Abstract
Maintenance of telomeres requires both DNA replication and telomere ‘capping’ by shelterin. These two processes employ two single-stranded DNA (ssDNA)-binding proteins, replication protein A (RPA) and protection of telomeres 1 (POT1). Although RPA and POT1 each have a critical role at telomeres, how they function in concert is not clear. POT1 ablation leads to activation of the ataxia telangiectasia and Rad3-related (ATR) checkpoint kinase at telomeres1, 2, suggesting that POT1 antagonizes RPA binding to telomeric ssDNA. Unexpectedly, we found that purified POT1 and its functional partner TPP1 are unable to efficiently prevent RPA binding to telomeric ssDNA. In cell extracts, we identified a novel activity that specifically displaces RPA, but not POT1, from telomeric ssDNA. Using purified protein, we show that the heterogeneous nuclear ribonucleoprotein A1 (hnRNPA1) recapitulates the RPA displacing activity. The RPA displacing activity is inhibited by the telomeric repeat-containing RNA (TERRA) in early S phase, but is then unleashed in late S phase when TERRA levels decline at telomeres3. Interestingly, TERRA also promotes POT1 binding to telomeric ssDNA by removing hnRNPA1, suggesting that the reaccumulation of TERRA after S phase helps to complete the RPA-to-POT1 switch on telomeric ssDNA. Together, our data suggest that hnRNPA1, TERRA, and POT1 act in concert to displace RPA from telomeric ssDNA following DNA replication, and promote telomere capping to preserve genomic integrity.
RPA binds ssDNA in a non-sequence specific manner4, whereas POT1 specifically recognizes ssDNA consisting of the telomeric repeats5. RPA plays a key role in DNA replication and activation of the ATR checkpoint6, and POT1 suppresses ATR activation at telomeres1, 2 (Fig. S1). In both yeast and humans, RPA associates with telomeres during S phase of the cell cycle7–9, and is implicated in telomere maintenance10–12. Furthermore, ATR transiently associates with telomeres and suppresses telomere instability7, 10, 13. These findings raise the question as to how the bindings of POT1 and RPA to telomeric ssDNA are orchestrated and, furthermore, how the interplay between POT1 and RPA affects DNA replication and ATR activation at telomeres.
Double-stranded DNA (dsDNA) with ssDNA overhangs activates ATR in human cell extracts14. To investigate how ATR activation is suppressed at telomeres, we tested whether telomeric ssDNA overhangs affect ATR activation in this assay. Resected dsDNA of random sequences, but not resected telomeric dsDNA, efficiently induced the phosphorylation of RPA2 by ATR (Fig. S2)14, suggesting that telomeric ssDNA overhangs do not support efficient ATR activation in cell extracts.
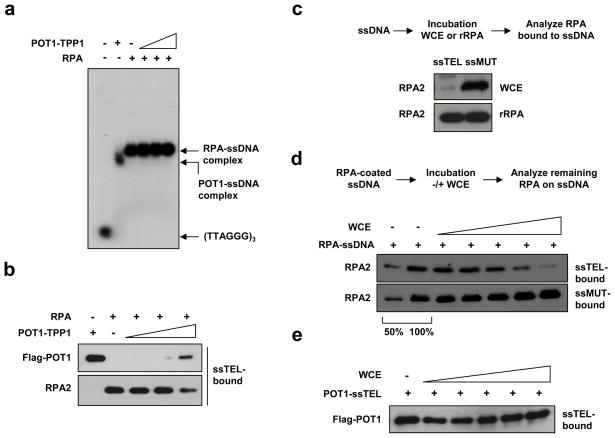
The absence of ATR activation by telomeric ssDNA suggests that POT1 may prevent RPA binding to telomeric ssDNA2. POT1 and TPP1 function as heterodimers in cells, and the complex binds to telomeric ssDNA more efficiently than POT1 alone15, 16. In gel-shift assays, the POT1-TPP1 complexes purified from insect or human cells and the RPA purified from E. coli efficiently bound to a telomeric ssDNA probe (Fig. 1a, S3a-b). POT1-TPP1 exhibited lower affinity for telomeric ssDNA compared to RPA (Fig. S3a). When POT1-TPP1 and RPA were coincubated with the probe, the RPA-ssDNA complex was readily detected, whereas no POT1-containing complexes were observed (Fig. 1b, S3b). In pull-down assays using biotinylated telomeric ssDNA (ssTEL), RPA also out-competed POT1-TPP1 for binding to ssTEL (Fig. 1b, S3c). Thus, RPA, which is more abundant than POT1-TPP1 in cells4, 17, out-competes POT1-TPP1 for binding to telomeric ssDNA when present at similar concentrations as POT1-TPP1. The E. coli ssDNA-binding protein SSB only modestly reduced POT1 binding to ssTEL (Fig. S3c), suggesting that the ability to out-compete POT1-TPP1 is unique to RPA.
