This study examines the long-term outcome of clinical stage II/III rectal cancer patients treated in a prospective phase II study of bevacizumab with chemoradiation and surgery. Neoadjuvant bevacizumab with standard chemoradiation and surgery shows promising long-term efficacy and safety profiles in locally advanced rectal cancer patients.
Keywords: Bevacizumab, Neoadjuvant, Rectal cancer, Chemoradiation, Toxicity
Abstract
Introduction.
Bevacizumab is increasingly being tested with neoadjuvant regimens in patients with localized cancer, but its effects on metastasis and survival remain unknown. This study examines the long-term outcome of clinical stage II/III rectal cancer patients treated in a prospective phase II study of bevacizumab with chemoradiation and surgery. As a benchmark, we used data from an analysis of 42 patients with locally advanced rectal cancer treated with a contemporary approach of preoperative fluoropyrimidine-based radiation therapy.
Materials and Methods.
Outcome analyses were performed on 32 patients treated prospectively with neoadjuvant bevacizumab, 5-fluorouracil, radiation therapy, and surgery as well as 42 patients treated with standard fluoropyrimidine-based chemoradiation.
Results.
Overall survival, disease-free survival, and local control showed favorable trends in patients treated with bevacizumab with chemoradiation followed by surgery. Acute and postoperative toxicity appeared acceptable.
Conclusions.
Neoadjuvant bevacizumab with standard chemoradiation and surgery shows promising long-term efficacy and safety profiles in locally advanced rectal cancer patients.
Introduction
Over the past 25 years, significant progress has been made in the treatment of patients with localized rectal carcinoma. Advances in surgery, neoadjuvant chemotherapy, and radiation therapy have significantly enhanced clinical outcome [1]. Despite these gains, important challenges remain in the management of patients with this malignancy. Rectal cancer has an insidious propensity for both local invasion with potential loss of anorectal function and systemic spread resulting in profound patient suffering and mortality. Following neoadjuvant treatment of localized disease and surgery, 36% of patients develop distant metastases, which are often uncontrollable and ultimately treatment refractory [1]. The use of bevacizumab (Avastin®; Genentech, Inc., South San Francisco, CA), which has been shown to have efficacy with chemotherapy in randomized phase III trials and is a current standard of care in first- and second-line metastatic colorectal cancer [2, 3], might improve neoadjuvant regimens and prevent or reduce metastatic dissemination.
Bevacizumab is a blocking antibody against human vascular endothelial growth factor (VEGF), a critical and highly pleiotropic factor that promotes new vessel formation in tumors [4, 5]. Bevacizumab and other anti-VEGF agents (e.g., sunitinib, sorafenib) are either approved or in late phases of development for multiple cancer types [6]. However, anti-VEGF therapies have shown benefit only in advanced/metastatic stages of disease, and it remains unknown to date if they will benefit patients with localized disease in the neoadjuvant setting. Multiple trials are under way in rectal cancer, breast cancer, sarcoma, etc., testing the feasibility and efficacy of bevacizumab with cytotoxics as neoadjuvant treatment. In rectal cancer, several trials of bevacizumab with chemoradiation have shown promising results [7–9]. But the lack of randomization and the bias associated with single-arm, phase II trials raises important concerns when interpreting these data. Moreover, in preclinical models, genetic deletion or transient high-dose pharmacologic blockade of VEGF has resulted in hypoxia, systemic inflammation, and acceleration of tumor metastasis in experimental metastasis models (i.e., after metastatic cell infusion), despite shrinkage of primary tumors [10–12]. In contrast, acceleration of lymphatic metastasis was not seen in a neoadjuvant model, of treatment with cediranib or vandetanib, after surgical removal of the primary tumor in mice [13]. More importantly, neither acceleration nor delay of metastasis has been reported in metastatic cancer patients after treatment with anti-VEGF agents, but no randomized study to date has tested the use of bevacizumab in the neoadjuvant setting for localized disease.
