We report whole-cell recording of kisspeptin neurons and give insight into how energy homeostasis (leptin) is tied to reproductive function.
Abstract
Hypothalamic kisspeptin neurons are critical for driving reproductive function, but virtually nothing is known about their endogenous electrophysiological properties and the effects of leptin on their excitability. Therefore, we used the slice preparation from female guinea pigs to study the endogenous conductances and the effects of leptin on kisspeptin neurons. We targeted the arcuate kisspeptin neurons using visualized-patch whole-cell recording and identified kisspeptin neurons using immuocytochemical staining for kisspeptin or single cell RT-PCR. We also harvested dispersed arcuate neurons for analysis of expression of channel transcripts. Kisspeptin neurons exhibited a relatively negative resting membrane potential, and eighty percent of the neurons expressed a pacemaker current (h-current) and a T-type Ca2+ current. Furthermore, the glutamate receptor agonist N-methyl D-aspartic acid depolarized and induced burst firing in kisspeptin neurons. Leptin activated an inward current that depolarized kisspeptin neurons and increased (burst) firing, but leptin hyperpolarized NPY neurons. Lanthanum, a TRPC-4,-5 channel activator, potentiated the leptin-induced inward current by 170%. The leptin-activated current reversed near −15 mV and was abrogated by the relatively selective TRPC channel blocker 2-APB. The leptin effects were also blocked by a Janus kinase inhibitor, a phosphatidylinositol 3 kinase inhibitor, and a phospholipase Cγ inhibitor. In addition, the majority of these neurons expressed TRPC1 and -5 and phospholipase Cγ1 based on single cell RT-PCR. Therefore, guinea pig kisspeptin neurons express endogenous pacemaker currents, and leptin excites these neurons via activation of TRPC channels. The leptin excitatory effects on kisspeptin neurons may be critical for governing the excitatory drive to GnRH neurons during different nutritional states.
A plethora of work has documented the critical role of kisspeptin to modulate GnRH release and hence control the mammalian reproductive cycle (1). Kisspeptin neurons are localized to the arcuate nucleus and periventricular preoptic area in a species-, sex-, and gonadal steroid–specific manner (2, 3). The Kiss1 gene encodes a 145–amino acid protein, which is proteolytically processed to kisspeptin-54 and several other smaller peptide fragments. Kisspeptin-54 is the endogenous ligand of GPR54, which is expressed in GnRH neurons, and kisspeptin has potent excitatory effects on GnRH neurons (4–7). Mutations in GPR54 cause autosomal recessive idiopathic hypogonadotropic hypogonadism in humans (8, 9), while deletion of GPR54 in mice produces defective sexual development and infertility (9). Targeted deletion of the Kiss1 gene in mice causes a similar phenotype as GPR54 gene mutation (10). Collectively, these findings suggest that kisspeptin and GPR54 are essential for normal reproductive physiology.
On the other hand, the adipocyte hormone leptin plays a key role in energy homeostasis and reproduction, and this hormone has an important role in the neuroendocrine adaptation to starvation (11). Studies reveal that low serum concentrations of leptin are important for signaling energy deficits to the hypothalamic-gonadal axis, whereas high leptin concentrations in obesity often are associated with leptin resistance (11). Leptin signals via its cognate receptors, leptin receptors (LRs), and there are several isoforms attributable to alternate splicing (12). The long isoform (leptin receptor b, LRb) is expressed abundantly in hypothalamic arcuate, ventromedial, and dorsomedial nuclei, and LRb is the predominant signaling form of the receptor (13, 14). LRb has been localized in proopiomelanocortin (POMC), neuropeptide Y (NPY), and kisspeptin neurons but not in GnRH neurons (15–18). Mutations in leptin or its receptor are associated with profound metabolic and physiological abnormalities such as extreme obesity and infertility (19).
Hypothalamic kisspeptin neurons are an obvious target for circulating leptin because kisspeptin neurons express LRb, Kiss1 mRNA is reduced in obese and infertile ob/ob mice (compared with wild-type), and the levels of Kiss1 mRNA increase after administration of leptin (17). In addition, a number of studies in mice and rats have demonstrated that negative energy balance can induce a decrease in Kiss1 gene expression in the hypothalamus (20–22). Because GnRH neurons do not express LRb and do not respond directly to leptin (unpublished data; see also Ref. 18), we explored the direct effects of leptin on kisspeptin neurons. Indeed, leptin depolarized via activation of TRPC channels and increased burst firing in kisspeptin neurons, which would increase the excitatory drive to GnRH neurons during satiated states.
Materials and Methods
Animals and treatments
All animal procedures described in this study are in accordance with institutional guidelines based on National Institutes of Health standards and approved by the Institutional Animal Care and Use Committee. Female Topeka guinea pigs (400–600 gm), bred in our institutional breeding facility, and female multicolor guinea pigs (400–500 gm; Elm Hill Breeding Labs, Chelmsford, MA) were used in these experiments. The guinea pigs were maintained under constant temperature (26 C) and light (on between 0630 and 2030 h) and had free access to food and water. One group of females were ovariectomized (OVX) for single cell (sc) harvesting under ketamine-xylazine anesthesia (33 and 6 mg/kg, respectively, sc) 5–7 d before experimentation, and they were given sesame oil vehicle (0.1 ml, sc) 24 h before experimentation. The oil vehicle treatment was used to compare the current animal treatment with that of our previous studies. Other females were used intact during the late follicular phase of the reproductive cycle for electrophysiology experiments. A small number of recordings were made in intact male Topeka guinea pigs to test for the effects of glutamate agonists on basal firing activity (Fig. 2, D and E) and the hyperpolarizing effects of leptin on NPY neurons (Fig. 3, B, C, and E).
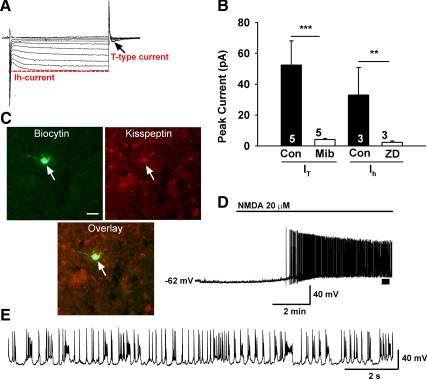
Fig. 2.
Endogenous conductances and NMDA-induced burst firing of guinea pig kisspeptin neurons. A, Ensemble of currents in response to depolarizing/hyperpolarizing steps from −50 to −140 mV illustrating the expression of a hyperpolarization-activated cation current (h-current) and a transient (T-type) Ca2+ current (arrow) in a representative kisspeptin neuron in the presence of TTX (1 μm). Vhold = −60 mV. B, Summary of the effects of selective h-current and T-type calcium current blockers in arcuate kisspeptin neurons. The peak h-current, measured as the difference between the instantaneous and steady-state current during a 1 s step to −140 mV, was completely blocked by ZD7288 (50 μm). The peak T-type Ca2+ current, measured as the rebound inward current after the step back from −140 to −60 mV, was blocked by mibefradil (10 μm). Cells were treated with ZD or mibefradil for 20 min to abolish the currents. C, Biocytin-streptavidin-Cy2 staining of a recorded cell and kisspeptin immunocytochemical Cy3 staining of the same cell. Bar, 10 μm. Initial studies used immunocytochemical staining for identifying the kisspeptin neurons, and then we moved exclusively to scRT-PCR to identify additional transcripts in the recorded cells. D, Current clamp recording in a kisspeptin neuron from an intact male showing the response to NMDA (20 μm). Similar responses were recorded in six of eight female kisspeptin neurons. RMP = −62 mV. E, The spiking above the bar in D was expanded to illustrate the pronounced effects of NMDA on burst firing activity of kisspeptin neurons with an ensemble of spikes riding on top of low threshold spikes. Cell continued to burst fire for 30 min until it was harvested for scRT-PCR.
