Abstract
Background
Understanding of the personal risks for rheumatoid arthritis (RA) and other rheumatic diseases remains poor, despite advances in knowledge of their pathogenesis, therapeutics, and clinical impact, in part because the personal lifetime risk of developing these diseases is unknown.
Objective
To estimate the lifetime risk of RA, as well as other inflammatory autoimmune rheumatic diseases, including systemic lupus erythematosus, psoriatic arthritis, polymyalgia rheumatica (PMR), giant cell arteritis, ankylosing spondylitis, and Sjögren’s syndrome, and to provide an overall estimate of the risk for developing inflammatory autoimmune rheumatic disease over a lifetime.
Methods
Using the incidence rates obtained from our population-based studies of rheumatic diseases among residents of Olmsted County, Minnesota, and mortality rates from life tables for the general population, we estimated sex-specific lifetime risk of rheumatic disease.
Results
The lifetime risk of RA developing in US adults is 3.6% for women and 1.7% for men, and the lifetime risk of rheumatoid factor positive RA is 2.4% for women and 1.1% for men. The second most common inflammatory autoimmune rheumatic disease is PMR with a lifetime risk of 2.4% for women and 1.7% among men. The overall lifetime risk of inflammatory autoimmune rheumatic disease was 8.4% for women and 5.1% for men.
Conclusion
One in 12 women and 1 in 20 men will develop inflammatory autoimmune rheumatic disease during their lifetime. These results can serve as useful guides in counseling patients regarding their lifetime risk of these conditions and have important implications for disease awareness campaigns.
Despite the increased visibility of rheumatoid arthritis and other rheumatic diseases resulting from the advent of new pharmacotherapies, understanding of the personal risk and public health impact of rheumatic disease remains poor. Rheumatic diseases are numerous with risks that vary in magnitude and differ by age and sex. Among the most common inflammatory autoimmune rheumatic diseases, in addition to rheumatoid arthritis (RA), are systemic lupus erythematosus (SLE), psoriatic arthritis (PsA), polymyalgia rheumatica (PMR), giant cell arteritis (GCA), ankylosing spondylitis (AS), and Sjögren’s syndrome (SS). Estimates of incidence or prevalence are available for these diseases, providing valuable public health information regarding the burden of disease at the population level.(1, 2) However, prevalence rates underestimate the individual risk for diseases which have increased mortality, and incidence rates report only yearly risk.
From an individual’s perspective, the absolute risk of developing disease over a lifetime, such as the well-known lifetime risk of breast cancer among women of 1 in 8, is a more useful and understandable measure of risk than these summary rates.(3, 4) In addition, such estimates provide a useful framework for quantification of the absolute risk of disease attributable to risk factors such as genetic variants. However, no estimates of lifetime risk of rheumatic diseases, separately or collectively, are available.
The purpose of our study was to estimate the lifetime risk of RA and other rheumatic diseases, including SLE, PsA, PMR, GCA, AS and SS, as well as the lifetime risk of developing any one of these rheumatic diseases.
METHODS
Study Subjects and Design
This retrospective population-based study of residents of Olmsted County, Minnesota, which includes the city of Rochester, was conducted using the resources of the Rochester Epidemiology Project, a medical records linkage system that allows ready access to the complete medical records from all community medical providers.(5) Cohorts of incident cases of RA, SLE, PsA, PMR, GCA, AS and SS in this population were previously assembled (Table 1). Approval for this study was obtained from the Mayo Clinic and Olmsted Medical Center institutional review boards and the need for informed consent was waived.
Table 1.
Characteristics of Cohorts for Various Adult-Onset Inflammatory Autoimmune Rheumatic Diseases
| Disease | Number of Cases (% Female) | Time Period |
|---|---|---|
| RA | 1179 (70) | 1955–2007 |
| SLE | 65 (86) | 1950–1992 |
| PsA | 147 (39) | 1970–1999 |
| PMR | 378 (67) | 1970–1999 |
| GCA | 207 (79) | 1950–2004 |
| AS | 158 (24) | 1935–1989 |
| Primary SS | 53 (93) | 1976–1992 |
RA = rheumatoid arthritis; SLE = systemic lupus erythematosus; PsA = psoriatic arthritis; PMR = polymyalgia rheumatica; GCA = giant cell arteritis; AS = ankylosing spondylitis; SS = Sjögrens syndrome;
The RA cohort included all 1179 residents age ≥ 18 years who first fulfilled 1987 American College of Rheumatology classification criteria for rheumatoid arthritis between 1/1/1955 and 12/31/2007.(6–8) In 1995–2007, the age-adjusted incidence per 100,000 population in women was 53.1 (95% confidence interval [CI]: 47.3, 58.9) and in men was 27.7 (95% CI: 23.1, 32.2). Results of rheumatoid factor tests were collected for all patients. Rheumatoid factor testing was performed by nephelometry (Beckman Auto ICS system, Beckman Coulter, Fullerton, CA, USA) for mostly immunoglobulin M rheumatoid factor, or latex agglutination assay (Dade RapiTex kit) for immunoglobulin G rheumatoid factor. Positive results were defined as ≥ 15 IU/ml or a semiquantitative titer of 1:80 or greater.
