Abstract
Objective
To determine the association between the frequencies of pharmaceutical exposures reported to a poison control center (PCC) and those seen in the emergency department (ED).
Design
A statewide population-based retrospective comparison of frequencies of ED pharmaceutical poisonings with frequencies of pharmaceutical exposures reported to a regional PCC. ED poisonings, identified by International Classification of Diseases, Version 9 (ICD-9) codes, were grouped into substance categories. Using a reproducible algorithm facilitated by probabilistic linkage, codes from the PCC classification system were mapped into the same categories. A readily identifiable subset of PCC calls was selected for comparison.
Measurements
Correlations between frequencies of quarterly exposures by substance categories were calculated using Pearson correlation coefficients and partial correlation coefficients with adjustment for seasonality.
Results
PCC reported exposures correlated with ED poisonings in nine of 10 categories. Partial correlation coefficients (rp) indicated strong associations (rp>0.8) for three substance categories that underwent large changes in their incidences (opiates, benzodiazepines, and muscle relaxants). Six substance categories were moderately correlated (rp>0.6). One category, salicylates, showed no association.
Limitations
Imperfect overlap between ICD-9 and PCC codes may have led to miscategorization. Substances without changes in exposure frequency have inadequate variability to detect association using this method.
Conclusion
PCC data are able to effectively identify trends in poisonings seen in EDs and may be useful as part of a pharmaceutical poisoning surveillance system. The authors developed an algorithm-driven technique for mapping American Association of Poison Control Centers codes to ICD-9 codes and identified a useful subset of poison control exposures for analysis.
Background
Over the past decade, abuse of prescription medications in the USA has become a major public health issue. While signs of this emerging epidemic were evident as early as 1999, the extent of the problem has only recently become apparent.1 This delay is partly the result of inherent limitations of current data sources. Many national patient samples, such as the National Health Interview Survey and the National Hospital Ambulatory Medical Care Survey, have limited sample size and lack the power to provide information on local trends or specific pharmaceutical substances.2–5 Administrative datasets from healthcare organizations and mortality records are more complete than sample-based data sources, but are limited by the timeliness of their data collection, and the specificity and accuracy of their coding systems.2 6–8 When information from any of these sources becomes broadly available, public health researchers and policy makers, who rely on these data, are unavoidably responding to a situation that is at least 1 to 2 years old. A more timely, sensitive system for surveillance of pharmaceutical poisonings is needed.2
Emergency departments (EDs) serve as the front-line medical resource in caring for patients with serious poisonings; thus, ED data reflect much of the morbidity associated with pharmaceutical poisonings.9 ED data are recognized as a valuable source of information regarding poisonings, are used by existing surveillance systems,4 10 11 and have the potential to be a sensitive indicator of pharmaceutical poisoning trends. However, population-based data from EDs are not available in many states and are generally not capable of tracking changes in individual drugs due to the coarseness and inconsistencies of the International Classification of Diseases, Version 9 (ICD-9)/E-code system.2 12–14
In the USA, poison control centers (PCCs) collect data about pharmaceutical poisonings at both regional and national levels. PCCs affiliated with the American Association of Poison Control Centers (AAPCC) serve as an immediately available resource for people who have had toxic exposures and as an information resource for medical providers.15 Since 1985, the AAPCC has been collecting exposure data from its member PCCs through the National Poison Data System (NPDS).15 The advantages of the NPDS are that it collects data in real time and is capable of capturing data on specific substances. The NPDS has previously been used to develop automated surveillance systems to identify potential episodes of bioterrorism, food contamination, and new product hazards.16 PCC data may be useful as the basis of a surveillance system for pharmaceutical poisonings, but the data must be shown to be related to community morbidity.
Our objective was to determine whether state-level morbidity represented by ED visits for pharmaceutical poisonings is reflected in exposure data collected by a regional PCC. We hypothesized that the frequency of exposures to specific categories of pharmaceutical substances in the ED is correlated with calls to the regional PCC for these same categories.
Methods
Pharmaceutical exposure data from the Utah Poison Control Center (UPCC) were compared with a Utah population-based statewide dataset of ED visits. This study was approved by the Institutional Review Board of the University of Utah.
Healthcare databases
All ED and hospital inpatient discharge records for the state of Utah from 1998 to 2005 were acquired from the Utah Health Data Committee, Office of Healthcare Statistics. In Utah, licensed emergency departments and hospitals are mandated to report discharge information to the Utah Department of Health. A dataset was created that contained all Utah ED visits by merging the ED database containing records for all visits to Utah EDs where the patient was not subsequently admitted to an inpatient facility and a hospital discharge database containing records that flagged inpatient encounters whose admission source was the ED. This combined dataset was then limited to records with an ICD-9 code for a pharmaceutical poisoning and a Utah billing zip code. Demographic information, including name, age, sex, and patient zip code, is captured as well as up to nine ICD-9 codes and one or more external cause of injury codes (E-codes).