In 2002, we initiated a phase I/II clinical trial (National Cancer Institute [NCI] #5642) incorporating neoadjuvant bevacizumab monotherapy for one 2-week cycle followed by three cycles of bevacizumab with standard 5-fluorouracil (5-FU), radiation therapy, and surgery in patients with locally advanced rectal cancer. Study results have shown the feasibility of this approach, promising clinical results, and the elucidation of a critical mechanism of action of bevacizumab [9, 14, 15]. This report summarizes the long-term outcomes of these 32 patients. As a benchmark, we used the data from an analysis of 42 patients with locally advanced rectal cancer treated with a contemporary approach of preoperative fluoropyrimidine-based radiation therapy.
Materials and Methods
Patients (NCI #5642)
NCI #5642 was a multicenter, phase I/II clinical trial of 32 patients (17 from Massachusetts General Hospital and 15 from Duke University Medical Center [DUMC]) that was approved by the Cancer Therapeutics Evaluation Program of the NCI as well as the internal review boards of the Massachusetts General Hospital (2002–2008) and DUMC (2004–2008) [9]. Informed written consent was obtained from all patients. Eligibility criteria included: histologically documented adenocarcinoma of the rectum, endorectal ultrasound or surface coil magnetic resonance imaging (MRI)-staged T3 or T4 primary rectal cancer, no evidence of metastatic disease, Karnofsky performance status score >70%, age >18 years, and normal hepatic, renal, and bone marrow function.
Study Treatment (NCI #5642)
Thirty-two patients received four cycles of therapy: bevacizumab infusion (5 or 10 mg/kg) on day 1 of each cycle, 5-FU infusion (225 mg/m2 over 24 hours) during cycles 2–4, external-beam radiation therapy to the pelvis (50.4 Gy in 28 fractions over 5.5 weeks), and surgery after completion of all therapy. Following recovery from surgery, 30 of the 32 (94%) patients received adjuvant chemotherapy at the discretion of the treating medical oncologist. Thirteen patients received 5-FU, leucovorin, and oxaliplatin, four patients received capecitabine and oxaliplatin, 10 patients received 5-FU and leucovorin, and three patients received capecitabine.
Patients (Duke #10254)
Duke #10254 was a contemporary study of 42 rectal cancer patients approved by the Internal Review Board of Duke University Health System. Data were collected retrospectively. These patients were treated in 2004–2008 at DUMC. This time period was chosen to match the time period of NCI #5642 accrual at DUMC. Eligibility criteria included: histologically documented adenocarcinoma of the rectum, endorectal ultrasound or surface coil MRI-staged T3 or T4 primary rectal cancer, no evidence of metastatic disease, Karnofsky performance status score >70%, age >18 years, and normal hepatic, renal, and bone marrow function.
Study Treatment (Duke #10254)
Thirty-six patients received capecitabine (850 mg/m2 twice daily Monday through Friday) and six patients received 5-FU infusion (225 mg/m2 over 24 hours, 7 days per week) concurrently with external-beam radiation therapy to the pelvis (50.4 Gy in 28 fractions over 5.5 weeks), and surgery following all therapy. No patients received oxaliplatin, bevacizumab, or any other agent concurrently with radiation therapy and a fluoropyrimidine. Following recovery from surgery, 30 of the 42 (71%) patients received adjuvant chemotherapy at the discretion of the medical oncologist. Four patients received 5-FU, leucovorin, and oxaliplatin, nine patients received capecitabine and oxaliplatin, three patients received 5-FU and leucovorin, 12 patients received capecitabine, one patient received 5-FU, leucovorin, oxaliplatin, and bevacizumab (hepatic metastases found and resected at resection), and one patient was treated with a regimen not specified at an outside hospital.
Results
Patient Characteristics (NCI #5642)
The characteristics of the 32 patients enrolled in the NCI #5672 study have been previously reported [9]. In brief, the patients had generally advanced T stage (28 T3, 88%; 4 T4, 12%) and N stage (23 N1–2, 71%; 9 N0, 21%) lesions. Median age was 51 (range, 35–72).