The SLE cohort included all 65 Rochester, Minnesota residents aged ≥18 who fulfilled the 1982 American College of Rheumatology criteria for the classification of SLE between 1/1/1950 and 12/31/1992.(9, 10) The incidence of SLE tripled in 1980–1992 compared to 1950–1979. The age- and sex-adjusted incidence of SLE was 5.6 (95% CI: 3.9, 7.2) per 100,000 with women having five to six fold higher rates than men.
The PsA cohort included 147 Olmsted County, Minnesota residents aged ≥18 years who first fulfilled the ClASsification of Psoriatic ARthritis (CASPAR) criteria between 1/1/1970 and 12/31/1999.(11) (12) The CASPAR criteria is defined as the presence of inflammatory articular disease (spinal, joint, entheseal) and a score of 3 or more from the following 5 areas: (1) current psoriasis (score of 2), personal history of psoriasis, or family history of psoriasis; (2) nail dystrophy; (3) negative rheumatoid factor; (4) current dactylitis; and (5) radiographic evidence of psoriatic bone changes. The age-adjusted incidence per 100,000 in men (9.1, 95% CI: 7.1, 11.0) was higher than in women (5.4, 95% CI: 4.0, 6.9). The age- and sex-adjusted incidence of PsA per 100,000 increased from 3.6 (95% CI: 2.0, 5.2) in 1970–1979 to 9.8 (95% CI: 7.7, 11.9) in 1990–1999.
The PMR cohort included 378 Olmsted County, Minnesota residents with incident PMR between 1/1/1970 and 12/31/1999.(13) Cases fulfilled the following 3 criteria: (1) age ≥ 50 years; (2) bilateral aching and morning stiffness (lasting ≥ 30 min) persisting for at least one month and involving 2 or more areas: neck or torso, shoulders or proximal regions of the arms, and hips or proximal aspects of the thighs; and (3) erythrocyte sedimentation rate elevated to > 40 mm/h (Westergren). However, patients who fulfilled only the first 2 criteria and also had documentation of a prompt response to corticosteroid therapy were also included. The incidence of PMR remained relatively stable over time with an age-adjusted incidence per 100,000 of 69.8 (95% CI: 61.2, 78.4) among women and 44.8 (95% CI: 37.0, 52.6) among men.
The GCA cohort included 207 Olmsted County, Minnesota residents with incident GCA between 1/1/1950 and 12/31/2004.(14, 15) Cases fulfilled the 1990 American College of Rheumatology criteria for GCA, which require 3 of the following 5 criteria: age ≥ 50 years, new headache, temporal artery abnormality, elevated erythrocyte sedimentation rate ≥ 50 mm/hr (Westergren), and abnormal artery biopsy.(16) The age-adjusted incidence of GCA per 100,000 was 24.4 (95% CI: 20.3, 28.6) among women and 10.3 (95% CI: 6.9, 13.6) among men for 1950–1999. The incidence was similar for 2000–2004 (25.1 among women and 8.6 among men per 100,000).
The AS cohort included 158 Rochester, Minnesota residents with incident AS between 1/1/1935 and 12/31/1989.(17) Cases fulfilled the modified New York criteria for definite AS (radiographic evidence of sacroiliitis, together with at least one of: inflammatory low back pain of three months duration, limitation of lumbar spine movement in sagittal and frontal planes, and reduced chest expansion).(18) The age-adjusted incidence per 100,000 in men (11.7; 95% CI: 9.6, 13.8) was four times that in women (2.9; 95% CI: 2.0, 3.9), and the incidence declined significantly over time.
The SS cohort included 53 Olmsted County, Minnesota residents with a physician diagnosis of primary SS (i.e. those without a previous diagnosis of RA or SLE) between 1/1/1976 and 12/31/1992.(19) The overall age- and sex-adjusted incidence of primary SS was 3.9 (95% CI: 2.8, 4.9) per 100,000.
Statistical Analysis
Using the most recent 10 year period available for each cohort, age- and sex-specific incidence rates were calculated by dividing the number of incident cases by the population estimates based on decennial census counts, with linear interpolation between census years.(20) Poisson regression models for each sex with smoothing splines for age were used to model the incidence rates, allowing interpolation for individual years of age and decreasing the effects of variability in the rates. For PMR and GCA, which only occur at ages ≥50 years, this reduced age range was used for Poisson modeling and rates for ages <50 years were set to zero. Primary inflammatory autoimmune rheumatic disease incidence rates were obtained by excluding patients who previously experienced another inflammatory autoimmune rheumatic disease from each cohort and adding together the incidence rates for each of these primary disease cohorts.