An ED visit for a pharmaceutical poisoning was defined by the presence of an ICD-9 code indicating Poisoning by Drugs, Medicinal or Biological Substance (960–979). Illicit drugs such as heroin and methamphetamine are classified as medicinal or biologic substances in the ICD-9 coding system and were included. Poisonings defined by ICD-9 codes for non-medicinal substances (980–989) and records containing E-codes indicating an adverse effect of therapeutic use of a pharmaceutical drug or device (E930–E949) were excluded.
Poison control database
Toxic substance exposure records for the years 1998 through 2005 were obtained from the UPCC, which serves the population of Utah and adjoining regions in the Intermountain West. This study was restricted to Utah callers. The UPCC database comprises telephone calls from both laypersons and healthcare providers. The UPCC consolidates multiple telephone calls referring to the same patient exposure into one record. For each call, patient characteristics including name, age, sex, and location, and exposure details including type and quantity of substance(s), and timing of the exposure are collected. Callers with potentially serious exposures are referred to a local healthcare facility for evaluation. With each referral, the UPCC notifies the receiving medical facility, provides the clinicians with information and advice, and records the patient's outcome. Most UPCC callers are reporting toxic exposures in another person; for this paper, caller characteristics and exposures refer to the exposed person.
Substance exposure data are collected using standard methodology established by the AAPCC for the NPDS. Exposures are categorized using a two-level classification scheme implemented nationwide by member PCCs. When available, the exact pharmaceutical formulation is coded using the Thompson/Micromedex POISINDEX system. All poisonings are then assigned another less specific AAPCC code.15 When an individual is exposed to multiple drugs, each substance is recorded as a separate exposure. For this analysis, multidrug drugs (eg, hydrocodone/acetaminophen) were recoded as an exposure to each component substance.
Category mapping
The ICD-9 codes used by the ED to document pharmaceutical poisonings do not correspond to AAPCC codes on a one-to-one basis. Therefore, we developed an algorithm-driven technique for mapping pharmaceutical codes used in the poison control dataset to ICD-9 codes used in the ED dataset. First, all ICD-9 codes representing pharmaceutical poisonings (960–979) were grouped into substance categories that generally represented four-digit ICD-9 codes. The 10 most common categories of pharmaceutical poisonings identified in the ED dataset were selected for comparison to the UPCC (table 1). Miscellaneous categories (eg, 969.9: other unspecified psychotropic agent) were not considered for comparison.
Table 1.
Ten most common substance categories in emergency department exposures
| Substance category | N (%) |
| Benzodiazepines | 7665 (12.0%) |
| Antidepressants | 6637 (10.4%) |
| Opiates | 6266 (9.8%) |
| Aromatic analgesics (including acetaminophen) | 5965 (9.3%) |
| Stimulants | 3980 (6.2%) |
| Non-steroidal anti-inflammatory drugs | 3534 (5.5%) |
| Antiallergics and antiemetics | 2913 (4.6%) |
| Salicylates | 2410 (3.8%) |
| Muscle relaxants | 2170 (3.4%) |
| Anticonvulsants | 1555 (2.4%) |
Each AAPCC code was mapped into an ICD-9 based substance category in a stepwise fashion. First, AAPCC codes that were an exact match with an ICD-9 code (eg, 007000: benzodiazepines) were assigned to that substance category. Next, AAPCC codes that corresponded to a substance found in the ICD-9 Table of Drugs and Chemicals were assigned directly to the same substance category as the corresponding ICD-9 code.
There was a set of AAPCC codes that could not be assigned to the ICD-9 based substance categories either by direct match to an ICD-9 code or by looking up the substance in the Table of Drugs and Chemicals. In order to categorize these substances, we used a dataset of records linked by patient factors as described below (ED to UPCC). Using these records, we were able to determine how this set of AAPCC codes corresponded to ICD-9 codes in the ED dataset. To ensure that the correct substances were being compared between datasets, only ICD-9 and AAPCC codes of patients who had a single substance exposure recorded in each dataset were compared. The AAPCC code could then be assigned to the same substance category as the most commonly associated ICD-9 code. A minimum of 20 linked records was required for each assignment to guard against coding errors and occasional incorrect linkages.