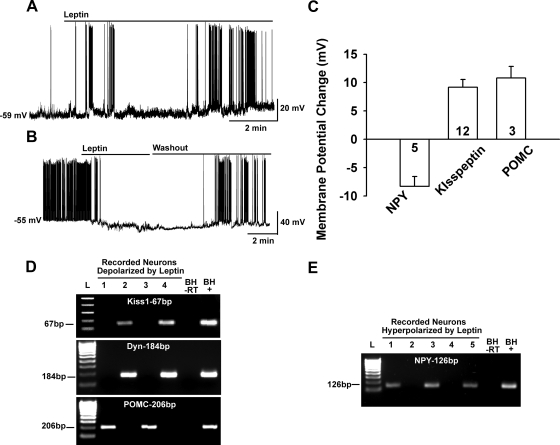
Fig. 3.
Leptin excites kisspeptin and POMC neurons but inhibits NPY neurons. A, Leptin (100 nm) depolarized a kisspeptin neuron and increased the firing frequency, which continued even after a long “washout” period for 30 min until the cell was harvested for scRT-PCR. Leptin had a similar long-lasting depolarizing effect on POMC neurons (data not shown). B, In contrast, leptin (100 nm) hyperpolarized NPY neurons (five of five) and abrogated firing, which fully recovered after several minutes of washing out leptin. C, Summary of the effects of leptin on NPY, kisspeptin, and POMC neurons. Leptin depolarized kisspeptin and POMC neurons, 9.8 ± 1.4 mV and 10.8 ± 2.0 mV, respectively. In contrast, leptin hyperpolarized both male (n = 2) and female (n = 3) NPY neurons (8.3 ± 1.8 mV, n = 5). D and E, Representative gels illustrating the expression of kisspeptin (Kiss-1), Dyn, and POMC mRNAs in leptin-depolarized neurons (D) or NPY mRNA expression in leptin-hyperpolarized neurons (E). Basal hypothalamic total RNA was also reverse transcribed in the presence or absence of RT (tissue controls, +/− respectively). Two different ladders (L) were used: one for Kiss1 (50-bp DNA ladder) and the other for Dyn, POMC, and NPY (100-bp DNA ladder).
Experimental design
For the kisspeptin-dynorphin mRNA colocalization experiments we used OVX animals to determine the maximum colocalization. This was based on findings in the mouse and on preliminary evidence in the guinea pig that both kisspeptin and dynorphin mRNA expression in the arcuate nucleus is increased in OVX compared with estradiol-treated animals. Most of the electrophysiology experiments were done in intact animals during the late follicular phase because we were interested in determining the basic characteristics of leptin signaling in kisspeptin neurons in intact animals before studying these characteristics in the presence and absence of 17β-estradiol. We chose the late follicular phase of the cycle because this is when serum E2 levels are elevated and when we would observe a maximal response to leptin based on our working model. To document LR mRNA expression in guinea pig kisspeptin neurons, we used cells harvested from an OVX estradiol-treated female as a model for late follicular phase animals.
Electrophysiological solutions/drugs
A standard artificial cerebrospinal fluid (aCSF) was used with the exception of when La3+ was added to the bath, a HEPES-buffered CSF solution was used (23). For experiments measuring the ramp current-voltage (I-V.), K+-gluconate in the normal internal solution was replaced with Cs+-gluconate (pH 7.35 with CsOH), and the extracellular solution contained Na+, K+, Ih (HCN), Ca2+, and GABAA channel blockers [in mm: NaCl, 126; 4-aminopyridine, 5; KCl, 2.5; MgCl2, 1.2; CsCl, 2; CaCl2, 1.4; CoCl2, 1; nifedipine, 0.01; HEPES, 20; NaOH, 8; glucose, 10; tetrodotoxin (TTX), 0.001; picrotoxin, 0.1].
All drugs were purchased from Calbiochem (La Jolla, CA) unless otherwise specified. Leptin was provided by Dr. Parlow (Harbor-UCLA Medical Center, Torrance, CA) through the National Hormone and Peptide Program. TTX (Alomone Labs, Jerusalem, Israel) (1 mm) was dissolved in H2O and further diluted with 0.1% acetic acid, pH 4–5. 4-Ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride (ZD7288) (Ascent, 50 mm) and mibefradil (Upjohn Pharmaceuticals, 10 mm), N-Methyl-d-aspartic acid (NMDA) (Sigma, 40 mm) and LaCl3 (100 mm) were dissolved in H20. The relatively selective TRPC channel blocker 2-aminoethyldiphenylborinate (2-APB, 100 mm) was dissolved in dimethylsulfoxide. The Janus kinase (Jak)2 inhibitor (E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)acrylamide (AG 490, 10 mm) and the phosphatidylinositol 3 kinase (PI3K) inhibitor wortmannin (Ascent, 100 μm) were dissolved in dimethylsulfoxide. The selective phospholipase C (PLC) γ inhibitor 1-O-octadecyl-2- O-methyl-rac-glycero-3-phosphorylcholine (ET-18-OCH3, 15 mm) was dissolved in H2O. Aliquots of the stock solutions were stored as appropriate until needed.
Preparation and maintenance of arcuate slices
Intact (late follicular) and OVX oil-treated female guinea pigs were killed by decapitation, the brain quickly removed from the skull, and a block containing the basal hypothalamus (BH) was immediately dissected. The BH block was submerged in cold (4 C) oxygenated (95% O2, 5% CO2) high sucrose aCSF (in mm): 208 sucrose, 2 KCl, 26 NaHCO3, 10 glucose, 1.25 NaH2PO4, 2 MgSO4, 1 CaCl2, 10 HEPES, (pH 7.4). Coronal slices (∼250 μm) were cut on a vibratome during which time (10 min) the slices were bathed in high sucrose CSF at 4 C. The slices were then transferred to an auxiliary chamber where they were kept at room temperature (25 C) in aCSF consisting of (in mm): 124 NaCl, 5 KCl, 2.6 NaH2PO4, 2 MgSO4, 2 CaCl2, 26 NaHCO3, 10 HEPES, 10 glucose (pH 7.4) until recording (recovery for 2 h). A single slice from the arcuate nucleus area was transferred to the recording chamber at a time and was kept viable by continually perfusing with warm (35 C) oxygenated aCSF at 1.5 ml/min.