Lifetime risk was estimated using established methods.(21) Using a hypothetical cohort of 100,000 persons of age 18 years and a particular sex, the age- and sex-specific incidence rates for disease and the age- and sex-specific mortality rates from the year 2000 for the U.S. were used to determine the numbers of persons expected to develop disease and expected to die for each year of age up to age 100 years. Cumulative lifetime risk refers to the percentage of persons who develop disease from age 18 years to a particular age. The lifetime risk is the percentage of persons who develop disease at any age. Residual lifetime risk, which is the risk for persons who reach a given age without yet developing the disease, was similarly estimated beginning with a hypothetical cohort of a particular age (e.g. 30 years). To examine the impact of changes in incidence and mortality rates, the lifetime risk of RA was estimated using only incidence rates from 1965–1974 with mortality rates from 1970 and also using only incidence rates from 1975–1984 with mortality rates from 1980. Finally, 95% confidence intervals for lifetime risk estimates were obtained based on 1000 bootstrap samples from each incidence cohort using bias corrected methods.(22)
RESULTS
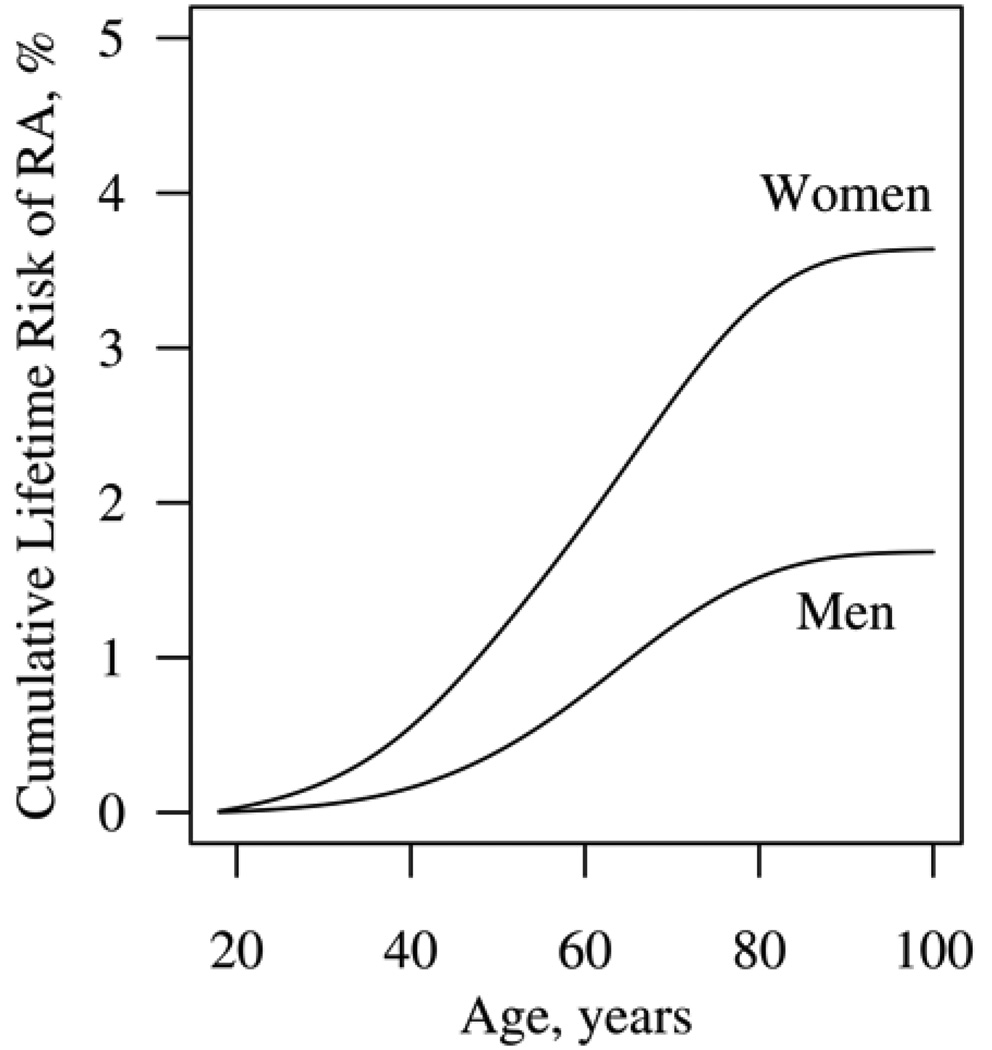
The cumulative lifetime risk of developing adult-onset RA in the year 2000 is higher for women (3.6%; 95% CI: 3.4%, 3.9%) than for men (1.7%; 95% CI: 1.4%, 1.9%; Figure 1). The cumulative risk is <1% before age 50 years reflecting the small incidence of RA during those ages. The cumulative risk of RA rises steeply in both genders around age 60 years, where the incidence of RA is highest, and flattens after age 80 years.
Figure 1.
Cumulative lifetime risk of rheumatoid arthritis for women and men.
