Abstract
There is an agreement that acute (in minutes) hydrolysis and accumulation of phosphatidylinositol 4,5-bisphosphate (PIP2) modulate TRPV1 and TRPA1 activities. Since inflammation results in PIP2 depletion, persisting for long periods (hours-to-days) in pain models and in clinic, we examined whether chronic depletion and accumulation of PIP2 affects capsaicin and mustard oil responses. In addition we also wanted to evaluate whether the effects of PIP2 depend on TRPV1 and TRPA1 co-expression, and whether the PIP2 actions vary in expression cells versus sensory neurons. Chronic PIP2 production was stimulated by over-expression of phosphatidylinositol-4-phosphate-5-kinase, while PIP2-specific phospholipid 5′-phosphatase was selected to reduce plasma membrane levels of PIP2. Our results demonstrate that capsaicin (100 nM; CAP) responses and receptor tachyphylaxis are not significantly influenced by chronic changes in PIP2 levels in wild-type (WT) or TRPA1 null-mutant sensory neurons, as well as CHO cells expressing TRPV1 alone or with TRPA1. However, low concentrations of CAP (20 nM) produced a higher response after PIP2 depletion in cells containing TRPV1 alone, but not TRPV1 together with TRPA1. Mustard oil (25 μM; MO) responses were also not affected by PIP2 in WT sensory neurons and cells co-expressing TRPA1 and TRPV1. In contrast, PIP2 reduction leads to pronounced tachyphylaxis to MO in cells with both channels. Chronic effect of PIP2 on TRPA1 activity depends on presence of the TRPV1 channel and cell type (CHO vs. sensory neurons). In summary, chronic alterations in PIP2 levels regulate magnitude of CAP and MO responses, as well as MO-tachyphylaxis. This regulation depends on co-expression profile of TRPA1 and TRPV1 and cell type.
Keywords: PIP2, TRPA1, TRPV1, nociceptor
Introduction
A plethora of inflammatory mediators affect phosphatidylinositol 4,5-bisphosphate (PIP2) levels in peripheral sensory neurons by activating Gq/11-protein coupled or tyrosine kinase receptors, and by elevating intracellular Ca2+ (Akopian et al. 2007; Gosselin et al. 2008; Huang et al. 2006; Miller et al. 2009). It has been well documented that acute PIP2 accumulation or depletion can regulate functional activities of a variety of voltage- and ligand-gated channels, including the TRPV1 and TRPA1 channels (Gamper et al. 2004; Qin 2007; Rohacs et al. 2008; Suh and Hille 2005). Two contrasting effects of PIP2 on TRPV1 and TRPA1 have been reported. Research presented in several publications demonstrates that PIP2 persistently inhibits the TRPV1 and TRPA1 channel activity (Chuang et al. 2001; Dai et al. 2007; Kim et al. 2008b; Prescott and Julius 2003). Thus the depletion of PIP2 levels induced by inflammatory mediators can lead to a release from this inhibition and subsequent sensitization of the TRPV1 and TRPA1 channels by inflammatory mediators (Chuang et al. 2001; Dai et al. 2007). In another set of reports, an enhancing potential of PIP2 on TRPV1 and TRPA1-mediated responses was illustrated. Thus, PIP2 was able to activate TRPV1 in excised patches (Stein et al. 2006). PIP2 also sensitizes TRPA1-mediated mustard oil (MO) responses (Karashima et al. 2008). Substantial elevation of intracellular Ca2+ generated by activation of TRPV1 and TRPA1 induces Ca2+-activated PLC isoforms, and the resulting depletion of PIP2 plays a role in pharmacological desensitization of the TRPV1 and TRPA1 channels to a variety of stimuli (Akopian et al. 2007; Akopian et al. 2008; Liu et al. 2005). Rohacs and colleagues suggested that the contradictory data on involvement of PIP2 depletion in sensitization and desensitization of TRP channels could be dependent on the experimental conditions (Lukacs et al. 2007; Rohacs et al. 2008; Wu et al. 2002). The experimental conditions could be defined by a difference in TRP channel agonist concentrations (Lukacs et al. 2007), basal amounts of PIP2 in cells, type of cells, or treatment period (minutes vs. hours or days) with low or high concentrations of PIP2. The last condition is especially important, since in inflammatory pain models and in clinic, inflammatory mediators bombard nociceptors for long periods of time (hours-days). By doing so, PIP2 amounts could also be altered within nociceptors for prolonged periods of times.
Regulation of TRPV1 and TRPA1 channels by PIP2 has often been studied by changing the amount of PIP2 acutely within TRP-expressing cells. This includes dialysis of PIP2 into cells (Akopian et al. 2007; Dai et al. 2007), PIP2 sequestering with antibodies (Chuang et al. 2001; Dai et al. 2007) or application of PIP2 to excised patches (Kim et al. 2008b; Stein et al. 2006). In this study we investigated whether the PIP2 depletion or accumulation within the cells for 1-2 days affects magnitude and pharmacological desensitization of capsaicin (CAP) and mustard oil (MO) response. We also examined whether the effects of PIP2 vary in heterologous expression system (CHO cells) versus sensory neurons, since the adapter protein Pirt which links TRPV1 and PIP2 is expressed only in sensory neurons but not in expression systems (Kim et al. 2008a). Finally, the contribution of TRPA1-TRPV1 co-expression on chronic PIP2 effects was assessed.
Material and Methods
Animals
Primary sensory neuron cultures were generated from trigeminal ganglia (TG) isolated from B6.129S4 (wild-type; WT), B6.129S4-trpV1tml/jul (TRPV1 null-mutant mice provided by Jackson Laboratory) or TRPA1 null-mutant mice generated on the B6129P1/F2J background (kindly provided by Dr. Kevin Kwan).