Visualized whole-cell patch recording
Whole-cell patch recordings were made in medial to caudal arcuate nucleus neurons using an Olympus BX51 W1 fixed stage scope out-fitted with IR-DIC video imaging as previously described (24). Patch pipettes (A-M Systems, Seattle; 1.5 mm O.D. borosilicate glass) were pulled on a Brown/Flaming puller (Sutter Instrument Co., Model P-97) and filled with the following solution (in mm): 128 potassium gluconate, 10 NaCl, 1 MgCl2, 11 EGTA, 10 HEPES, 2 ATP, 0.25 GTP; adjusted to pH 7.3 with KOH; 295 mOsm. For some of the recordings, 0.25% biocytin was included in the internal solution for post hoc identification of kisspeptin neurons (see below). Pipette resistances ranged from 3–5 mΩ. In whole cell configuration, access resistance was less than 20 mΩ; the access resistance was 80% compensated. The input resistance (Rin) was calculated by measuring the slope of the current-voltage relationship curve between −70 and −50 mV. Standard whole-cell patch recording procedures and pharmacological testing were followed as previously described (23, 25, 26). Electrophysiological signals were digitized with Digidata 1322A (Axon Instruments), and the data were analyzed using p-Clamp software (version 9.2, Molecular Devices, Foster City, CA). Steady-state current/voltage (I-V) plots were constructed with step command potentials from −140 to −50 mV with a step of 10 mV (holding potential was −60 mV) and duration of 1s. I-V relationships of the ligand-sensitive (i.e., leptin) currents were obtained by subtracting the I-V curve in control conditions from that in the presence of drug. The liquid junction potential was corrected for all data analysis.
Immunocytochemical identification of hypothalamic arcuate neurons
For whole-cell recording we used a standard internal solution that included biocytin, then after the electrophysiological recording the slices were fixed with 4% paraformaldehyde and prepared for kisspeptin immunocytochemistry as described previously (27) with the following modifications: Biocytin-filled neurons were labeled with streptavidin-Cy2 (1:7500; Jackson ImmunoResearch, West Grove, PA), and the labeled cells were reacted for kisspeptin using a rabbit polyclonal antibody (kindly provided by Dr. Alan Caraty, Nouzilly, France) (28) at 1:2500 dilution. Kisspeptin-positive cells were visualized with Cy3 as described (27). Some kisspeptin neurons were identified using sc RT-PCR post hoc after recording (see below). Because there is no available NPY antisera for staining cell bodies in non–colchicine-treated animals, NPY neurons were identified using scRT-PCR (see below). Recorded POMC neurons were also identified using scRT-PCR. Two control experiments were performed for kisspeptin immunocytochemical staining: 1) Absorption control using the kisspeptin antiserum (1:5,000- 1:10,000 dilution) with added kisspeptin (1–10) peptide (1–10 μm); 2) Replacement of primary kisspeptin antiserum with PBS. For colocalization of kisspeptin and TRPC5, double-label immunocytochemistry was performed as described previously (29) using the rabbit kisspeptin antibody from Caraty (1:20,000) and a mouse monoclonal TRPC5 antibody (1:500, UC Davis/NIH NeuroMab Facility, Davis, CA). Immunoreactive kisspeptin was visualized using biotinylated IgG followed by streptavidin-conjugated to Cy2 and TRPC5 with IgG-conjugated to Cy3. Immunostained cells were photographed using a Nikon E800 microscope equipped with a digital Nikon DS camera. Brightness and contrast of digitized images were adjusted using Adobe Photoshop (Mountain View, CA) software to match images observed in the microscope.
Harvesting of arcuate neurons and scRT-PCR
Two to three 300-μm basal hypothalamic slices were cut on a vibratome and placed in an auxiliary chamber containing oxygenated aCSF (25). The slices were allowed to recover for 1–2 h in the chamber before dispersion. The cells were dispersed according to established protocols (26). The cells were visualized using a Leitz inverted microscope, patched, and then harvested by applying negative pressure to the pipette. Also after electrophysiological recording in the slice, the content of the cell was aspirated into the patch-pipette under visual control. In all cases, the contents of the pipette were expelled into a test tube containing a solution with 15 U of RNasin and 10 mm of dithiothreitol in a total of 5 μl. Each harvested cell was reverse transcribed as described previously (26) with a modification in which both random primers (100 ng per cell, Promega, Madison, WI) and anchored oligo(dT)20 primer (400 ng per cell, Invitrogen, Carlsbad, CA), and superscript III transcriptase (100 U per cell, Invitrogen) were used. Cells and tissue RNA used as negative controls were processed as described above but without reverse transcriptase (RT). Primers for the scPCR were designed using the Clone Manager software (Sci Ed Software, Cary, NC) and optimized for each reaction (Table 1). The PCR was performed using 1–4 μl of cDNA template from each RT reaction in a 20-μl PCR mix. Fifty cycles of amplification were performed using a BIO-RAD C1000 Thermal Cycler (Bio-Rad, Hercules, CA) according to established protocols (26). PCR products were visualized with ethidium bromide on a 2% agarose gel. In addition to the controls described above, harvested aCSF in the vicinity of the dispersed cells was also used as a control in the RT-PCR.
Table 1.
Primer sequences used in guinea pig arcuate scRT-PCR
| Gene name | Accession number or scaffold number | Product size (bp) | Primer sequence | Primer position |
|---|---|---|---|---|
| Kiss1 | 1321987 | 67 | GTGTCACCTCCTTGGGAGAAC | Forward 7-27 |
| TGGCCTGTGGGTCTAGGAT | Reverse 73-55 | |||
| Dyn | U38912 | 184 | AAGTGCGAGAGGGATGGTGTG | Forward 147-167 |
| CTCAGAGGGCAGCAAGGTTTC | Reverse 330-310 | |||
| POMC | S78260 | 206 | CTGGCCTTGCTGCTTCAGAT | Forward 40-59 |
| AAGTGGCCCGTGACGTACTT | Reverse 245-226 | |||
| NPY | M15789 | 126 | CTGCGACACTACATCAACC | Forward 168-186 |
| GTCTTCAAGCCGAGTTCTG | Reverse 293-275 | |||
| LRb | scaffold_2.549.1 | 124 | ATGCTGGGCGCACTGTTAATAG | Forward 2947-2968 |
| GCTCAAATGTTTCGGGCTTCTG | Reverse 3070-3049 | |||
| TRPC1 | scaffold_8.413.1 | 167 | TGCCCAAGCCCGGAATTCAC | Forward 1256-1275 |
| GCTGGCAGTTAGACTGGGACAC | Reverse 1422-1401 | |||
| TRPC4 | DQ008965 | 136 | GGTTAAGCTGCAAAGGCATACG | Forward 1580-1601 |
| CCATGCTGTGCTTTCACATTGG | Reverse 1715-1694 | |||
| TRPC5 | DQ008966 | 167 | GGCTTCTCAGCACATTGTC | Forward 1131-1149 |
| CCATCAGGTTCCACCAATC | Reverse 1297-1279 | |||
| PLCγ1 | scaffold_45.086.1 | 226 | GATGAAGAGGAGCCCAAAG | Forward 1307-1325 |
| CATTTCGCCAGAAGGACAG | Reverse 1532-1514 |
Primer design
The primer pairs for guinea pig Kiss1 were designed based on a partial cDNA sequence with 310 bp (gene bank accession number 1321987) cloned in our Laboratory (Ronnekleiv, manuscript in preparation). This guinea pig sequence was blasted against the gene bank and validated to be the Kiss1 aligning with human, cow, mouse, and rat Kiss1 sequences. The primer pairs for guinea pig LR were designed based on the predicted mRNA (3882 bp) from genomic sequence in UCSC Genome Browser on Guinea Pig Feb. 2008 Assembly (cavPor3) (scaffold_2.549.1, http://genome.ucsc.edu/cgi-bin/hgGateway). This predicted sequence was aligned with human, mouse, and rat LR mRNA. The 124-bp product was for detecting the long form (LRb) of the LR. The NPY primers were previously designed based on human NPY mRNA sequence (30) and are listed in Table 1. The guinea pig TRPC1 primers were also designed based upon the predicted mRNA (3137bp) from genomic sequence in UCSC Genome Browser on Guinea Pig Feb. 2008 Assembly (cavPor3) (scaffold_8.413.1, http://genome.ucsc.edu/cgi-bin/hgGateway). This sequence had high homology with several other major mammalian species including the human, mouse and rat. The guinea pig PLCγ1 primers were designed based upon the predicted mRNA (3598bp) from genomic sequence in UCSC Genome Browser on Guinea Pig Feb. 2008 Assembly (cavPor3) (scaffold_45.086.1, http://genome.ucsc.edu/cgi-bin/hgGateway). This predicted sequence was aligned with human and mouse PLCγ1 mRNA. The primers for guinea pig prodynorphin (Dyn), POMC, TRPC4, and TRPC5 were designed based upon the existing guinea pig mRNA sequences from Gene Bank and are listed in Table 1. All of the primers were designed to cross an intron to eliminate any genomic DNA contaminations. In all cases, the PCR product in sc was sequenced to confirm the correct product.