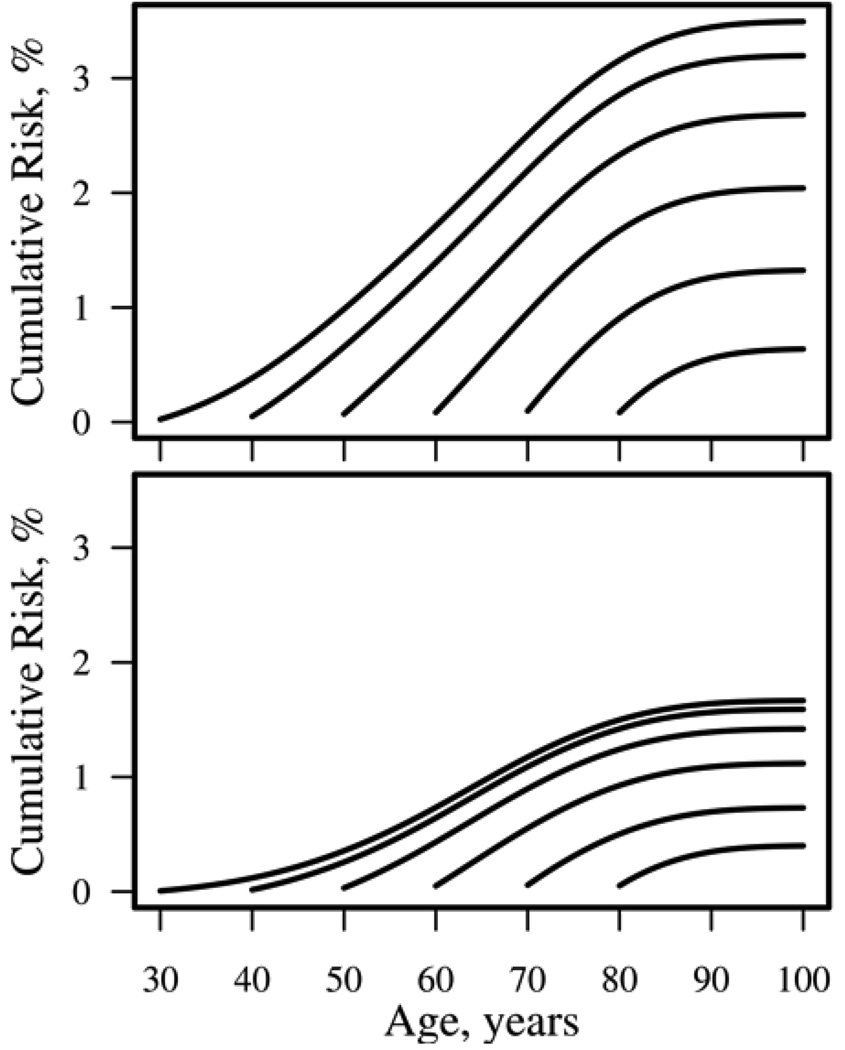
The residual lifetime risk of RA is the risk of developing RA for persons who have not yet developed RA at various ages. Figure 2 displays the residual lifetime risk of RA for women (top panel) and men (bottom panel) who are alive and have not yet developed RA at ages 30, 40, 50, 60, 70, or 80 years. The residual lifetime risks for individuals age 30, 40, and 50 years are similar because the risk of RA is small in younger ages. Thus the risk of developing RA for persons who live to age 50 years without developing RA is only minimally reduced (2.7% for women and 1.4% for men) compared to the overall lifetime risk in Figure 1.
Figure 2.
Residual lifetime risk of rheumatoid arthritis for women (top panel) and men (bottom panel) without rheumatoid arthritis at ages 30, 40, 50, 60, 70, and 80 years.
Sensitivity analyses of the lifetime risk of RA in 1970, when both the incidence and mortality rates were substantially higher than current rates, revealed similar lifetime risks among women (3.8%) and men (1.6%) compared to those in the year 2000. Estimates for 1980, when RA incidence rates and the general population mortality rates were declining, were also very similar to those for the year 2000 (3.6% among women and 1.5% among men).
Table 2 displays the lifetime risk of adult-onset RA (as shown in Figure 1) and other rheumatic diseases for women and men. The lifetime risk of developing rheumatoid factor positive RA is 2.4% for women and 1.1% for men. The lifetime risk of SLE is much higher for women (0.9%) compared to men (0.2%). The lifetime risk of PsA is slightly higher for men (0.6%) than for women (0.5%).
Table 2.
Lifetime Risk of Developing Various Adult-Onset Inflammatory Autoimmune Rheumatic Diseases according to Sex
| Lifetime risk (95% CI) | ||
|---|---|---|
| Disease | Women | Men |
| RA | 3.64 (3.37, 3.90) | 1.68 (1.41, 1.94) |
| RF+ RA | 2.39 (2.18, 2.60) | 1.13 (0.91, 1.33) |
| SLE | 0.91 (0.75, 1.06) | 0.21 (0.06, 0.42) |
| PsA | 0.46 (0.33, 0.59) | 0.62 (0.51, 0.72) |
| PMR | 2.43 (2.15, 2.72) | 1.66 (1.32, 2.00) |
| GCA | 1.04 (0.90, 1.16) | 0.51 (0.27, 0.64) |
| AS | 0.08 (0.00, 0.19) | 0.63 (0.53, 0.74) |
| Primary SS | 0.75 (0.67, 0.83) | 0.04 (0.00, 0.10) |
| Primary inflammatory autoimmune rheumatic disease* | 8.42 (7.98, 8.83) | 5.13 (4.64, 5.64) |
RA = rheumatoid arthritis; RF+ = rheumatoid factor positive; SLE = systemic lupus erythematosus; PsA = psoriatic arthritis; PMR = polymyalgia rheumatica; GCA = giant cell arteritis; AS = ankylosing spondylitis; SS = Sjögrens syndrome; CI = confidence interval
Primary inflammatory autoimmune rheumatic disease is defined as the first of any of these rheumatic diseases
The lifetime risk of PMR is slightly higher for women (2.4%) than for men (1.7%), as is the lifetime risk of GCA (1.0% for women and 0.5% for men). The lifetime risk of AS is almost entirely among men (0.63% among men compared to 0.08% among women), whereas the lifetime risk of SS is almost exclusively among women (0.75% compared to 0.04% among men).
