, Massachusetts: 2003. pp. 227–250. [Google Scholar]
- 23.Holgert H, et al. PACAP in the adrenal gland--relationship with choline acetyltransferase, enkephalin and chromaffin cells and effects of immunological sympathectomy. NeuroReport. 1996;20:297–301. doi: 10.1097/00001756-199612200-00059. [DOI] [PubMed] [Google Scholar]
- 24.Moller K, Sundler F. Expression of pituitary adenylate cyclase-activating peptide (PACAP) and PACAP type I receptors in the rat adrenal medulla. Regul Peptides. 1996;63:129–139. doi: 10.1016/0167-0115(96)00033-x. [DOI] [PubMed] [Google Scholar]
- 25.Hamelink C, et al. Pituitary adenylate cyclase activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci USA. 2002;99:461–466. doi: 10.1073/pnas.012608999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stroth N, Eiden LE. Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience. 2010;165:1025–1030. doi: 10.1016/j.neuroscience.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kvetnansky R, et al. Sympathoadrenal system in stress. Interaction with the hypothalamic-pituitary-adrenocortical system. Ann N Y Acad Sci. 1995:131–158. doi: 10.1111/j.1749-6632.1995.tb44676.x. [DOI] [PubMed] [Google Scholar]
- 28.Ait-Ali D, et al. Neuropeptides, growth factors and cytokines: A cohort of informational molecules whose expression is up-regulated by the stress-associated slow transmitter PACAP in chromaffin cells. Cell Mol Neurobiol. 2010:1–7. doi: 10.1007/s10571-010-9620-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang KZ, et al. High expression of stanniocalcin in differentiated brain neurons. Am J Pathol. 1998;153:439–445. doi: 10.1016/S0002-9440(10)65587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang K, et al. Stanniocalcin: A molecular guard of neurons during cerebral ischemia. Proc Natl Acad Sci USA. 2000;97:3637–3642. doi: 10.1073/pnas.070045897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teplova V, et al. Differentiated Paju cells have increased resistance to toxic effects of potassium ionophores. Acta Biochim Pol. 2004;51:539–544. [PubMed] [Google Scholar]
- 32.Koizumi K, et al. Stanniocalcin 1 prevents cytosolic Ca2+ overload and cell hypercontracture in cardiomyocytes. Circ J. 2007;71:796–801. doi: 10.1253/circj.71.796. [DOI] [PubMed] [Google Scholar]
- 33.Westberg JA, et al. Hypoxic preconditioning induces elevated expression of stanniocalcin-1 in the heart. Am J Physiol Heart Circ Physiol. 2007;293:H1766–1771. doi: 10.1152/ajpheart.00017.2007. [DOI] [PubMed] [Google Scholar]
- 34.Westberg JA, et al. Hypoxic preconditioning induces neuroprotective stanniocalcin-1 in brain via IL-6 signaling. Stroke. 2007;38:1025–1030. doi: 10.1161/01.STR.0000258113.67252.fa. [DOI] [PubMed] [Google Scholar]
- 35.Vaudry D, et al. Analysis of the PC12 cell transcriptome after differentiation with pituitary adenylate cyclase-activating polypeptide (PACAP) J Neurochem. 2002;83:1272–1284. doi: 10.1046/j.1471-4159.2002.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eiden LE, et al. Discovery of PACAP-related genes through microarray analyses in cell culture and in vivo. Ann N Y Acad Sci. 2008;1144:6–20. doi: 10.1196/annals.1418.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravni A, et al. A cAMP-dependent, PKA-independent signaling pathway mediating neuritogenesis through Egr1 in PC12 cells. Mol Pharmacol. 2008;73:1688–1708. doi: 10.1124/mol.107.044792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamelink C, et al. Coincident elevation of cyclic AMP and calcium influx by PACAP-27 synergistically regulates VIP gene transcription through a novel PKA-independent signaling pathway. J Neurosci. 2002;22:5310–5320. doi: 10.1523/JNEUROSCI.22-13-05310.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kageyama K, et al. Pituitary adenylate cyclase-activating polypeptide stimulates corticotropin-releasing factor, vasopressin and interleukin-6 gene transcription in hypothalamic 4B cells. J Endocrinol. 2007;195:199–211. doi: 10.1677/JOE-07-0125. [DOI] [PubMed] [Google Scholar]
