Abstract
BACKGROUND
Characterizing short-term detection patterns of young women’s incident alpha-genus human papillomavirus (HPV) infections may further understanding of HPV transmission.
METHODS
Between 2000–2007, we followed 18–22 year old female university students with triannual HPV DNA and Papanicolau testing. Using Kaplan-Meier methods, we estimated: duration of detectable, type-specific incident infections; time to re-detection (among infections that became undetectable); and time to cervical lesion development after incident infection. We evaluated risk factors for short-term persistent versus transient infection with logistic regression.
RESULTS
303 incident type-specific infections were detected in 85 sexually active women. Median time to first negative test after incident infection was 9.4 (95%CI:7.8–11.2) months; 90.6% of infections became undetectable within two years. 19.4% of infections that became undetectable were re-detected within one year. Cervical lesions were common, and 60% were positive for multiple HPV types in concurrent cervical swabs. Incident HPV detection in the cervix only (versus the vulva/vagina only or both sites) was associated with short-term transience.
CONCLUSIONS
While most incident infections became undetectable within two years, re-detection was not uncommon. Cervical lesions were a common early manifestation of HPV infection.
IMPACT
It remains unclear whether potentially modifiable risk factors can be identified to reduce infection duration (and transmission likelihood).
Keywords: human papillomavirus, incidence, duration, persistence, women, epidemiology
INTRODUCTION
While it is well-established that long-term persistent infection with high-risk human papillomavirus (HPV) is necessary for the development of cervical cancer (1), our earlier work suggests that sporadic detection in the early course of an HPV infection is not incompatible with long-term persistence (2). While early short-term detection patterns may be unrelated to the risk of carcinogenic progression, characterizing the early course of new HPV infections may further our understanding of infectivity and provide estimates that can be used in models of HPV transmission. Assuming a positive relationship between persistent HPV detection and duration of infectivity, it is reasonable to surmise that repeat HPV detection suggests a longer window of infectivity and therefore a greater likelihood of transmission to new sex partners (based on the positive relationship between duration of infectivity and the reproductive rate of an infection (3)). Therefore, by identifying risk factors for repeat HPV detection, it is possible to identify potentially modifiable behavioral risk factors for HPV transmission.
The goal of the present study was to characterize the early natural history of incident, type-specific HPV infections. By enrolling a cohort of newly sexually active young women with minimal prior exposure to HPV, we were able to maximize the likelihood of detecting (and following) truly incident infections. The aims of the study were to describe detection patterns of newly acquired infections; to identify both behavioral and viral determinants of repeat versus transient detection in the first eight months of an infection; and to estimate the incidence of low-grade cervical squamous intraepithelial lesions (SILs) following incident HPV infection.
METHODS
Study population and design
From December 2000 to March 2007, we enrolled 18 to 22 year old female University of Washington students into a longitudinal study of HPV infections. Recruitment and data collection procedures were described previously (4). Women were eligible to enroll if they had never had vaginal intercourse with a male partner or had their first intercourse with one male partner within the past three months. We mailed 34,079 letters of invitation to women who met the age criterion and released their names to the registrar. Of 290 women who responded, 250 were enrolled. All participants provided informed consent, and the protocol was approved by University of Washington’s Institutional Review Board.
Every 2 weeks, women completed a Web-based diary designed to capture daily sexual behavior information. Detailed diary features have been described elsewhere (4, 5). Every 4 months, women were followed with clinical visits (4). At each visit, self-collected vaginal swabs were collected for HPV DNA testing. Following the self-collection, the nurse practitioner performed a standardized pelvic examination. Separate cervical and vulvar/vaginal swabs were collected with Dacron-swabs into specimen transport medium for HPV DNA testing. For Papanicolaou (Pap) testing, a cytobrush was used to collect cells from the endocervix and a plastic spatula was used to collect cells from the squamocolumnar junction and ectocervix. All women with cytologic or colposcopic evidence of a high-grade squamous intraepithelial lesion (SIL) were referred for colposcopically-directed biopsy. Women with repeated cytologic test results showing a low-grade SIL or atypical squamous cells of undetermined significance (ASCUS) were also referred.
Laboratory Methods
Specimens were tested for HPV DNA using polymerase chain reaction (PCR)-based methods. DNA was isolated using the QIAamp DNA blood mini column (Qiagen, Inc., Valencia, CA) according to the manufacturer’s protocol. One two-hundred-fiftieth of each sample was amplified and ten microliters of PCR products were dotted onto nylon filters and probed with a biotin-labeled generic probe. As described previously, specimens testing positive by a generic probe were typed using a reverse line-blot assay (Roche Molecular Systems) for 37 HPV types (4). Unless otherwise noted, PCR results for self-collected vaginal swabs and clinician-collected cervical and vulvar/vaginal swabs were combined to analyze duration of type-specific genital HPV infections.
Variant specificity was determined on pairs of type-specific positive samples separated by at least one intercurrent negative test. Only high- or probable high-risk infections (HPV types 16/18/26/31/33/35/39/45/51/52/53/56/58/59/66/68/73/82/IS39 (6)) or HPV-6 infections were selected for variant characterization. Sequence analysis of the E6 gene was performed using type-specific primers. The PCR product of HPV16 E6 was cloned using a TOPO TA Cloning® Kit (Invitrogen). Clones containing target inserts were identified by digestion with appropriate restriction enzymes followed by electrophoreses on 1.5% agarose gel. Three clones per sample were selected for sequencing. Plasmid DNAs were purified with a QIAprep Spin Miniprep Kit (Qiagen, Valencia, CA). The purified DNA templates were sequenced by Functional Biosciences (Functional Biosciences, Madison, WI). Sequence variations that were found at least twice were counted as variants and those found only once were counted as potential PCR errors. If the sequence of the variant in the first sample showed 100% sequence identity when aligned with the variant in the second sample, it was considered to represent a single persistent infection. If available, the last positive type-specific sample prior to the first intercurrent negative was selected for variant testing. Otherwise, an earlier type-specific positive sample was selected. Likewise, the first type-specific re-detection was selected for variant characterization. If not available, a subsequent type-specific positive sample was selected. Given previous findings that individual pairs of cervical and vulvar/vaginal samples were positive for identical HPV-16 variants (Long Fu Xi, personal communication), sequence analysis for a given HPV type was performed on only one genital sample from a given visit.