Figure 1. A novel telomere-specific RPA displacing activity in human cell extracts.
a, POT-TPP1 (60 nM; purified from insect cells), RPA (60 nM), and mixtures of POT1-TPP1 and RPA (60, 120, 180 nM of POT1-TPP1 mixed with 60 nM of RPA) were incubated with 20 nM of the ssDNA probe and analyzed by gel-shift. b, POT1-TPP1 (2.4 nM), RPA (2.4 nM), and mixtures of POT1-TPP1 and RPA (2.4, 4.8, 7.2 nM of POT1-TPP1 mixed with 2.4 nM of RPA) were incubated with 0.8 nM of biotinylated ssTEL [(TTAGGG)8]. The proteins bound to ssTEL were retrieved by streptavidin beads and analyzed by Western blot. c, Biotinylated ssTEL or ssMUT [(TTTGCG)8] was incubated with WCEs or recombinant RPA (rRPA). d, ssTEL or ssMUT precoated with RPA was incubated with increasing concentrations of HeLa WCEs (0.08, 0.19, 0.36, 0.8, 1.3 μg/μl). The RPA2 remaining on ssTEL was analyzed as in b. e, ssTEL precoated with POT1 was incubated with increasing concentrations of HeLa WCEs (0.07, 0.18, 0.33, 0.66, 1.3 μg/μl).
The ability of RPA to out-compete POT1-TPP1 raises the question of how ATR activation is suppressed in cell extracts. Purified RPA bound to ssTEL and mutated telomeric repeats (ssMUT) efficiently (Fig. 1c). In stark contrast to purified RPA, the endogenous RPA in HeLa whole-cell extracts (WCEs) was largely excluded from ssTEL, however, still associated with ssMUT (Fig. 1c). The sequence-specific exclusion of RPA from ssTEL in WCEs suggests that RPA may be out-competed by other proteins or actively displaced from telomeric ssDNA.
To assess if RPA is actively displaced from ssTEL, we precoated ssTEL and ssMUT with RPA and then incubated them in extracts. The levels of RPA on ssTEL gradually declined with increasing concentrations of WCEs from HeLa, HEK293E, U2OS, and MEF cells (Fig. 1d, S4a). In addition, HeLa nuclear extracts (NEs), but not the cytoplasmic extracts, efficiently displaced RPA from ssTEL (Fig. S4b). In marked contrast to the RPA on ssTEL, the RPA bound to ssMUT remained constant regardless of WCE concentrations (Fig. 1d). When POT1-coated ssTEL was incubated in extracts, POT1 remained stably bound to ssTEL even in high concentrations of WCEs (Fig. 1e). Furthermore, RPA was rapidly displaced from ssTEL within 5 min, whereas no POT1 was displaced after 60 min (Fig. S4c). Thus, the activity that displaces RPA from telomeric ssDNA is sequence-specific, protein-specific, and localized within the nucleus.
