Abstract
Renovascular disease remains among the most prevalent and important causes of secondary hypertension and renal dysfunction. Many lesions reduce perfusion pressure including fibromuscular diseases and renal infarction, but most are caused by atherosclerotic disease. Epidemiologic studies establish a strong association between with atherosclerotic renal artery stenosis and cardiovascular risk. Hypertension develops in patients with renovascular disease from a complex set of pressor signals, including activation of the renin-angiotensin system, recruitment of oxidative stress pathways, and sympatho-adrenergic activation. Although the kidney maintains function over a broad range of autoregulation, sustained reduction in renal perfusion leads to disturbed microvascular function, vascular rarefaction and ultimately development of interstitial fibrosis. Advances in antihypertensive drug therapy and intensive risk factor management including smoking cessation and statin therapy can provide excellent blood pressure control for many individuals. Despite extensive observational experience with renal revascularization in patients with renovascular hypertension, recent prospective randomized trials fail to establish compelling benefits either with endovascular stents or surgery when added to effective medical therapy. These trials are limited and exclude many patients most likely to benefit from revascularization. Meaningful recovery of kidney function after revascularization is limited once fibrosis is established. Recent experimental studies indicate that mechanisms allowing repair and regeneration of parenchymal kidney tissue may lead to improved outcomes in the future. Until additional staging tools become available, clinicians will be forced to individualize therapy carefully to optimize the potential benefits regarding both blood pressure and renal function for such patients.
Keywords: Renovascular Hypertension, renal artery stenosis, renin, BOLD MR, angioplasty, stent, atheroemboli
Introduction
Management of patients with hypertension and renovascular disease has never been more complex—or ambiguous. Continued emphasis on achieving lower goal blood pressures to reduce cardiovascular risk forces clinicians caring for hypertensive patients to intensify therapy and consider secondary factors, such as renovascular occlusive disease, more than ever before. At the same time, however, a confusing array of prospective, randomized clinical trials published in the prominent general medical literature fail to establish major benefits from revascularizing patients with atherosclerotic renal arterial disease 1;2. Many of these trials are seriously flawed 3. While these trial results indeed reflect major improvements in medical management and antihypertensive drug therapy, most clinicians in the field recognize the need to optimize both endovascular and medical interventions for renovascular disease. In some cases, critical opportunities to preserve renal function are missed, allowing patients to drift into advanced kidney failure. The goal of this review is to summarize the current state of affairs regarding renovascular hypertension and ischemic nephropathy for the clinician with a major interest in hypertension.
Definition and epidemiology
Renovascular hypertension refers to the rise in arterial pressure attributable to reduced perfusion of the kidney. Most often this is from main renal arterial obstruction from either atherosclerotic occlusion or fibromuscular dysplasia. A variety of other lesions can produce the same syndrome, however, some of which are identified in TABLE 1. As a clinical phenomenon, these lesions sometimes produce an unexpected rise in blood pressure in younger subjects, e.g. less than 30 or contribute to resistant hypertension in previously normotensive or treated hypertensive subjects Recent imaging studies indicate that “incidental” vascular lesions can be identified in 3-5% of normotensive subjects, e.g. potential living kidney donors with normal kidney function and blood pressure 4. Hence, radiographic identification of a vascular occlusive lesion alone does not establish its physiologic role.
TABLE 1. Major Causes of vascular occlusion producing Renovascular Hypertension.
| Unilateral disease |
| Unilateral atherosclerotic renal artery stenosis |
| Unilateral fibromuscular dysplasia (FMD) |
| Medial fibroplasia |
| Perimedial fibroplasia |
| Intimal fibroplasia |
| Medial hyperplasia |
| Renal artery aneurysm |
| Arterial embolus |
| Arteriovenous fistula (congential / traumatic) |
| Segmental arterial occlusion (post-traumatic) |
| Extrinsic compression of renal artery, e.g pheochromocytoma |
| Renal compression, e.g. metastatic tumor |
| Bilateral Disease or Solitary Functioning Kidney |
| Stenosis to a solitary functioning kidney |
| Bilateral renal arterial stenosis |
| Aortic coarctation |
| Systemic vasculitis (e.g. Takayasu's, Polyarteritis) |
| Atheroembolic disease |
| Vascular occlusion due to endovascular aortic stent graft |
A variety of fibromuscular lesions are recognized, most commonly in young women. These sometimes first appear as unexplained rises in arterial pressure, e.g. during pregnancy. These lesions may have varied appearance including the “string-of-beads” associated with medial fibroplasia or focal banding in the mid-renal artery 5. Remarkably, these lesions often trigger renin release without loss of renal parenchymal volume or glomerular filtration rate unless they lead to dissection or thrombosis of the entire kidney.
By far the most common renovascular lesion is atherosclerotic renal artery stenosis (ARAS). Atherosclerosis leading to elevated arterial velocities suggesting more than 60% stenosis can be detected in 6.8% of community based subjects above age 65 6. The prevalence of ARAS rises with age and with clinically manifest disease in the coronary arteries (18-20%), the aorta, or peripheral vascular beds (35-50%), as recently reviewed 7. Development of atherosclerotic disease relates to the magnitude of cardiovascular risk factors, including smoking, dyslipidemia, diabetes and age, among others. Epidemiologic associations have been proposed to suggest that individuals with refractory congestive heart failure and/or end-stage renal disease may have demonstrable of ARAS in 40-50% of cases 7. Whether these lesions are active causal factors in these conditions or represent an associated “biomarker” of atherosclerosis remains controversial. Development and use of aortic endovascular stent grafts, often placed adjacent to the renal arteries has produced a new class of patients with “acquired” renal artery stenosis related to occlusive effects of the stent graft 8. This may become more common as these devices become more widely used in patients at ever older age ranges.
Pathophysiology: Renovascular hypertension and the role of renin-angiotensin system
Seminal studies demonstrating the link between vascular perfusion to the kidney and the development of hypertension remain fundamental to the field of blood pressure research 9-11. Models using renal arterial lesions have been reproduced in multiple species including rat, dog, rabbit and swine. Studies in these models facilitated discovery and elucidation of the renin-angiotensin system. Unilateral experimental renovascular disease with a functioning “contralateral kidney” that excretes sodium as function of “pressure natriuresis” (identified as 2-kidney-1-clip hypertension) remains a premier model of angiotensin-dependent hypertension. When a solitary kidney is present or both kidneys are affected, angiotensin-dependence is temporary and dependent upon sodium depletion 12. Targeted blockade of the renin-angiotensin system 13;14 or its activation in animals without angiotensin receptors protects against the development of renovascular hypertension 15. Studies using kidney transplants from angiotensin receptor knockout mice indicate that both systemic and renal effects of angiotensin participate in pressure and target organ injury in this disorder 16. Remarkably, activation of the circulating renin-angiotensin system is transient and leads to recruitment of additional pressor pathways, including oxidative stress, sympatho-adrenergic activation, and impaired vasodilatory responses both within the renal and systemic microcirculation 17. It should be emphasized that the release of circulating renin depends upon a substantial reduction in kidney perfusion pressure. Studies using balloon occlusion in humans indicate that this process requires development of a gradient across the lesion such that distal pressures fall at least by 10-20% below aortic pressure 18. Such pressure differences correspond to translesional peak systolic gradients of 15-25 mm and develop only when the cross-sectional area of occlusion approaches 70-80% as illustrated in FIGURE 1 19. An important corollary of this observation is that vascular lesions that fail to generate such a gradient are unlikely to participate in renovascular hypertension and do not benefit from measures to open the vessel. Conversely, revascularization of vessels with lesions that produce a gradient does not invariably resolve hypertension and renal dysfunction, which may be caused by alternative or co-existing mechanisms.
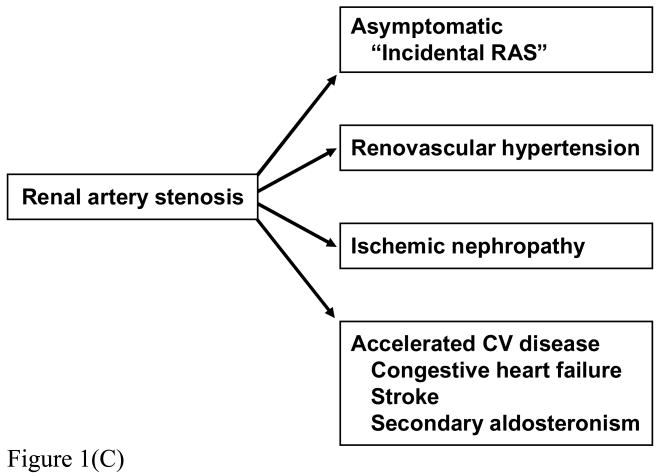
FIGURE 1.