A cytotechnologist examined all Pap smears and the pathologist reviewed all abnormal smears. Findings were classified according to the Bethesda system (7) as normal, ASCUS, low-grade SIL, or high-grade SIL. Biopsy tissue was diagnosed as showing cervical intraepithelial neoplasia (CIN) grade 1, 2, or 3. None of the women developed cytologic or histologic evidence of invasive cervical cancer.
Statistical Methods
Incident HPV infection was defined as the first positive result for a specific HPV type (in either the cervical sample, the vulvar/vaginal sample, or the self-collected vaginal sample). Kaplan-Meier methods were used to estimate the duration of detectable type-specific HPV infections after incident detection. At-risk time was calculated from the date of incident type-specific detection to the first negative test for that type. Infections were censored at the last visit date or date of treatment for CIN 2+. To account for correlation within subjects due to the assessment of multiple potential HPV types per individual, a 95% confidence interval (CI) for the median failure time was computed using percentile bootstrap methods with 1,000 repetitions. Kaplan-Meier methods were also used to estimate time to HPV re-detection; at-risk time was calculated from the date of the first negative test (after incident detection) to the first subsequent re-detection of that type.
Logistic regression was used to evaluate risk factors for repeatedly detected incident type-specific HPV infections. Cases were defined as incident type-specific infections immediately followed by at least two consecutive positive visits (i.e. positive for at least 8 months) (+++). Controls were defined as incident type-specific infections immediately followed by two consecutive negative visits (+−−).
Potential predictors of repeatedly-detected infections included variables assessed at the time of incident infection and variables assessed in the time interval between incident detection and the subsequent 4-month visit. Variables assessed at the time of incident detection included: site of incident infection (cervix only, vulva/vagina only, or both sites), HPV risk type (high- or probable high-risk [types 16/18/26/31/33/35/39/45/51/52/53/56/58/59/66/68/73/82/IS39 (6) versus low- or undetermined risk [types 6/11/40/42/54/55/61/70/72/81/CP6108]), number of concurrent HPV types detected, current smoking (yes/no), current use of hormonal contraceptives (yes/no), timing of last reported vaginal intercourse (>2 days before HPV testing/≤2 days before HPV testing), sex partners reported in the prior 8 months (0, non-new partner(s) only [e.g. partners reported more than 8 months prior to incident detection], ≥1 new partner), reported total number of sex acts in the prior 8 months (0 and approximate tertiles above 0: 1–16, 17–41, 42+), and reported number of condom-unprotected sex acts in the prior 8 months (0 and approximate tertiles above 0: 1–6, 7–25, 26+; excluding infections detected in women reporting no vaginal intercourse in the prior 8 months). Variables assessed in the interval between incident detection and the subsequent 4-month visit included: sex partners reported (0, non-new partner(s) only, ≥1 new partner), reported total number of sex acts (0 and approximate tertiles above 0: 1–11, 12–27, 28+), and number of condom-unprotected sex acts with non-new partner(s) (e.g. partner(s) reported in the 8 months prior to incident detection) (0 and approximate tertiles above 0: 1–8, 9–22, 23+; excluding infections detected in women reporting no vaginal intercourse in the interval between incident detection and the subsequent 4-month visit). Variables were first tested univariately. Robust variance estimates were used to account for correlation between multiple HPV types within a woman. Statistically significant (p< .10) variables were considered for entry into a multivariate model. However, because only one variable was statistically significantly associated with persistence in univariate analysis, multivariate analysis was not performed.
Kaplan-Meier methods were used to estimate time to development of cervical SIL (low-grade or high-grade SIL; based on cytology); at-risk time was calculated both from a) the date of first incident HPV detection at any site (any type) to the date of detection of first SIL (including the incident HPV positive visit) and b) the date of first incident HPV detection at the cervix (any type; ignoring any HPV results from previously collected vulvar/vaginal samples) to the date of detection of first SIL (including the incident HPV positive visit).
RESULTS
Study population characteristics
Twenty-six women were excluded from all analyses because their reported date of first intercourse was more than three months before the enrollment visit (due to scheduling delays). At enrollment, the mean age of the remaining 224 women was 19.2 (SD,1.5) years, and they were followed for a mean of 30.7 (SD,22.7) months. The majority (64.3%) was white, 23.2% were Asian and the remaining 12.5% self-reported a different race. Fifty-eight women were sexually active at enrollment. One hundred and eight (65.1%) of the 166 women who were not sexually active at enrollment reported their first vaginal intercourse with a male partner while on study (the remaining 58 women remained virgins throughout follow-up).
Incident type-specific HPV infections
Over follow-up, 303 incident type-specific HPV infections were detected in 85 sexually active women, with a median of 3 types (range,1 to 14 types) per woman. In addition, seven infections were detected in five women before their first reported vaginal intercourse with a male partner and seven infections were detected in five women who reported no vaginal intercourse throughout follow-up. (Of nine such infections that were detected prior to the last follow-up visit, six were re-detected at ≥1 follow-up visit.) All analyses described below were restricted to infections detected in sexually active women.