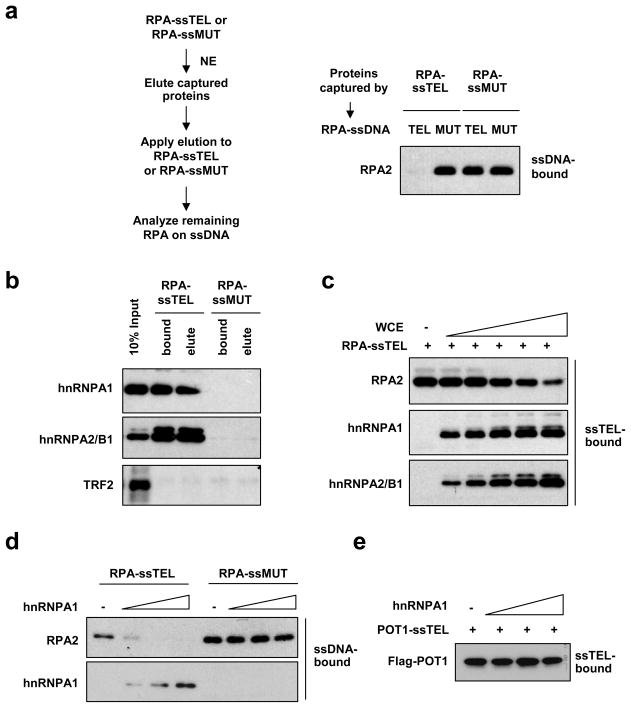
The specific displacement of RPA, but not POT1, from telomeric ssDNA prompted us to test if POT1 is the RPA displacing factor. When incubated with RPA-ssTEL, POT1-TPP1 did not significantly reduce the levels of ssTEL-bound RPA (Fig. S5a). To identify the RPA displacing factors, we sought to capture the RPA displacing activity from extracts using RPA-ssTEL as bait. The proteins captured and eluted from RPA-ssTEL, but not RPA-ssMUT, recapitulated the RPA displacing activity (Fig. 2a). Mass spectrometry analysis of the proteins specifically captured by RPA-ssTEL identified hnRNPA1 and hnRNPA2/B1 (Fig. S5b-c), both of which are known to bind telomeric ssDNA18, 19. The presence of hnRNPA1 and A2/B1 in the eluted fraction with RPA displacing activity was confirmed by Western blot (Fig. 2b). Moreover, hnRNPA1 and A2/B1 gradually bound to ssTEL as RPA was displaced in WCEs (Fig. 2c). These results suggest that hnRNPA1 and A2/B1 may play a role in RPA displacement.
Figure 2. RPA displacement by hnRNPA1.
a, ssTEL and ssMUT precoated with RPA were incubated with NEs. After the incubation, the proteins bound to DNA were retrieved, eluted, and applied to RPA-coated ssTEL or ssMUT (see Supplemental Methods). After the second incubation, the remaining RPA2 on DNA was analyzed by Western blot. b, Proteins captured by RPA-ssTEL or RPA-ssMUT and eluted by salt were analyzed by Western blot using antibodies to hnRNPA1, hnRNPA2/B1, and TRF2. c, RPA-coated ssTEL (0.8 nM) was incubated with increasing concentrations of WCEs (0.06, 0.24, 0.96 μg/μl). The hnRNPA1 and hnRNPA2/B1 bound to DNA and the remaining RPA2 on DNA were analyzed by Western blot. d, RPA-coated ssTEL or ssMUT (0.8 nM) was incubated with increasing concentrations of purified hnRNPA1 (2.4, 4.8, 7.2 nM). The remaining RPA2 on DNA was analyzed as in a. e, POT1-coated ssTEL (0.8 nM) was incubated with increasing concentrations of purified hnRNPA1 (2.4, 4.8, 7.2 nM).
hnRNPA1 has been implicated in telomere maintenance20, 21. Extracts from hnRNPA1 knockdown cells exhibited reduced activity in RPA displacement (Fig. S6a). Purified hnRNPA1 efficiently displaces RPA from ssTEL, but not ssMUT (Fig. 2d). Furthermore, hnRNPA1 did not displace POT1 from ssTEL (Fig 2e). hnRNPA1 only displaces RPA from ssTEL containing 4 or more telomeric repeats (Fig. S6b), indicating that a DNA length-dependent binding mode of hnRNPA1 may be needed to displace RPA22. Given that hnRNPA1 and A2/B1 are highly homologous in the RRM domains that bind telomeric ssDNA, both of these hnRNPs may contribute to RPA displacement.
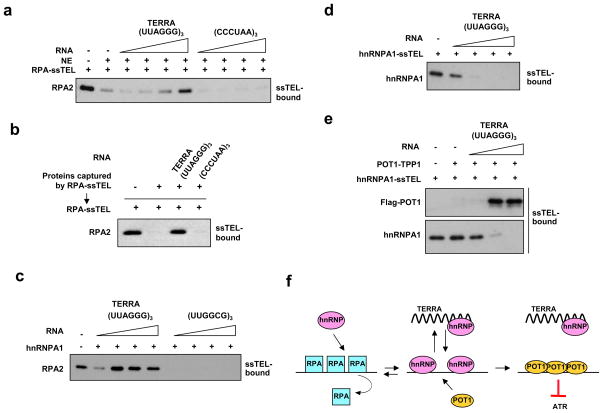
hnRNPA1 not only binds telomeric ssDNA but also TERRA23–26. To test if TERRA affects the ability of hnRNPA1 to displace RPA from ssTEL, we added increasing concentrations of TERRA or control RNA to NEs, and then incubated the extracts with RPA-ssTEL. RPA displacement was virtually abolished by TERRA, but not control RNA (Fig. 3a). The RPA displacing activity captured by RPA-ssTEL was also specifically inhibited by TERRA (Fig. 3b). Furthermore, the ability of purified hnRNPA1 to bind ssTEL and to displace RPA from ssTEL was specifically inhibited by TERRA (Fig. 3c, S6c). Thus, TERRA is a potent inhibitor of the RPA displacing activity of hnRNPA1.