- 40.Hashimoto H, et al. Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP) Proc Natl Acad Sci USA. 2001;98:13355–13360. doi: 10.1073/pnas.231094498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otto C, et al. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res Mol Brain Res. 2001;92:78–84. doi: 10.1016/s0169-328x(01)00153-x. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, et al. Expression profiling of cerebrocortical transcripts during middle cerebral artery occlusion and treatment with pituitary adenylate cyclase-activating polypeptide (PACAP) in the mouse. In: Krieglstein J, Klumpp S, editors. Pharmacology of cerebral ischemia. Medpharm Scientific Publishers Stuttgart; Stuttgart, Germany: 2004. pp. 267–277. [Google Scholar]
- 43.Chen Y, et al. Neuroprotection by endogenous and exogenous PACAP following stroke. Regul Pept. 2006;137:4–19. doi: 10.1016/j.regpep.2006.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reglodi D, et al. Effects of pretreatment with PACAP on the infarct size and functional outcome in rat permanent focal cerebral ischemia. Peptides. 2002;23:2227–2234. doi: 10.1016/s0196-9781(02)00262-0. [DOI] [PubMed] [Google Scholar]
- 45.Ohtaki H, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc Natl Acad Sci USA. 2006;103:7488–7493. doi: 10.1073/pnas.0600375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Legradi G, Hannibal J, Lechan RM. Pituitary adenylate cyclase-activating polypeptide-nerve terminals densely innervate corticotropin-releasing hormone-neurons in the hypothalamic paraventricular nucleus of the rat. Neurosci Lett. 1998;246:145–148. doi: 10.1016/s0304-3940(98)00255-9. [DOI] [PubMed] [Google Scholar]
- 47.Agarwal A, Halvorson LM, Legradi G. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Res Mol Brain Res. 2005;138:45–57. doi: 10.1016/j.molbrainres.2005.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grinevich V, Fournier A, Pelletier G. Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on corticotropin-releasing hormone (CRH) gene expression in the rat hypothalamic paraventricular nucleus. Brain Res. 1997;773:190–196. doi: 10.1016/s0006-8993(97)01011-1. [DOI] [PubMed] [Google Scholar]
- 49.Hannibal J. Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol. 2002;453:389–417. doi: 10.1002/cne.10418. [DOI] [PubMed] [Google Scholar]
- 50.Hannibal J, et al. PACAP gene expression in neurons of the rat hypothalamo-pituitary-adrenocortical axis is induced by endotoxin and interleukin-1beta. Neuroendocrinology. 1999;70:73–82. doi: 10.1159/000054461. [DOI] [PubMed] [Google Scholar]
- 51.Girard BM, et al. Regulation of neurotrophic peptide expression in sympathetic neurons: quantitative analysis using radioimmunoassay and real-time quantitative polymerase chain reaction. Regul Pept. 2002;109:89–101. doi: 10.1016/s0167-0115(02)00191-x. [DOI] [PubMed] [Google Scholar]
- 52.Elenkov IJ, et al. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 53.Kvetnansky R, et al. Stressor specificity of peripheral catecholaminergic activation. Adv Pharmacol. 1998;42:556–560. doi: 10.1016/s1054-3589(08)60811-x. [DOI] [PubMed] [Google Scholar]
- 54.Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev. 2009;89:535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]
- 55.Hammack SE, et al. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34:833–843. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hammack SE, et al. Roles for Pituitary Adenylate Cyclase-Activating Peptide (PACAP) Expression and Signaling in the Bed Nucleus of the Stria Terminalis (BNST) in Mediating the Behavioral Consequences of Chronic Stress. J Mol Neurosci. 2010 doi: 10.1007/s12031-010-9364-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]