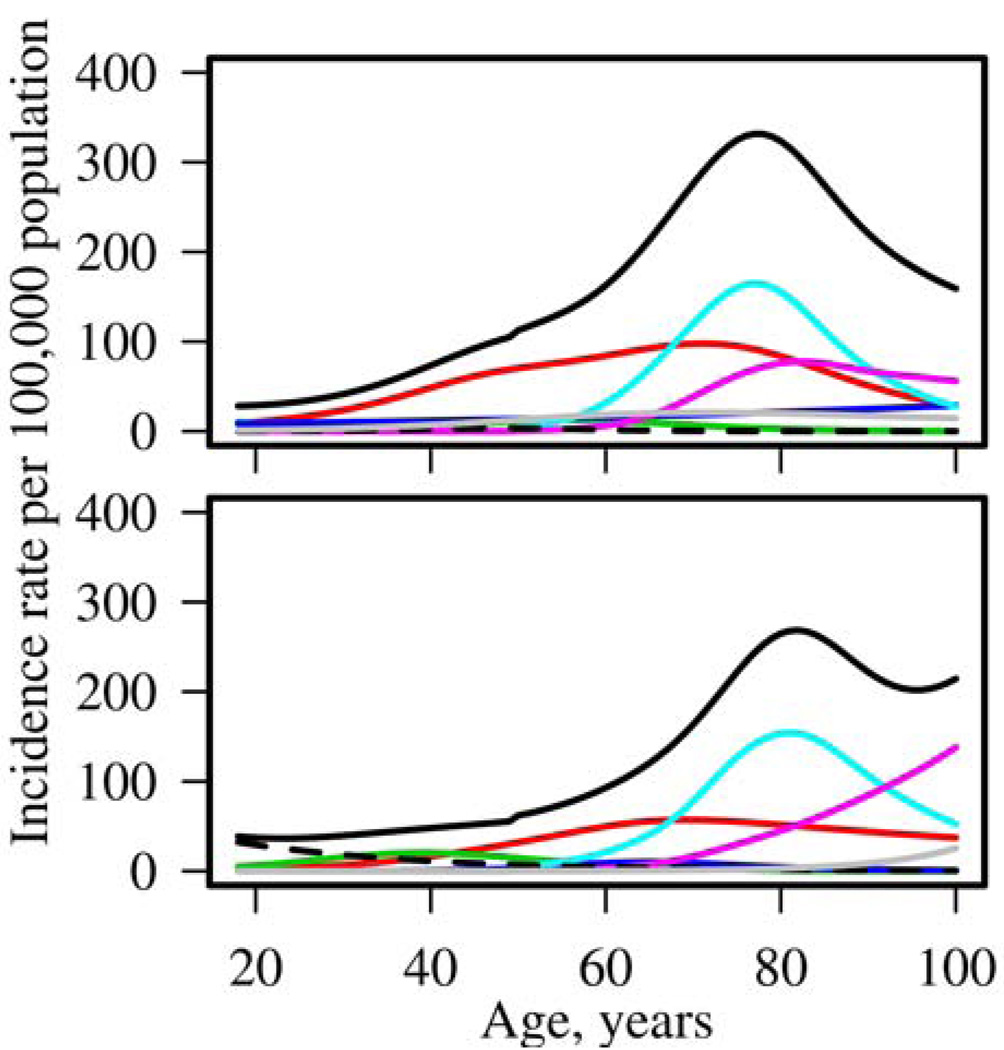
The lifetime risk of primary inflammatory autoimmune rheumatic disease, defined as the first inflammatory autoimmune rheumatic disease per person, was estimated as 8.42% (95% CI: 7.98%, 8.83%) for women and 5.13% (95% CI: 4.64%, 5.64%) for men (Table 2). This estimate was obtained by removing the overlap between cohorts caused by the occurrence of more than one rheumatic disease during a person’s lifetime. The most common co-occurrence of inflammatory autoimmune rheumatic diseases occurred between patients with PMR and GCA, with 3.8% of PMR patients having GCA previously and 8.2% of GCA patients having PMR previously. Among RA patients 2.4% experienced SS and 1.2% had PMR or GCA prior to developing RA. The incidence rates for each rheumatic disease and for primary inflammatory autoimmune rheumatic disease are shown in Figure 3 for women (top panel) and men (bottom panel). The incidence of primary inflammatory autoimmune rheumatic disease is greater for women than for men and peaks near age 80 years in both sexes. Sensitivity analyses revealed the estimates of lifetime risk ignoring the overlap (8.96% among women and 5.21% among men) were not much larger than those for primary inflammatory autoimmune rheumatic disease.