Repeat-detection of incident type-specific HPV infections
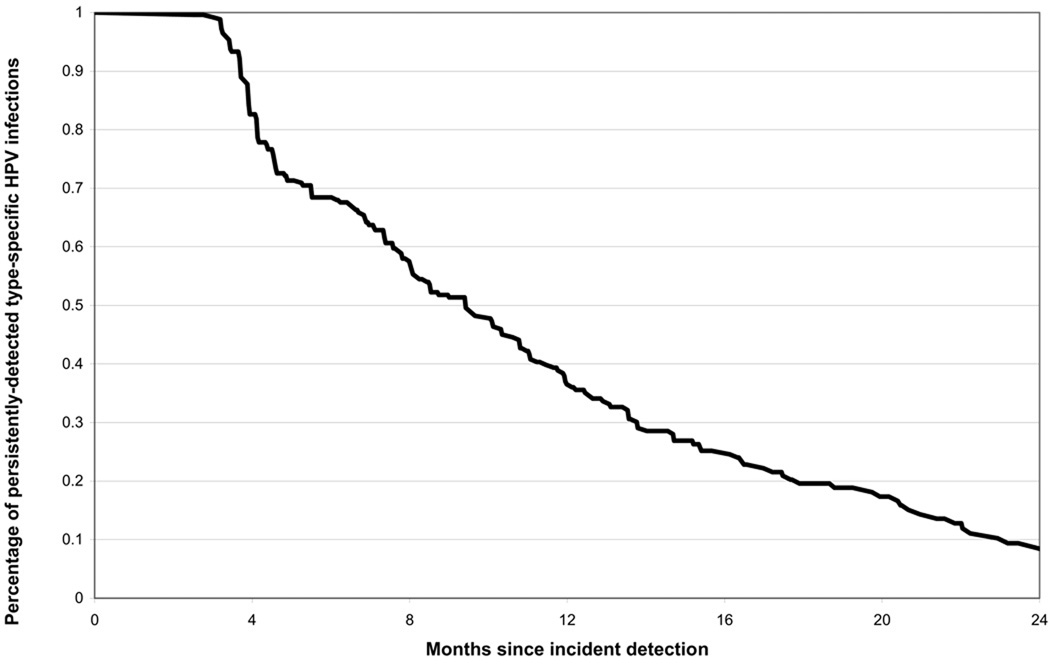
Infections detected before the last follow-up visit (n=257) were followed for a mean of 24.3 (SD,15.7) months after incident detection. The median time to the first negative test after the first positive test for a given type was 9.4 (95%CI:7.8–11.2) months, with 90.6% of infections becoming undetectable within two years (figure 1).
Figure 1.
Percentage of persistently-detected type-specific HPV infections, after incident type-specific HPV detection (n=257 type-specific HPV infections). A failure was defined as the first type-specific negative test after the incident positive test. Infections were censored at the last follow-up visit or the date of treatment for CIN 2+.
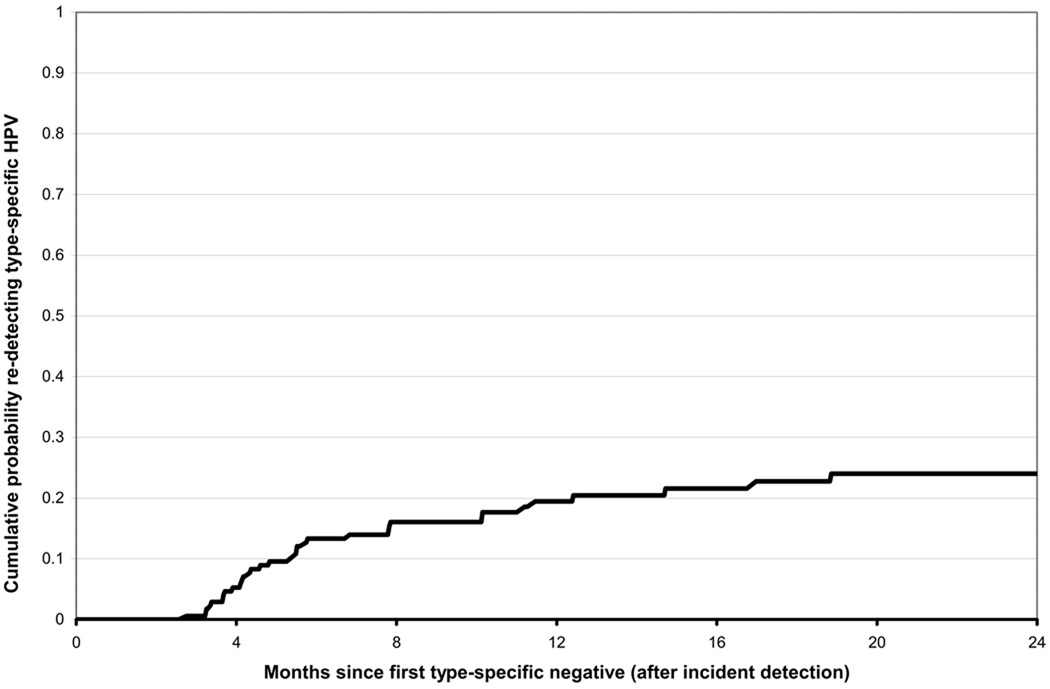
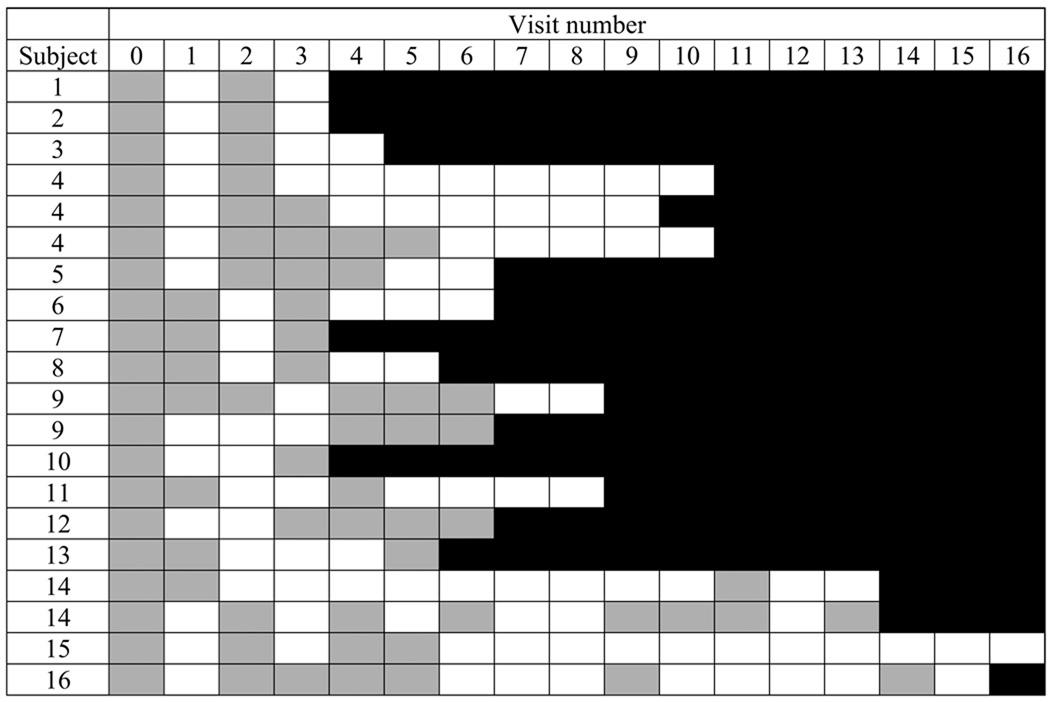
Of 173 incident type-specific infections that became undetectable before the last follow-up visit, 19.4% were re-detected again within one year (figure 2). The 39 type-specific infections that were re-detected included 20 infections with high- or probable high-risk types and 19 infections with low- or undetermined risk types (including one HPV-6 infection). (Detection patterns for the 20 high- or probable high-risk type infections are shown in figure 3.) For 17 infections, pairs of positive samples collected at two time points separated by at least one intercurrent negative were available for variant characterization. For 4 infections, one sample in the pair tested negative by PCR-based DNA sequencing. In the remaining 13 pairs, all displayed the same variant in both samples. Overall, 13 variants of 9 HPV types were detected, with the number of variants for individual types ranging from 1–3.