Data analysis
Comparisons between different drug treatments were performed using a one-way ANOVA analysis with the Newman-Keuls's post hoc test. Differences were considered statistically significant if P < 0.05. All data are presented as mean ± sem.
Results
Identification and characterization of arcuate kisspeptin neurons in guinea pig
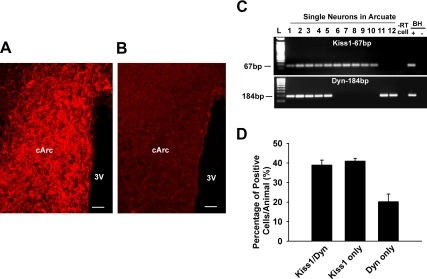
Kisspeptin neurons were found to be highly expressed in the medial to caudal regions of the arcuate nucleus of guinea pig hypothalamus (Fig. 1A). Extensive cell and fiber stain were observed primarily in the arcuate nucleus. Importantly, all immunoreactivity was eliminated in sections reacted with the absorbed antiserum (Fig. 1B) or in sections reacted without the primary antiserum (data not shown). In a number of animal models, kisspeptin neurons in the arcuate nucleus are almost exclusively colocalized with dynorphin (31, 32). Therefore, we dispersed neurons from the microdissected arcuate nucleus and harvested individual cells from four OVX female guinea pigs. The number of cells expressing Kiss1 and/or Dyn mRNA was determined using scRT-PCR (Fig. 1C,D). Of the 248 cells harvested, 141 cells (58%) expressed Kiss1 and 103 cells (42%) expressed Dyn. Among the Kiss1 identified cells, 51% expressed only Kiss1 and 49% coexpressed Dyn. Among the Dyn identified cells, 67% coexpressed Kiss1 and 33% expressed only dynorphin. Therefore, in OVX female guinea pigs, a subpopulation of kisspeptin neurons express dynorphin.
Fig. 1.
Coexpression of kisspeptin and Dyn in guinea pig arcuate neurons. A, Representative image of immunoreactive kisspeptin in the caudal arcuate (cArc) nucleus of an OVX, female guinea pig. Note that the kisspeptin cell bodies and the fibers are densely distributed in the Arc nucleus. B, Adjacent section showing the absorption control (see Materials and Methods). 3V, third ventricle. Bar, 50 μm. C, Representative gel images illustrating the mRNA expression of Kiss1 and dynorphin (Dyn) in harvested Arc neurons. Three subgroups were found: Kiss1/Dyn coexpressed cells, Kiss1 only expressed cells, and Dyn only expressed cells in hypothalamic Arc single neurons harvested from four OVX female guinea pigs. The expected size of each PCR product is indicated. The −RT cell (negative control) was reacted without RT. The BH+, BH− represent basal hypothalamic total RNA processed with and without RT, respectively. Two different ladders (L) were used: one for Kiss1 (50 bp DNA Ladder) and the other for Dyn (100 bp DNA ladder). D, Bar graph represents the mean ± sem of the percentage of Kiss1 and/or Dyn in 175 cells from four animals. An average of 44 cells was analyzed from each animal.
Basic characteristics of arcuate kisspeptin neurons
We targeted the more caudal arcuate nucleus area in slices primarily from intact, late follicular females using blind patch recording. We recorded from 190 neurons and identified 69 (36%) of these post hoc as kisspeptin neurons using either immunocytochemistry or scRT-PCR (Figs. 2–4). Female arcuate kisspeptin neurons exhibited a resting membrane potential of −58.5 ± 1.0 mV (n = 69) and Rm = 770.0 ± 62.2 mΩ, and the majority (80%) of these cell expressed both pacemaker (h-current) and T-type Ca2+ currents (n = 55, Fig. 2, A and B). In a subset of these kisspeptin neurons we blocked the T-type calcium current with mibefradil (10 μm, n = 5) and the h-current with ZD 7288 (50 μm, n = 3) (Fig. 2B). The expression of h-current and T-type calcium currents are critical for generating burst firing in hypothalamic (33, 34) and thalamic neurons (35). For comparison, the h-current was expressed in 58% of the nonkisspeptin neurons and the T-type calcium current in 50% of the cells.
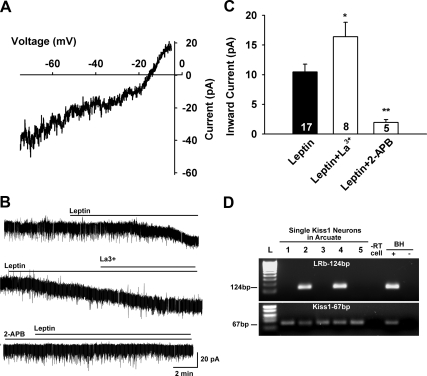
Fig. 4.