Outcome (NCI #5642)
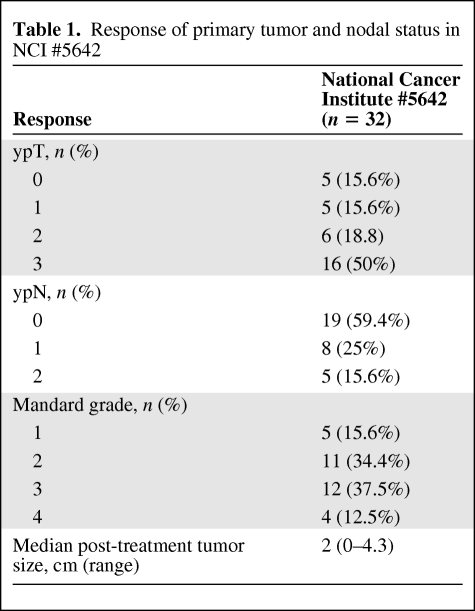
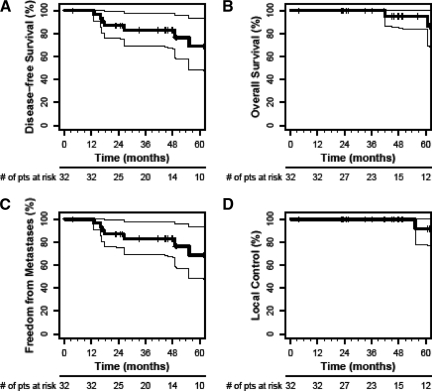
Mandard grades of the primary tumor and pathological downstaging rates following neoadjuvant therapy are summarized in Table 1. Primary tumor (cT3–4 to ypT0–2) downstaging occurred in 19 of 32 patients (59.4%). Of the 23 patients judged to have nodal involvement by clinical staging, 13 were ultimately found to have pathological evidence of nodal metastases. Complete pathological response of the primary tumor was observed in five (15.6%) of the 32 patients treated in the NCI #5642 study. The 5-year actuarial overall survival rate was 95.0%, the disease-free survival and freedom from distant metastases rates were 68.9%, and the local control rate was 91.7% (Fig. 1).
Table 1.
Response of primary tumor and nodal status in NCI #5642
Figure 1.
Overall survival, disease-free survival, freedom from distant metastases, and local control from the National Cancer Institute #5642 study (bevacizumab group). Shown are Kaplan–Meier estimates of disease-free survival (A), overall survival (B), freedom from distant metastases (C), and local control (D).
Safety (NCI #5642)
Most of the acute adverse events in patients treated in the NCI #5642 study were limited to grade 1 and 2 toxicities [9]. There were no grade 4 or 5 toxicities. Grade 3 diarrhea was observed in seven patients, likely a reflection of the 7-day continuous infusion of 5-FU during the entire course of radiation therapy. Although the overall acute toxicities seemed typical of fluoropyrimidine-based chemoradiation therapy, there were also unique toxicities associated with bevacizumab, such as hypertension.
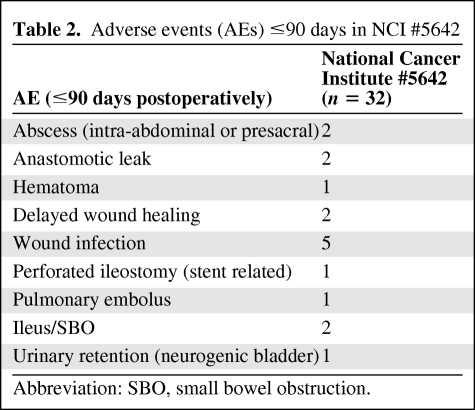
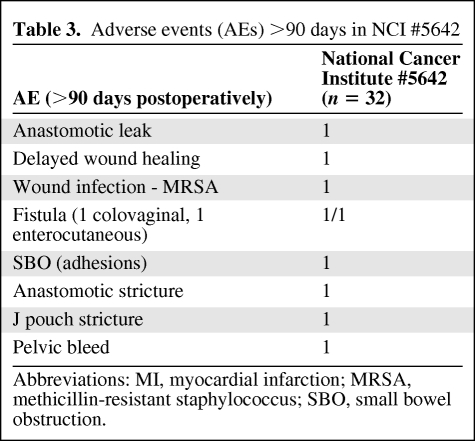
Adverse events within 90 days of surgery and after 90 days are summarized in Tables 2 and 3 There were no postoperative deaths. As these data indicate, surgical complication rates appeared consistent with data from other series. With longer term follow-up, two patients developed anastomotic strictures requiring surgical intervention.