The linkage of ED and UPCC patient records was performed using probabilistic linkage, a technique in which records in separate databases are determined to refer to the same person or event using variables common to both datasets.17 Where present, the following variables were used for the linkage: last name, first name, gender, age, arrival date/call date, presenting ED/referral facility, and postal code. The linkage also utilized flags indicating the presence of a hospitalization, poisoning, or death. Since the relationship of AAPCC codes to ICD-9 codes was of interest, individual codes were not used to determine linkage.
Two toxicologists independently reviewed the codes assigned by the linkage, as well as the assignment of ICD-9 codes to the 10 most common categories of ED pharmaceutical poisonings.
Selection of a comparison subset
A substantial fraction of callers to PCCs inquire about low-toxicity exposures not necessitating evaluation in the ED.15 We hypothesized that a subset of UPCC callers would better represent patients seen in the ED for pharmaceutical poisonings. In a surveillance system, this subset would be expected to better model clinically significant poisoning trends. Ideally, the patients in the subset would reflect the age and sex distribution of the ED population and retain most pharmaceutical poisoning exposures that require urgent medical evaluation. Three non-exclusive subsets of UPCC callers were created a priori for consideration: (1) patients referred for medical evaluation by the UPCC or whose calls originated from a healthcare facility (high-risk subset); (2) patients with age >12 years (adolescent/adult subset); and (3) patients coded by the UPCC as suicidal or intentionally abusing substances (intentional subset). All UPCC callers (the reference group) and each subset were compared to ED patients on the basis of sex distribution, age ranges, and exposure type. These patient features were chosen for comparison because they illustrate important differences between the poison control reference population, poison control subsets, and ED patients. For example, exposures nationally reported to poison control have a slight male preponderance, while a substantial majority of ED patients with pharmaceutical poisonings in Utah are female.16 18
Statistical analysis
All UPCC callers (reference group) and each of the three subsets of UPCC callers were compared to the ED patients with pharmaceutical poisonings by examining the percent in each age category (<6 years old, 6–12 years old, 13–19 years old, and 20+ years old), sex, and proportion exposed to one of the substance categories in table 1. χ2 Tests were used to evaluate categorical variables.
For each substance category, Pearson correlation coefficients were calculated between quarterly ED exposures and quarterly poison control exposures in the high-risk subset. To account for seasonal fluctuations in calls to the UPCC and visits to the ED, partial correlation coefficients, adjusted for season, were calculated for each substance category. Graphical and numeric residual diagnostics were evaluated for each model.
Over the study period, ED visits and calls to the UPCC have risen in concert.19 20 Because relationships could be inferred solely on the basis of increased medical service utilization, a secondary analysis was performed to ensure any associations found were not due to population growth alone. Each quarterly frequency was converted into a proportion consisting of the number of exposures to an individual substance category divided by the number of exposures in all 10 substance categories from table 1. As above, each proportion from the poison control high-risk subset was compared to the ED data by quarter.
Statistical analyses were performed using SAS 9.2.
Results
From March 21st, 1998, to December 20th, 2005, there were 48 693 patient visits to Utah emergency departments due to poisoning by pharmaceutical substances. As multiple substances are often ingested concomitantly, these encounters represented 63 807 pharmaceutical exposures. Over the same time period, the UPCC reported 134 175 telephone calls for pharmaceutical poisonings representing 164 987 exposures.
ICD-9 codes from the ED database were assigned to 92 distinct substance categories. The 10 most frequent substance categories represented 67.5% (n=43 095) of the total ED pharmaceutical exposures. Counts and frequencies for these categories are shown in table 1. Of the 48 693 patient visits to the emergency department for a pharmaceutical poisoning, 34 482 (70.8%) involved an exposure to at least one of these substance categories.
Code mapping
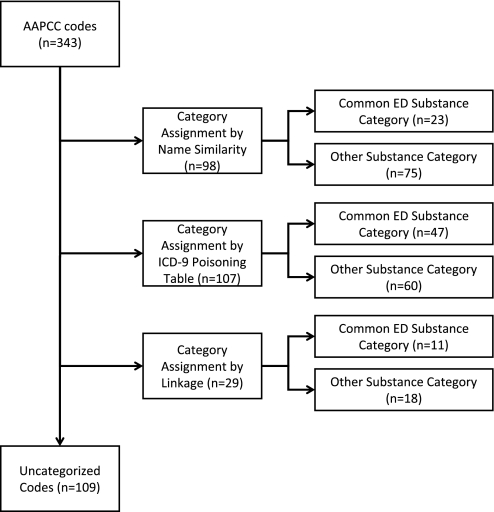
Of 398 AAPCC pharmaceutical codes, 55 represented combination drugs. An exposure to one of these combinations was recoded as separate exposures to the component parts. The assignment algorithm was applied to the remaining 343 codes. Successful mapping of 234 codes (68.2%) representing 93.7% of exposures was achieved: 205 codes (59.8%) were mapped based on name similarity or by a match in the ICD-9 poisoning table; 29 codes (8.4%) were mapped via the linkage mechanism (figure 1). The remaining 109 AAPCC codes (31.8%) represented 6.3% of poison control exposures and were largely from miscellaneous categories or rare exposures such as 77160: Other Topical Antiseptic.