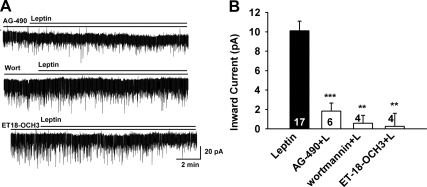
Leptin activation of nonselective cation current. A, The I-V relationship for the leptin-induced current was obtained by digital subtraction of the control I-V from the I-V in the presence of leptin (100 nm) using a Cs+-based internal solution and K+ channel blockers in the extracellular CSF (see Materials and Methods). The reversal potential of the nonselective cation current was −15 mV. B, Representative traces of the leptin-induced currents in the presence or absence of the TRPC4,5 activator La3+ (100 μm) or the relatively selective TRPC channel blocker 2-APB (100 μm). In voltage clamp, leptin induced an inward current in kisspeptin neurons (upper trace, 10.4 ± 1.3 pA), which was potentiated by La3+ (middle trace, 16.4 ± 2.4 pA). In another kisspeptin neuron leptin induced an inward current that was abrogated by 2-APB (lower trace, 1.9 ± 0.5 pA), applied 15 min before the application of leptin (100 nm). C, Summary of the effects of 2-APB and La3+ on the leptin-induced inward currents in guinea pig arcuate (including kisspeptin) neurons. *, P < 0.05; **, P < 0.01; significantly different from the maximum current induced by leptin alone. Cell numbers are indicated. D, Representative gel illustrating LRb mRNA expression in kisspeptin neurons. −RT cell and BH+, BH− represent controls processed with (+) without (−) RT.
Because the majority of kisspeptin neurons express these pacemaker currents, it was of interest to see whether these critical relay neurons in the reproductive circuit would exhibit burst firing behavior. Therefore, we bath perfused the glutamate receptor agonist NMDA (20 μm) and found that seven of nine kisspeptin neurons exhibited burst firing activity similar to subthalamic neurons (Fig. 2, D and E) (36). Hence, kisspeptin neurons express the endogenous pacemaker conductances (T-type calcium and h-currents) and can be driven via glutamate inputs to generate burst firing activity, which would be critical for governing GnRH neuronal output and fertility.
Leptin activates a nonselective cationic current in kisspeptin neurons
Because mouse kisspeptin neurons are known to express LRb (17), we hypothesized that leptin depolarizes kisspeptin neurons via activation of a nonselective cationic current (24). Indeed, leptin (100 nm) depolarized (9.2 ± 1.4 mV, n = 12) and increased the firing frequency of female kisspeptin neurons (2.5 to 5 Hz, n = 7, Fig. 3A). Similarly, as has been described previously in mouse POMC neurons (24, 37), leptin depolarized and increased the firing of guinea pig female POMC neurons (10.8 ± 2.0 mV, n = 3). Because we characterized the leptin depolarizing effects in mouse POMC neurons, we did not specifically target POMC neurons in the guinea pig, but they were included in the subsequent signaling pathway analysis (see below). In contrast, leptin hyperpolarized and inhibited NPY neurons (−8.3 ± 1.8 mV, n = 5) (Fig. 3B). This hyperpolarizing effect of leptin has been described in rat arcuate neurons and is mediated by the opening of K-ATP channels (38). Interestingly, the onset and washout of leptin's inhibitory effects on NPY neurons appeared to be more rapid compared with the depolarizing effects of leptin (Fig. 3B). However, we did not pursue the characterization of this signaling pathway to augment K-ATP channel activity.
To characterize the leptin-mediated depolarizing response in kisspeptin neurons, we voltage-clamped arcuate neurons (Vhold = −60 mV) and perfused TTX (500 nm) to synaptically isolate the cells. Leptin (100 nm) activated an inward current (10.4 ± 1.3 pA) in 28 of the neurons (Fig. 4), and 17 were identified using scRT-PCR as kisspeptin-positive. Three kisspeptin neurons did not respond to leptin (i.e., no change in the holding current). Interestingly, the magnitude of the response in all kisspeptin neurons (10.4 ± 1.3 pA, n = 17) did not differ from the overall mean excitatory response in guinea pig arcuate neurons or the mean leptin response (same concentration) of mouse POMC neurons (24). Indeed, six of the neurons in which leptin induced an inward current (10.5 ± 1.9 pA) were identified as POMC neurons based on post hoc scRT-PCR. Five of the “leptin-excited” arcuate neurons were not identified by scRT-PCR. Leptin (100 nm) induced an outward current (inhibition) in 13 arcuate neurons, five of which were identified as NPY neurons based on scRT-PCR.
To characterize the ionic mechanism(s) underlying the leptin-induced depolarization, we analyzed the reversal potential of the leptin-induced current. To examine the current-voltage relationship of the leptin-activated NSCC currents over a wider range of membrane potentials, experiments were conducted using a Cs+-gluconate–based internal solution to block voltage-gated K+ channels. Also, 4-AP (5 mm) and Cs+ (1 mm) were included in a HEPES-buffered CSF to block the A-type K+ and h-currents, respectively. Similar to mouse POMC neurons (24), the leptin-activated current reversed near −15 mV (Fig. 4A), indicative of the activation of a nonselective cationic current.
Because in heterologous expression systems micromolar concentrations of La3+ potentiate TRPC4 and TRPC5 (39) but inhibit TRPC1, 3, 6, and 7 channels (40), we tested the effect of this lanthanide on the leptin-activated current. Indeed, 100 μm La3+ greatly potentiated the leptin-induced current by 170% in all of the neurons tested (16.4 ± 2.4, n = 8, P < 0.05 vs. control) (Fig. 4, B and C). In addition we tested the relatively selective TRPC channel blocker 2-APB (41) on the leptin-induced inward current and found that in five of five neurons, including three kisspeptin neurons, 2-APB (100 μm) completely abrogated leptin's effects. Therefore, these physiological and pharmacological characteristics indicated that TRPC4 and 5 channels were involved in the leptin-mediated depolarization of kisspeptin neurons.
LRb is expressed in kisspeptin neurons and coupled to a JAK-PI3K-PLCγ pathway
Because it was evident that leptin activates TRPC channels in guinea pig kisspeptin neurons, we used scRT-PCR to identify the expression of LRb in kisspeptin neurons harvested from an OVX, estradiol (benzoate)-treated female guinea pig. We found that 36% of the neurons (5 out of 14) expressed LRb (Fig. 4D). Previously, we showed that the LR is coupled to the Jak2-PI3K signaling pathway to activate TRPC channels in mouse POMC neurons (24). In guinea pig kisspeptin neurons, the Jak inhibitor AG490 (10 μm) potently blocked the effects of leptin (1.8 ± 0.8 pA, n = 6, P < 0.001 vs. control) (Fig. 5, A and B). PI3K is essential for leptin-induced activation (24, 42), but it is also critical for the membrane insertion of TRPC channels (43). Therefore, we examined the role of PI3K in the leptin-induced inward current. Indeed, the selective PI3K inhibitor wortmannin (100 nm) blocked the leptin-induced inward current by 95% (0.6 ± 0.8 pA, n = 4, P < 0.001 vs. control) (Fig. 5, A and B). This indicates that the LRb couples to Jak2 to activate PI3K in guinea pig kisspeptin neurons. Because leptin activates PLCγ1 in mouse POMC neurons (24), we perfused the selective PLCγ inhibitor ET-18-OCH3 (15 μm) and found that it potently blocked the effects of leptin (0.2 ± 1.4 pA, n = 4, P < 0.001 vs. control) (Fig. 5, A and B). Finally, because LRb is coupled through PLCγ1 in the mouse POMC neurons (24), we used scRT-PCR to identify the expression of this transcript in guinea pig kisspeptin neurons (Fig. 6, A and B). We found that the PLCγ1 was expressed in 78% of guinea pig kisspeptin neurons. Overall, it appears that leptin couples to TRPC channel activation in guinea pig kisspeptin neurons through LRb-Jak2-PI3K and PLCγ1 signaling pathways.