Table 2.
Adverse events (AEs) ≤90 days in NCI #5642
Abbreviation: SBO, small bowel obstruction.
Table 3.
Adverse events (AEs) >90 days in NCI #5642
Abbreviations: MI, myocardial infarction; MRSA, methicillin-resistant staphylococcus; SBO, small bowel obstruction.
Patient Characteristics (Duke #10254)
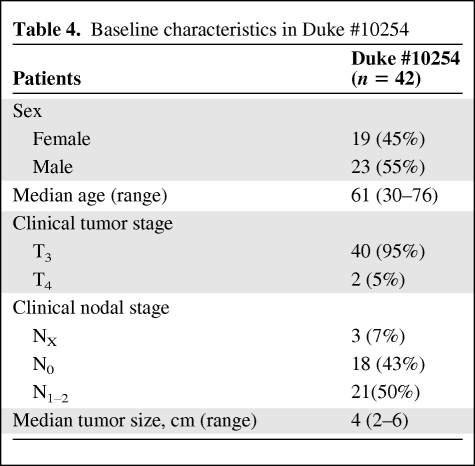
The characteristics of the 42 patients enrolled in the Duke #10254 study are summarized in Table 4. As described, all patients had at least T3 tumors, with half of the patients having nodal involvement by radiological evaluation.
Table 4.
Baseline characteristics in Duke #10254
Outcome (Duke #10254)
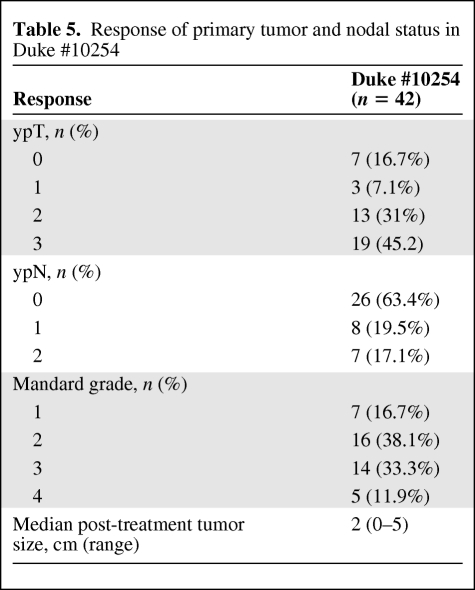
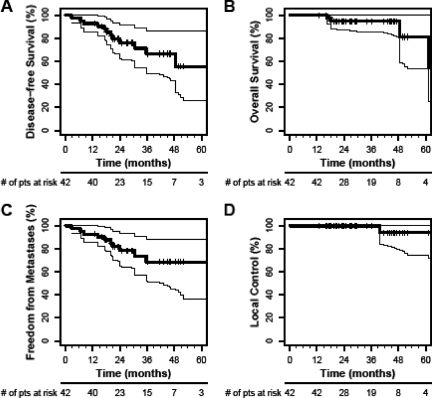
Mandard grades of the primary tumor and pathological downstaging rates following neoadjuvant therapy are summarized in Table 5. Primary tumor (cT3–4 to ypT0–2) downstaging occurred in the tumors of 24 of the 42 patients (57.1%). Complete pathological response of the primary tumor was observed in seven (16.7%) of the 42 patients treated in the Duke #10254 study. The 5-year actuarial overall survival rate was 81.3%, the disease-free survival rate was 55.5%, the freedom from distant metastases rate was 68.7%, and the local control rate was 94.1% (Fig. 2).
Table 5.
Response of primary tumor and nodal status in Duke #10254
Figure 2.
Overall survival, disease-free survival, freedom from distant metastases, and local control from the Duke #10254 study (control group). Shown are Kaplan–Meier estimates of disease-free survival (A), overall survival (B), freedom from distant metastases (C), and local control (D).