Figure 3.
Incidence rates for women (top panel) and men (bottom panel) according to age for rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases (solid black: primary autoimmune inflammatory rheumatic disease; red: rheumatoid arthritis; blue: systemic lupus erythematosus; green: psoriatic arthritis; cyan: polymyalgia rheumatica; magenta: giant cell arteritis; dashed: ankylosing spondylitis; grey: Sjögrens syndrome).
The residual lifetime risks of RA and other rheumatic diseases are shown in Table 3 (also shown in Figure 2 for RA). For PMR and GCA, the largest residual risks occur for those alive at age 60 years without disease. Since there is no risk of PMR or GCA prior to age 50 years, the chance of dying before reaching age 50 years affects the lifetime risk and results in lower lifetime risk estimates at age 18 years compared to the residual risk at age 50 years.
Table 3.
Residual Lifetime Risk of Developing Various Adult-onset Inflammatory Autoimmune Rheumatic Diseases according to Sex and Age
| Sex/ age |
RA | RF+ RA |
SLE | PsA | PMR | GCA | AS | Primary SS |
Primary inflammatory autoimmune rheumatic disease * |
|---|---|---|---|---|---|---|---|---|---|
| Women | |||||||||
| 30 | 3.49 | 2.33 | 0.81 | 0.38 | 2.44 | 1.05 | 0.08 | 0.73 | 8.11 |
| 40 | 3.20 | 2.13 | 0.71 | 0.33 | 2.47 | 1.06 | 0.07 | 0.69 | 7.68 |
| 50 | 2.68 | 1.75 | 0.60 | 0.25 | 2.52 | 1.08 | 0.04 | 0.61 | 7.00 |
| 60 | 2.04 | 1.30 | 0.48 | 0.14 | 2.51 | 1.11 | 0.01 | 0.48 | 6.07 |
| 70 | 1.32 | 0.79 | 0.35 | 0.05 | 2.12 | 1.06 | <0.01 | 0.32 | 4.67 |
| 80 | 0.64 | 0.32 | 0.23 | 0.01 | 1.05 | 0.72 | <0.01 | 0.18 | 2.60 |
| Men | |||||||||
| 30 | 1.67 | 1.11 | 0.21 | 0.52 | 1.68 | 0.51 | 0.33 | 0.04 | 4.78 |
| 40 | 1.59 | 1.05 | 0.21 | 0.34 | 1.71 | 0.52 | 0.19 | 0.04 | 4.44 |
| 50 | 1.42 | 0.91 | 0.20 | 0.17 | 1.78 | 0.54 | 0.10 | 0.04 | 4.11 |
| 60 | 1.11 | 0.68 | 0.16 | 0.07 | 1.81 | 0.57 | 0.05 | 0.05 | 3.69 |
| 70 | 0.73 | 0.43 | 0.08 | 0.02 | 1.71 | 0.60 | 0.03 | 0.05 | 3.11 |
| 80 | 0.40 | 0.23 | <0.01 | <0.01 | 1.12 | 0.56 | 0.01 | 0.05 | 2.08 |
RA = rheumatoid arthritis; RF+ = rheumatoid factor positive; SLE = systemic lupus erythematosus; PsA = psoriatic arthritis; PMR = polymyalgia rheumatica; GCA = giant cell arteritis; AS = ankylosing spondylitis; SS = Sjögrens syndrome
Primary inflammatory autoimmune rheumatic disease is defined as the first of any of these rheumatic diseases
DISCUSSION
The overall lifetime risk of developing an inflammatory autoimmune rheumatic disease in U.S. adults is 8.4% for women and 5.1% for men, which is equivalent to 1 in 12 for women and 1 in 20 for men. The lifetime risk of developing RA is 3.6% (or 1 in 28) for women and 1.7% (or 1 in 59) for men, and the lifetime risk of developing rheumatoid factor positive RA was 2.4% for women and 1.1% for men. The second most common rheumatic disease was PMR with a lifetime risk of 2.4% for women and 1.7% among men.