Constructs and Heterologous Expression in CHO cells and sensory neurons
We used the following expression constructs: enhanced green fluorescent protein (pEGFP-N1 from Clontech); TRPV1 (accession number - NM031982) in pcDNA3 (Invitrogen, Carlsbad, CA); TRPA1 (NM177781) in pcDNA5/FRT (Invitrogen); PIP2-specific phospholipid 5′-phosphatase (Lyn-PP) plasmid consisting of the 5′-phosphatase of yeast Inp54p; (Stolz et al. 1998) which is expressed as a fusion protein to GFP and to a myristoylation-palmitoylation sequence taken from the Src-family tyrosine kinase Lyn to achieve plasma membrane localization (Raucher et al. 2000); and mouse phosphatidylinositol-4-phosphate-5-kinase-Iβ (PI5-K) in pcDNA 3.1 containing GFP sequence (Winks et al. 2005).
The expression constructs listed above were delivered into Chinese hamster ovary (CHO) cells using PolyFect (Qiagen, Valencia, CA) according to the manufacturers’ protocol. CHO cells were subjected to experimental procedures within 24-48 h after transfection. Electroporation, by using the Amaxa nucleofector, was carried out according to the manufacturer protocol to deliver pEGFP-N1, Lyn-PP and PI5-K into trigeminal ganglion (TG) sensory neurons. In brief, plasimds were mixed with the provided transfection solution and dispersed sensory neurons, and then electroporated at the G013 setting on the nucleofector. Cells were plated onto glass coverslips coated with poly-D-lysine and laminin and cultured in presence of 100ng/ml nerve growth factor (NGF-7.02S; Harlan, Indianapolis, IN) as previously described (Diogenes et al. 2007). Sensory neurons were subjected to experimental procedures within 24-48 h post transfection.
Ca2+ imaging
The Ca2+ imaging experiments were performed in standard solution (i.e. SES) as previously described (Diogenes et al. 2006). The net changes in Ca+2 influx were calculated by subtracting the basal [Ca+2]i (mean value collected for 60 s prior to agonist addition) from the peak [Ca+2]i value achieved after exposure to the agonists. Ca2+ increases above 50 nM were considered positive. This minimal threshold criterion was established by application of 0.1% DMSO as a vehicle. Ratiometric data were converted to [Ca+2]i (in μM) by using the equation [Ca+2]i = K* (R – Rmin)/(Rmax – R), where R is the 340/380 nm fluorescence ratio. Rmin, Rmax and K* were measured according to a previously described method (Gamper and Shapiro 2003).
Electrophysiology
All recordings were made in perforated patch voltage clamp (holding potential (Vh) of −60mV) configuration at 22-24 °C from the somata of neurons (15-40 pF) or CHO cells. Data were acquired and analyzed using Axopatch 200B or MultiClamp700 amplifiers and pCLAMP10 software (Axon Instruments, Union City, CA). Recording data were filtered at 0.5-2.5 kHz and sampled at 2-10 kHz depending on current kinetics. Borosilicate pipettes (Sutter, Novato, CA) were polished to resistances of 7-10 MΩ in the perforated patch pipette solution. Access resistance (Rs) was compensated (40-80%) when appropriate up to the value of 20-25MΩ. Data were rejected when Rs changed >20% during recording, leak currents were >70pA, or input resistance was < 200 MΩ. Currents were considered positive when their amplitudes were 5-fold larger than displayed noise (in root mean square).
Standard external solution (SES) contained (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 D-glucose and 10 HEPES, pH 7.4. Bath solution for measurement of voltage-gated Ca2+ channels was 160mM TEA-Cl, 5mM CaCl2, 10mM glucose, 10mM HEPES pH 7.4 (by TEA-OH). The pipette solution for the perforated patch configurations consisted of (in mM): 140 KCl, 1 MgCl2, 10 HEPES pH 7.3 and 250 mg/ml amphotericin B (Sigma, St. Louis, MO). In a set of experiments designed to suppress K+ currents, KCl was equimolarly substituted with CsCl. Drugs were applied using a fast, pressure-driven and computer controlled 8-channel system (AutoMate Scientific, San Francisco, CA).
Data analysis
For statistical analysis, GraphPad Prism 5.0 (GraphPad, San Diego, CA) was used. The data in Figures were given as mean ± standard error of the mean (SEM), with the value of n referring to the number of analyzed cells. All experiments were performed at least in triplicates. The significant difference between groups was assessed by one-way analysis of variance (ANOVA) with Bonferroni multiple comparison post-hoc-test. A difference was accepted as significant when p<0.05, <0.01 or <0.001 and are identified by *, ** and ***, respectively.
Results
Positive control experiments: effects of chronic PIP2 on run-down of voltage-gated Ca2+ currents in sensory neurons
The production of PIP2 in sensory neurons was stimulated by over-expression of phosphatidylinositol-4-phosphate-5-kinase (PI5-K); while PIP2-specific phospholipid 5′-phosphatase (Lyn-PP) was selected to reduce plasma membrane PIP2 concentrations in sensory neurons, but not other intracellular pools of PIP2 or other plasma membrane phosphatidylinositol phosphates. To evaluate the potential chronic effects of PIP2 on CAP and MO responses, we first employed a positive control to verify functionally relevant over-expression of PI5-K and Lyn-PP in sensory neurons. Previous studies have shown that the depletion of membrane bound PIP2 contributes to run-down of N-type and P/Q-type Ca2+ channels (Gamper et al. 2004; Suh and Hille 2005; Wu et al. 2002) which are major components of high voltage activated (HVA) Ca2+ currents (ICa) in sensory neurons (Grigaliunas et al. 2002; Nowycky et al. 1985). Therefore, as a positive control, we evaluated run-down of ICa in sensory neurons expressing GFP, PI5-K or Lyn-PP.