Figure 3. Regulation of RPA displacement by TERRA.
a, NEs (34 ng/μl) were treated with increasing concentrations (2, 4, 10, 20 nM) of TERRA (UUAGGG)3, control RNA (CCCUAA)3, or mock treated. The treated NEs were then incubated with RPA-coated ssTEL (2 nM), and the remaining RPA2 on ssTEL was analyzed after the incubation. b, The RPA displacing factors were captured with RPA-ssTEL as in Fig. 2a. The elution was incubated with TERRA or control RNA, and then applied to RPA-ssTEL. c, Purified hnRNPA1 (4.8 nM) was incubated with increasing concentrations of TERRA or control RNA (2, 4, 10, 20 nM), and then incubated with RPA-ssTEL (0.8 nM). d, hnRNPA1-coated ssTEL (0.8 nM) was incubated with increasing concentrations of TERRA (2, 20, 200, 2000 nM). The remaining hnRNPA1 on ssTEL was analyzed by Western blot. e, hnRNPA1-coated ssTEL (0.8 nM) was incubated with increasing concentrations of TERRA (2, 20, 200 nM) in the presence of POT1-TPP1 (2.4 nM). The hnRNPA1 and POT1 on ssTEL were analyzed by Western blot. f, A model for RPA displacement.
If hnRNPA1 displaces RPA from telomeric ssDNA, how can POT1 bind to telomeric ssDNA? Given that hnRNPA1 has affinity for both telomeric ssDNA and TERRA, the presence of TERRA at telomeres may promote the dissociation of hnRNPA1 from telomeric ssDNA. Indeed, when hnRNPA1-coated ssTEL was incubated with TERRA, hnRNPA1 was stripped from ssTEL (Fig. 3d), showing that hnRNPA1 binds telomeric ssDNA dynamically. Furthermore, when hnRNPA1-ssTEL was incubated with TERRA and POT1-TPP1, POT1 efficiently bound to ssTEL as hnRNPA1 was removed by TERRA (Fig. 3e).
The in vitro results above suggest that the initial displacement of RPA from telomeric ssDNA may be carried out by hnRNPs when TERRA levels are low at telomeres (Fig. 3f). However, if TERRA levels rise at telomeres, hnRNPA1 may shuttle between telomeric ssDNA and TERRA dynamically. In this situation, both RPA and POT1 may have the chance to bind telomeric ssDNA. Because hnRNPA1 only displaces RPA, but not POT1, this dynamic process will eventually promote POT1 occupancy at telomeric ssDNA.
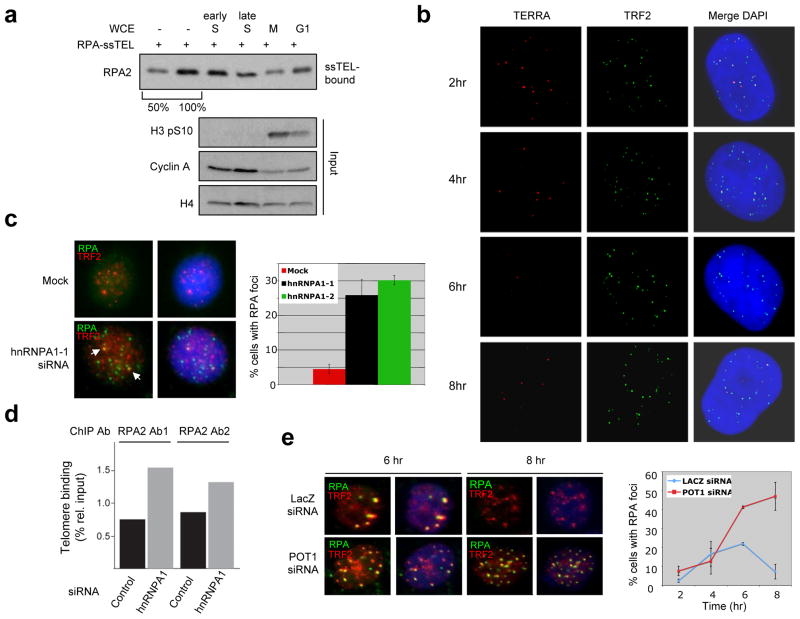
This model raises the possibility that the RPA displacing activity may be regulated by TERRA during the cell cycle. To test this, we generated WCEs from cells in G1, early S, late S, and M phases of the cell cycle. RPA was more efficiently displaced in the late Sand M-phase extracts than in the G1- and early S-phase extracts (Fig. 4a, S7a-b). Thus, the RPA displacing activity is low in G1 and early S phase, but up regulated in late S phase.