Figure 1.
American Association of Poison Control Centers (AAPCC) codes, mapped into one of 92 International Classification of Diseases, Version 9 (ICD-9) based substance categories using a stepwise algorithm as shown above. The 10 most common categories of exposures in the emergency department (ED; table 1) were used for the analysis and are labeled ‘Common ED Substance Category’; the remainder are labeled ‘Other Substance Category.’
A total of 81 AAPCC codes were mapped into the substance categories listed in table 1: 23 (28.4%) were assigned by name similarity, 47 (58.0%) were assigned by ICD-9 poisoning tables, and 11 (13.6%) were assigned based on the linkage (figure 1; also see Appendix A, available as an online data supplement at www.jamia.org/). On review of the linkage assigned codes, there was no disagreement between the two toxicologists. In each instance, the category assignment based on the linkage was felt to be the best possible selection.
Poison control subset selection
A total of 134 175 UPCC calls comprised the reference group: 35 361 (26.4%) were in the high-risk subset; 43 067 (32.1%) were in the adolescent/adult subset; and 19 651 (14.7%) were in the intentional subset. Of the reference group, 78 764 (58.7%) callers did not belong to any subset. Over 99% of the exposures to callers not included in a subset resulted in no effects or minimal effects, or were judged by the UPCC to be non-toxic.
We compared the distributions of age, sex, and proportion of exposures to the commonly identified ED substance categories (table 1) between ED patients, all UPCC callers, and three subsets of UPCC callers (table 2). UPCC callers were more frequently under 13 years old than ED patients (65%, 15% respectively). Age distributions for the high-risk and intentional subsets were more similar to ED visits, with 30% and 2% of callers under 13 years old. The adolescent/adult subset did not contain children under 13 years old by definition. The gender distributions of all UPCC callers and the three subsets were similar to the ED patients. Pharmaceutical exposures reported by the three subsets of UPCC callers were more similar to exposures of the ED patients than exposures reported by the all callers group.
Table 2.
Frequency of age, sex, and exposures in the emergency department (ED), the poison control reference group, and three subgroups of poison control callers
| ED | Poison control center callers† | ||||
| All callers (reference group) | High-risk | Adolescent/adult | Intentional | ||
| Age | |||||
| <6 | 6743 (13.8%) | 81 539 (60.8%) | 9555 (27.0%) | 0 (0%) | 29 (0.1%) |
| 6–12 | 660 (1.4%) | 6309 (4.7%) | 905 (2.6%) | 0 (0%) | 286 (1.5%) |
| 13–19 | 10 464 (21.5%) | 11 188 (8.3%) | 7356 (20.8%) | 11 188 (24.2%) | 6686 (34.0%) |
| 20+ | 30 637 (62.9%) | 31 879 (23.8%) | 15 907 (45.0%) | 31 879 (68.8%) | 11 305 (57.5%) |
| Unknown | 189 (0.4%) | 3260 (2.4%) | 1638 (4.6%) | 3260 (7.0%) | 1345 (6.8%) |
| Sex | |||||
| Female | 23 502 (60.6%) | 70 552 (52.6%) | 20 565 (58.2%) | 29 420 (63.5%) | 12 374 (63.0%) |
| Male | 18 890 (38.8%) | 63 412 (47.3%) | 14 676 (41.5%) | 16 719 (36.0%) | 7180 (36.5%) |
| Missing | 301 (0.6%) | 211 (0.2%) | 120 (0.3%) | 188 (0.4%) | 97 (0.5%) |
| Common ED substance* | |||||
| No | 14 211 (29.2%) | 75 886 (56.6%) | 12 782 (36.1%) | 19 904 (43.0%) | 4722 (24.0%) |
| Yes | 34 482 (70.8%) | 58 289 (43.4%) | 22 579 (63.9%) | 26 423 (57.0%) | 14 929 (76.0%) |
The proportion of total exposures in each subgroup due to one of the 10 most common categories of exposures in the ED (categories listed in table 1).
All comparisons between the ED group and each poison control group differ significantly (p<0.001).