We delivered expression plasmids into sensory neurons using electroporation with Amaxa nucleofector (Fig 1A and 1B). This method is effective with 5-15% transfection ratio and leaves undamaged neurons suitable for reliable patch clamp recording. It is noteworthy that Lyn-PP fused to GFP is effectively expressed on the plasma membrane (Fig 1B). The recording of ICa was conducted from small-to-medium neurons (25-35 pF), since a size of cells somewhat affects run-down rate of Ca2+-currents (i.e. run-down is lesser in large cells). In GFP-containing neurons, repetitive application of voltage steps produced approximately 25% run-down of ICa within 30 min (Fig 1C and 1D). Accumulation of PIP2 generated by PI5-K over-expression in sensory neurons virtually stopped run-down process for ICa (Fig 1C, 1D and 1E). In contrast, putative reduction of PIP2 amounts in sensory neurons transfected with Lyn-PP significantly enhanced run-down rate (70%) of ICa (Fig 1C, 1D and 1F). Further, ICa density was substantially reduced by over-expression of Lyn-PP in sensory neurons, and slightly increased by introduction of PI5-K into sensory neurons. Thus, measurements are: GFP in TG 69.35±8.54 pA/pF (n=8); PI5-K in TG 89.5±9.15 pA/pF and Lyn-PP in TG 37.7±5.62 pA/pF (GFP in TG vs. Lyn-PP in TG p<0.05; and PI5-K in TG vs Lyn-PP in TG p<0.001; one-way ANOVA). These data is in accordance with previously published results (Gamper et al. 2004; Suh et al. 2010). In summary, chronic accumulation and depletion of PIP2 in sensory neurons can be induced by over-expression of PI5-K and Lyn-PP, respectively.
Figure 1. Positive control experiments: chronic PIP2-dependend run-down of voltage-gated Ca2+ channels (VGCaCh) in sensory neurons.
Chronic accumulation and depletion of PIP2 affects run-down of VGCaCh in sensory neurons. (A) and (B) panels illustrate expression of GFP-containing PI5-K (A) and Lyn-PP (B) plasmids in sensory neurons transfected by electroporation. Cells containing plasmids are marked with yellow arrows. The pictures were captured in bright-field DIC (upper panels) and fluorescent modes on TE2000-U (Nikon) microscope controlled with Metafluor 6.0 software (Molecular Devices). (C) Time-dependent run-down of VGCaCh within 35 min. VGCaCh were activated by voltage step from −60 mV to 0 mV for 40msec. The voltage step was applied to cells in interval of 15 sec. Expression plasmids are indicated. Ca2+-current was normalized to the peak of Ca2+-current recorded at a time point of 0 min. n=7-9. (D) Bar graph of normalized voltage-gated Ca2+-current recorded at a 30 min- time point. n=7-9. (E) and (F) panels illustrate representative traces of voltage-gated Ca2+-current recorded at 0 min- and 30 min- time points from sensory neurons expressing PI5-K (E) and Lyn-PP (F) plasmids.
Effect of chronic PIP2 depletion and accumulation on capsaicin responses
The modulation of the TRPV1 channel by acute application or depletion of PIP2 has been well documented (Chuang et al. 2001; Liu et al. 2005). However, it appears that acute production and hydrolysis PIP2 has a dual effect on TRPV1 activity (Lukacs et al. 2007). This difference was attributed to experimental conditions, namely stimulus strength for TRPV1 (Rohacs et al. 2008). Here, we investigated roles of other experimental conditions such as chronic alteration in PIP2 levels, co-expression of TRPV1 with TRPA1 and cell type.
Effects of low concentrations of CAP (20 nM) in cells with chronically low or high amounts of PIP2 were examined using Ca2+-imaging, since 20nM CAP-evoked current (ICAP) is too small (≈75 pA), percentage of responsive neurons is low, and the current’s noise/flickering is relatively high (≈25 pA) to reliably study its regulation. Figure 2A illustrates that chronic PIP2 accumulation and reduction in CHO cells expressing only TRPV1 results in inhibition and potentiating of CAP-evoked Ca2+ influx. This regulation is similar to acute effects of PIP2 on low-concentration of CAP responses (Chuang et al. 2001; Lukacs et al. 2007). TRPV1 is extensively co-expressed with TRPA1 in sensory neurons (Diogenes et al. 2007; Katsura et al. 2007; Kobayashi et al. 2005), and both channels undergo an interaction with each other (Salas et al. 2009; Staruschenko et al. 2010). Therefore, we evaluated effects of PIP2 levels on TRPV1 activity in TRPV1-TRPA1 containing cells (responding to both CAP and MO). Co-expression of TRPA1 with TRPV1 led to the eradication of chronic PIP2 effect on 20nM CAP responses (Fig 2A).
Figure 2. Chronic effects of PIP2 on CAP responses.
Accumulation and depletion of PIP2 for periods of 1-2 days affect CAP responses in certain cell lines. (A) 20nM CAP-evoked Ca2+-influx in CHO cells expressing TRPV1 (V1) or TRPV1 with TRPA1 (V1/A1), as well as GFP (visual marker), PI5-K (PI5) or Lyn-PP (Lyn). n=54-59 for TRPV1 expressing CHO cells; n=38-56 for TRPV1-TRPA1 co-expressing cells. (B) 100nM CAP-gated current density in CHO cells expressing TRPV1 or TRPV1 with TRPA1, as well as GFP (visual marker), PI5-K or Lyn-PP. n=6-10 for TRPV1 expressing CHO cells; n=7 for TRPV1-TRPA1 co-expressing cells. (C) 100nM CAP-gated current in wild-type (WT) and TRPA1 null-mutant (TRPA1 KO) TG sensory neurons expressing GFP, PI5-K or Lyn-PP. n=9-10 for WT sensory neurons; n=6-8 for TRPA1 KO sensory neurons.