Figure 4. hnRNPA1 and POT1 suppress the accumulation of RPA at telomeres.
a, RPA-coated ssTEL was incubated with WCEs from cells in G1, early S, late S, and M phases of the cell cycle (see Supplemental Methods). The remaining RPA2 on ssTEL was analyzed after incubation. Cyclin A and phospho-histone H3 serve as cell-cycle markers, and histone H4 as a loading control. b, TERRA was analyzed by RNA FISH in HeLa cells following thymidine release. TRF2 severs as a marker of telomeres. c, HeLa cells were treated with hnRNPA1 siRNA or mock treated, and then immunostained with antibodies to RPA2 and TRF2 (left panel). The cells with RPA foci (>5) were quantified (right panel). Mean+s.d., n=3 for mock and sihnRNPA1-1, n=2 for sihnRNPA1-2. d, Chromatin immunoprecipitation of RPA was performed with two different RPA2 antibodies. The association of RPA with telomeres was analyzed by dot blot using a telomere probe and quantified. e, HeLa cells transfected with POT1 or LacZ siRNA were released from a thymidine block. At the indicated times, the G2/M population was determined by FACS (see Fig. S10c). Cells were immunostained for RPA2 and TRF2 (left panel). The cells with RPA foci (>5) were quantified (right panel). Mean+s.d., n=3.
If TERRA inhibits the RPA displacing activity, its levels should inversely correlate with the activity. Furthermore, removal of TERRA in early S phase should alleviate the inhibition. Indeed, a recent study showed that TERRA levels significantly decrease in late S phase and increase again after S phase3. Consistently, telomeric TERRA foci declined as cells progressed from early to late S phase (Fig. 4b, S7c-d). In addition, RNase A treatment of early S-phase extracts significantly enhanced the RPA displacing activity (Fig. S7e). Together, these results suggest that TERRA inhibits RPA displacement in early S phase, and its decline in late S phase may provide a window for RPA displacement.
The model above also predicts that hnRNPs are necessary for RPA displacement from telomeres. Depletion of hnRNPA1 using two independent siRNAs significantly increased the fraction of cells displaying RPA foci (Fig. 4c, S8a-d). Notably, a fraction of the RPA foci in hnRNPA1 knockdown cells closely associated with TRF2 foci. Furthermore, increased RPA binding at telomeres was detected in hnRNPA1 knockdown cells by chromatin immunoprecipitation (Fig. 4d). In synchronized hnRNPA1 knockdown cells, RPA binding to telomeres was enhanced in early S phase (Fig. S9a-b), indicating that even during this period some hnRNPA1 remains free from TERRA and limits RPA binding to telomeres9. In late S/G2, RPA still declined at telomeres in hnRNPA1 knockdown cells, possibly owing to the redundancy among hnRNPs.
If the displacement of RPA by hnRNPA1 is a prerequisite for POT1 binding, POT1 should be needed for RPA exclusion after late S phase. To assess this possibility, we treated cells with POT1 siRNA and synchronized the cells with thymidine as POT1 levels declined (Fig. S10a-b). After POT1 knockdown cells and control cells were synchronously released, RPA foci appeared in both cell populations (Fig. 4e). As control cells entered G2, RPA foci rapidly declined. In contrast, the POT1 knockdown cells containing RPA foci that colocalized with TRF2 continued to increase. Concomitantly, modest Chk1 phosphorylation was detected in POT1 knockdown cells (Fig. S10c). Thus, reduction of POT1 compromises the exclusion of RPA from telomeres following replication27.