Figure 2.
Cumulative probability of re-detecting type-specific HPV, after the first negative test following incident detection (n=173 type-specific infections).
Figure 3.
Patterns of type-specific high-risk HPV DNA detection in sexually active young women. Each row represents an incident type-specific HPV infection characterized by a detection pattern that included intercurrent negative tests between two or more positive tests. Visit number is in relation to the first detection of type-specific HPV DNA (visit 0) (e.g. visit 5 refers to the 5th visit after first detection of type-specific HPV DNA). A gray box indicates type-specific HPV detection in at least one genital sample. A white box indicates that all samples were negative for the specific HPV type. Black indicates the end of study follow-up.
Risk factor analyses were restricted to type-specific incident infections followed by at least two additional visits (n=224). An additional 34 infections were excluded because the second follow-up visit was more than one year after incident detection (n=32) or sufficient HPV testing results were recorded at <2 genital sites at one of the two follow-up visits (n=2), leaving 190 infections in 59 women for analysis. 40.0% of infections were persistently detected (+++), 31.0% were sporadically detected (++−/+−+), and 29.0% were transiently detected (+−−). Compared to incident detection in the cervix only, incident detection in the cervix and vulva/vagina or in the vulva/vagina only was associated with an increased likelihood of persistent versus transient detection (table 1).
Table 1.