We next examined whether PIP2 affects 100nM CAP-activated responses (CAP-gated current; ICAP), which are considered as “strong CAP responses” (Rohacs et al. 2008). The results presented in Figure 2B indicate that ICAP generated by 100nM CAP is not regulated by chronic accumulation and hydrolysis of PIP2 in either TRPV1 or TRPV1-TRPA1 expressing CHO cells. The cell type could influence the regulatory action of chronic PIP2 accumulation and hydrolysis. Accordingly, PIP2 effects on ICAP (100nM) were investigated in sensory neurons containing TRPV1 with TRPA1 (a subset of WT sensory neurons) and TRPV1 alone (TRPA1 KO sensory neurons). It appears that like in CHO cells, chronic PIP2 does not regulate ICAP (100 nM) in sensory neurons (Fig 2B and 2C).
In a TRPV1 expression system, acute pharmacological blockage of PIP2 hydrolysis can significantly reduce tachyphylaxis generated by 1μM CAP (Liu et al. 2005). However, this PIP2 action was not observed in sensory neurons, in which CAP tachyphylaxis is mainly regulated by dephosphorylation (Akopian et al. 2007; Jung et al. 2004; Koplas et al. 1997). Here, we examined whether chronic accumulation and depletion of PIP2 controls ICAP tachyphylaxis generated by 100nM CAP in TRPV1 as well as TRPV1-TRPA1 expressing cells. Low concentrations of CAP were not selected for these experiments, since CAP tachyphylaxis was insignificant or absent for <50nM CAP (Koplas et al. 1997). Neither the accumulation nor depletion of PIP2 for 1-2 days altered CAP-induced tachyphylaxis in TRPV1 and TRPV1-TRPA1 expressing CHO cells (Fig 3A). Further, over-expression of PI5-K and Lyn-PP in TRPA1 KO and WT sensory neurons expressing both TRPV1 and TRPA1 channels did not change CAP (100 nM) tachyphylaxis (Fig 3B and 3C). Altogether, chronic enhanced production and hydrolysis of PIP2 has limited action on TRPV1 activity. Thus, the PIP2 effects were only observed for low concentration (20 nM) of CAP responses in TRPV1, but not in the TRPV1-TRPA1 expressing CHO cells (Fig 2A). Otherwise, chronic PIP2 does not affect CAP (100nM) responses and tachyphylaxis in TRPV1 as well as TRPV1-TRPA1 expressing cells, including sensory neurons (Fig 2B, 2C and 3).
Figure 3. Chronic effects of PIP2 on CAP pharmacological desensitization (tachyphylaxis).
Accumulation and depletion of PIP2 for periods of 1-2 days does not influence CAP tachyphylaxis. (A) Tachyphylaxis produced by 100nM CAP in CHO cells expressing TRPV1 (V1) or TRPV1 with TRPA1 (V1/A1), as well as GFP, PI5-K (PI5) or Lyn-PP (Lyn). 4 min was between CAP applications. n=7 for TRPV1 expressing CHO cells; n=7 for TRPV1-TRPA1 co-expressing cells. (B) Tachyphylaxis produced by 100nM CAP in WT and TRPA1 KO TG sensory neurons expressing GFP, PI5-K or Lyn-PP. n=7 for WT sensory neurons; n=7-9 for TRPA1 KO sensory neurons. (C) Representative traces illustrate CAP (100 nM)-induced tachyphylaxis in WT TG sensory neurons expressing GFP, PI5-K or Lyn-PP. Application period for CAP is marked with a horizontal bar. There was a 4 min interval between CAP applications. The peak responses were used for the ratio.
Effect of chronic PIP2 depletion and accumulation on mustard oil responses
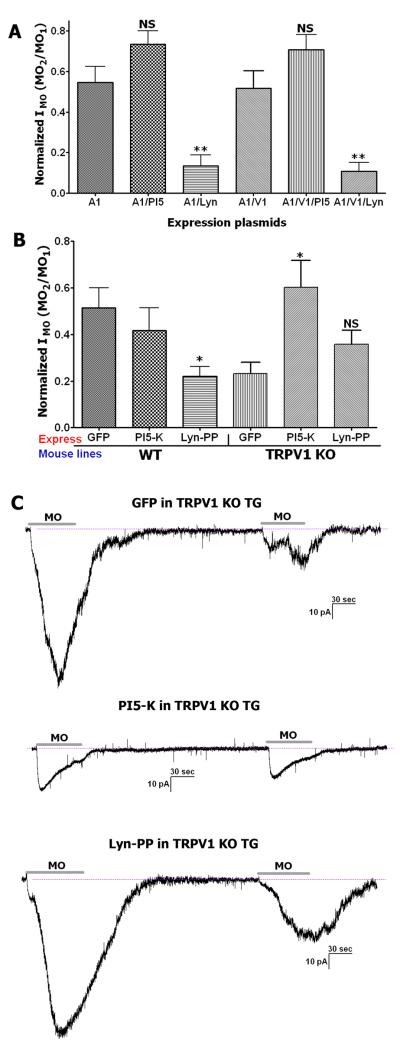
There are many parallels between the regulation of TRPV1 and TRPA1 by acute alteration in PIP2 levels. Like with TRPV1, both stimulatory and inhibitory effects of acute PIP2 have been reported for the TRPA1 channel (Akopian et al. 2007; Dai et al. 2007; Karashima et al. 2008; Kim et al. 2008b). Here, we examined whether chronic alteration in PIP2 levels, co-expression of TRPA1 with TRPV1 and selection of cell type contribute to regulation of TRPA1 by PIP2. TRPA1 was activated with 25 μM MO, which is strong stimuli (≈EC70) for TRPA1-expressing CHO (or HEK) cells (Jordt et al. 2004), but could be considered moderate stimuli for sensory neurons (Dai et al. 2007; Salas et al. 2009). Data presented in Figure 4A and 4B suggest that MO responses in TRPA1 expressing CHO cells are significantly increased after chronic PIP2 depletion for 1-2 days. This is very similar to reported acute effects of PIP2 in the expression system (Dai et al. 2007). Co-expression of TRPV1 with TRPA1 abolished PIP2 effect on MO responses (Fig 4A and 4B). Independence of TRPA1 activity on chronic accumulation and depletion of PIP2 was also observed in a subset of wild-type TG sensory neurons that express both channels (i.e. responsive for MO and CAP; Fig 4C and 4D). However, in TG neurons expressing only TRPA1, PIP2 accumulation substantially inhibited MO responses (Fig 4C and 4D). This data indicates that there is a resemblance in chronic PIP2 regulation of TRPA1 activity in the expression system and sensory neurons: increase in PIP2 production inhibits, whereas PIP2 depletion enhances MO responses. However, difference between expression system and sensory neurons could be noted. Thus, in sensory neurons, PIP2 accumulation, rather than PIP2 depletion was required to suppress MO responses (Fig 3A and 3B vs. 3C and 3D). In summary, chronic PIP2 affects MO responses only in TRPA1 expressing CHO cells and TRPV1 KO TG sensory neurons. Further, PIP2 action varies in CHO cells compared to sensory neurons.