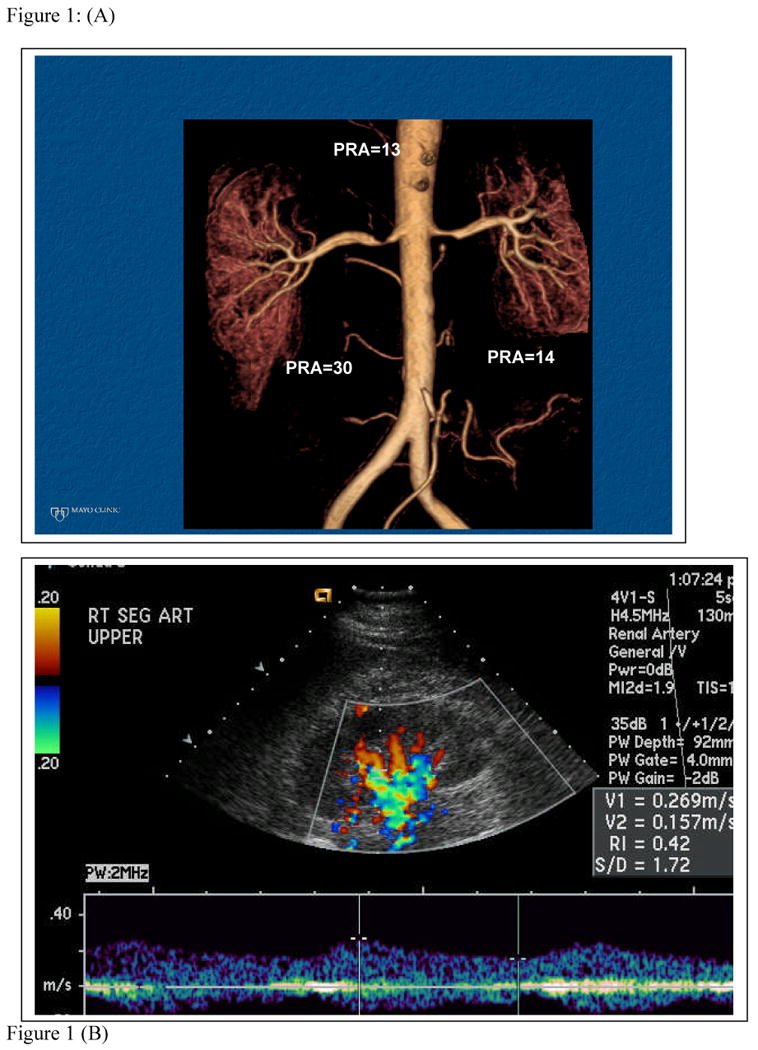
(a) MR Angiogram of High grade renal artery stenosis with renal vein renin measurements: lateralization suggests high probability of pressor activity.
(b) Renal artery duplex study of distal segments on the right kidney illustrating “parvus tardus” waveform and low resistive index (RI=0.42). These data suggest excellent distal blood flow “runoff' and limited parenchymal fibrosis. Severe hypertension had developed over a three month period that was reversed by successful revascularization.
(c) Manifestations of renal arterial disease
The kidney is relatively overperfused relative to its metabolic requirements, a feature consistent with its role as a filtering organ. Hence, blood flow and filtration may fall considerably without jeopardizing tissue viability. Recent studies indicate that reduction of renal blood flow sufficient to reduce kidney size and produce renin release may develop despite preserving normal overall levels of both cortical and medullary tissue oxygenation 20. These data imply that renovascular hypertension does not depend upon true renal “ischemia” per se.
Clinical manifestations of renovascular hypertension include high rates of target organ injury as compared to similar levels of “essential hypertension” 21. Importantly, the presence of ARAS also increases cardiovascular risk 22. Some target organ injury may be related directly to activation of the renin-angiotensin-aldosterone axis. For this reason, the U.S. Cardiovascular Outcomes for Renal Artery Lesions (CORAL) trial specifies angiotensin-blockade as part of medical therapy for all patients in the study 23. Patients with ARAS commonly have disturbed day-night blood pressures with loss of nocturnal BP fall, increased sympathetic nerve traffic as measured in efferent nerve fibers, more severe left ventricular hypertrophy and lower glomerular filtration rate (GFR) as compared to essential hypertension with similar clinic blood pressures 24;25.
Loss of renal function and viability
Although the kidney overall receives more blood than needed strictly for its metabolic activity, impaired blood flow eventually leads to tissue fibrosis. Remarkably, parenchymal fibrosis rarely develops in patients with fibromuscular disease with the exception of those experiencing renal infarction. This suggests that activation of remodeling mechanisms in the post-stenotic kidney is related to the atherosclerotic milieu itself 26. Recent experimental studies also underscore the development of renal microvascular changes distal to a stenosis in the renal artery 27;28. An example of microvascular proliferation induced by cholesterol feeding (a surrogate for early atherosclerosis) and subsequent “rarefaction” of renal small vessels beyond a main renal artery lesion is illustrated in FIGURE 2. Numerous signaling pathways lead to upregulation of cytokines and inflammatory mediators, including transforming growth factor (TGF)-beta, within the post stenotic kidney 29;30. Over time, rarefaction of the distal arterioles develops, associated with fibrogenesis and loss of viable function 31. 32-34 When vascular occlusion reaches severe proportions, overt tissue ischemia can be demonstrated as illustrated using Blood Oxygen Level Dependent (BOLD) magnetic resonance imaging (FIGURE 3).
FIGURE 2.
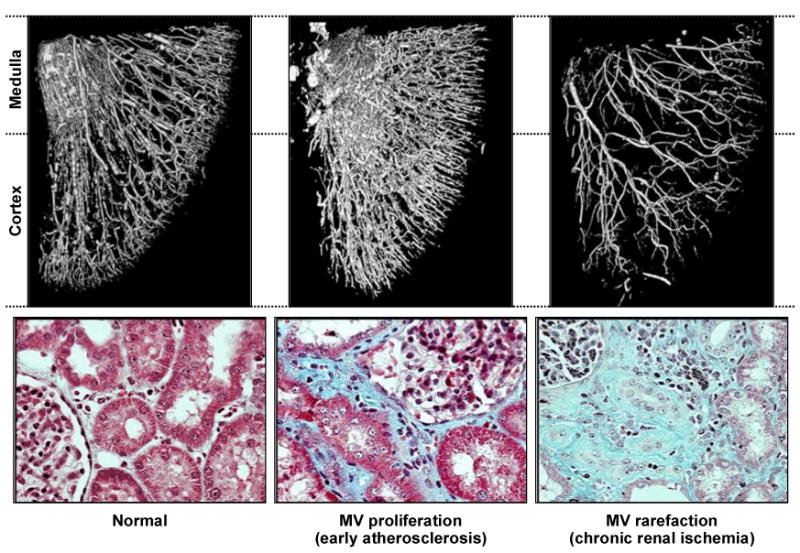
Micro-CT imaging of vascular casts obtained from a swine model of atherosclerotic renal artery stenosis. Atherosclerosis produced by cholesterol feeding induces small vessel proliferation and disturbed endothelial function (middle panel). The kidney beyond a main renal arterial occlusive lesion induced by copper stent experiences dropout (“rarefication”) of small vessels within both cortex and medulla and accelerated tissue fibrosis. From Lerman and Chade, with permission 90.
FIGURE 3.