Odds ratios for the association between persistent* (n=76) versus transient† (n=55) detection of incident type-specific HPV infections and selected characteristics
| Crude Odds Ratio (95% CI) |
No. persistent/ no. transient |
|
|---|---|---|
| Site of incident detection (n=125‡) | ||
| Cervix only | 1.00 | 1/8 |
| Vulva/vagina only | 10.78 (1.20–96.67) | 31/23 |
| Both cervix and vulva/vagina | 15.62 (1.89–129.25) | 41/21 |
| HPV risk group | ||
| Low- or undetermined-risk | 1.00 | 31/24 |
| High-risk | 1.12 (0.51–2.48) | 45/31 |
| Number of concurrent HPV types detected§ | ||
| 0 | 1.00 | 18/14 |
| ≥1 | 1.10 (0.47–2.60) | 58/41 |
| Current smoker§ | ||
| No | 1.00 | 68/53 |
| Yes | 3.12 (0.37–26.37) | 8/2 |
| Currently using hormonal contraceptives§ | ||
| No | 1.00 | 35/17 |
| Yes | 0.52 (0.24–1.16) | 41/38 |
| Last reported vaginal intercourse§ (n=127‖) | ||
| >2 days before testing | 1.00 | 17/16 |
| ≤2 days before testing | 0.72 (0.31–1.69) | 56/38 |
| Sex partner(s) in the prior 8 months§ |
||
| No sex partners | 1.00 | 1/3 |
| Non-new sex partners only | 2.25 (0.16–31.11) | 9/12 |
| ≥1 new sex partner(s) | 4.95 (0.41–60.15) | 66/40 |
| Total no. of sex acts in the prior 8 months§ (n=123)** | ||
| 0 | 1.00 | 1/3 |
| 1–16 | 4.69 (0.39–55.96) | 25/16 |
| 17–41 | 4.80 (0.35–65.64) | 24/15 |
| 42+ | 3.16 (0.24–41.89) | 20/19 |
| No. of condom-unprotected sex acts in the prior 8 months§ (n=119)**†† |
||
| 0 | 1.00 | 9/6 |
| 1–6 | 1.19 (0.39–3.67) | 25/14 |
| 7–25 | 1.47 (0.35–6.10) | 22/10 |
| 26+ | 0.43 (0.12–1.57) | 13/20 |
| Sex partner(s) after incident infection‡‡ | ||
| No sex partners | 1.00 | 15/14 |
| Non-new sex partners only | 1.14 (0.47–2.74) | 39/32 |
| ≥1 new sex partner(s) | 2.28 (0.82–6.38) | 22/9 |
| Total no. of sex acts after incident infection‡‡ | ||
| 0 | 1.00 | 15/14 |
| 1–11 | 1.44 (0.54–3.79) | 20/13 |
| 12–27 | 1.87 (0.70–4.95) | 24/12 |
| 28+ | 0.99 (0.32–3.05) | 17/16 |
| No. of condom-unprotected sex acts with a non-new partner after incident infection‡‡ (n=102) §§ |
||
| 0 | 1.00 | 18/12 |
| 1–8 | 2.00 (0.52–7.63) | 18/6 |
| 9–22 | 0.73 (0.21–2.49) | 12/11 |
| 23+ | 0.73 (0.25–2.08) | 13/12 |
Persistent detections included all incident type-specific positives that were followed by two consecutive type-specific positives (+++).
Transient detections included all incident type-specific positives that were followed by two consecutive type-specific negatives (+−−).
6 infections that were first detected in the self-collected sample only were excluded.
At time of incident detection.
4 infections were detected in women who reported vaginal intercourse on the day of HPV testing. Because data on time of vaginal intercourse was not collected, it was not possible to determine whether vaginal intercourse occurred before or after testing. Therefore, those 4 infections were excluded.