Figure 4. Chronic effects of PIP2 on MO responses.
Chronic accumulation and depletion of PIP2 for 1-2 days affects MO responses. (A) 25μM MO-evoked Ca2+-influx in CHO cells expressing TRPA1 (A1) or TRPA1 with TRPV1 (A1/V1), as well as GFP (visual marker), PI5-K (PI5) or Lyn-PP (Lyn). n=51-84 for TRPA1 expressing CHO cells; n=61-85 for TRPA1-TRPV1 co-expressing cells. (B) 25μM MO-gated current density in CHO cells expressing TRPA1 or TRPA1 with TRPV1, as well as GFP, PI5-K or Lyn-PP. n=7-9 for TRPA1 expressing CHO cells; n=7-9 for TRPA1-TRPV1 co-expressing cells. (C) 25μM MO-evoked Ca2+-influx in WT and TRPV1 null-mutant (TRPV1 KO) TG neurons mock transfected (control), expressing GFP, PI5-K or Lyn-PP. n=143 for control in WT TG neurons; n=20-25 for WT sensory neurons; n=34-58 for TRPV1 KO sensory neurons. (D) 25μM MO-gated current in WT and TRPV1 KO TG neurons expressing GFP, PI5-K or Lyn-PP. n=7-10 for WT sensory neurons; n=10-11 for TRPV1 KO sensory neurons.
Acute PIP2 regulates MO tachyphylaxis in expression systems (Akopian et al. 2007; Karashima et al. 2008). In sensory neurons, there is a tendency for the same regulation; however, the effect is not statistically significant (Akopian et al. 2007). Further, it appears that substantial Ca2+-influx by strong stimuli is required to activate PLC that bring about PIP2 depletion and pharmacological desensitization of MO responses (Akopian et al. 2007). Here, we stimulated production and hydrolyzed PIP2 for 1-2 days, which is independent from Ca2+-influx. Therefore, chronic PIP2 depletion resulted in significantly stronger MO tachyphylaxis in TRPA1 expressing CHO cells (Fig 5A) as well as TRPA1-TRPV1 expressing CHO cells and TG neurons (Fig 5A and 5B). It should be noted that the effect is less pronounced in WT sensory neurons, but is still statistically significant (Fig 5A and 5B). In sensory neurons expressing only TRPA1, chronic PIP2 accumulation led to a miniscule IMO (−32.3±9.4pA; Fig 4D), which undergoes pharmacological desensitization in only 4 of 8 recorded neurons (Fig 5B and 5C). Interestingly, all of “non-desensitizing neurons” had a small IMO (<30 pA). Reasons for small MO responses in TRPV1 KO sensory neurons are not clear. Thus, functional loss of TRPA1 activities could be attributed to either conformational modifications of the TRPA1 channel or reduced density of TRPA1 on the plasma membrane. Overall, cells with depleted amount of PIP2 have greater MO tachyphylaxis. The exceptions are TRPA1 alone expressing TG neurons, in which MO responses were suppressed and MO tachyphylaxis dramatically diminished by over-expression of PI5-K and chronic PIP2 accumulation.
Figure 5. Chronic effects of PIP2 on MO desensitization (tachyphylaxis).
Accumulation and depletion of PIP2 for 1-2 days influence MO tachyphylaxis. (A) Tachyphylaxis produced by 25μM MO in CHO cells expressing TRPA1 (A1) or TRPA1 with TRPV1 (A1/V1), as well as GFP, PI5-K (PI5) or Lyn-PP (Lyn). n=6-7 for TRPA1 expressing CHO cells; n=6-8 for TRPA1-TRPV1 co-expressing cells. (B) Tachyphylaxis produced by 25μM MO in WT and TRPV1 KO TG sensory neurons expressing GFP, PI5-K or Lyn-PP. n=7-10 for WT sensory neurons; n=9-10 for TRPV1 KO sensory neurons. (C) Representative traces illustrate MO (25μM)-induced tachyphylaxis in TRPV1 KO TG sensory neurons expressing GFP, PI5-K or Lyn-PP. Application period for MO is marked with a horizontal bar. There was a 5 min interval between MO applications. The peak responses were used for the ratio.