Because no caller subset was clearly superior to the others in terms of demographic or exposure similarity to the ED patients, the high-risk caller subset was chosen for comparison to the ED dataset. This choice allowed the inclusion of pediatric poisonings while eliminating most callers with non-toxic or low-risk exposures. While the intentional subset also tended to have exposures to substances frequently seen in the ED, it was smaller than the other two groups and would have resulted in substantively less data for analysis.
Correlation of exposures between ED and UPCC
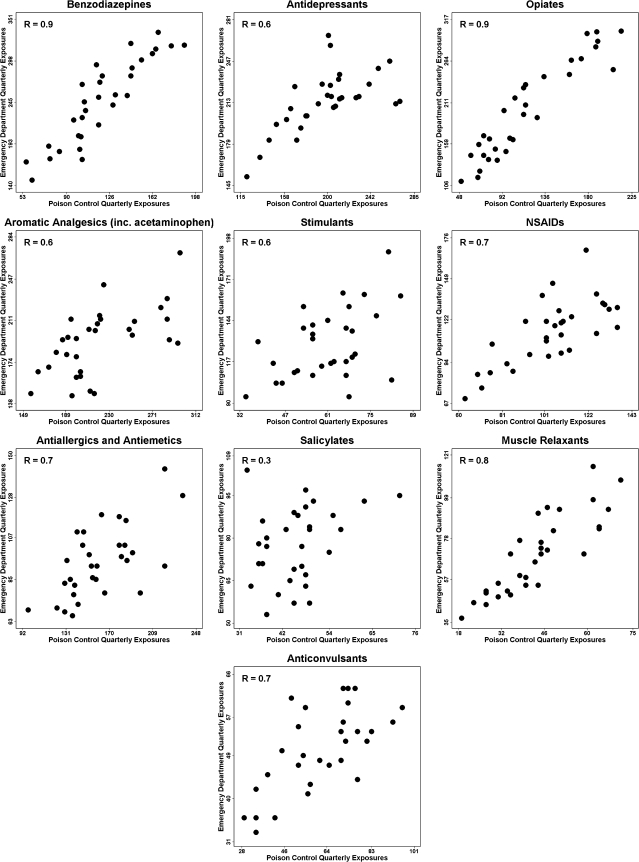
Table 3 displays the Pearson correlation coefficients for the comparison of quarterly frequency of poison control exposures to ED exposures by substance category. All categories except salicylates had statistically significant correlations. In figure 2, the scatter plots of quarterly PCC exposures are compared with quarterly ED exposures among high-risk callers. Coefficients adjusted for the effect of season on the frequency of exposures were calculated using partial correlation coefficients (rp). Significant associations (p<0.001) were seen for all substances except salicylates (rp=0.292, p=0.290). Three substance categories, opiates (rp=0.929), benzodiazepines (rp=0.895), and muscle relaxants (rp=0.827) were strongly associated in the two datasets. Antidepressants, aromatic analgesics, stimulants, NSAIDs, antiallergics and antiemetics, and anticonvulsants showed moderate associations (rp=0.608–0.716). Residual diagnostics including residuals over time provided no evidence of heteroscedasticity, non-normality, or time dependence.
Table 3.
Correlation of poison control exposures to emergency department exposures by quarter
| Substance category | Pearson correlation coefficient | Partial correlation coefficient* |
| Benzodiazepines | 0.895 | 0.895 (<0.0001) |
| Antidepressants | 0.647 | 0.655 (<0.0001) |
| Opiates | 0.929 | 0.929 (<0.0001) |
| Aromatic analgesics | 0.616 | 0.618 (<0.001) |
| Stimulants | 0.609 | 0.608 (<0.001) |
| Non-steroidal anti-inflammatory drugs | 0.729 | 0.716 (<0.0001) |
| Antiallergics and antiemetics | 0.652 | 0.648 (<0.001) |
| Salicylates | 0.292 | 0.290 (0.1197) |
| Muscle relaxants | 0.827 | 0.827 (<0.0001) |
| Anticonvulsants | 0.670 | 0.670 (<0.0001) |
After adjustment for season with p values in parentheses.
Figure 2.
Scatter plots of poison control exposures versus emergency department exposures by quarter. NSAIDs, non-steroid anti-inflammatory drugs.
In order to account for the concurrent increase in PCC calls and ED visits over time, each substance category was considered as a proportion of the total exposures to the substances listed in table 1.The association between the UPCC and ED proportional exposures remained significant for all substance categories except anticonvulsants (table 4).
Table 4.