Cervical SIL
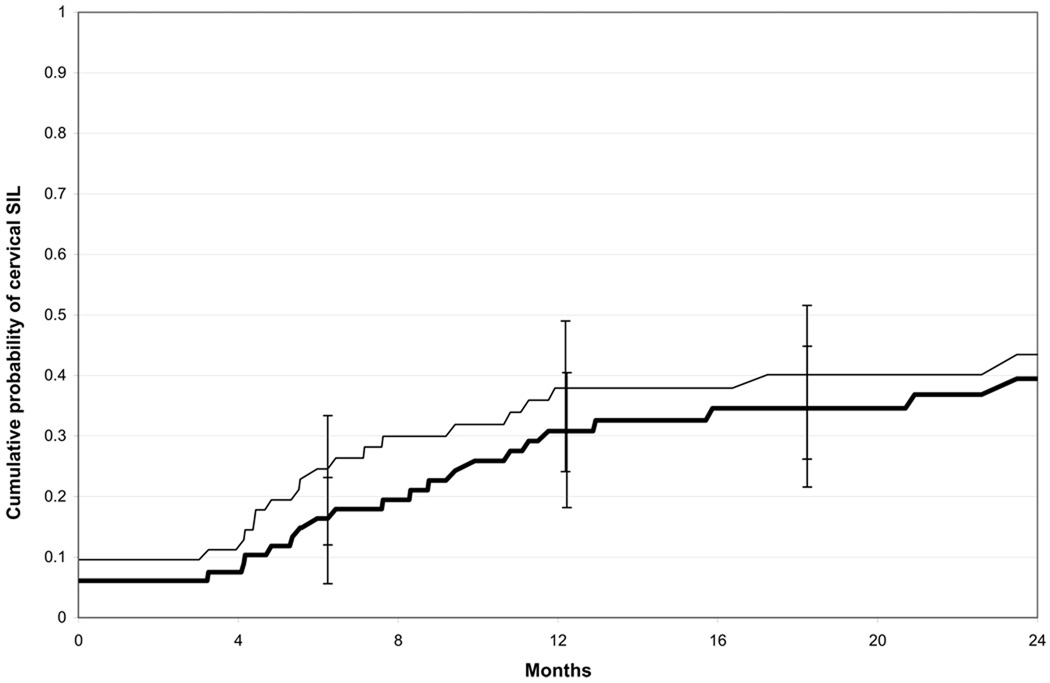
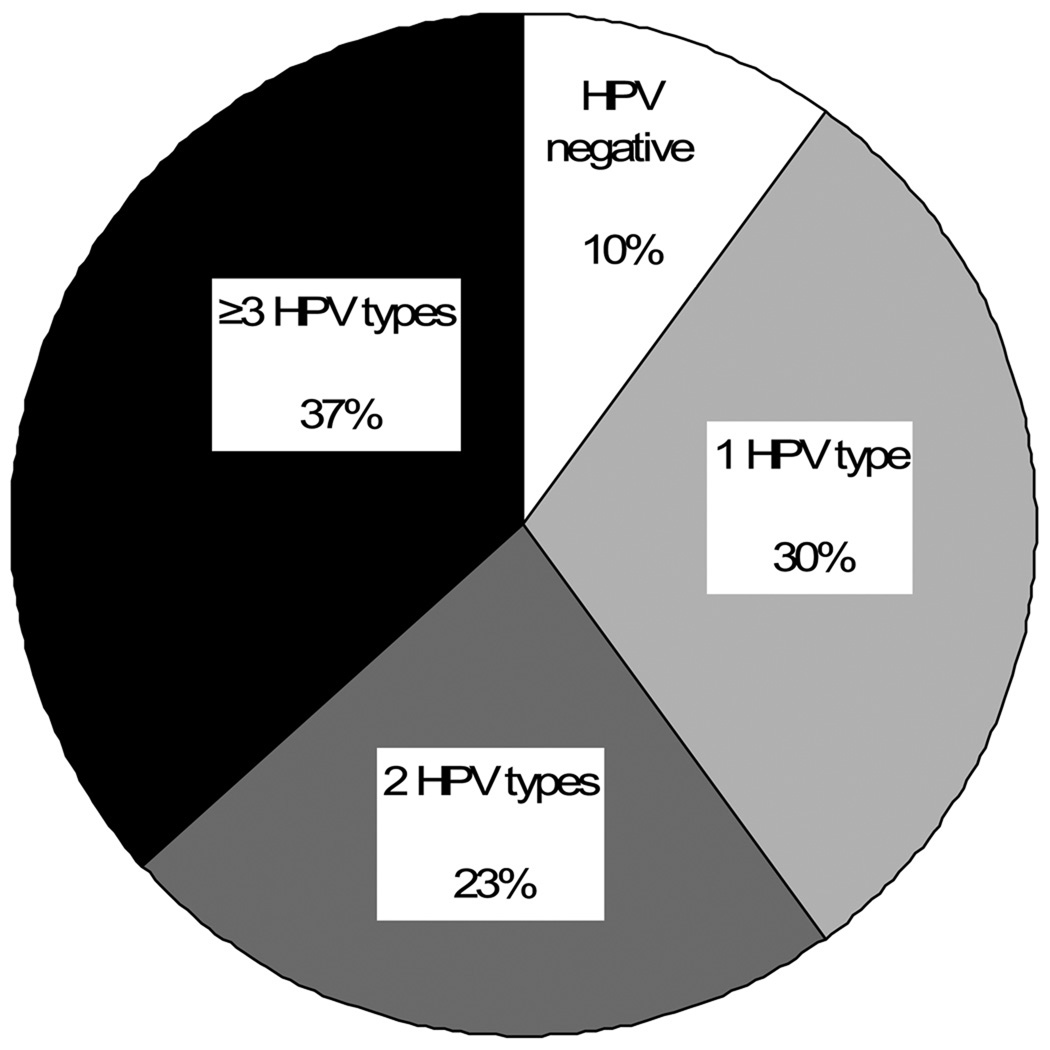
Thirty out of 85 women with incident HPV infections developed cervical SIL during study follow-up. Three women received a SIL diagnosis prior to first incident detection of HPV infection, and were excluded from the subsequent analyses. Among the remaining 82 women, the 12-month cumulative incidence of developing a cervical SIL after first incident HPV infection (at any site) was 30.8% (95%CI:21.2–43.5). Among 73 women with incident cervical HPV infections (ignoring any HPV results from previously collected vulvar/vaginal samples), the 12-month cumulative incidence of developing a cervical SIL after first incident HPV infection (at any site) was 37.9% (95%CI:26.8−51.7) (figure 4). The median time to cervical SIL could not be estimated from our data because of limited follow-up. However, among those who developed SIL within 12 months, the median time to development was 5.6 months after first incident HPV infection at any site, and 4.6 months after first incident cervical HPV infection. Sixty percent of newly-detected cervical SILs (18/30 cases) was positive for >1 HPV types in the cervical swab sample collected at the same visit (figure 5).
Figure 4.
Cumulative probability of developing cervical SIL among women with incident HPV infection at any site (thick black line; n=82 sexually active women with incident HPV infection at any site; three sexually active women who developed cervical SIL prior to first incident HPV detection were excluded from the analysis) and women with incident HPV infection at the cervix (thin black line; n=73 women with incident HPV infection at the cervix [ignoring any HPV results from previously collected vulvar/vaginal samples]; three sexually active women who developed cervical SIL prior to first incident cervical HPV detection were excluded from the analysis). Of 27 cases of newly-detected cervical SIL, 25 were low-grade and 2 were high-grade. Eleven of the 25 women with LSIL were subsequently referred for colposcopically-directed biopsy; 2 women had biopsy-confirmed CIN 2 (1 woman had biopsy-confirmed CIN 3 diagnosed by her primary care provider). One of the 2 women with HSIL had biopsy-confirmed CIN 2 diagnosed at a subsequent visit. The time between first incident HPV infection (at any site) and CIN 2 or CIN 3 ranged from 4 to 32 months.
Figure 5.
Distribution of the number of HPV types detected in the cervical swab sample collected at the same visit as the first SIL diagnosis (n=30).