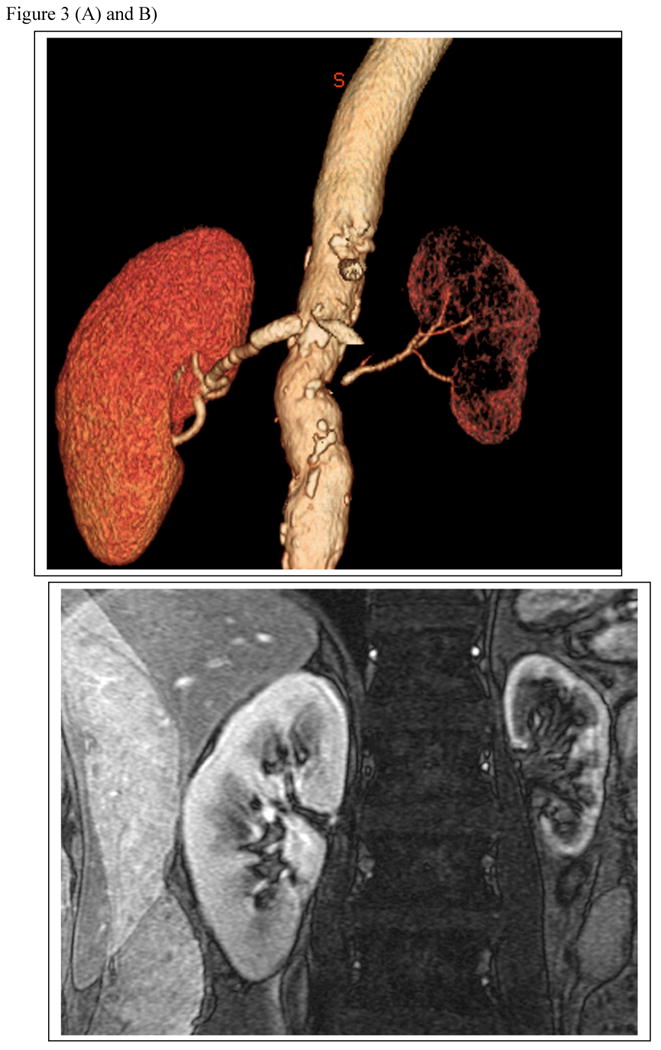
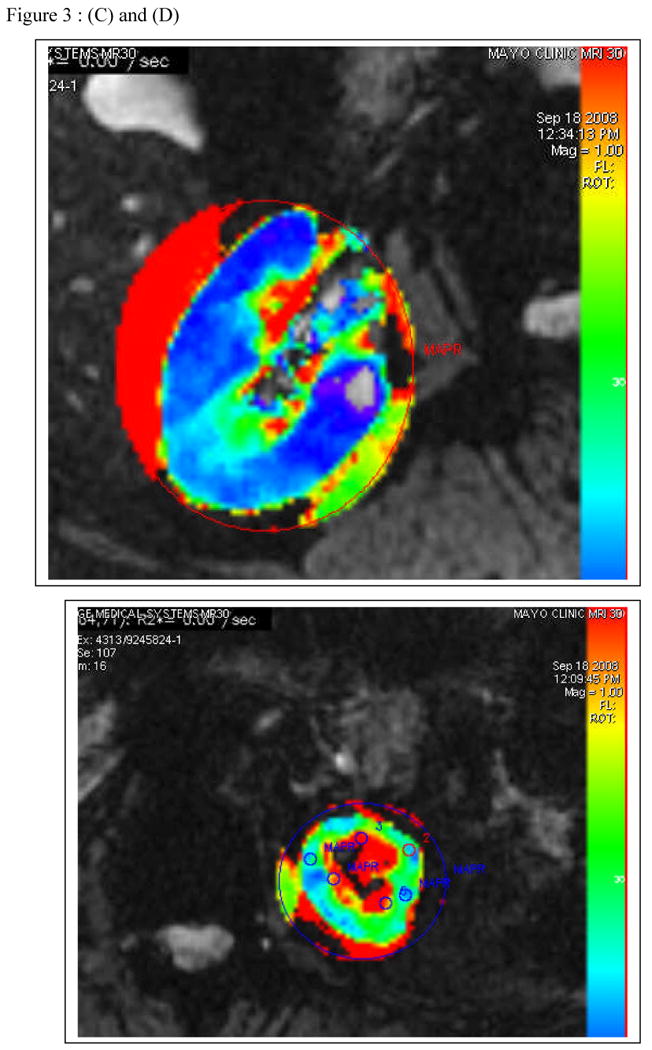
(a) T2 imaging by MR and CT angiogram demonstrating high grade renal artery stenosis to the left kidney and a normal nephrogram on the right (with a vascular stent in place and distal fibromuscular disease). (b) Parametric maps of R2* (reflecting the level of deoxyhemoglobin) from Blood Oxygen Level Dependent (BOLD) MR at 3 Tesla from the same kidneys are shown below. The right kidney has low cortical R2* (blue) with small areas of medullary deoxygenation typical of a normal kidney. Moderate vascular stenosis such as observed with fibromuscular disease is associated with well-preserved tissue oxygenation as shown here 20. The small left kidney has higher levels of cortical R2* and a large, deep area of medullary deoxygenation (red) illustrating physiologic oxygen deprivation as a result of extreme vascular compromise. Hence, progressive occlusive disease ultimately overrides compensatory changes within the kidney to produce ischemic injury.
For many of these patients, restoring kidney blood flow alone has little effect on improving kidney function, as emphasized by the results from recent clinical trials 35. However, some injurious pathways responsible for renal remodeling can be modified using intensive therapy with either anti-oxidants or statins 36, leading to improved blood flow, vascular integrity and reduced kidney injury 32. Clinical observations suggest that statins slow the progression of tissue injury in ARAS and are associated with substantially less interstitial fibrosis in kidneys removed for total arterial occlusion 33;34. Remarkably, infusion of autologous endothelial progenitor cells in a swine model produces increases in renal blood flow and glomerular filtration rate even without renal revascularization 37. Whether additional maneuvers, such as delivering undifferentiated progenitor cells to the kidney or mobilization of resident kidney stem cells, can facilitate recovery of viable tissue in humans under these circumstances remains to be seen.
Clinical manifestations and the Decision to undertake Diagnostic studies
Stenotic occlusive disease in the renal arteries is associated with a broad range of clinical manifestations as illustrated in FIGURE 1. Many of these lesions reflect incidental or modest disease with little clinical impact regarding renal perfusion, blood pressure or glomerular filtration. Results from recent trials underscore the fact that the severity of many vascular lesions, i.e. the degree of actual vessel occlusion, is overestimated from non-invasive diagnostic imaging, e.g. MR angiography, Doppler ultrasound or CT angiography. When subjected to quantitative angiography, many of these lesions remain below the threshold warranting vascular intervention. In the STent placement for Atherosclerotic Renal artery stenosis (STAR) trial, for example, 28% of stenotic lesions were not subjected to intervention despite assignment to this therapy, primarily due to identifying only clinically trivial disease after closer scrutiny during angiography 2;3.
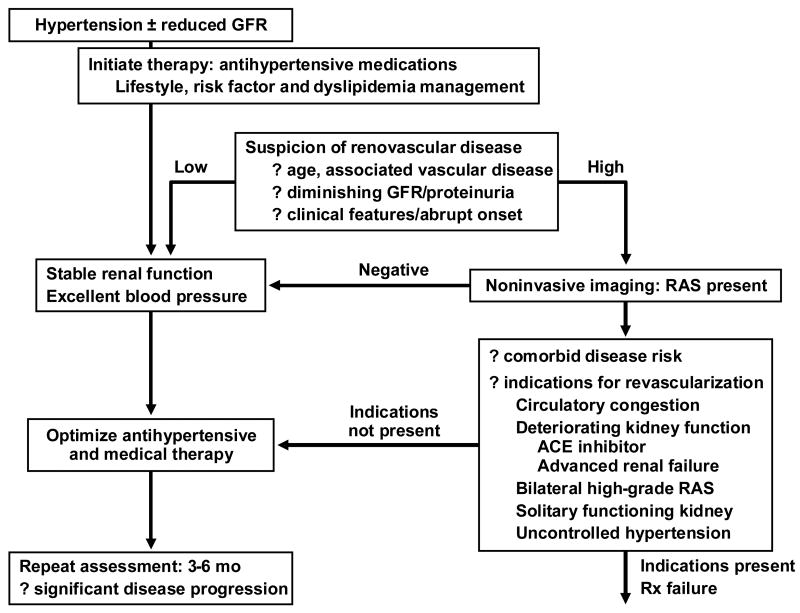
When reduced kidney perfusion activates pressor mechanisms, blood pressure often rises, sometimes leading to acceleration of pre-existing essential hypertension. As a result, the clinical manifestations of ARAS most commonly develop in previously treated hypertensive subjects. A rapid rise in arterial pressure can be associated with target injury such as a stroke, or when combined with reduced renal perfusion to the entire renal mass and enhanced sodium reabsorption can lead to rapidly developing circulatory congestion (sometimes termed “flash pulmonary edema”). Sodium retention in patients with left-ventricular heart dysfunction contributes to refractory congestive failure, particularly when both kidneys or the entire renal mass is affected. Finally, loss of blood flow produces irreversible kidney fibrosis as noted above. As a practical matter, the decision whether to initiate diagnostic studies often depends directly upon the clinical likelihood that renovascular disease is an important contributor to the disease state of the patient. It also depends upon whether these manifestations can be managed readily with medical therapy alone. It may be argued that if blood pressure and kidney function are treated satisfactorily and remain stable, little is to be gained by embarking on a path of expanded diagnostic studies. Hence, the decision to undertake expanded diagnostic testing should be based upon the commitment to consider renal revascularization if studies are positive. A commonly applied algorithm is summarized in FIGURE 4.
FIGURE 4.
Algorithm for evaluation and intervention in renovascular disease (from Textor, in Brenner's Textbook, with permission 91
It is worth emphasizing that this approach is more conservative than proposed even a decade ago. Formal reviews of both the observational studies 38;39 and prospective trials emphasize that surgical and endovascular renal revascularization procedures carry expense and morbidity risks. Outcomes of these trials are summarized below (TABLE 2). The benefit to intensive investigation and vascular intervention for most patients is modest and should be reserved for those most likely to experience real clinical benefit.
TABLE 2.