Fig. 5.
The leptin response in arcuate neurons requires Jak2, PI3K, and PLCγ activation. A, Representative traces of the leptin-induced currents in the presence of signaling pathway inhibitors. Upper trace, Jak2 inhibitor AG490 (10 μm); middle trace, PI3K inhibitor wortmannin (100 nm); lower trace, PLCγ inhibitor ET-18-OCH3 (15 μm). Blockers were applied for 15 min before the application of leptin (100 nm). Vhold = −60 mV. B, Summary of the effects of the Jak2 inhibitor AG490, PI3K inhibitor wortmannin, and the PLCγ inhibitor ET-18-OCH3 on the leptin-induced inward current in arcuate neurons (including kisspeptin neurons). **, P < 0.01; ***, P < 0.001; significantly different from the leptin control group. Cell numbers tested are indicated.
Fig. 6.
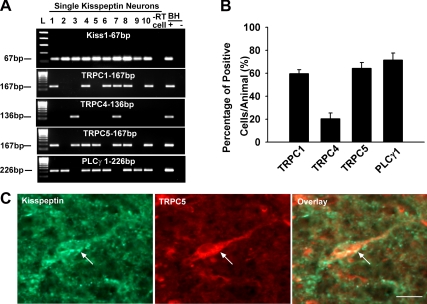
TRPC channel and PLCγ transcript expression in arcuate kisspeptin neurons as measured by scRT-PCR. A, Representative gel image illustrating mRNA expression of Kiss1, TRPC1, TRPC4, TRPC5, and PLCγ1 in hypothalamic arcuate single neurons harvested from OVX female guinea pigs. The expected size of each PCR product is indicated. The −RT cell (negative control) was reacted without RT. The BH+, BH− represent basal hypothalamic total RNA processed with and without RT, respectively. Two different ladders (L) were used: one for Kiss1 (50-bp DNA ladder) and the other (100-bp DNA ladder) for TRPC1, TRPC4, TRPC5, and PLCγ1. B, Summary of the expression of TRPC1, TRPC4, TRPC5, and PLCγ1 transcripts (mean ± sem of the percentage) in 114 kisspeptin neurons from four guinea pig females. An average of 28 cells was analyzed from each animal. C, Double-label immunocytochemical staining for kisspeptin and the TRPC5 channel subunit protein (in a guinea pig arcuate nucleus) illustrating the presence of TRPC5 channel protein in arcuate kisspeptin neurons. Bar, 10 μm.
TRPC channel transcripts are expressed in kisspeptin neurons
Based on the physiological and pharmacological properties, we measured the expression of TRPC1, 4, and 5 channel transcripts in kisspeptin neurons. We analyzed 114 cells that expressed Kiss1 mRNA. We detected the expression of TRPC1 mRNA in 60.0 ± 3.5% of the cells, TRPC4 mRNA in 20.3 ± 5.0% of the cells, and TRPC5 mRNA in 64.3 ± 4.9% of the cells (Fig. 6, A and B), indicating that TRPC1 and 5 are the primary subunits in kisspeptin neurons. Therefore, we used immunocytochemical staining to identify TRPC5 channel protein in kisspeptin neurons. Consistent with the physiological findings, the immunocytochemical analysis revealed that TRPC 5 protein was expressed in kisspeptin neurons (Fig. 6C).
Discussion
Using whole-cell patch clamp recordings and scRT-PCR, we have established that arcuate nucleus kisspeptin neurons express the pacemaker (h- and T-type calcium) currents that invariably contribute to burst firing in central nervous system neurons. In addition, NMDA potently depolarized kisspeptin neurons and induced bursting activity. Most importantly for linking energy homeostasis with reproduction, the adipocyte hormone leptin induced an inward (nonselective cation) current in kisspeptin neurons and also increased burst firing. Based on current-voltage relationship, the block by 2-APB, the potentiating effects of La3+, and the mRNA expression in individual neurons, LRb appears to be coupled to activation of the TRPC1 and TRPC5 channels in kisspeptin neurons.
Kisspeptin neurons are highly expressed in the guinea pig arcuate nucleus, fifty percent of which coexpress the opioid peptide dynorphin in comparison with the near 80–90% colocalization reported in mouse and sheep arcuate neurons (31, 32). Based on findings in the mouse (32), both kisspeptin and dynorphin mRNAs are increased in OVX animals and highly reduced with E2 treatment. Therefore, we used the OVX guinea pig for this coexpression analysis to determine the maximum coexpression of the two peptides in the guinea pig. The functional significance of the colocalization differences is currently not well understood but may be related to a differential physiological role for arcuate nucleus kisspeptin/dynorphin neurons in the various species (44, 45). The other subpopulation of guinea pig arcuate kisspeptin neurons may colocalize a different transmitter, but further experimentation is needed to tease out the physiological significance of this colocalization in guinea pig arcuate neurons.
Although there are a plethora of publications that implicate kisspeptin neurons as vital for initiating pubertal development and controlling the mammalian reproductive cycle, virtually nothing is known about the excitability of these neurons. A recent report using cell-attached recordings of mouse anteroventral periventricular kisspeptin neurons, identified post hoc by immunohistochemistry, suggested that a small subset (25%) of these neurons generate burst-like activity (46). Because the guinea pig does not have a prominent anteroventral periventricular population of kisspeptin neurons (Ronnekleiv, manuscript in preparation), we targeted the arcuate kisspeptin neurons for our recordings using the intact, late follicular phase female as an animal model. The majority of guinea pig kisspeptin neurons exhibited both a hyperpolarization-activated cation (HCN, pacemaker) current and a T-type calcium current. Both of these conductances play critical roles in generating burst firing in hypothalamic neurosecretory neurons (34) and thalamic relay neurons (35). Indeed, in the presence of the glutamate receptor agonist NMDA, the majority of kisspeptin neurons depolarized and exhibited burst firing activity, emphasizing the importance of the expression of the pacemaker (h and T) currents in kisspeptin neurons during this stage of the ovulatory cycle when E2 levels are increased.