DISCUSSION
By enrolling a cohort of newly sexually active young women with minimal previous exposure to HPV, we were able to observe the early natural history of truly incident HPV infections in the female genital tract. Consistent with previous longitudinal studies (of both prevalent and incident infections) (8), we observed that 90% of incident type-specific infections in our cohort became undetectable within two years (with half of all infections becoming undetectable within 9.4 months). Re-detection after a period of negativity was not uncommon. Nineteen percent of type-specific infections that became undetectable were re-detected within one year, and sequencing data indicated the presence of the same type-specific variant in samples collected before and after the intercurrent negative period. Re-detection of the same HPV type after intercurrent negative periods may reflect fluctuations in viral levels (or low-level viral shedding (2)), sampling inconsistencies, intercurrent false-negative test results, or new infection. Regardless, estimates of duration or clearance that are based on one or two negative HPV tests following a string of positive tests may be insufficient for distinguishing between persistent and transient infections. Furthermore, while our data do not address whether repeat detection in the early course of an HPV infection is predictive of long-term viral persistence, we previously reported that it was not uncommon for long-term persistent infections (defined as infections detected four to twelve years after incident detection) to have been detected sporadically in the first several years following incident detection (2).
Characterizing the early course of new HPV infections may further our understanding of infectivity and provide estimates that can be used in models of HPV transmission. Presumably, there is a positive correlation between duration of detectable HPV DNA and duration of infectivity, suggesting that repeat DNA detection is associated with a longer window of infectivity and therefore a greater probability of transmission to new sex partners (based on the positive relationship between the duration of infectivity and the reproductive rate of an infection (3)). Therefore, in order to identify factors that might increase an infected woman’s risk of transmitting her infection to a new sex partner, we sought to identify risk factors for repeat versus transient detection (in the first eight months after incident infection).
Smoking was positively associated with repeat HPV detection, but the proportion of smokers in our cohort was small and the association did not reach statistical significance. Although smoking is a clear risk factor for cervical cancer (6), the relationship between smoking and HPV persistence in the literature is inconsistent and the stage at which smoking exerts an effect on carcinogenic progression is unclear (8).
Though not statistically-significant, report of new sex partners in the 8 months prior to incident detection was associated with an increased likelihood of repeat detection compared to report of only non-new sex partners (2.5-fold increase) or no sex partners (5-fold increase). We previously showed that report of more than one new partner in the eight months prior to incident HPV-16 or HPV-18 infection was associated with increased E7 mRNA viral levels (9) (and higher viral levels have been linked with persistent detection (10–12)). No statistically significant sexual behavioral determinants of persistent versus transient infections were identified.
We also evaluated viral determinants of repeat detection, and found that site of initial infection was a strong predictor of repeat detection. Compared to infections that were first detected in the cervix only, those that were detected in the vulva/vagina only were twelve times as likely to be repeatedly detected and those that were detected in both the cervix and the vulva/vagina were seventeen times as likely to be repeatedly detected. Alpha-genus HPV infection that involves the entire genital tract (versus one site only) may be associated with higher viral loads. It is also possible that our results may partially reflect the potential for vulvar/vaginal infections to precede cervical infections, as incident infections following this trajectory would be detected at at least two consecutive visits (first in the vulva/vagina and subsequently in the cervix) (13–17).
While it is well-established that persistent high-risk HPV infection is a necessary step in cervical carcinogenesis (1) (with a one-year minimum proposed as a clinically-relevant threshold for identifying persistent high-risk infections that are likely to progress to precancer (CIN 3+) (18)), the role of early repeated detection of the same high-risk HPV type on the infection-to-precancer continuum is unclear. Our results do suggest, however, that in the early stages of infection, development of clinically-evident cervical lesions has little to do with persistent detection of HPV. Consistent with our findings in a previous cohort (19), we found that low-grade cervical SILs were a common manifestation in the year following incident HPV infection. In those who developed SIL in that time frame, the median induction time was less than six months (shorter than the median duration of continuously detectable infections in this cohort). Most of these early, low-grade SILs regress spontaneously and are unrelated to cervical precancer (19).
One limitation of our study is that we did not dissect cervical tissue, and therefore could not accurately attribute lesions to specific HPV types. Co-infection with multiple HPV types was common, as was sequential infection with new HPV types. Furthermore, sixty percent of cervical SILs were positive for multiple HPV types in concurrently-collected cervical swab samples, a finding that is consistent with data from an Oklahoma colposcopy clinic showing that 75% of women with ASCUS or low-grade SIL tested positive for multiple HPV types in the cervix (in that study, younger age was also associated with multiple type infections in all cervical disease categories) (20). Therefore, our estimates of the median time from first incident HPV detection (with any type) to cervical SIL among women who developed SILs within 12 months of first incident HPV infection were likely overestimates, as the HPV type(s) present in the cervical swab at the time of SIL detection were not necessarily the same type(s) present at the time of first incident HPV detection. While we could not estimate the unconstrained median time to SIL development (due to limited follow-up), restricting our estimate to women who developed SILs within 12 months of their first incident HPV infection was a reasonable analytical approach, as all cervical HPV types detected concurrently with new SILs were first detected within the prior 12 months (data not shown). SILs detected more than 12 months after first incident HPV infection were likely due to more recently acquired HPV types. Another limitation is the potential for false-positive or false-negative results in either our HPV or cytologic testing. (The three cases of SIL that were detected prior to first incident HPV infection likely indicate either false-positive Pap results or false-negative HPV results.)
In summary, our results indicate that sporadic detection of incident HPV infections is common in newly sexually active young women. Whether or not potentially modifiable risk factors can be identified to reduce the duration of infectivity (and the likelihood of transmission to new sex partners) remains unclear. Finally, clinically-evident low-grade cervical lesions were a common early manifestation of HPV infections, but did not appear to be associated with early HPV persistence.
Acknowledgments
Financial support was provided by grants from the National Institute of Allergy and Infectious Diseases (R01-A138383 and T32AI007140-24).
Dr. Koutsky has received commercial research grant support from Merck.