Summary of Prospective, Randomized trials of medical therapy vs renal revascularization in atherosclerotic renal artery stenosis.
| Author/No. Patients | Inclusion/BP measurement | Outcome | Weaknesses/Limitations |
|---|---|---|---|
| The ASTRAL Investigators— | “Uncertainty”-- “Patient's doctor was uncertain”. Primary endpoint. 20% change in renal function. |
No difference in BP, serum creatinine, mortality, CHF at 33 months (median). | Enrollment bias: “uncertainty” not defined |
| NEJM 361;1953, 2009 | 20-22% renal event | Non-standard imaging 42% “<70%” 58% ≥70% |
|
| ASTRAL * N=806 |
49-51% Cardiovascular event | Nonstandard medical therapy | |
| Only 335 of 403 patients assigned to stents treated | |||
| 6% Crossover | |||
| Bax, et al: STAR* | RAS>50% Clearance <80 mL/min/1.73m2 Endpoint ≥20% change in clearance |
No difference in rates of developing a fall in GFR between groups. | Minor disease 28% of assigned stent group not treated, mainly due to minimal stenosis |
| Ann Int Med 150:840, 2009 | Med failure: 4/74 (46/64 randomized to stent were actually treated) | ||
| Uzzo, et all Transplant Proc. 34:723, 2002 | High-grade stenosis entire renal mass (>75% stenosis) Creatinine ≤ 4 mg/dL |
Stop points BP, doubling Cr ESRD, CV event or death. | Enrollment bias? |
| N=52 | Median f/u: 74mo: No difference between groups: 67% reached stop point. | All patients required to be surgical candidates. | |
| Van Jaarsveld, et al NEJM 342, 2000 | Resistant: 2 drugs DBP>95 mm Hg or creatinine rise with ACEI RAS>50% |
No difference in BP outcomes at 3 mos between groups: Fewer drugs: 1.9 vs 2.4 in PTRA group p<.01 | Large crossover for BP Control (44%) |
| N=106 ASO | Exclusion: Creat ≥2.3 Solitary kidney/total occlusion Kidney <8 cm |
Creat Clearance (3 mos) ml/min: PTRA: 70 Med Rx: 59 (p=.03) |
Few/no stents. |
| BP automated oscillometric | Abnormal renograms PTRA: 36% Med Rx: 70% (p=.002) |
Short follow-up. | |
| Renal artery occlusion PTRA 0 Med Rx 8 |
Inadequate BP control in both groups | ||
| Plouin, et al Hypertension 31:822, 1998 | <75 years Normal contralateral kidney RAS>75% or >60%, lateralizing study |
No difference in BP or Creatinine clearance between groups: Fewer drugs (DDD) in PTRA: PTRA 1.0 Med Rx: 1.78, p<.01 |
Excluded RAAS blockade. |
| N=49 (unilateral ASO) | Exclusion: Malig HTN CVA, CHF, MI within 6 mos | 27% crossover in Medical group | |
| BP: Automated Sphygomanometer, ABPM at 6 mos | Enrollment selection bias (exclusion). | ||
| Webster, et al J. Human Hypertens 12:329, 1998 | DBP ≥95, 2 drugs | No BP Difference in Unilateral ARAS: | Improved BP in bilateral disease. |
| N=55 (unilateral=27) N=135 eligible |
Exclusion: CVA, MI within 3 mos: Creat>500(mcmol/L) RAS>50% |
Lower BP in Bilateral ARAS: PTRA: 152/83 Med Rx: 171/91 p<.01 |
Limited BP control by current standards. |
| BP: Random Zero Device No ACEI allowed | |||
DDD=defined daily doses
ASTRAL: = Angioplasty and Stent Therapy for Renal Artery Lesions
STAR = Stent placement in patients with Atherosclerotic Renal Artery Stenosis
Overview of prospective randomized trials comparing medical therapy for renovascular hypertension to percutaneous renal artery angioplasty (PTRA) with and without stenting or surgery. These studies contained selected patient populations that excluded highest risk groups. Each was different, but all found less blood pressure or renal functional benefits in interventional groups than reported from previous observational studies alone. These data highlight advances in medical therapy and relatively modest rates of renal functional loss for patients with limited disease. Crossover rates from medical to angioplasty arms were significant in the early trials mainly related to blood pressure control, however, and emphasize the importance of restoring blood supply in selected patients, particularly those with bilateral disease.
ASTRAL 1
STAR 85
Surgery: Uzzo 86
Webster 87
Plouin 88
Van Jaarsveld 89
Diagnostic Studies for Renovascular Disease
Exhaustive consideration of vascular imaging of the kidney is beyond the scope of this review. Before initiating any of these procedures, clinicians would do well to define precisely the goals to be achieved, as we have discussed elsewhere 40. In general, imaging procedures are undertaken to define the presence and/or bilateral location of hemodynamically important vascular occlusion. Optimally, one would like an estimate of the severity, accessibility, and location of these lesions, and the functional status of the kidney beyond. Even more importantly, one would like to define the degree to which these vascular occlusive lesions actually are responsible for the clinical manifestations present and the likelihood that restoring vascular patency would reverse this process. Achieving all of these goals is difficult with any single study.
Renal artery Doppler ultrasound is a widely available method that reliably defines velocity changes consistent with vascular narrowing. It requires attention to detail and varies considerably between institutions, particularly in light of operator expertise, patient preparation, and the time allowed for satisfactory study. Prospective series suggest sensitivity and specificity above 90% in dedicated laboratories 41;42. Others have reported sensitivity in the 60% range with more than 20% technically inadequate studies, often due to obesity or poor preparation 43. In our experience, duplex ultrasound rarely produces “false positives”, but can miss important vascular lesions, resulting in “false negatives”. It can be helpful to follow previously identified lesions regarding progression of disease. Identification of low resistive index in the affected kidney has been proposed as a marker of likely benefit from revascularization 44, although this has not been observed universally 45. Velocity thresholds for significant disease vary considerably (classically considered at 180-200 cm/sec Peak Systolic Velocity for “hemodynamically significant lesions” above 60% occlusion 46). To avoid overdiagnosis of modest lesions some authors advocate setting the threshold at 300 cm/sec, currently the required threshold for enrollment in the Cardiovascular Outcomes for Renal Atherosclerotic Lesions (CORAL) trial in the U.S..
CT angiography (CTA) and MR angiography (MRA) provide ever more sophisticated imaging, allowing construction of three dimensional vascular images and estimates of individual kidney function. Use of contrast MRA in subjects with reduced GFR has fallen off drastically in this disease since the concerns regarding development of nephrogenic systemic fibrosis (NSF) and the putative role of gadolinium contrast 47. While gadolinium contrast may be safe for patients with preserved GFR, newer techniques allow improved vascular imaging without gadolinium and may become more widely available 47. CT angiography has both radiation and contrast exposure, but can provide excellent definition of both vascular tree and renal function.
Functional testing based upon activation of the renin angiotensin-system
Renovascular hypertension is more closely associated with activation of the renin-angiotensin system (RAAS) than any other form of hypertension. While intuitively attractive as diagnostic tools, measurement of plasma renin activity alone and/or the blood pressure response to angiotensin blockade are limited by interactions with drug effects, sodium intake, renal dysfunction and possible alterations dependent upon age and the duration of renovascular disease.
Angiotensin is an important determinant of filtration pressure in the kidney. Most patients with renovascular disease tolerate RAAS blockade without evident adverse effects, although some fall in GFR may accrue inevitably from functional removal of the angiotensin II action that supports filtration 48. This fall in GFR is exaggerated in the presence of pre-glomerular vascular disease and is the basis for examining changes in isotope renograms before and after captopril. Several series derived from clinically selected populations suggest that captopril renography can predict blood pressure and/or renal function improvements after renal revascularization with predictive values exceeding 75% and sensitivity above 90% 49-51. These observations have not been confirmed when applied to larger populations and may be confounded by previous drug therapy, bilateral disease and reduced kidney function 52;53. Captopril renography is less commonly used than before, but has advocates within the hypertension community 54. It had little predictive value in the Dutch Renal Artery Stenosis Intervention Cooperative study group (DRASTIC) trial other than to identify individuals with progressive total occlusion 53. Renography does provide functional data regarding blood flow and filtration, but no direct imaging regarding the renal vasculature itself. Hence, it provides an estimate of relative function when total occlusion is present that may validate consideration of nephrectomy of a “pressor kidney”, as recently revisited 55;56.
Intra-arterial angiography and renal vein renin measurements remain the “gold standard”. Angiographic core laboratories indicate that visual estimates of stenosis commonly overstate the severity of vascular occlusive lesions. As a result, some authors recommend confirmation with translesional gradient measurement using low profile pressure transducers 57. Without a measurable gradient, it is hard to justify vascular intervention. While measurement of renal vein renin levels has fallen into less common use, a considerable body of data supports a predictive role of lateralizing values regarding the likely benefit of revascularization, at least regarding improved blood pressure control 58. Careful studies of renal vein renin levels obtained after withholding drug therapy for two weeks indicate that lateralizing values (more than 1.7) accurately predict the fall in arterial pressure following nephrectomy in patients with total occlusion 59. The authors argue that lateralization for non-occluded vessels performed best (by receiver operating curves) at a level of 1.55, which corresponded to a sensitivity of 54% with a false positive rate of 15%. In practice, many clinicians are unwilling to withhold drug therapy before testing and results may be less consistent. As with most diagnostic studies in this disorder, the results are most useful when positive. Failure to demonstrate lateralization can result for many reasons, including volume expansion commonly employed during angiography.