Safety (Duke #10254)
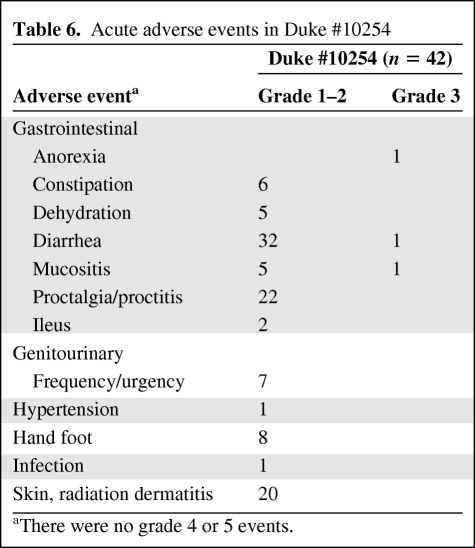
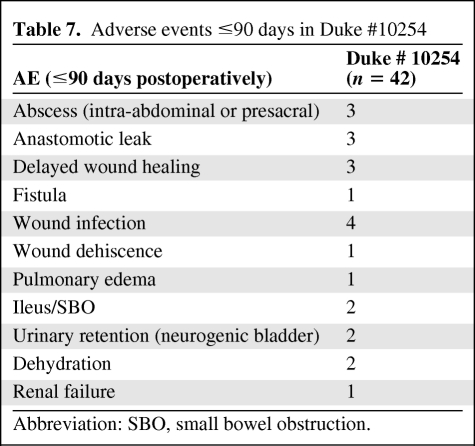
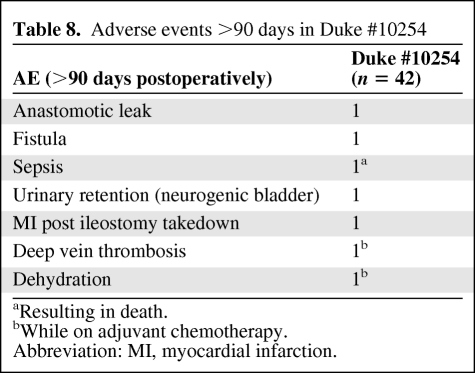
Most of the adverse events in patients treated in the Duke #10254 study were limited to grade 1 and 2 toxicities (Table 6). There were no grade 4 or 5 toxicities. Acute toxicity rates appeared acceptable. Adverse events within 90 days of surgery and after 90 days are summarized in Tables 7 and 8 Anastomotic leaks occurred in four patients.
Table 6.
Acute adverse events in Duke #10254
aThere were no grade 4 or 5 events.
Table 7.
Adverse events ≤90 days in Duke #10254
Abbreviation: SBO, small bowel obstruction.
Table 8.
Adverse events >90 days in Duke #10254
aResulting in death.
bWhile on adjuvant chemotherapy.
Abbreviation: MI, myocardial infarction.
Discussion
The addition of bevacizumab to chemoradiation produced a pathologic complete response rate of 16%, and the 5-year overall survival, disease-free survival, freedom from distant metastases, and local control rates of patients treated in the NCI #5642 trial were 95%, 69%, 69%, and 92%, respectively. These overall survival and disease-free survival results compare favorably with the outcome of other patients with locally advanced rectal cancer treated with chemoradiation and surgery. Although the patients who received bevacizumab tended to have more advanced lesions, the population was younger, on average, which may have also positively affected the overall survival rate. No marked effects were observed with the addition of neoadjuvant bevacizumab on early or late adverse events postoperatively.
The potential role of neoadjuvant bevacizumab in improving survival will have to be established prospectively in randomized studies (the ongoing phase II trial NCT00865189 is comparing two bevacizumab-containing chemotherapy regimens with radiation). This is particularly important because adjuvant bevacizumab (for 1 year) with chemotherapy (for 6 months) did not significantly change the disease-free survival rate at 3 years in a randomized phase III trial in localized colorectal cancer patients [16]. In addition, the use of biomarkers, such as soluble VEGF receptor 1, may help identify patients with rectal cancer likely to have a complete pathological response when employing bevacizumab with standard chemoradiation [17].
As experience increases with the use of targeted agents, such as bevacizumab and other anti-VEGF agents, in the early treatment of patients with cancer, it is imperative to establish how this therapy will impact the long-term outcome. Our exploratory study suggests an acceptable safety profile and a potential long-term benefit of bevacizumab in the neoadjuvant setting in rectal cancer patients, and supports the design of further studies of bevacizumab incorporation in early-stage disease to address this issue.