Footnotes
None of the other authors have commercial or other associations that might pose a conflict of interest.
REFERENCES
- 1.Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, Tortolero-Luna G, Kjaer SK, Munoz N. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26 Suppl 10:K1–K16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 2.Sycuro LK, Xi LF, Hughes JP, Feng Q, Winer RL, Lee SK, O'Reilly S, Kiviat NB, Koutsky LA. Persistence of genital human papillomavirus infection in a long-term follow-up study of female university students. J Infect Dis. 2008;198:971–978. doi: 10.1086/591625. [DOI] [PubMed] [Google Scholar]
- 3.Garnett GP. The geographical and temporal evolution of sexually transmitted disease epidemics. Sex Transm Infect. 2002;78 Suppl 1:i14–i19. doi: 10.1136/sti.78.suppl_1.i14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winer RL, Hughes JP, Feng Q, O'Reilly S, Kiviat NB, Holmes KK, Koutsky LA. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645–2654. doi: 10.1056/NEJMoa053284. [DOI] [PubMed] [Google Scholar]
- 5.Baer A, Saroiu S, Koutsky LA. Obtaining sensitive data through the Web: an example of design and methods. Epidemiology. 2002;13:640–645. doi: 10.1097/00001648-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Munoz N, Castellsague X, de Gonzalez AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006;24S3:S1–S10. doi: 10.1016/j.vaccine.2006.05.115. [DOI] [PubMed] [Google Scholar]
- 7.Kurman RJ, Henson DE, Herbst AL, Noller KL, Schiffman MH. Interim guidelines for management of abnormal cervical cytology. The 1992 National Cancer Institute Workshop. Jama. 1994;271:1866–1869. [PubMed] [Google Scholar]
- 8.Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: Updating the natural history of HPV and anogenital cancer. Vaccine. 2006;24 Suppl 3:S42–S51. doi: 10.1016/j.vaccine.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Winer RL, Harris TG, Xi LF, Jansen KU, Hughes JP, Feng Q, Welebob C, Ho J, Lee SK, Carter JJ, Galloway DA, Kiviat NB, Koutsky LA. Quantitative human papillomavirus 16 and 18 levels in incident infections and cervical lesion development. J Med Virol. 2009;81:713–721. doi: 10.1002/jmv.21450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho GYF, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 11.Munoz N, Hernandez-Suarez G, Mendez F, Molano M, Posso H, Moreno V, Murillo R, Ronderos M, Meijer C, Munoz A. Persistence of HPV infection and risk of high-grade cervical intraepithelial neoplasia in a cohort of Colombian women. Br J Cancer. 2009;100:1184–1190. doi: 10.1038/sj.bjc.6604972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brisson J, Bairati I, Morin C, Fortier M, Bouchard C, Christen A, Bernard P, Roy M, Meisels A. Determinants of persistent detection of human papillomavirus DNA in the uterine cervix. J Infect Dis. 1996;173:794–799. doi: 10.1093/infdis/173.4.794. [DOI] [PubMed] [Google Scholar]
- 13.Castle PE, Jeronimo J, Schiffman M, Herrero R, Rodriguez AC, Bratti MC, Hildesheim A, Wacholder S, Long LR, Neve L, Pfeiffer R, Burk RD. Age-related changes of the cervix influence human papillomavirus type distribution. Cancer Res. 2006;66:1218–1224. doi: 10.1158/0008-5472.CAN-05-3066. [DOI] [PubMed] [Google Scholar]
- 14.Castle PE, Rodriguez AC, Porras C, Herrero R, Schiffman M, Gonzalez P, Hildesheim A, Burk RD. A comparison of cervical and vaginal human papillomavirus. Sex Transm Dis. 2007;34:849–855. doi: 10.1097/OLQ.0b013e318064c8c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castle PE, Schiffman M, Bratti MC, Hildesheim A, Herrero R, Hutchinson ML, Rodriguez AC, Wacholder S, Sherman ME, Kendall H, Viscidi RP, Jeronimo J, Schussler JE, Burk RD. A population-based study of vaginal human papillomavirus infection in hysterectomized women. J Infect Dis. 2004;190:458–467. doi: 10.1086/421916. [DOI] [PubMed] [Google Scholar]
- 16.Winer RL, Hughes JP, Feng Q, O'Reilly S, Kiviat NB, Koutsky LA. Comparison of incident cervical and vulvar/vaginal human papillomavirus infections in newly sexually active young women. J Infect Dis. 2009;199:815–818. doi: 10.1086/597118. [DOI] [PubMed] [Google Scholar]
- 17.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Incident infection with genital human papillomavirus: rates and risk factors among a cohort of female university students. Am J Epidemiol. 2003;157:218–226. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 18.Castle PE. Invited commentary: is monitoring of human papillomavirus infection for viral persistence ready for use in cervical cancer screening? Am J Epidemiol. 2008;168:138–144. doi: 10.1093/aje/kwn037. discussion 145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winer RL, Kiviat NB, Hughes JP, Adam DE, Lee SK, Kuypers JM, Koutsky LA. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731–738. doi: 10.1086/427557. [DOI] [PubMed] [Google Scholar]
- 20.Wentzensen N, Schiffman M, Dunn T, Zuna RE, Gold MA, Allen RA, Zhang R, Sherman ME, Wacholder S, Walker J, Wang SS. Multiple human papillomavirus genotype infections in cervical cancer progression in the study to understand cervical cancer early endpoints and determinants. Int J Cancer. 2009;125:2151–2158. doi: 10.1002/ijc.24528. [DOI] [PMC free article] [PubMed] [Google Scholar]