One might argue that ambiguous trial results should encourage more careful documentation and evaluation before undertaking renal revascularization procedures. There is a pressing need to refine the tools for defining the clinical impact of vascular occlusion in the kidney, as we have discussed 40.
Medical Therapy for Renovascular Hypertension and Ischemic Nephropathy
As noted above, much of the commitment for extensive diagnostic studies and vascular intervention depends upon the results of medical therapy as an initial step. Particularly for patients with accelerated or extreme hypertension, blood pressure reduction to safe levels should be achieved before invasive diagnostic procedures. Pharmacologic therapy of renovascular hypertension follows the basic principles of all antihypertensive drug therapy, but especially depends on effective blockade of the renin-angiotensin-aldosterone system (RAAS). Italian and Canadian registry data suggest considerable survival advantages for patients able to employ angiotensin converting enzyme (ACE) inhibition or angiotensin receptor blockers (ARB's) as part of their regimen 60;61. Few data address the role of direct renin inhibition, although this is a rational alternative. Analysis of subgroups from the HOPE and PEACE trials suggest the greatest clinical benefits from RAAS blockade accrue to those with some degree of renal dysfunction 62;63. These groups are likely to include some patients with unrecognized atherosclerotic RAS. A clinically important fall in GFR associated with a rise in serum creatinine and potassium sometimes occurs with major preglomerular vascular occlusion that precludes the use of these agents. It should be emphasized that such a rise in creatinine develops both because blood flow is threatened and because angiotensin II supports filtration under those conditions 64. Hence, the loss of GFR reflects a functional signal that blood flow is threatened and filtration requires the supportive action of angiotensin. Filtration can recover when blockade of RAAS is removed. A corollary of this observation is that other forms of antihypertensive drug therapy may also threaten blood flow, which in some cases constitutes a legitimate reason to move forward to restore renal blood flow. Remarkably, most patients with hemodynamically important ARAS tolerate RAAS blockade without difficulty. Withdrawal of ACE / ARB in the heart failure trials occurred in less than 5% of subjects 65. Recommendations for the use of these agents include re-evaluation of serum creatinine and potassium level soon (within a week) after initiating therapy, particularly in patients with reduced kidney function 48. It remains essential for the clinician to define what increase in serum creatinine can be tolerated, but some authors indicate that a rise of 30% may be expected 66. Some investigators argue that the cardiovascular risk is magnified by the neuro-hormonal activation associated with ARAS 57. As a result, the authors of CORAL advocate universal blockade of the RAAS as a central component of managing this disorder.
At least equally important is the intensive and rigorous management of atherosclerosis. Discontinuing tobacco use, statin therapy, aspirin, and diabetes management are major components that require attention. Most of the adverse outcomes for patients with atherosclerotic renovascular disease derive from non-renal vascular complications, including stroke, coronary events, and peripheral vascular disease 67.
The role of Renal Revascularization
Restoring blood flow to kidneys that actively trigger pressor mechanisms in renovascular hypertension is an intuitively rational approach 68. Indeed, both surgical or endovascular revascularization occasionally produce normalization of arterial pressures in such cases, leading to “cure”. Most cases of fibromuscular dysplasia producing hypertension in younger individuals can be considered for angioplasty at low procedural risk, so long as no aneurysms are present. Considering the potential need for many years of antihypertensive therapy and the limitations on using either ACE inhibitors or ARBs in pregnancy, most clinicians favor angioplasty as initial therapy for younger women, who are most likely to be treated for this disorder. It is important to recognize that even with FMD, rates of “cure” are limited. Recent series suggest that achieving normal blood pressures without antihypertensive drug therapy occurs in less than 30% of subjects, although some improvement may occur in an additional 50% or more 5. Complex disease including aneurysmal dilatation should be approached using either surgical or medical therapy primarily. Restenosis may occur with FMD and lead to repeat procedures in 11-23% 5;69.
Atherosclerotic RAS poses a different set of concerns, as noted above. These lesions primarily develop in subjects with pre-existing hypertension, so revascularization rarely lowers blood pressure to normal. Nonetheless, restoring blood flow to kidneys at critically reduced perfusion offers the potential to recover kidney function, protect from further degradation, and improve blood pressure control. Observational studies including both surgical and endovascular repair emphasize that important improvements in kidney function and reduced antihypertensive drug requirements can be obtained in such patients 70-74. A formal review from the Agency for Health Quality Research (AHQR) indicates that while prospective studies fail to define unequivocal strategies for management of atherosclerotic RAS, clinically important improvements in kidney function and reversal of hypertension developed only in patients with successful revascularization 38. Major improvements do not occur frequently, however, and these procedures have both costs and risks. Because the advances in medical therapy have been substantial, numerous prospective, randomized trials comparing intensive medical therapy with and without renal revascularization procedures have been undertaken in recent years 39. Some of these have been completed and published. The main results from these trials are summarized in TABLE 2. It is notable that these trials vary enormously in size and power estimates, ranging from less than 100 to more than 1000 for target populations. Because patients with ARAS are older and commonly have widespread atherosclerosis, clinical events are subject to major confounding with other comorbid diseases. Some authors argue that such populations are inherently so heterogeneous as to be impossible to study as a single population 75. A major limitation of these initial trials including STAR 2 and ASTRAL 1 has been the inconsistency in defining “critical” vascular occlusion and the degree of stenosis. Hence, the power of several trials has been undermined by realization that patients selected for treatment often have trivial disease. Another key observation has been the recognition that current optimal medical therapy appears to allow stabilization of atherosclerotic disease and renal dysfunction to a much greater degree than originally predicted. Hence, the ability to achieve reasonably good blood pressure control has been much better than reported in studies a decade ago 76. Perhaps for these reasons, the results of the recent trials fail to demonstrate major advantages to renal revascularization as a primary intervention. The largest and most carefully performed study in this field in the U.S., the Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL), remains in progress and will not provide outcome data for several more years.
It must be recognized that revascularization procedures carry some risks, although modern techniques have reduced these substantially. Moderate complications including bleeding, local dissection, and branch occlusion can develop, but are reported in less than 10% of cases 77. Restenosis remains a concern and can develop in 10-15% of subjects 78. ARAS lesions commonly develop as part of generalized atherosclerosis, particularly in the abdominal aorta. As a result, either surgical or endovascular manipulation carry some risk of releasing atheroemboli. While a small amount of embolic debris may be released in nearly any vascular procedure, including guidewire placement 79;80, the incidence of clinically important renal infarction or systemic atheroemboli remains low, reported between 1 and 4% 77;81. When the full syndrome of systemic emboli occurs, however, it can be devastating. Risk factors for emboli include extensive aortic plaque and/or aneurysm, uncontrolled hypertension, and direct renal artery manipulation 82. In a series of 43 patients developing advanced renal failure from this procedure, 31 required long-term dialysis and 33% did not survive the first year. Of those requiring dialytic support, only 12 (28%) ultimately recovered sufficiently to withdraw from dialysis. Whether use of embolic protection devices will improve these outcomes is uncertain. Results from a recent trial suggest that simply having the device in place during the procedure offers little benefit for recovery of kidney function after renal artery stenting 83. Many embolic events develop in a “stuttering” fashion for days and weeks after the procedure, suggesting that temporary vascular protection will have limited value.
Future Developments to recover renal function
ARAS initiates complex changes by which disturbed signaling pathways activate inflammatory and fibrogenic processes at different times 29;30. Although the kidney microvasculature is complex and remarkably resilient despite local environments with reduced oxygen tension, it is likely that either recurrent or critical levels of ischemia participate in triggering some of these responses. While restoring large vessel perfusion alone is rarely sufficient, future studies may elucidate a role for adjunctive or alternative measures that overcome hypoxic stimuli and allow for restoring the microvasculature. Some may also support regeneration of functional cells to repair injured tubules and glomeruli. Experimental studies suggest that undifferentiated cells, either local progenitor cells or those recruited from elsewhere in the body, may foster a microenvironment where such repair can occur. We are optimistic that newer tools to monitor tissue conditions and improved technology to stimulate local repair mechanisms will play an important role in this process.
Summary: An integrated view of managing renovascular disease
Renovascular disease remains one of the most prevalent and important causes of secondary hypertension and renal dysfunction. Advances in antihypertensive drug therapy and intensive risk factor management including statin therapy can provide excellent blood pressure control in many individuals. Despite these advances, timely recognition of vascular occlusive disease is important to avoid progressive renal functional loss. Recent studies emphasize important interactions between large vessel occlusive disease and changes in the milieu or microvascular environment in the kidney. Changes in microvascular disease appear to activate multiple mechanisms of tissue injury and repair that remain incompletely understood 31;84. Improved therapy for this disorder will depend upon more precise definition of the role of post-stenotic kidneys in sustaining hypertension and identification of kidneys that are threatened by vascular occlusive disease but remain viable and salvageable. Based on the ambiguous results of prospective treatment trials to date, clinicians will need to examine each patient closely to consider if limited net benefits warrant the expense and potential hazard of invasive procedures and vascular repair. We anticipate the need for more refined tools to define the degree of actual tissue ischemia within an individual subject and the role of adjunctive maneuvers to assist in tissue repair. Effective patient care for renovascular disease likely will benefit from close collaboration among clinicians directly managing medical therapy and those providing vascular intervention.