The lifetime risks of RA and other rheumatic diseases have not been estimated previously. The lifetime risk of RA is commonly misunderstood and miscommunicated as a risk of about 1 in 100 based upon the prevalence of RA, which is 0.5% to 1%. The overall prevalence is a poor estimate of individual risk, particularly for diseases like RA which occur more frequently at older ages and are associated with increased mortality. The overall prevalence is an average across all ages, whereas the age-specific prevalence of RA increases with age (to >2% among women age > 65 years). In addition, prevalence includes only persons living with the disease, so prevalence underestimates disease occurrence in diseases with increased mortality. For example, the age-adjusted prevalence of breast cancer in U.S. women in 2006 is 1.1%, which is comparable to the prevalence of RA among women, whereas the lifetime risk of breast cancer is much larger (12% or 1 in 8).(4)
While the lifetime risks of RA and primary inflammatory autoimmune rheumatic disease are greater than generally understood, they are still smaller than lifetime risks for diseases that are considered to be common. The residual lifetime risk of hip fracture at age 50 years in the U.S., which is reported to be 15.8% for women and 6% for men, is greater than the residual lifetime risk of rheumatic disease at age 50 years of 7.0% for women and 4.1% for men.(23) Many rheumatic diseases are known to be more common at older ages, thus the residual risk of rheumatic disease remains high at age 70 years (4.6% for women and 3.1% for men). In comparison, dementia, another disease which occurs predominantly in older ages, has a residual lifetime risk of about 10% at age 70 years.(24)
Lifetime risk estimates are averages that are generally applicable to persons of a particular age and sex. An individual’s risk could be higher or lower than these estimates depending on their personal set of risk factors (e.g. smoking, family history, genetics). For example, persons with a family history of RA in a first-degree relative have a 3 to 5 times higher risk of developing RA.(25) For these persons, the lifetime risk of RA could be as high as 18% (or 1 in 6) for women and 8.5% (or 1 in 12) for men. Similarly, these lifetime risk estimates can be used to translate relative risks for rare genetic polymorphisms associated with development of RA into absolute risks, which are more easily understood.(3)
Strengths of our study include the use of multiple well-characterized cohorts from the same underlying population. Each cohort was defined using established classification criteria, where available, applied during a comprehensive review of all inpatient and outpatient medical records for all persons in the community with relevant diagnoses. The population-based nature of our study is an advantage over lifetime risk estimates obtained from cohorts comprised of longitudinally tracked volunteers, which are influenced by participation bias and temporal trends in disease risk.
Our estimates of the lifetime risk of primary inflammatory autoimmune rheumatic disease are likely conservative because our reliance on classification criteria excluded patients with undifferentiated spondyloarthopathies or polyarthritis. In addition, several rare rheumatic diseases (e.g. dermatomyositis, polymyositis, scleroderma, non-giant cell arteritis vasculitides and other undifferentiated connective tissue diseases) were not included in our study. In addition, the SS cohort was based on physician diagnosis and may underestimate the true incidence, as well as the lifetime risk, of SS. A few more common rheumatic diseases, namely osteoarthritis, fibromyalgia and gout, were intentionally excluded as they are not autoimmune rheumatic diseases.
Potential limitations of our study include the assumption that the most recent incidence rates for each disease will remain stable into the future. This assumption is questionable for cohorts that have not demonstrated stable incidence rates in the past, such as RA, SLE, AS and PsA. However, sensitivity analyses for RA revealed estimated lifetime risks for 1970 and 1980 were reassuringly similar to those for 2000. In addition, the cohorts for SLE, AS and SS did not include the most recent decade. While the population of Olmsted County, Minnesota has historically been predominantly white, the proportion of minorities has steadily increased and is now >10%; however, our results may not be generalizable to other more diverse populations. The incidence rates for rheumatic diseases are similar in North America and Northern Europe, but are generally smaller in Southern Europe and the rest of the world, with the exception of higher rates of systemic lupus erythematosus among persons of African ancestry.(26–30) Therefore, our lifetime risk estimates are likely generalizable to Northern European populations, but may overestimate the lifetime risks for rheumatic diseases in the rest of the world. In addition, evidence of geographical variation in RA within the U.S. suggests the issue of generalizability is complex as both genetic and environmental exposures likely impact the development of rheumatic diseases.(31) Finally, changes in life expectancy in the future cannot be anticipated. If advances in other diseases, such cardiovascular disease or cancer, significantly impact the life expectancy of this population, the lifetime risks for rheumatic diseases could increase.
In conclusion, the lifetime risk of inflammatory autoimmune rheumatic disease development is substantial at 1 in 12 for women and 1 in 20 for men. The lifetime risk of RA is larger than previously thought at 1 in 28 for women and 1 in 59 for men. These results can serve as useful guides in counseling patients regarding their lifetime risk of these conditions and have important implications for disease awareness campaigns such as those of the Arthritis Foundation (U.S.) and the National Rheumatoid Arthritis Society (United Kingdom). These findings underscore the need for further investigations to determine whether identification of persons with high genetic risk for rheumatoid arthritis might justify early detection and intervention to prevent progression to overt clinical disease.
Acknowledgments
Funding: This work was partially funded by a grant from the National Institutes of Health, NIAMS (R01 AR46849) and made possible by a grant from the National Institutes of Health, NIAMS (AR-30582).
REFERENCES
- 1.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crowson CS, Therneau TM, Matteson EL, Gabriel SE. Primer: demystifying risk-understanding and communicating medical risks. Nat Clin Pract Rheumatol. 2007;3(3):181–187. doi: 10.1038/ncprheum0397. [DOI] [PubMed] [Google Scholar]
- 4.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, et al. Bethesda, MD: National Cancer Institute; SEER Cancer Statistics Review, 1975–2006. 2009 http://seer.cancer.gov/csr/1975_2006/ based on November 2008 SEER data submission, posted to the SEER web site, In.