During early to mid S phase, TERRA sequesters hnRNPs and allows RPA to bind telomeric ssDNA at replication forks or telomere ends (Fig. S1). When TERRA levels decline in late S phase, hnRNPs are unleashed to displace RPA from telomeric ssDNA. The dynamic binding of hnRNPs to telomeric ssDNA is gradually antagonized by TERRA when TERRA reaccumulates at telomeres, providing a window for both RPA and POT1 to bind. Because only POT1, but not RPA, binds to telomeric ssDNA irreversibly in the presence of hnRNPs, this dynamic process favors the formation of POT1-coated telomeric ssDNA. Unlike RPA, POT1 kinks telomeric ssDNA and induces its self-recognition28, 29. These unique properties of POT1 may confer resistance to hnRNP-mediated displacement. The cell cycle-regulated RPA displacement may allow RPA to transiently associate with telomeric ssDNA during replication, and prevent persistent ATR activation at telomeres after S phase. Once coated by POT1, telomeric ssDNA may remain capped until the arrival of replication forks in the next S phase. Together, TERRA and hnRNPs orchestrate a cell cycle regulated RPA-to-POT1 switch on telomeric ssDNA, ensuring orderly telomere replication and capping.
Methods Summary
For analyzing the bindings of RPA and POT1-TPP1 to ssDNA, biotinylated ssDNA was attached to streptavidin-coated magnetic beads. Biotinylated ssDNA (1 pmol) was incubated with purified protein in 500 μl of binding buffer [10 mM Tris-HCl (pH 7.5), 100 mM NaCl, 10 μg/ml BSA, 10% glycerol, 0.05% NP-40].
Supplementary Material
Acknowledgments
We thank Drs. T. de Lange, A. Krainer, B. Chabot, M. Wold for reagents, and member of the Zou lab for discussion. L. Z. is an Ellison New Scholar on Aging. R. L. F. is supported by the NIH fellowship 5T32CA009216-28 and ACS fellowship 0902501. R. C. C. is supported by the NIH fellowship F32-GM089150. R.J.O. is supported by the George E. Hewitt Foundation for Medical Research. This work is supported by NIH grants CA129037 to S. C., GM06525 and AG025837 to J.K., and GM076388 to L. Z.
Footnotes
Full Methods and any associated references are available in the Supplementary Information.
Author contribution
R.L.F. and L.Z. conceived the project. R.L.F., R.C.C., R.J.O, R.R., A.T., and L.Z. performed the experiments. Z.S. contributed the POT1-TPP1 complex purified from insect cells. R.R. and S.C. performed the combined RNA-FISH and immunostaining analysis. R.J.O. and J.K. performed the RPA chromatin immunoprecipitation and Northern blot. R.L.F. and L.Z. wrote the paper.
References
- 1.Guo X, et al. Dysfunctional telomeres activate an ATM-ATR-dependent DNA damage response to suppress tumorigenesis. Embo J. 2007;26:4709–19. doi: 10.1038/sj.emboj.7601893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denchi EL, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448:1068–71. doi: 10.1038/nature06065. [DOI] [PubMed] [Google Scholar]
- 3.Porro A, Feuerhahn S, Reichenbach P, Lingner J. Molecular dissection of TERRA biogenesis unveils the presence of distinct and multiple regulatory pathways. Mol Cell Biol. doi: 10.1128/MCB.00460-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wold MS. Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu Rev Biochem. 1997;66:61–92. doi: 10.1146/annurev.biochem.66.1.61. [DOI] [PubMed] [Google Scholar]
- 5.Baumann P, Cech TR. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science. 2001;292:1171–5. doi: 10.1126/science.1060036. [DOI] [PubMed] [Google Scholar]
- 6.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–8. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 7.Verdun RE, Karlseder J. The DNA damage machinery and homologous recombination pathway act consecutively to protect human telomeres. Cell. 2006;127:709–20. doi: 10.1016/j.cell.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 8.Moser BA, et al. Differential arrival of leading and lagging strand DNA polymerases at fission yeast telomeres. Embo J. 2009;28:810–20. doi: 10.1038/emboj.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGee JS, et al. Reduced Rif2 and lack of Mec1 target short telomeres for elongation rather than double-strand break repair. Nat Struct Mol Biol. doi: 10.1038/nsmb.