Acknowledgments
The project described was supported by Award number P01HL085307 from National Heart, Lung and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the National Institutes of Health.
Contributor Information
Stephen C. Textor, Division of Nephrology and Hypertension, Mayo Clinic, Rochester, Minnesota, USA.
Lilach Lerman, Division of Nephrology and Hypertension, Mayo Clinic, Rochester, Minnesota, USA.
References
- 1.The ASTRAL Investigators Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361:1953–1962. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 2.Bax L, Woittiez AJ, Kouwenberg HJ, Mali PTM, Buskens E, Beek FJA, Braam B, Huysmans FTM, Kool LJS, Rutten MJCM, Doorenbos CJ, Aarts JCNM, Rabelink TJ, Plouin PF, Raynaud A, van Montfrans GA, Reekers JA, van den Meiracker AH, Pattynama PMT, van de Ven PJG, Vroegindeweij D, Kroon AA, de Haan MW, Postma CT, Beutler JJ. Stent Placement in Patients with atherosclerotic renal artery stenosis and impaired renal function. Ann Int Med. 2009;150:999. doi: 10.7326/0003-4819-150-12-200906160-00119. [DOI] [PubMed] [Google Scholar]
- 3.Mann SJ, Sos TA. Misleading results of randomized trials: the example of renal artery stenting. J Clin Hypertens. 2010;12:1–2. doi: 10.1111/j.1751-7176.2009.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenz EC, Vrtiska TJ, Lieske JC, Dillon JJ, Stegall MD, Li X, Bergstralh EJ, Rule AD. Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clin J Am Soc Nephrol. 2010;5:431–438. doi: 10.2215/CJN.07641009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slovut DP, Olin JW. Current concepts: Fibromuscular dysplasia. N Engl J Med. 2004;350:1862–1871. doi: 10.1056/NEJMra032393. [DOI] [PubMed] [Google Scholar]
- 6.Hansen KJ, Edwards MS, Craven TE, Cherr GS, Jackson SA, Appel RG, Burke GL, Dean RH. Prevalence of renovascular disease in the elderly: a population based study. J Vasc surg. 2002;36:443–451. doi: 10.1067/mva.2002.127351. [DOI] [PubMed] [Google Scholar]
- 7.de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systematic literature review. J Hypertens. 2009;27:1333–1340. doi: 10.1097/HJH.0b013e328329bbf4. [DOI] [PubMed] [Google Scholar]
- 8.Kramer SC, Seifarth H, Pamler R, Fleiter T, Buhring J, Sunder-Plassmann L, Brambs HJ, Gorich J. Renal infarction following endovascular aortic aneurysm repair: incidence and clinical consequences. J Endoovasc Therapy. 2002;9:98–102. doi: 10.1177/152660280200900116. [DOI] [PubMed] [Google Scholar]
- 9.Loesch J. Ein Beitrag zur experimentellen Nephritis und zum arteriellen Hochdruck I. Die Veranderungen im Blutdruck II. Die Veranderungen in der Blutchemie. Zentralblatt fur Innere Medizin. 1933;7:144–169. [Google Scholar]
- 10.Goldblatt H, Lynch J, Hanzal RE, Summerville WW. Studies on experimental hypertension I: the production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347–379. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero JC, Feldstein AE, Rodriguez-Porcel MG, Cases-Amenos A. New Insights into the pathophysiology of renovascular hypertension. Mayo Clin Proc. 1997;72:251–260. doi: 10.4065/72.3.251. [DOI] [PubMed] [Google Scholar]
- 12.Brunner HR, Kirshmann JD, Sealey JE, Laragh JH. Hypertension of renal origin: Evidence for two different mechanisms. Science. 1971;174:1344–1346. doi: 10.1126/science.174.4016.1344. [DOI] [PubMed] [Google Scholar]
- 13.Brunner HR, Gavras H, Laragh JH, Keenan T. Angiotensin II blockade in man by Sar-1-ala-8-angiotensin II for understanding and treatment of high blood pressure. Lancet. 1973;2:1045–1048. doi: 10.1016/s0140-6736(73)92657-3. [DOI] [PubMed] [Google Scholar]
- 14.DeForrest JM, Knappenberger RC, Antonaccio MJ, Ferrone RA, Creekmore JS. Angiotensin II is a necessary component for the development of hypertension in the two-kidney, one clip rat. Am J Cardiol. 1982;49:1515–1517. doi: 10.1016/0002-9149(82)90373-3. [DOI] [PubMed] [Google Scholar]
- 15.Cervenka L, Horacek V, Vaneckova I, Hubacek JA, Oliverio MI, Coffman TM, Navar LG. Essential role of AT1-A receptor in the development of 2K1C hypertension. Hypertension. 2002;40:735–741. doi: 10.1161/01.hyp.0000036452.28493.74. [DOI] [PubMed] [Google Scholar]
- 16.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lerman LO, Nath KA, Rodriguez-Porcel M, Krier JD, Schwartz RS, Napoli C, Romero JC. Increased oxidative stress in experimental renovascular hypertension. Hypertension. 2001;37:541–546. doi: 10.1161/01.hyp.37.2.541. [DOI] [PubMed] [Google Scholar]
- 18.De Bruyne B, Manoharan G, Pijls NHJ, Verhamme K, Madaric J, Bartunek J, vanderheyden M, Heyndrickx GR. Assessment of renal artery stenosis severity by pressure gradient measurements. J Am Coll Cardiol. 2006;48:1851–1855. doi: 10.1016/j.jacc.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 19.Simon G. What is critical renal artery stenosis? Implications for treatment. Am J Hyper. 2000;13:1189–1193. doi: 10.1016/s0895-7061(00)01179-1. [DOI] [PubMed] [Google Scholar]
- 20.Gloviczki ML, Glockner JF, Lerman LO, McKusick MA, Misra S, Grande JP, Textor SC. Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension. 2010;55:961–966. doi: 10.1161/HYPERTENSIONAHA.109.145227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Losito A, Fagugli RM, Zampi I, Parente B, de Rango P, Giordano G, Cao P. Comparison of target organ damage in renovascular and essential hypertension. Am J Hyper. 1996;9:1062–1067. doi: 10.1016/0895-7061(96)00199-9. [DOI] [PubMed] [Google Scholar]
- 22.Edwards MS, Craven TE, Burke GL, Dean RH, Hansen KJ. Renovascular disease and the risk of adverse coronary events in the elderly: a prospective, population-based study. Arch Int Med. 2005;165:207–213. doi: 10.1001/archinte.165.2.207. [DOI] [PubMed] [Google Scholar]
- 23.Murphy TP, Cooper CJ, Dworkin LD, Henrich WL, Rundback JH, Matsumoto AH, Jamerson KA, D'Agostino RB. The Cardiovascular Outcomes with Renal Atherosclerotic Lesions (CORAL) Study: Rationale and Methods. J Vasc Interv Radiol. 2005;16:1295–1300. doi: 10.1097/01.RVI.0000176301.69756.28. [DOI] [PubMed] [Google Scholar]
- 24.Johansson M, Herlitz H, Jensen G, Rundqvist B, Friberg P. Increased cardiovascular mortality in hypertensive patients with renal artery stenosis. Relation to sympathetic activation, renal function and treatment regimens. J Hypertens. 1999;17:1743–1750. doi: 10.1097/00004872-199917120-00012. [DOI] [PubMed] [Google Scholar]
- 25.Miyajima E, Yamada Y, Yoshida Y, Matsukawa T, Shionoiri H, Tochikubo O, Ishii M. Muscle sympathetic nerve activity in renovascular hypertension and primary aldosteronism. Hypertension. 1991;17:1057–1062. doi: 10.1161/01.hyp.17.6.1057. [DOI] [PubMed] [Google Scholar]
- 26.Chade AR, Rodriquez-Porcel M, Grande JP, Zhou XJ, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arteroscler Thromb Vasc Biol. 2003;23:1295–1301. doi: 10.1161/01.ATV.0000077477.40824.52. [DOI] [PubMed] [Google Scholar]
- 27.Feltrin GP, Rossi G, Talenti E, Pessina AC, Miotto D, Thiene G, Dal Palu C. Prognostic value of nephrography in atherosclerotic occlusion of the renal artery. Hypertension. 1986;8:962–964. doi: 10.1161/01.hyp.8.10.962. [DOI] [PubMed] [Google Scholar]
- 28.Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A, Lerman LO. Cortical microvascular remodeling in the stenotic kidney: role of increased oxidative stress. Arterioscler Thromb Vasc Biol. 2004;24:1854–1859. doi: 10.1161/01.ATV.0000142443.52606.81. [DOI] [PubMed] [Google Scholar]