- 5.Maradit Kremers H, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheum Dis Clin North Am. 2004:819–834. doi: 10.1016/j.rdc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 7.Doran MF, Pond GR, Crowson CS, O'Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum. 2002;46(3):625–631. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- 8.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the Incidence of Rheumatoid Arthritis Rising? Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62(6):1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis & Rheumatism. 1982;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 10.Uramoto KM, Michet CJJ, Thumboo J, Sunku J, O'Fallon WM, Gabriel SE. Trends in the incidence and mortality of systemic lupus erythematosus (SLE) - 1950–1992. Arthritis & Rheumatism. 1999;42(1):46–50. doi: 10.1002/1529-0131(199901)42:1<46::AID-ANR6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 12.Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Maradit Kremers H. Time trends in epidemiology and characteristics of psoriatic arthritis over 3 decades: A population-based study. The Journal of Rheumatology. 2009;36(2):361–367. doi: 10.3899/jrheum.080691. PMC2717703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doran MF, Crowson CS, O'Fallon WM, Hunder GG, Gabriel SE. Trends in the incidence of polymyalgia rheumatica over a 30 year period in Olmsted County, Minnesota, USA. Journal of Rheumatology. 2002;29:1694–1697. [PubMed] [Google Scholar]
- 14.Salvarani C, Crowson CS, O'Fallon WM, Hunder GG, Gabriel SE. Reappraisal of the epidemiology of giant cell arteritis in Olmsted County, Minnesota, over a fifty-year period. Arthritis & Rheumatism. 2004;51(2):264–268. doi: 10.1002/art.20227. [DOI] [PubMed] [Google Scholar]
- 15.Kermani TA, Schafer VS, Crowson CS, Hunder GG, Gabriel SE, Matteson EL, et al. Increase in Age at Onset of Giant Cell Arteritis: A Population-based Study. Ann Rheum Dis. 2009 doi: 10.1136/ard.2009.111005. [DOI] [PubMed] [Google Scholar]
- 16.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis & Rheumatism. 1990;33(8):1122–1128. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 17.Carbone LD, Cooper C, Michet CJ, Atkinson EJ, O'Fallon WM, Melton LJd. Ankylosing spondylitis in Rochester, Minnesota, 1935–1989. Is the epidemiology changing? Arthritis & Rheumatism. 1992;35(12):1476–1482. doi: 10.1002/art.1780351211. [DOI] [PubMed] [Google Scholar]
- 18.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4):361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 19.Pillemer SR, Matteson EL, Jacobsson LT, Martens PB, Melton LJ, 3rd, O'Fallon WM, et al. Incidence of physician-diagnosed primary Sjogren syndrome in residents of Olmsted County, Minnesota. Mayo Clin Proc. 2001;76(6):593–599. doi: 10.4065/76.6.593. [DOI] [PubMed] [Google Scholar]
- 20.Bergstralh EJ, Offord KP, Chu CP, Beard CM, O'Fallon WM, Melton LJ., III Calculating incidence, prevalence and mortality rates for Olmsted County, Minnesota: An update. Technical Report Series 1992;No. 49, April
- 21.Wun LM, Merrill RM, Feuer EJ. Estimating lifetime and age-conditional probabilities of developing cancer. Lifetime Data Anal. 1998;4(2):169–186. doi: 10.1023/a:1009685507602. [DOI] [PubMed] [Google Scholar]
- 22.Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med. 2000;19(9):1141–1164. doi: 10.1002/(sici)1097-0258(20000515)19:9<1141::aid-sim479>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Kanis JA, Johnell O, De Laet C, Jonsson B, Oden A, Ogelsby AK. International variations in hip fracture probabilities: implications for risk assessment. J Bone Miner Res. 2002;17(7):1237–1244. doi: 10.1359/jbmr.2002.17.7.1237. [DOI] [PubMed] [Google Scholar]
- 24.Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, et al. Lifetime risk of dementia and Alzheimer's disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49(6):1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 25.Hemminki K, Li X, Sundquist J, Sundquist K. Familial associations of rheumatoid arthritis with autoimmune diseases and related conditions. Arthritis Rheum. 2009;60(3):661–668. doi: 10.1002/art.24328. [DOI] [PubMed] [Google Scholar]
- 26.Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther. 2009;11(3):229. doi: 10.1186/ar2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182–188. doi: 10.1016/j.semarthrit.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Alamanos Y, Voulgari PV, Drosos AA. Incidence and Prevalence of Psoriatic Arthritis: A Systematic Review. J Rheumatol. 2008;35(7):1354–1358. [PubMed] [Google Scholar]
- 29.Gonzalez-Gay MA, Vazquez-Rodriguez TR, Lopez-Diaz MJ, Miranda-Filloy JA, Gonzalez-Juanatey C, Martin J, et al. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Rheum. 2009;61(10):1454–1461. doi: 10.1002/art.24459. [DOI] [PubMed] [Google Scholar]
- 30.Bae SC, Fraser P, Liang MH. The epidemiology of systemic lupus erythematosus in populations of African ancestry: a critical review of the "prevalence gradient hypothesis". Arthritis Rheum. 1998;41(12):2091–2099. doi: 10.1002/1529-0131(199812)41:12<2091::AID-ART2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 31.Costenbader KH, Chang SC, Laden F, Puett R, Karlson EW. Geographic variation in rheumatoid arthritis incidence among women in the United States. Arch Intern Med. 2008;168(15):1664–1670. doi: 10.1001/archinte.168.15.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]