Discussion
A plethora of channels, metabotrophic receptors, adaptor proteins and enzymes control nociceptive transmission during inflammation. There is an agreement that the TRPV1 and TRPA1 channels play critical roles in regulation of certain types of inflammatory hyperalgesia at the peripheral site (Bautista et al. 2006; Caterina et al. 2000; Dai et al. 2007; Davis et al. 2000; Kwan et al. 2006; Obata et al. 2005). In this respect, studies on modulation of TRPA1 and TRPV1 activity by inflammatory mediators are of special interest, because this modulation could underline molecular mechanisms of inflammatory hyperalgesia. Almost every inflammatory mediator can in principle deplete PIP2 which is a known regulator of many channels (Suh and Hille 2005). The TRPV1 and TRPA1 channels are not exceptions to this rule, and the control of TRPA1 and TRPV1 activity by PIP2 is well documented. However, it appears that these regulations strictly depend on experimental conditions (Lukacs et al. 2007; Rohacs et al. 2008). Keeping this in mind, we have examined regulation of the TRPV1 and TRPA1 activities by PIP2 in conditions closely relevant to peripheral inflammation. In animal models and in clinic, inflammation usually lasts for many hours and days. However, a majority of the data on effects of PIP2 on TRPV1 and TRPA1 activities was obtained after acute (seconds-to-minutes) alterations of PIP2 levels (Akopian et al. 2007; Chuang et al. 2001; Dai et al. 2007; Karashima et al. 2008; Liu et al. 2005; Lukacs et al. 2007). Therefore, we have investigated whether changes (i.e. over-production or hydrolysis) in PIP2 levels for 1-2 days could affect capsaicin and mustard oil responses. Inflammation also affects excitability of sensory neurons. However, there is no systematic data addressing difference in PIP2 regulation of TRPV1 and TRPA1 in expression system versus sensory neurons. This is especially important, since recent identification of a novel protein Pirt that is specifically expressed in sensory neurons, but not in expression systems and is required for the stimulatory effect of PIP2 on TRPV1 (Kim et al. 2008a). Accordingly, we have examined whether chronic changes in PIP2 levels regulates TRPV1 and TRPA1 in the similar fashion in an expression system and sensory neurons. In sensory neurons, inflammation functionally up-regulates both TRPV1 and TRPA1 channels and leads to co-expression of these channels in ≈80% neurons (Diogenes et al. 2007; Obata et al. 2005). It is known that TRPA1 and TRPV1 are mutually involved in nociceptive transmission and undergo interaction (Bautista et al. 2006; McMahon and Wood 2006; Salas et al. 2009; Staruschenko et al. 2010). Further, a PIP2 interaction domain is closely localized to the tetramerization region on C-terminal portion of the TRPV1 channel (Garcia-Sanz et al. 2004; Prescott and Julius 2003). Consequently, here we evaluated regulation of CAP and MO responses in chronic alteration of PIP2 in TRPA1 or TRPV1 alone expressing and TRPV1-TRPA1 co-expressing cells, including sensory neurons. Finally, pharmacological desensitization of receptors and channels could dramatically affect responsiveness of neurons to stimuli (i.e. tolerance) (Akopian et al. 2009; Bhave et al. 2002; Docherty et al. 1996; Ruparel et al. 2008; Ueda and Ueda 2009). In this context, we studied not only CAP and MO responses at different conditions listed above, but also pharmacological desensitization of the TRPV1 and TRPA1 to CAP and MO, respectively.
It is well accepted that TRPV1 activity is regulated by phosphorylation/ dephosphorylation processes. Thus, a variety of inflammatory mediators upon treatment on sensory neurons lead to phosphorylation and subsequent sensitization of TRPV1 via different pathways involving protein kinase C (PKC), A (PKA), Src-kinase and PI3-kinase (Cesare et al. 1999; Lopshire and Nicol 1998; Numazaki et al. 2002; Sugiura et al. 2002; Zhang et al. 2005; Zhuang et al. 2004). Phosphorylation provides physiologically relevant mechanism for TRPV1 sensitization, since involvement of these kinases in inflammatory thermal hyperalgesia has been reported multiple times (Aley et al. 2000; Dai et al. 2004; Katsura et al. 2007; Yajima et al. 2003; Zhuang et al. 2004). Pharmacological and functional desensitization of the TRPV1 channel is also regulated by dephosphorylation with PP2B/calcineurin that can be rescued by calcineirin blockers and PKA phosphorylation (Bhave et al. 2002; Docherty et al. 1996; Jeske et al. 2006; Jung et al. 2004; Ruparel et al. 2008). Despite all these compelling evidence, there is still a viewpoint that PIP2 could also be involved in physiologically relevant sensitization and desensitization of TRPV1 in sensory neurons (Chuang et al. 2001; Liu et al. 2005). Our data generated in sensory neurons expressing both TRPV1 and TRPA1 channels and chronically over- or under-producing PIP2 suggests that PIP2’s role in regulation of TRPV1 during inflammatory thermal hyperalgesia and pharmacological desensitization of the TRPV1 channel could be minimal if any. Involvement of sensory neuronal PIP2 in inflammatory hyperalgesia was not directly evaluated by a behavioral test, since it is technically difficult task. However, indirect evidence suggests that partial involvement of PIP2 in pain processing from periphery could still occur. Thus, prostatic acid phosphatase (PAP) applied intrathecally modulates several cellular pathways, including reduction PIP2 levels for 1-2 hours. This PIP2 depletion correlates with inhibition of thermo-sensation as well as thermal and mechanical inflammatory hyperalgesia, which have been measured 6-48h post-PAP application (Sowa et al. 2010). These data indicate that changes in PIP2 levels could correlate with initiation of thermal and mechanical hyperalgesia mediated by TRPV1. Similarly, ablation of Pirt that serves as a bridge between PIP2 and TRPV1, only partially reverses bradykinin-induced sensitization of CAP (100 nM) responses (Kim et al. 2008a). Contribution of Pirt in inflammatory hyperalgesia has not been examined as yet (Kim et al. 2008a). In summary, the physiological role of PIP2 in inflammatory thermal hyperalgesia still remains an open question.