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verdun RE, Crabbe L, Haggblom C, Karlseder J. Functional human telomeres are recognized as DNA damage in G2 of the cell cycle. Mol Cell. 2005;20:551–61. doi: 10.1016/j.molcel.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Kibe T, Ono Y, Sato K, Ueno M. Fission yeast Taz1 and RPA are synergistically required to prevent rapid telomere loss. Mol Biol Cell. 2007;18:2378–87. doi: 10.1091/mbc.E06-12-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schramke V, et al. RPA regulates telomerase action by providing Est1p access to chromosome ends. Nat Genet. 2004;36:46–54. doi: 10.1038/ng1284. [DOI] [PubMed] [Google Scholar]
- 13.McNees CJ, et al. ATR suppresses telomere fragility and recombination but is dispensable for elongation of short telomeres by telomerase. J Cell Biol. 188:639–52. doi: 10.1083/jcb.200908136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiotani B, Zou L. Single-stranded DNA orchestrates an ATM-to-ATR switch at DNA breaks. Mol Cell. 2009;33:547–58. doi: 10.1016/j.molcel.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F, et al. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature. 2007;445:506–10. doi: 10.1038/nature05454. [DOI] [PubMed] [Google Scholar]
- 16.Xin H, et al. TPP1 is a homologue of ciliate TEBP-beta and interacts with POT1 to recruit telomerase. Nature. 2007;445:559–62. doi: 10.1038/nature05469. [DOI] [PubMed] [Google Scholar]
- 17.Takai KK, Hooper S, Blackwood S, Gandhi R, de Lange T. In vivo stoichiometry of shelterin components. J Biol Chem. 285:1457–67. doi: 10.1074/jbc.M109.038026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikawa F, Matunis MJ, Dreyfuss G, Cech TR. Nuclear proteins that bind the pre-mRNA 3′ splice site sequence r(UUAG/G) and the human telomeric DNA sequence d(TTAGGG)n. Mol Cell Biol. 1993;13:4301–10. doi: 10.1128/mcb.13.7.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKay SJ, Cooke H. hnRNP A2/B1 binds specifically to single stranded vertebrate telomeric repeat TTAGGGn. Nucleic Acids Res. 1992;20:6461–4. doi: 10.1093/nar/20.24.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaBranche H, et al. Telomere elongation by hnRNP A1 and a derivative that interacts with telomeric repeats and telomerase. Nat Genet. 1998;19:199–202. doi: 10.1038/575. [DOI] [PubMed] [Google Scholar]
- 21.Zhang QS, Manche L, Xu RM, Krainer AR. hnRNP A1 associates with telomere ends and stimulates telomerase activity. Rna. 2006;12:1116–28. doi: 10.1261/rna.58806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding J, et al. Crystal structure of the two-RRM domain of hnRNP A1 (UP1) complexed with single-stranded telomeric DNA. Genes Dev. 1999;13:1102–15. doi: 10.1101/gad.13.9.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng Z, Norseen J, Wiedmer A, Riethman H, Lieberman PM. TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol Cell. 2009;35:403–13. doi: 10.1016/j.molcel.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Silanes IL, d’Alcontres MS, Blasco MA. TERRA transcripts are bound by a complex array of RNA-binding proteins. Nat Commun. 1:1–9. doi: 10.1038/ncomms1032. [DOI] [PubMed] [Google Scholar]
- 25.Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007;318:798–801. doi: 10.1126/science.1147182. [DOI] [PubMed] [Google Scholar]
- 26.Redon S, Reichenbach P, Lingner J. The non-coding RNA TERRA is a natural ligand and direct inhibitor of human telomerase. Nucleic Acids Res. 38:5797–806. doi: 10.1093/nar/gkq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong Y, de Lange T. A Shld1-Controlled POT1a Provides Support for Repression of ATR Signaling at Telomeres through RPA Exclusion. Mol Cell. 40:377–87. doi: 10.1016/j.molcel.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lei M, Podell ER, Cech TR. Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nat Struct Mol Biol. 2004;11:1223–9. doi: 10.1038/nsmb867. [DOI] [PubMed] [Google Scholar]
- 29.Bochkarev A, Pfuetzner RA, Edwards AM, Frappier L. Structure of the single-stranded-DNA-binding domain of replication protein A bound to DNA. Nature. 1997;385:176–81. doi: 10.1038/385176a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.