Acknowledgments
This study was partially supported by NIH grants R21-CA99237, P01-CA80124, R01-CA115767, R01-CA85140, and R01-CA126642 and Federal Share/NCI Proton Beam Program Income grants, and by the National Foundation for Cancer Research.
Author Contributions
Conception/Design: Dan G. Duda, Rakesh K. Jain, Christopher G. Willett
Financial support: Rakesh K. Jain, Christopher G. Willett
Administrative support: Madeline Carroll, Rakesh K. Jain, Christopher G. Willett
Provision of study material or patients: Paul Shellito, Daniel C. Chung, Jeffrey W. Clark, Rakesh K. Jain, Christopher G. Willett, Brian Czito
Collection and/or assembly of data: Dan G. Duda, Hiroshi Fujita, Paul Shellito, Gregory Lauwers, Madeline Carroll, Jeffrey W. Clark, Douglas Tyler, Christopher G. Willett, Marek Ancukiewicz, Mira Shah, Brian Czito, Rex Bentley, Christopher Mantyh, Martin Poleski
Data analysis and interpretation: Dan G. Duda, Hiroshi Fujita, Gregory Lauwers, Daniel C. Chung, Jeffrey W. Clark, Douglas Tyler, Rakesh K. Jain, Christopher G. Willett, Marek Ancukiewicz, Mira Shah, Brian Czito, Rex Bentley, Christopher Mantyh, Martin Poleski
Manuscript writing: Dan G. Duda, Rakesh K. Jain, Christopher G. Willett, Marek Ancukiewicz
Final approval of manuscript: Dan G. Duda, Hiroshi Fujita, Paul Shellito, Gregory Lauwers, Daniel C. Chung, Madeline Carroll, Jeffrey W. Clark, Douglas Tyler, Rakesh K. Jain, Christopher G. Willett, Marek Ancukiewicz, Mira Shah, Brian Czito, Rex Bentley, Christopher Mantyh, Martin Poleski
References
- 1.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 2.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 4.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 6.Jain RK, Duda DG, Clark JW, et al. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 7.Crane CH, Eng C, Feig BW, et al. Phase II trial of neoadjuvant bevacizumab, capecitabine, and radiotherapy for locally advanced rectal cancer [abstract 4091] Int J Radiat Oncol Biol Phys. 2010;76:824–830. doi: 10.1016/j.ijrobp.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 8.DiPetrillo TA, Pricolo V, Sikov WM, et al. Neoadjuvant bevacizumab, oxaliplatin, 5-fluorouracil, and radiation in clinical stage II-III rectal cancer. J Clin Oncol 2008. 26(15 suppl):15041. doi: 10.1016/j.ijrobp.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: A multidisciplinary phase II study. J Clin Oncol. 2009;27:3020–3026. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebos JM, Lee CR, Cruz-Munoz W, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loges S, Mazzone M, Hohensinner P, et al. Silencing or fueling metastasis with VEGF inhibitors: Antiangiogenesis revisited. Cancer Cell. 2009;15:167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Pàez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padera TP, Kuo AH, Hoshida T, et al. Differential response of primary tumor versus lymphatic metastasis to VEGFR-2 and VEGFR-3 kinase inhibitors cediranib and vandetanib. Mol Cancer Ther. 2008;7:2272–2279. doi: 10.1158/1535-7163.MCT-08-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willett CG, Boucher Y, Duda DG, et al. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: Continued experience of a phase I trial in rectal cancer patients. J Clin Oncol. 2005;23:8136–8139. doi: 10.1200/JCO.2005.02.5635. [DOI] [PubMed] [Google Scholar]
- 16.Wolmark N, Yothers G, O’Connell MG, et al. A phase III trial comparing mFOLFOX6 to mFOLFOX6 plus bevacizumab in stage II or III carcinoma of the colon: Results of NSABP Protocol C-08. J Clin Oncol. 2009;27(18 suppl):LBA4. [Google Scholar]
- 17.Duda DG, Willett CG, Ancukiewicz M, et al. Plasma soluble VEGFR-1 is a potential dual biomarker of response and toxicity for bevacizumab with chemoradiation in locally advanced rectal cancer. The Oncologist. 2010;15:577–583. doi: 10.1634/theoncologist.2010-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]