TRPA1 controls certain aspects of inflammatory hyperalgesia. Unlike for TRPV1, there is no agreement on the molecular mechanisms that connect the TRPA1 channel to inflammatory hyperalgesia. However, there are several possible pathways. First, TRPA1 could be sensitized by inflammatory mediator-induced depletion of PIP2 which provides tonic inhibition of TRPA1 (Dai et al. 2007). Interesting, our results indicate that such inhibition can take place in sensory neurons expressing TRPA1 alone, but not in WT neurons co-expressing TRPA1 with TRPV1 (Fig 4D). Second, TRPA1 could be sensitized via PKA pathway that leads to increase in membrane levels of the TRPA1 channel (Schmidt et al. 2009). In contrast, it was reported that activation of PKC could not lead to TRPV1 phosphorylation and desensitization (Dai et al. 2007). Third, TRPA1 sensitization could be controlled by associated TRPV1 channels, which are in turn modulated by inflammatory mediators (Akopian 2010). This pathway in the regulation TRPA1 is a possibility, since TRPA1-mediated responses (such as MO, WIN55,212-2 and especially AM1241) are closely controlled by TRPV1-co-expression (Akopian et al. 2008; Salas et al. 2009).
Pharmacological desensitization of MO engages several mechanisms, which includes Ca2+-dependent PIP2 depletion and covalent modification of TRPA1 by agonists (Akopian et al. 2007; Macpherson et al. 2007; Schmidt et al. 2009). Therefore, it is not surprising that MO tachyphylaxis is more pronounced in cells with strong MO responses (Akopian et al. 2007). This could explain the less evident MO tachyphylaxis in TRPA1 alone expressing sensory neurons (Fig 5B and 5C). However, TRPA1 recovers poorly from MO induced desensitization in cells with depleted PIP2 (Fig 5). In native sensory neurons, PIP2 depletion and MO desensitization occurs only after substantial Ca2+ influx triggered with strong stimuli such as CAP (Akopian et al. 2007). These data suggest that recovery from desensitization and sensitization of TRPA1 activity during inflammation could require some other pathway independent from PIP2, since inflammation leads to PIP2 depletion. Thus, PLC induced PKA phosphorylation could be one of possible pathways (Schmidt et al. 2009). Interestingly, TRPV1 recovery from desensitization is also controlled by PKA (Bhave et al. 2002; Jeske et al. 2006). One confusing point is that the desensitization of the TRPV1, but not TRPA1 depends on dephosphorylation (Akopian et al. 2007). In summary, PIP2 could play a role in recovery of TRPA1 pharmacological desensitization in native sensory neurons; however, TRPA1 sensitization during inflammation is probably controlled through some other signaling pathways.
Acknowledgements
We would like to thank Dr. Mark Shapiro (UTHSCSA, San Antonio, TX) for the kind gift of PI5-kinase and Lyn-PIP2-phosphotase cDNAs. We would also like to thank Jie Li for technical assistance. Research was supported by NIH/NIDCR DE019311 and DE017696 (to ANA).
References
- Akopian AN. Regulation of Nociceptive Transmission at the Periphery via TRPA1-TRPV1 Interactions. Curr Pharm Biotechnol. 2010 doi: 10.2174/138920111793937952. [DOI] [PubMed] [Google Scholar]
- Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol. 2007;583(Pt 1):175–193. doi: 10.1113/jphysiol.2007.133231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian AN, Ruparel NB, Jeske NA, Patwardhan A, Hargreaves KM. Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia. Trends Pharmacol Sci. 2009;30(2):79–84. doi: 10.1016/j.tips.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM. Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J Neurosci. 2008;28(5):1064–1075. doi: 10.1523/JNEUROSCI.1565-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20(12):4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 Mediates the Inflammatory Actions of Environmental Irritants and Proalgesic Agents. Cell. 2006;124(6):1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35(4):721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23(3):617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411(6840):957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, Tominaga M, Noguchi K. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J Neurosci. 2004;24(18):4293–4299. doi: 10.1523/JNEUROSCI.0454-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117(7):1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405(6783):183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Diogenes A, Akopian AN, Hargreaves KM. NGF up-regulates TRPA1: implications for orofacial pain. J Dent Res. 2007;86(6):550–555. doi: 10.1177/154405910708600612. [DOI] [PubMed] [Google Scholar]
- Diogenes A, Patwardhan AM, Jeske NA, Ruparel NB, Goffin V, Akopian AN, Hargreaves KM. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci. 2006;26(31):8126–8136. doi: 10.1523/JNEUROSCI.0793-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch. 1996;431(6):828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- Gamper N, Reznikov V, Yamada Y, Yang J, Shapiro MS. Phosphatidylinositol [correction] 4,5-bisphosphate signals underlie receptor-specific Gq/11-mediated modulation of N-type Ca2+ channels. J Neurosci. 2004;24(48):10980–10992. doi: 10.1523/JNEUROSCI.3869-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper N, Shapiro MS. Calmodulin mediates Ca2+-dependent modulation of M-type K+ channels. J Gen Physiol. 2003;122(1):17–31. doi: 10.1085/jgp.200208783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sanz N, Fernandez-Carvajal A, Morenilla-Palao C, Planells-Cases R, Fajardo-Sanchez E, Fernandez-Ballester G, Ferrer-Montiel A. Identification of a tetramerization domain in the C terminus of the vanilloid receptor. J Neurosci. 2004;24(23):5307–5314. doi: 10.1523/JNEUROSCI.0202-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin RD, Dansereau MA, Pohl M, Kitabgi P, Beaudet N, Sarret P, Parsadaniantz S. Melik. Chemokine network in the nervous system: a new target for pain relief. Curr Med Chem. 2008;15(27):2866–2875. doi: 10.2174/092986708786242822. [DOI] [PubMed] [Google Scholar]
- Grigaliunas A, Bradley RM, MacCallum DK, Mistretta CM. Distinctive neurophysiological properties of embryonic trigeminal and geniculate neurons in culture. J Neurophysiol. 2002;88(4):2058–2074. doi: 10.1152/jn.2002.88.4.2058. [DOI] [PubMed] [Google Scholar]