- 29.Cheng J, Zhou W, Warner GM, Knudsen BE, Garovic VD, Gray CE, Lerman LO, Platt JL, Romero JC, Textor SC, Nath KA, Grande JP. Temporal analysis of signaling pathways activated in a murine model of 2-Kidney,1-Clip hypertension. Am J Physiol Renal Physiol. 2009;297:F1055–F1068. doi: 10.1152/ajprenal.90439.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lerman LO, Textor SC, Grande JP. The Mechanisms of tissue Injury in Renal Artery Stenosis: Ischemia and Beyond. Prog Cardiovasc Dis. 2009;52:196–203. doi: 10.1016/j.pcad.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation. 2002;106:1165–1171. doi: 10.1161/01.cir.0000027105.02327.48. [DOI] [PubMed] [Google Scholar]
- 32.Chade AR, Krier JD, Rodgriguez-Porcel M, Breen JF, McKusick MA, Lerman A, Lerman LO. Comparison of acute and chronic antioxidant interventions in experimental renovascular disease. Am J Physiol. 2004;286:F1079–F1086. doi: 10.1152/ajprenal.00385.2003. [DOI] [PubMed] [Google Scholar]
- 33.Keddis M, Garovic V, Bailey K, Wood C, Raissian Y, Grande J. Ischemic nephropathy secondary to atherosclerotic renal artery stenosis: Clinical and histopathological correlates. Nephrol Dial Transplant. 2010;99:999. doi: 10.1093/ndt/gfq269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung CM, Patel A, Shaheen N, Cain S, Eddington H, Hegarty J, Middleton RJ, Cowie A, Mamtora H, Kalra PA. The effects of statins on the progression of atherosclerotic renovascular disease. Nephron Clin Pract. 2007;107:c35–c42. doi: 10.1159/000107552. [DOI] [PubMed] [Google Scholar]
- 35.Textor SC, McKusick MM, Misra S, Glockner J. Timing and selection for renal revascularization in the era of negative trials: what to do? Prog Cardiovasc Dis. 2009;52:220–228. doi: 10.1016/j.pcad.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva VS, Martin LC, Franco RJS, Carvalho FC, Bregagnollo EA, Castro JH, Gavras I, Gavras H. Pleiotropic effects of statins may improve outcomes in atherosclerotic renovascular disease. Am J Hypertens. 2008;21:1163–1168. doi: 10.1038/ajh.2008.249. [DOI] [PubMed] [Google Scholar]
- 37.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119:557. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balk E, Raman G, Chung M, Ip S, Tatsioni A, Alonso A, Chew P, Gilbert SJ, Lau J. Effectiveness of management strategies for renal artery stenosis: a systematic review. Annals of Internal Medicine. 2006;145:901–912. doi: 10.7326/0003-4819-145-12-200612190-00143. [DOI] [PubMed] [Google Scholar]
- 39.Textor SC, Lerman L, McKusick M. The uncertain value of Renal Artery Interventions: Where are we now? J Am Coll Cardiol Cardiovasc Interv. 2009;2:175–182. doi: 10.1016/j.jcin.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Textor SC. Atherosclerotic renal artery stenosis: overtreated, but underrated? J Am Soc Nephrol. 2008;19:656–659. doi: 10.1681/ASN.2007111204. [DOI] [PubMed] [Google Scholar]
- 41.Napoli V, Pinto S, Bargellini I, Vignali C, Cioni R, Petruzzi P, Salvetti A, Bartolozzi C. Duplex ultrasonographic study of the renal arteries before and after renal artery stenting. Europ Radiol. 2002;12:796–803. doi: 10.1007/s003300101121. [DOI] [PubMed] [Google Scholar]
- 42.Olin JW, Piedmonte MR, Young JR, DeAnna S, Grubb M, Childs MB. The utility of duplex ultrasound scanning of the renal arteries for diagnosing significant renal artery stenosis. Ann Intern Med. 1995;122:833–838. doi: 10.7326/0003-4819-122-11-199506010-00004. [DOI] [PubMed] [Google Scholar]
- 43.Postma CT, van Aalen J, de Boo T, Rosenbusch G, Thien T. Doppler ultrasound scanning in the detection of renal artery stenosis in hypertensive patients. Brit J Radiol. 1992;65:857–860. doi: 10.1259/0007-1285-65-778-857. [DOI] [PubMed] [Google Scholar]
- 44.Radermacher J, Chavan A, Bleck J, Vitzthum A, Stoess B, Gebel MJ, Galanski M, Koch KM, Haller H. Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Engl J Med. 2001;344:410–417. doi: 10.1056/NEJM200102083440603. [DOI] [PubMed] [Google Scholar]
- 45.Krumme B, Hollenbeck M. Doppler sonography in renal artery stenosis--does the Resistive Index predict the success of intervention? Nephrol Dial Transplant. 2007;22:692–696. doi: 10.1093/ndt/gfl686. [DOI] [PubMed] [Google Scholar]
- 46.Fisher JEE, Olin JW. Renal Artery Stenosis: Clinical Evaluation. In: Creager MA, Loscalzo J, editors. Vascular Medicine: A Companion to Braunwald's Heart Disease. chap 22. Philadelphia: 2006. pp. 335–347. [Google Scholar]
- 47.Glockner JF, Vrtiska TJ. Renal MR and CT angiography: current concepts. Abdominal Imaging. 2007;32:407–420. doi: 10.1007/s00261-006-9066-3. [DOI] [PubMed] [Google Scholar]
- 48.Schoolwerth AC, Sica DA, Ballermann BJ, Wilcox CS. Renal considerations in angiotensin converting enzyme inhibitor therapy. Circulation. 2001;104:1985–1991. doi: 10.1161/hc4101.096153. [DOI] [PubMed] [Google Scholar]
- 49.Elliott WJ, Martin WB, Murphy MB. Comparison of two noninvasive screening tests for renovascular hypertension. Arch Intern Med. 1993;153:755–764. [PubMed] [Google Scholar]
- 50.Geyskes G, Oei HY, Puylaert CB, Mees EJ. Renovascular hypertension identified by captopril-induced changes in the renogram. Hypertension. 1987;9:451–458. doi: 10.1161/01.hyp.9.5.451. [DOI] [PubMed] [Google Scholar]
- 51.Setaro JF, Saddler MC, Chen CC, Hoffer PB, Roer D, Markowitz DM, Meier GH, Gusberg RJ, Black HR. Simplified captopril renography in diagnosis and treatment of renal artery stenosis. Hypertension. 1991;18:289–298. doi: 10.1161/01.hyp.18.3.289. [DOI] [PubMed] [Google Scholar]
- 52.Postma CT, van Oijen AH, Barentsz JO, de Boo T, Hoefnagels WH, Corstens FH, Thien T. The value of tests predicting renovascular hypertension in patients with renal artery stenosis treated by angioplasty. Arch Int Med. 1991;151:1531–1535. [PubMed] [Google Scholar]
- 53.van Jaarsveld BC, Krijnen P, Derkx FHM, Oei HY, Postma CT, Schalekamp MADH. The place of renal scintigraphy in the diagnosis of renal artery stenosis. Arch Intern Med. 1997;157:1226–1234. doi: 10.1001/archinte.157.11.1226. [DOI] [PubMed] [Google Scholar]
- 54.Taylor A. Functional testing: ACEI Renography. Semin Nephrol. 2000;20:437–444. [PubMed] [Google Scholar]
- 55.Kane GC, Textor SC, Schirger A, Garovic VD. Revisiting the role of nephrectomy for advanced renovascular disease. Am J Med. 2003;114:729–735. doi: 10.1016/s0002-9343(03)00161-x. [DOI] [PubMed] [Google Scholar]
- 56.Safian RD, Madder RD. Refining the approach to Renal Artery Revascularization. J Am Coll Cardiol Intv. 2009;2:161–174. doi: 10.1016/j.jcin.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 57.Cooper CJ, Murphy TP, Matsumoto A, Steffes M, Cohen DJ, Jaff M, Kuntz R, Jamerson K, Reid D, Rosenfield K, Rundback J, D'Agostino R, Henrich W, Dworkin L. Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J. 2006;152:59–66. doi: 10.1016/j.ahj.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 58.Textor SC. Renovascular Hypertension and Ischemic Nephropathy. In: Brenner BM, editor. Brenner and Rector's: The Kidney. chap 46. Philadelphia: 2004. pp. 2065–2108. [Google Scholar]
- 59.Rossi GP, Cesari M, Chiesura-Corona M, Miotto D, Semplicini A, Pessina AC. Renal vein renin measurements accurately identify renovascular hypertension caused by total occlusion of the renal artery. J Hypertens. 2002;20:975–984. doi: 10.1097/00004872-200205000-00033. [DOI] [PubMed] [Google Scholar]
- 60.Losito A, Gaburri M, Errico R, Parente B, Cao PG. Survival of patients with renovascular disease and ACE inhibition. Clin Nephrol. 1999;52:339–343. [PubMed] [Google Scholar]