Correlation of proportional poison control exposures to emergency department exposures by quarter
| Substance category | Pearson correlation coefficient | Partial correlation coefficient* |
| Benzodiazepines | 0.730 | 0.730 (<0.001) |
| Antidepressants | 0.489 | 0.505 (0.005) |
| Opiates | 0.870 | 0.869 (<0.001) |
| Aromatic analgesics | 0.393 | 0.386 (0.035) |
| Stimulants | 0.603 | 0.618 (<0.001) |
| Non-steroidal anti-inflammatory drugs | 0.713 | 0.672 (<0.001) |
| Antiallergics and antiemetics | 0.554 | 0.532 (0.003) |
| Salicylates | 0.551 | 0.552 (0.002) |
| Muscle relaxants | 0.639 | 0.639 (<0.001) |
| Anticonvulsants | 0.272 | 0.271 (0.147) |
After adjustment for season with p values in parentheses.
Discussion
In this study, a subset of callers to a regional PCC reflected community pharmaceutical poisoning morbidity as measured by ED visits. Pharmaceutical exposures from an easily identifiable subset of poison control callers were significantly associated with the frequency of poisonings by these same drugs in patients treated in the ED. Significant associations in the frequency of exposures between the UPCC and the ED existed for nine of the top 10 types of drugs responsible for ED visits due to poisoning. Our results suggest that PCC exposure data can be used to construct a real-time pharmaceutical poisoning surveillance system.
Poison control data are attractive as a potential surveillance system due to the large volume of available data and the timeliness of the collection system. The use of trained poison information specialists to document calls presents an opportunity for high specificity and granularity within the data. However, the utility of the NPDS has been questioned because of perceived data limitations.
Previous research examining the relationship of PCC data to community data suggests that poison control exposure records do not correlate well with other indicators of pharmaceutical poisonings.12 21–23 However, unlike our study, prior studies only examined fatal poisonings. Surveillance of pharmaceutical poisonings using only death registries may misrepresent community morbidity, since only a small fraction of poisoning patients die from their exposure. Surveillance using death registries alone also shifts the focus away from substances that contribute substantially to the morbidity of poisoning but are rarely fatal.
In contrast, we found that a selection of UPCC data reflected ED morbidity for the most common pharmaceutical poisonings. We found significant associations between nine of the 10 substance categories examined. One of these categories, opiates, has been largely responsible for the increase in fatalities from prescription drug abuse.24 In our analysis, a strong positive association was seen in the frequency of poisonings from opiates between the PCC and the ED. Exposures from another drug category prone to abuse, benzodiazepines,25 were also strongly correlated. The only category not to display significant correlation was salicylates. This category did not undergo a large change in the frequency of exposures and therefore may not have contained enough variability for statistical detection.
One concern of using PCC data for surveillance is that the high volume of low-toxicity, low-morbidity calls15 could influence a surveillance system's ability to detect clinically important poisoning trends. To screen out these calls, we explored three UPCC caller subsets. The UPCC caller subset that was referred for medical evaluation provided both similar demographic and exposure characteristics to the ED patients and enough data for a substance category comparison on a statewide basis. This ‘high-risk’ group was readily identified from the UPCC data fields; thus, this group's exposure information could be easily selected for use in a surveillance system.
One advantage of PCC data is their potential sensitivity to regional trends. Existing sample-based datasets, such as the National Hospital Ambulatory Medical Care Survey and National Health Interview Survey, could be used to examine poisoning trends over time.2–4 26 However, these systems cannot detect regional changes or changes within individual categories of substances. Much of the responsibility for developing public health policy and legislation to prevent poisonings is at the state and local levels making these systems less helpful. The problem of prescription drug abuse varies widely between states and regions.27 28 A system capable of identifying regional trends would have key advantages in influencing policy. In our study, we were able to show that associations can be made for individual substance categories in a state with a population of 2.2 million people.29 As of 2006, the entire population of the USA is served by regional PCCs submitting data to the NPDS,15 making this a potentially comprehensive surveillance system for the USA.
According to the Centers for Disease Control and Prevention's Framework for Program Evaluation in Public Health, there are nine key attributes to be considered when evaluating a surveillance system: simplicity, flexibility, data quality, acceptability, sensitivity, positive predictive value, representativeness, timeliness, and stability.30 PCC exposure data have inherent advantages over current sample-based and administrative systems in terms of three of these attributes: timeliness, flexibility, and acceptability. While the system is complex due to its nationwide scope and mission, its fundamental structure and function are easily understandable. To date, most questions about using the NPDS for pharmaceutical poisoning surveillance have centered on concerns about sensitivity, positive predictive value, and representativeness. Our study makes a significant contribution toward addressing these issues.