Most importantly, we found that leptin depolarized kisspeptin neurons via activation of an inward, nonselective cation current that increased burst firing. These findings are consistent with our previous findings in mouse EGFP-POMC neurons (24). We now have confirmed these effects in guinea pig “native” POMC neurons. In contrast, leptin hyperpolarized and inhibited the firing of guinea pig NPY neurons, similar to what has been described in rat arcuate neurons (38). This hyperpolarizing effect has been ascribed to the activation of K-ATP channels (47). Leptin also inhibits high-voltage activated calcium currents in NPY neurons (48), further contributing to inhibition of these orexigenic neurons. The divergence of the excitatory (kisspeptin and POMC neurons) and inhibitory (NPY neurons) effects of leptin occurs downstream of the hormone binding to its receptor and activating (phosphorylating) Jak2 tyrosine kinase. One critical downstream signaling pathway is the activation of signal transducer and activator of transcription 3 (STAT3), which is important for gene activation (49, 50). Some of the major metabolic effects of leptin are believed to be mediated by STAT3 because neuronal depletion of STAT3 results in an obese phenotype similar to LR-deficient mice (49, 50). However, mice containing a mutant LRb incapable of STAT3 signaling are obese but remain fertile (51), which would suggest that STAT3 signaling in LRb-expressing neurons does not regulate fertility. Therefore, other leptin signaling pathways, such as the insulin receptor substrate (IRS)–PI3K signaling pathway (52), must be involved. Indeed as we and others have described in mouse POMC neurons (24, 42), the PI3K pathway appears to be critical for the rapid excitatory effects of leptin in guinea pig kisspeptin neurons. The PI3K inhibitor wortmannin potently blocked the effects of leptin. Furthermore, activation of PI3K generates phosphatidylinositol-3,4,5-triphosphate (PIP3), which contributes to the translocation and activation of PLCγ at the plasma membrane (53). The PLCγ inhibitor ET-18-OCH3 completely abrogated the effects of leptin, similar to what we have described in mouse POMC neurons (24). Finally, using scRT-PCR, we found that the majority of kisspeptin neurons express PLCγ1 transcript. PLCγ plays a critical role in augmenting TPRC channel activity in hippocampal CA1 neurons (43) and arcuate POMC neurons (24). Therefore, the leptin “excitatory” signaling pathway is similar in guinea pig kisspeptin neurons and mouse POMC neurons.
Similar to the excitatory effects of leptin in POMC neurons (24), we have found that leptin depolarizes kisspeptin neurons via activation of TRPC 4,5 channels. The tell-tale sign that the leptin-induced current in kisspeptin neurons is mediated by TRPC channels is the characteristic I-V relationship in the presence of a K+ channel blocker (i.e., Cs+). Indeed, the I-V relationship in kisspeptin neurons resembles the I-V relationship of heteromeric complexes of TRPC1 + 4 or TRPC1 + 5 subunits expressed in HEK cells with a negative slope conductance and pronounced outward rectification (39). Similar I-V relationships have been obtained with the leptin-activated currents in mouse POMC neurons (24). Moreover, the effects of leptin were blocked by the relatively selective TRPC blocker 2-APB (41). Previously, we found that these blocking effects were not attributable to the intracellular blockade of the inositol-1,4,5-trisphophate (IP3) receptor because intracellular dialysis with 2-APB had no effect on leptin's depolarizing response (24). Most importantly, La3+ greatly potentiated the leptin-induced current in kisspeptin neurons, which is a signature response of TRPC4 and 5 channel activation (54). In support of the electrophysiological and pharmacological studies, the majority of kisspeptin neurons expressed the TRPC1 plus 5 channel subunit combination, which is similar to the findings in arcuate POMC neurons (24), and in addition TRPC5 channel protein was expressed in the guinea pig kisspeptin neurons.
Presently, we have found that leptin excites the majority of kisspeptin neurons in slices obtained from fed animals during the late follicular phase of the ovulatory cycle when plasma levels of E2 are elevated. This may indicate that leptin can override the effects of E2, which are hypothesized to be inhibitory to arcuate kissppeptin neurons (55–57). However, it is not known how E2 affects the leptin responsiveness of kisspeptin neurons, which will be the focus of future experiments.
In summary, we have found that kisspeptin neurons express critical pacemaker (h and T-type) currents that contribute to burst firing. In addition, leptin depolarizes kisspeptin neurons via activation of TRPC channels, which may also contribute to burst firing activity and provide an excitatory (pacemaker) drive to GnRH neurons, thereby linking energy states with fertility. Although more studies are needed to fully characterize kisspeptin neurons, it is evident that guinea pig arcuate kisspeptin neurons contain the endogenous armament necessary for driving GnRH neuronal excitability.
Acknowledgments
This work was supported by United States Public Health Service Grants NS 38809, NS 43330, and DK 68098.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- aCSF
- Artificial cerebrospinal fluid
- BH
- basal hypothalamus
- Dyn
- prodynorphin
- JAK
- Janus kinase
- LR
- leptin receptor
- LRb
- leptin receptor b
- NMDA
- N-Methyl-d-aspartic acid
- NPY
- neuropeptide Y
- OVX
- ovariectomized
- PI3K
- phosphatidylinositol 3 kinase
- PLC
- phospholipase C
- POMC
- proopiomelanocortin
- RT
- reverse transcriptase
- sc
- single cell
- STAT3
- signal transducer and activator of transcription 3
- TTX
- tetrodotoxin.
References
- 1. Kauffman AS, Clifton DK, Steiner RA. 2007. Emerging ideas about kisspeptin-GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci 30:504–511 [DOI] [PubMed] [Google Scholar]
- 2. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. 2006. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26:6687–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ. 2006. Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotrophin-releasing hormone/lutenising hormone surge in the ewe suggests a stimulatory role for kisspeptin in oestrogen-positive feedback. J Neuroendocrinol 18:806–809 [DOI] [PubMed] [Google Scholar]
- 4. Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden J-M, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. 2001. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 276:34631–34636 [DOI] [PubMed] [Google Scholar]
- 5. Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. 2004. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuronendocrinology 80:264–272 [DOI] [PubMed] [Google Scholar]
- 6. Han S-K, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. 2005. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MBL, Colledge WH, Caraty A, Aparicio SAJR. 2005. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Roux N, Genin E, Carel J-C, Matsuda F, Chaussain J-L, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS 1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MBL, Crowley WF, Aparicio SAJR, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. The New England Journal of Medicine 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 10. d'Anglemont de Tassigny X, Fagg LA, Dixon JPC, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MBL, Aparicio SAJR, Colledge WH. 2007. Hypogonadotropic hypogonadism in mice lacking a functional KiSS 1 gene. Proc Natl Acad Sci USA 104:10714–10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan JL, Mantzoros CS. 2005. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorrexia nervosa. Lancet 366:74–85 [DOI] [PubMed] [Google Scholar]
- 12. Myers MG., Jr 2004. Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res 59:287–304 [DOI] [PubMed] [Google Scholar]
- 13. Bjørbæk C, Uotani S, da Silva B, Flier JS. 1997. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem 272:32686–32695 [DOI] [PubMed] [Google Scholar]
- 14. Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. 1998. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395:535–547 [PubMed] [Google Scholar]
- 15. Håkansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. 1998. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci 18:559–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meister B, Håkansson ML. 2001. Leptin receptors in hypothalamus and circumventricular organs. Clin Exp Pharmacol Physiol 28:610–617 [DOI] [PubMed] [Google Scholar]