- 61.Hackam DG, Duong-Hua ML, Mamdani M, Li P, Tobe SW, Spence JD, Garg A. Angiotensin inhibition in renovascular disease: a population-based cohort study. Am Heart J. 2008;156:549–555. doi: 10.1016/j.ahj.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 62.Mann JFE, Gerstein HC, Pogue J, Bosch J, Yusuf S. HOPE investigators: Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Int Med. 2001;134:629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 63.Solomon SD, Rice MM, Jablonski KA, Powell J, Domanski M, Sabatine M, Gersh BJ, Rouleau J, Pfeffer MA, Braunwald E. Renal function and effectiveness of angiotensin-converting enzyme inhibitor therapy in patients with chronic stable coronary disease in the Prevention of Events with ACE inhbition (PEACE) trial. Circulation. 2006;114:26–31. doi: 10.1161/CIRCULATIONAHA.105.592733. [DOI] [PubMed] [Google Scholar]
- 64.Hall JE, Guyton AC, Jackson TE, Coleman TG, Lohmeier TE, Trippodo NC. Control of glomerular filtration rate by renin-angiotensin system. Am J Physiol. 1977;233:F366–F372. doi: 10.1152/ajprenal.1977.233.5.F366. [DOI] [PubMed] [Google Scholar]
- 65.Textor SC. Renal failure related to ACE inhibitors. Semin Nephrol. 1997;17:67–76. [PubMed] [Google Scholar]
- 66.Bakris GL, Weir MR. Angiotensin-Converting enzyme inhibitor-associated elevations in serum creatinine. Arch Intern Med. 2000;160:685–688. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]
- 67.Kalra PA, Guo H, Kausz AT, Gilbertson DT, Liu J, Chen SC, Ishani A, Collins AJ, Foley RN. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization and prognosis. Kidney Int. 2005;68:293–301. doi: 10.1111/j.1523-1755.2005.00406.x. [DOI] [PubMed] [Google Scholar]
- 68.White CJ. Management of renal artery stenosis: the case for intervention, defending current guidelines, and screening (drive-by) renal angiography at the time of catheterization. Prog Cardiovasc Dis. 2009;52:229–237. doi: 10.1016/j.pcad.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 69.Birrer M, Do DD, Mahler F, Triller J, Baumgartner I. Treatment of renal artery fibromuscular dysplasia with balloon angioplasty: a prospective follow-up study. Eur J Vasc & Endovasc Surg. 2002;23:146–152. doi: 10.1053/ejvs.2001.1559. [DOI] [PubMed] [Google Scholar]
- 70.Stanley JC. Surgical Treatment of Renovascular Hypertension. Am J Surg. 1997;174:102–110. doi: 10.1016/s0002-9610(97)00094-9. [DOI] [PubMed] [Google Scholar]
- 71.Novick AC. Long-term results of surgical revascularization for renal artery disease. Urol Clin N Am. 2001;827 doi: 10.1016/s0094-0143(01)80037-9. [DOI] [PubMed] [Google Scholar]
- 72.Balzer KM, Pfeiffer T, Rossbach S, Voiculescu A, Modder U, Godehardt E, Sandmann W. Prospective randomized trial of operative vs interventional treatment for renal artery ostial occlusive disease. J Vasc surg. 2009;49:667–674. doi: 10.1016/j.jvs.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 73.Bloch MJ, Pickering T. Renal Vascular Disease: Medical Management, Angioplasty and Stenting. Semin Nephrol. 2000;20:474–488. [PubMed] [Google Scholar]
- 74.Isles CG, Robertson S, Hill D. Management of renovascular disease: a review of renal artery stenting in ten studies. Q J Med. 1999;92:159–167. doi: 10.1093/qjmed/92.3.159. [DOI] [PubMed] [Google Scholar]
- 75.Main J. The problem with ASTRAL. J Renovascular Dis. 2002;1:19–23. [Google Scholar]
- 76.Nordmann AJ, Woo K, Parkes R, Logan AG. Balloon angioplasty or medical therapy for hypertensive patients with atherosclerotic renal artery stenosis? a meta-analysis of randomized controlled trials. Am J Med. 2003;114:44–50. doi: 10.1016/s0002-9343(02)01396-7. [DOI] [PubMed] [Google Scholar]
- 77.Rocha-Singh K, Jaff MR, Rosenfield K. Aspire-2 Trial Investigators: Evaluation of the safety and effectiveness of renal artery stenting after unsuccessful balloon angioplasty: the ASPIRE-2 study. J A C C. 2005;46:776–783. doi: 10.1016/j.jacc.2004.11.073. [DOI] [PubMed] [Google Scholar]
- 78.Zeller T, Rastan A, Rothenpieler U, Muller C. Restenosis after stenting of atherosclerotic renal artery stenosis: is there a rationale for the use of drug-eluting stents? Catheterization & Cardiovascular Interventions. 2006;68:125–130. doi: 10.1002/ccd.20773. [DOI] [PubMed] [Google Scholar]
- 79.Topol EJ, Yadav JS. Recognition of the importance of embolization in atherosclerotic vascular disease. Circulation. 2000;101:570–580. doi: 10.1161/01.cir.101.5.570. [DOI] [PubMed] [Google Scholar]
- 80.Modi KS, Rao VK. Atheroembolic renal disease. J Am Soc Nephrol. 2001;12:1781–1787. doi: 10.1681/ASN.V1281781. [DOI] [PubMed] [Google Scholar]
- 81.Hiramoto J, Hansen KJ, Pan XM, Edwards MS, Sawhney R, Rapp JH. Atheroemboli during renal artery angioplasty: an ex-vivo study. J Vasc surg. 2005;41:1026–1030. doi: 10.1016/j.jvs.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 82.Scolari F, Ravani P, Pola A, Guerini S, Zubani R, Movilli E, Savoldi S, Malberti F, Maiorca R. Predictors of renal and patient outcomes in atherembolic renal disease: a prospective study. J Am Soc Nephrol. 2003;14:1584–1590. doi: 10.1097/01.asn.0000069220.60954.f1. [DOI] [PubMed] [Google Scholar]
- 83.Cooper CJ, Haller ST, Colyer W, Steffes M, Burket MW, Thomas WJ, Safian R, Reddy B, Brewster P, Ankenbrandt MA, Vimnani R, Dippel E, Rocha-Singh K, Murphy TP, Kennedy DJ, Shapiro JI, D'Agostin RD, Pencina MJ, Khuder S. Embolic protection and platelet inhibition during renal artery stenting. Circulation. 2008;117:2752–2760. doi: 10.1161/CIRCULATIONAHA.107.730259. [DOI] [PubMed] [Google Scholar]
- 84.Chade AR, Mushin O, Zhu X, Rodriguez-Porcel M, Textor SC, Grande JP, Lerman A, Lerman LO. Pathways of renal fibrosis and modulation of matrix turnover in experimental hypercholesterolemia. Hypertension. 2005;46:772–779. doi: 10.1161/01.HYP.0000184250.37607.da. [DOI] [PubMed] [Google Scholar]
- 85.Bax L, Mali WP, Buskens E, Koomans HA, Beutler JJ. The benefit of STent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by Atherosclerotic ostial stenosis of the Renal Artery: the STAR study: rationale and study design. J Nephrol. 2003;16:807–812. [PubMed] [Google Scholar]
- 86.Uzzo RG, Novick AC, Goormastic M, Mascha E, Pohl M. Medical versus surgical management of atherosclerotic renal artery stenosis. Transplantation Proc. 2002;34:723–725. doi: 10.1016/s0041-1345(02)02623-4. [DOI] [PubMed] [Google Scholar]
- 87.Webster J, Marshall F, Abdalla M, Dominiczak A, Edwards R, Isles CG, Loose H, Main J, Padfield P, Russell IT, Walker B, Watson M. Randomised comparsion of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. J Hum Hypertens. 1998;12:329–335. doi: 10.1038/sj.jhh.1000599. [DOI] [PubMed] [Google Scholar]
- 88.Plouin PF, Chatellier G, Darne B, Raynaud A. Blood Pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Hypertension. 1998;31:822–829. doi: 10.1161/01.hyp.31.3.823. [DOI] [PubMed] [Google Scholar]
- 89.van Jaarsveld BC, Krijnen P, Pieterman H, Derkx FHM, Deinum J, Postma CT, Dees A, Woittiez AJJ, Bartelink AKM, Man in't Veld AJ, Schalekamp MADH for the Dutch Renal Artery Stenosis Intervention Cooperative Study Group. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. N Engl J Med. 2000;342:1007–1014. doi: 10.1056/NEJM200004063421403. [DOI] [PubMed] [Google Scholar]
- 90.Lerman LO, Chade AR. Angiogenesis in the kidney: a new therapeutic target? Curr Opin Nephrol Hyper. 2009;18:160–165. doi: 10.1097/MNH.0b013e32831ec1db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Textor SC. Renovascular Hypertension and Ischemic Nephropathy. In: Brenner BM, editor. Brenner and Rector's: The Kidney. chap 43. Philadelphia: 2008. pp. 1528–1566. [Google Scholar]