Limitations and strengths
There are several limitations to this study. The primary limitation was the low granularity and lack of specificity of the ICD-9 coding system for pharmaceutical exposures. Approximately 12.6% of ED visits were coded as ‘unspecified poisoning.’ These visits likely contained some exposures that could have been included in our substance categories. While the poor specificity of the ICD-9 coding system was the primary limiting factor in the granularity of our analysis, limitations of the AAPCC coding schema also exist. Unlike the ICD-9 system, the AAPCC coding schema has not been widely validated. While the poison control data have the advantage that exposures can be classified very finely using POISINDEX codes (Thompson/Micromedex), many exposures are recorded using the more generic AAPCC codes. These generic codes are not uniformly based on ingredient-level data and often represent entire classes of medications; as a result, some information available to the PCC specialist is lost. To be used effectively as part of a surveillance system, the AAPCC coding system would need to permit ingredient-level coding for substances, even when the manufacturer and formulation are unknown. Ideally, PCCs and EDs would code exposures based on a multilevel pharmaceutical taxonomy that permits use of granular ingredient-level data when available but is capable of accepting less granular information as needed. To minimize the loss of information in this process and promote data comparisons, a robust pharmaceutical ontology linked to a national medication standard such as RxNorm would be the most effective.31
We attempted to overcome some of these limitations by using an assignment algorithm augmented with information gained from a probabilistically linked dataset. While the majority of AAPCC codes were assigned using name similarity and ICD-9 poisoning tables, a small percentage of AAPCC codes required information from linkage. Despite the advantages of this technique, in some cases AAPCC codes and ICD-9 codes represented imperfectly overlapping sets of drugs, and some misclassification may have occurred.
Another potential limitation is that substance categories which have stable underlying community poisoning rates would be expected to have inadequate variability to detect an association using our analysis technique. While this problem is a limitation of our study, it should not limit the ability of a surveillance system to detect significant changes in exposure frequency.
These data represent only one state which potentially limits generalizability. Most poison control centers have adopted a uniform software system for assisting with the classification of poisonings; however, regional differences in PCC coding practices may exist.32 Any surveillance system developed would need to keep these possible regional differences in mind.
Finally, fatalities are likely under-represented in these data. While there is substantial overlap between substances causing morbidity and substances causing mortality, there are also significant differences. A surveillance system that combines both morbidity and mortality data would be stronger.
Strengths of this study include the utilization of population-based statewide datasets to compare pharmaceutical poisonings between a regional PCC and EDs. This is the first population-based study to evaluate the association between emergency department exposures and calls to a PCC. Our ability to detect significant associations at the state level suggests the feasibility of regional substance-specific trending.
Second, we developed an empirical system for mapping AAPCC codes to ICD-9 codes in which difficult-to-assign codes were resolved using probabilistic linkage and a defined algorithm. By including in our analysis the 10 most common exposures seen in the ED instead of an individually selected set of substances, we evaluated a representative picture of clinically important poisonings.
Finally, we identified a subset of poison control callers that would be potentially useful in the construction of a surveillance system by excluding many exposures that rarely contribute to community morbidity.
Conclusion
In this study, we have shown that a select subset of poison control center data is able to effectively identify trends in poisonings seen in emergency departments. This suggests that poison control center exposure data may be useful as part of a pharmaceutical poisoning surveillance system.
Acknowledgments
We wish to thank B Crouch, G Oderna, and the Utah Poison Control Center for their assistance in making this project possible.
Footnotes
Competing interests: None.
Ethics approval: This study was conducted with the approval of the University of Utah.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Centers for Disease Control and Prevention (CDC) Unintentional poisoning deaths—United States, 1999–2004. Morb Mortal Wkly Rep 2007;56:93–6 [PubMed] [Google Scholar]
- 2.Guyer B, Mavor A; Institute of Medicine Committee on Poison Prevention and Control Forging a poison prevention and control system: report of an Institute of Medicine committee. Ambul Pediatr 2005;5:197–200 [DOI] [PubMed] [Google Scholar]
- 3.Middleton K, Hing E, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 outpatient department summary. Adv Data 2007;389:1–34 [PubMed] [Google Scholar]
- 4.Nawar EW, Niska RW, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv Data 2007;386:1–32 [PubMed] [Google Scholar]
- 5.Adams PF, Barnes PM, Vickerie JL. Summary health statistics for the US population: National Health Interview Survey, 2007. Vital Health Stat 10 2008;238:1–104 [PubMed] [Google Scholar]
- 6.Flanagan RJ, Rooney C. Recording acute poisoning deaths. Forensic Sci Int 2002;128:3–19 [DOI] [PubMed] [Google Scholar]
- 7.Langley J, Stephenson S, Thorpe C, et al. Accuracy of injury coding under ICD-9 for New Zealand public hospital discharges. Inj Prev 2006;12:58–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13 2007;165:1–209 [PubMed] [Google Scholar]
- 9.Corso P, Finkelstein E, Miller T, et al. Incidence and lifetime costs of injuries in the United States. Inj Prev 2006;12:212–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control Web-based Injury Statistics Query and Reporting System (WISQARS). http://www.cdc.gov/ncipc/wisqars (accessed 19 Feb 2008).