- Huang J, Zhang X, McNaughton PA. Inflammatory pain: the cellular basis of heat hyperalgesia. Curr Neuropharmacol. 2006;4(3):197–206. doi: 10.2174/157015906778019554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Patwardhan AM, Gamper N, Price TJ, Akopian AN, Hargreaves KM. Cannabinoid WIN 55,212-2 regulates TRPV1 phosphorylation in sensory neurons. J Biol Chem. 2006;281(43):32879–32890. doi: 10.1074/jbc.M603220200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427(6971):260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, Oh U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem. 2004;279(8):7048–7054. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- Karashima Y, Prenen J, Meseguer V, Owsianik G, Voets T, Nilius B. Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5-biphosphate manipulators. Pflugers Arch. 2008;457(1):77–89. doi: 10.1007/s00424-008-0493-6. [DOI] [PubMed] [Google Scholar]
- Katsura H, Obata K, Mizushima T, Sakurai J, Kobayashi K, Yamanaka H, Dai Y, Fukuoka T, Sakagami M, Noguchi K. Activation of extracellular signal-regulated protein kinases 5 in primary afferent neurons contributes to heat and cold hyperalgesia after inflammation. J Neurochem. 2007;102(5):1614–1624. doi: 10.1111/j.1471-4159.2007.04698.x. [DOI] [PubMed] [Google Scholar]
- Kim AY, Tang Z, Liu Q, Patel KN, Maag D, Geng Y, Dong X. Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell. 2008a;133(3):475–485. doi: 10.1016/j.cell.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Cavanaugh EJ, Simkin D. Inhibition of transient receptor potential A1 channel by phosphatidylinositol-4,5-bisphosphate. Am J Physiol Cell Physiol. 2008b;295(1):C92–99. doi: 10.1152/ajpcell.00023.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493(4):596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci. 1997;17(10):3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50(2):277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25(19):4835–4843. doi: 10.1523/JNEUROSCI.1296-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopshire JC, Nicol GD. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: whole-cell and single-channel studies. J Neurosci. 1998;18(16):6081–6092. doi: 10.1523/JNEUROSCI.18-16-06081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci. 2007;27(26):7070–7080. doi: 10.1523/JNEUROSCI.1866-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445(7127):541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Wood JN. Increasingly irritable and close to tears: TRPA1 in inflammatory pain. Cell. 2006;124(6):1123–1125. doi: 10.1016/j.cell.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009;(194):417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem. 2002;277(16):13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115(9):2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300(5623):1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- Qin F. Regulation of TRP ion channels by phosphatidylinositol-4,5-bisphosphate. Handb Exp Pharmacol. 2007;(179):509–525. doi: 10.1007/978-3-540-34891-7_30. [DOI] [PubMed] [Google Scholar]
- Raucher D, Stauffer T, Chen W, Shen K, Guo S, York JD, Sheetz MP, Meyer T. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell. 2000;100(2):221–228. doi: 10.1016/s0092-8674(00)81560-3. [DOI] [PubMed] [Google Scholar]
- Rohacs T, Thyagarajan B, Lukacs V. Phospholipase C mediated modulation of TRPV1 channels. Mol Neurobiol. 2008;37(2-3):153–163. doi: 10.1007/s12035-008-8027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain. 2008;135(3):271–279. doi: 10.1016/j.pain.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas MM, Hargreaves KM, Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur J Neurosci. 2009;29(8):1568–1578. doi: 10.1111/j.1460-9568.2009.06702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron. 2009;64(4):498–509. doi: 10.1016/j.neuron.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa NA, Street SE, Vihko P, Zylka MJ. Prostatic acid phosphatase reduces thermal sensitivity and chronic pain sensitization by depleting phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2010;30(31):10282–10293. doi: 10.1523/JNEUROSCI.2162-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staruschenko A, Jeske NA, Akopian AN. Contribution of TRPV1-TRPA1 interaction to the single channel properties of the TRPA1 channel. J Biol Chem. 2010;285(20):15167–15177. doi: 10.1074/jbc.M110.106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128(5):509–522. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz LE, Kuo WJ, Longchamps J, Sekhon MK, York JD. INP51, a yeast inositol polyphosphate 5-phosphatase required for phosphatidylinositol 4,5-bisphosphate homeostasis and whose absence confers a cold-resistant phenotype. J Biol Chem. 1998;273(19):11852–11861. doi: 10.1074/jbc.273.19.11852. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Tominaga M, Katsuya H, Mizumura K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol. 2002;88(1):544–548. doi: 10.1152/jn.2002.88.1.544. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol. 2005;15(3):370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Suh BC, Leal K, Hille B. Modulation of high-voltage activated Ca(2+) channels by membrane phosphatidylinositol 4,5-bisphosphate. Neuron. 2010;67(2):224–238. doi: 10.1016/j.neuron.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Ueda M. Mechanisms underlying morphine analgesic tolerance and dependence. Front Biosci. 2009;14:5260–5272. doi: 10.2741/3596. [DOI] [PubMed] [Google Scholar]
- Winks JS, Hughes S, Filippov AK, Tatulian L, Abogadie FC, Brown DA, Marsh SJ. Relationship between membrane phosphatidylinositol-4,5-bisphosphate and receptor-mediated inhibition of native neuronal M channels. J Neurosci. 2005;25(13):3400–3413. doi: 10.1523/JNEUROSCI.3231-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Bauer CS, Zhen XG, Xie C, Yang J. Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2. Nature. 2002;419(6910):947–952. doi: 10.1038/nature01118. [DOI] [PubMed] [Google Scholar]
- Yajima Y, Narita M, Shimamura M, Kubota C, Suzuki T. Differential involvement of spinal protein kinase C and protein kinase A in neuropathic and inflammatory pain in mice. Brain Res. 2003;992(2):288–293. doi: 10.1016/j.brainres.2003.08.042. [DOI] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. Embo J. 2005;24(24):4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci. 2004;24(38):8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]