- 17. Smith JT, Acohido BV, Clifton DK, Steiner RA. 2006. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol 18:298–303 [DOI] [PubMed] [Google Scholar]
- 18. Quennell JH, Mulligan AC, Tups A, Liu X, Phipps SJ, Kemp CJ, Herbison AE, Grattan DR, Anderson GM. 2009. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 150:2805–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Montez JM, Soukas A, Asilmaz E, Fayzikhodjaeva G, Fantuzzi G, Friedman JM. 2005. Acute leptin deficiency, leptin resistance, and the physiologic response to leptin withdrawal. Proc Natl Acad Sci USA 102:2537–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Castellano JM, Navarro VM, Fernández-Fernández R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. 2005. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the repoductive axis by kisspeptin in undernutrition. Endocrinology 146:3917–3925 [DOI] [PubMed] [Google Scholar]
- 21. Yamada S, Uenoyama Y, Kinoshita M, Iwata K, Takase K, Matsui H, Adachi S, Inoue K, Maeda K-I, Tsukamura H. 2007. Inhibition of metastin (kisspeptin-54)-GPR54 signaling in the arcuate nucleus-median eminence region during lactation in rats. Endocrinology 148:2226–2232 [DOI] [PubMed] [Google Scholar]
- 22. Xu J, Kirigiti MA, Grove KL, Smith MS. 2009. Regulation of food intake and gonadotropin-releasing hormone/luteinizing hormone during lactation: role of insul and leptin. Endocrinology 150:4231–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. 2008. Kisspeptin depolarizes GnRH neurons through activation of TRPC-like cationic channels. J Neurosci 28:4423–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qiu J, Fang Y, Rønnekleiv OK, Kelly MJ. 2010. Leptin exceites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci 30:1560–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Rønnekleiv OK, Low MJ, Kelly MJ. 2003. Hypothalamic proopiomelanocortin neurons are glucose-responsive and express K-ATP channels. Endocrinology 144:1331–1340 [DOI] [PubMed] [Google Scholar]
- 26. Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ. 2007. Gonadotropin-releasing hormone neurons express KATP Channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci 27:10153–10164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kelly MJ, Rønnekleiv OK. 1994. Electrophysiological analysis of neuroendocrine neuronal activity in hypothalamic slices. In: Levine JE. ed. Methods in neurosciences: pulsatility in neuroendocrine systems. 1st ed. San Diego: Academic Press, Inc.; 47–67 [Google Scholar]
- 28. Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. 2006. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett 401:225–230 [DOI] [PubMed] [Google Scholar]
- 29. Dziennis S, Jia T, Rønnekleiv OK, Hurn PD, Alkayed NJ. 2007. Role of signal transducer and activator of transcription-3 in estradiol-mediated neuroprotection. J Neurosci 27:7268–7274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roepke TA, Malyala A, Bosch MA, Kelly MJ, Rønnekleiv OK. 2007. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology 148:4937–4951 [DOI] [PubMed] [Google Scholar]
- 31. Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CVR, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. 2007. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148:5752–5760 [DOI] [PubMed] [Google Scholar]
- 32. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. 2009. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Erickson KR, Rønnekleiv OK, Kelly MJ. 1993. Electrophysiology of guinea-pig supraoptic neurones: Role of a hyperpolarization-activated cation current in phasic firing. J Physiol (Lond) 460:407–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Erickson KR, Rønnekleiv OK, Kelly MJ. 1993. Role of a T-type calcium current in supporting a depolarizing potential, damped oscillations, and phasic firing in vasopressinergic guinea pig supraoptic neurons. Neuroendo 57:789–800 [DOI] [PubMed] [Google Scholar]
- 35. McCormick DA, Huguenard JR. 1992. A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol 68:1384–1400 [DOI] [PubMed] [Google Scholar]
- 36. Bevan MD, Wilson CJ. 1999. Mechanisms underlying spontaneous oscillation and rhythmic firing in rat subthalamic neurons. J Neurosci 19:7617–7628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. 2001. Leptin activates anorexigenic POMC neurons through a neural network in arcuate nucleus. Nature 411:480–484 [DOI] [PubMed] [Google Scholar]
- 38. Van den Top M, Lyons DJ, Coderre E, Renaud LP, Spanswick D. 2007. Pharmacological and molecular characterization of ATP-sensitive K+ conductances in CART and NPY/AgRP expressing neurons of the hypothalamic arcuate nucleus. Neurosci 144:815–824 [DOI] [PubMed] [Google Scholar]
- 39. Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. 2001. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29:645–655 [DOI] [PubMed] [Google Scholar]
- 40. Clapham DE. 2007. Snapshot: mammalian TRP channels. Cell 129:220. [DOI] [PubMed] [Google Scholar]
- 41. Clapham D, Julius D, Montell C, Schultz G. 2005. International union of pharmacology. XLIX. nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev 57:427–450 [DOI] [PubMed] [Google Scholar]
- 42. Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. 2008. Acute effects of leptin require PI3K signalng in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest 118:1796–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. 2004. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol 6:709–720 [DOI] [PubMed] [Google Scholar]
- 44. Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. 2007. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci 27:12088–12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Caraty A, Franceschini I, Hoffman GE. 2010. Kisspeptin and the preovulatory GnRH/LH surge in the ewe: basic aspects and potential applications in the control of ovulation. J Neuroendocrinology 22:710–715 [DOI] [PubMed] [Google Scholar]
- 46. Ducret E, Gaidamaka G, Herbison AE. 2010. Electrical and morophological characteristics of anteroventral periventricular nucleus kisspeptin and other neurons in the female mouse. Endocrinology 151:2223–2232 [DOI] [PubMed] [Google Scholar]
- 47. Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford MLJ. 1997. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390:521–525 [DOI] [PubMed] [Google Scholar]
- 48. Wang J-H, Wang F, Yang M-J, Yu D-F, Wu W-N, Liu J, Ma L-Q, Cai F, Chen J-G. 2008. Leptin regulated calcium channels of NPY and POMC neurons by activation of different signal pathways. Neurosci 156:89–98 [DOI] [PubMed] [Google Scholar]
- 49. Bates SH, Dundon TA, Seifert M, Carlson M, Maratos-Flier E, Myers MG., Jr 2008. LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes 53:3067–3073 [DOI] [PubMed] [Google Scholar]
- 50. Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu X-Y. 2004. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci USA 101:4661–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Piper ML, Unger EK, Myers MG, Jr, Xu AW. 2008. Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol Endo 22:751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. 2006. Central nervous system control of food intake and body weight. Nature 443:289–295 [DOI] [PubMed] [Google Scholar]
- 53. Bae YS, Cantley LG, Chen C-S, Kim S-R, Kwon K-S, Rhee SG. 1998. Activation of phospholipase Cγ by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273:4465–4469 [DOI] [PubMed] [Google Scholar]
- 54. Blair NT, Kaczmarek JS, Clapham DE. 2009. Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J Gen Physiol 133:525–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smith JT, Clay CM, Caraty A, Clarke IJ. 2007. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 148:1150–1157 [DOI] [PubMed] [Google Scholar]
- 56. Gottsch MI, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Lameson JL, Clifton DK, Levine JE, Steiner RA. 2009. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci 29:9390–9395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Eghlidi DH, Haley GE, Noriega NC, Kohama SG, Urbanski HF. 2010. Influence of age and 17β-estradaiol on kisspeptin, neurokinin B, and prodynorphin gene expression in the arucate-median eminence of female Rhesus Macaques. Endocrinology 151:3783–3794 [DOI] [PMC free article] [PubMed] [Google Scholar]