- 11.Prosser JM, Perrone J, Pines JM. The epidemiology of intentional non-fatal self-harm poisoning in the United States: 2001–2004. J Med Toxicol 2007;3:20–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoppe-Roberts JM, Lloyd LM, Chyka PA. Poisoning mortality in the United States: comparison of national mortality statistics and poison control center reports. Ann Emerg Med 2000;35:440–8 [PubMed] [Google Scholar]
- 13.Coben JH, Steiner CA, Barrett M, et al. Completeness of cause of injury coding in healthcare administrative databases in the United States, 2001. Inj Prev 2006;12:199–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overview of the State Emergency Department Databases (SEDD) http://www.hcup-us.ahrq.gov/seddoverview.jsp (accessed 1 Dec 2010).
- 15.Bronstein AC, Spyker DA, Cantilena LR, Jr, et al. 2006 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS). Clin Toxicol (Phila) 2007;45:815–917 [DOI] [PubMed] [Google Scholar]
- 16.Watson WA, Litovitz TL, Belson MG, et al. The Toxic Exposure Surveillance System (TESS): Risk assessment and real-time toxicovigilance across United States poison centers. Toxicol Appl Pharmacol 2005;207(2 Suppl):604–10 [DOI] [PubMed] [Google Scholar]
- 17.Cook LJ, Knight S, Junkins EP, Jr, et al. Repeat patients to the emergency department in a statewide database. Acad Emerg Med 2004;11:256–63 [DOI] [PubMed] [Google Scholar]
- 18.IBIS-PH, Utah Department of Health http://ibis.health.utah.gov/query/result/ed/InjEDCntyHospED/Count.html (accessed 29 Dec 2009).
- 19.Utah Poison Control Center Annual Reports, University of Utah http://uuhsc.utah.edu/poison/ (accessed 29 May 2009).
- 20.Utah Emergency Department Utilization and Charge Profile Reports 1996–2005, Utah Bureau of Emergency Medical Services. http://health.utah.gov/ems/data/er/ (accessed 29 May 2009).
- 21.Soslow AR, Woolf AD. Reliability of data sources for poisoning deaths in Massachusetts. Am J Emerg Med 1992;10:124–7 [DOI] [PubMed] [Google Scholar]
- 22.Linakis JG, Frederick KA. Poisoning deaths not reported to the regional poison control center. Ann Emerg Med 1993;22:1822–8 [DOI] [PubMed] [Google Scholar]
- 23.Blanc PD, Kearney TE, Olson KR. Underreporting of fatal cases to a regional poison control center. West J Med 1995;162:505–9 [PMC free article] [PubMed] [Google Scholar]
- 24.Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf 2006;15:618–27 [DOI] [PubMed] [Google Scholar]
- 25.Drug Abuse Warning Network Substance Abuse and Mental Health Services Administration (SAMHSA), Office of Applied Studies. http://dawninfo.samhsa.gov/ (accessed 14 May 2009).
- 26.Pleis JR, Lucas JW. Summary health statistics for US adults: National Health Interview Survey, 2007. Vital Health Stat 10 2009;240:1–159 [PubMed] [Google Scholar]
- 27.Huang B, Dawson DA, Stinson FS, et al. Prevalence, correlates, and comorbidity of nonmedical prescription drug use and drug use disorders in the United States: Results of the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry 2006;67:1062–73 [DOI] [PubMed] [Google Scholar]
- 28.Manchikanti L. National drug control policy and prescription drug abuse: facts and fallacies. Pain Physician 2007;10:399–424 [PubMed] [Google Scholar]
- 29.US Census Bureau 2000 population estimates. http://quickfacts.census.gov/qfd/states/49000.html (accessed 17 Mar 2009).
- 30.German RR, Lee LM, Horan JM, et al. ; Guidelines Working Group Centers for Disease Control and Prevention (CDC) Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Recomm Rep 2001;50:1–35; quiz CE1-7. [PubMed] [Google Scholar]
- 31.RxNorm documentation, NLM. http://www.nlm.nih.gov/research/umls/rxnorm/index.html (accessed 22 Feb 2010).
- 32.Toxicall© http://www.cas-co.com/products/toxicall/index.htm (accessed 3 Dec 2010).