Abstract
Objective
We determined the postoperative pharmacokinetics (PK), safety and analgesic effects of ketorolac in 14 infants (aged <6 months) receiving a single intravenous (IV) administration of racemic ketorolac or placebo.
Background
Information on the PK of ketorolac in infants is limited. Unblinded studies suggest ketorolac may be useful in infants.
Methods
This double-blind, placebo-controlled study enrolled 14 infants (aged <6 months) postoperatively. At 6–18 hours after surgery, infants were randomized to receive placebo, 0.5 mg/kg, or 1 mg/kg ketorolac IV. All infants received morphine sulfate as needed for pain control. Blood was collected up to 12-hours post-dosing. Analysis used non-compartmental and compartmental population modeling methods.
Results
In addition to noncompartmental and empirical Bayes PK modeling, data were integrated with a previously studied dataset comprising 25 infants and toddlers (aged 6–18 months). A two-compartmental model described the comprehensive data set. The population estimates of the R (+) isomer were (%CV): central volume of distribution 1130 (10%) ml, peripheral volume of distribution 626 (25%) ml, clearance from the central compartment 7.40 (8%) ml/min. Those of the S (−) isomer were 1930 (15%) ml, 319 (58%) ml, 39.5 (13%) ml/min. Typical elimination half-lives were 191, and 33 min respectively. There was a trend for increased clearance and central volume with increasing age and weight. The base model suggested that clearance of the S (−) isomer was weakly related to age; however, when body size adjustment was added to the model, no covariates were significant. Safety assessment showed no changes in renal or hepatic function tests, surgical drain output, or continuous oximetry between groups. Cumulative morphine administration showed large interpatient variability and was not different between groups.
Conclusion
Stereo-isomer specific clearance of ketorolac in infants (aged 2–6 months) shows rapid elimination of the analgesic S (−) isomer as reported in infants aged 6–18 months. No adverse effects were seen after a single IV ketorolac dose.
Keywords: ketorolac pharmacokinetics, stereo-isomers, infants, post-operative analgesia, safety
Introduction
Nonsteroidal anti-inflammatory agents (NSAIDs) have been useful in treating postoperative pain in children (1, 2). These drugs work via blockade of the cyclo-oxygenase (COX) system, decreasing prostaglandin synthesis and diminishing the inflammatory cascade. The COX system has at least 2 components. COX-1 is present in many cells and is expressed at all times; it serves important roles in the maintenance of gastric mucosal function, renal perfusion and platelet aggregation. COX-2 activity is increased in association with inflammation. Most investigations in pediatric patients have involved the COX-1 or non-specific COX agents. The COX-2 specific agents are not available for intravenous use in the USA, so pediatric use, at least in the perioperative period, will continue to be limited to the non-selective COX-blocking agents for some time (3).
A survey of British anesthetists over 10 years ago, in 1996, reported use of NSAIDs postoperatively in 11% of neonates, increasing to 59% in infants 3–12 months of age (4). The only parenteral NSAID currently available in the USA is ketorolac tromethamine, which has both COX-1 and COX-2 effects. A small case series of infants who received ketorolac after abdominal surgery reported a decrease in morphine use (5).
Information on the pharmacokinetics of ketorolac in infants is sparse, making dosing problematic (6–11). Ketorolac is administered as a racemic mixture with the S (−) isomer responsible for the analgesic effect in animal models. We previously reported on the stereo-specific pharmacokinetics of S (−) and R (+) isomers of ketorolac for 37 infants aged 6–18 months studied after surgery (12). The infants 6–18 months rapidly clear the active S (−) isomer of ketorolac (elimination half-life of 50 min), while the R (+) isomer clearance is slower. Modeling showed steady accumulation of the R (+) isomer with multiple dosing. No adverse effects regarding renal function, hepatic function, bleeding or continuous oximetry were seen in this study with single IV dosing. Extrapolation of dosing guidelines from data for older children or adults may put infants at risk for inadequate effect or increased toxicity. Multiple examples of the error of such extrapolations exist, including chloramphenicol and morphine (13, 14), and suggest that investigation of infant pharmacokinetic parameters and safety assessments are the most favorable course to evaluate drugs being administered to this population.
We are reporting results from a randomized, blinded, placebo-controlled study of ketorolac pharmacokinetics, safety and efficacy when used in infants postoperatively. This report includes infants aged 2–6 months. While safety and efficacy data are reported for the 2–6 month old infants, to maximize the pharmacokinetic information, the dataset previously reported for infants 6–18 months (12) receiving single dose IV ketorolac (25 of 37 infants) was combined with the pharmacokinetic values from these 2–6 month old infants, as has been proposed previously (15).
Methods
Infants, aged <6 months, who were scheduled for hospital admission following surgery, were considered. Prematurity (gestational age <36 weeks at birth), history of gastrointestinal bleeding, coagulopathy (in the infant or a positive family history), and hepatic or renal impairment were exclusion criteria. Institutional Review Board approval of the protocol was obtained prior to the study. Informed consent was obtained from parents of each eligible infant by research personnel who were not directly involved in the infant’s clinical care. After anesthesia induction, a second peripheral intravenous (IV) catheter was placed to draw screening renal and hepatic function blood samples, and was heparin-locked for sampling on the evening after surgery or on postoperative day 1. If clinical care required arterial catheter placement, blood samples were drawn from the arterial catheter while it was in place. If the screening blood results and urinalysis were normal, the infant was enrolled in the study.
On the evening of surgery (at least 6 hours following surgery completion) for infants who are usually discharged the day after surgery (cleft lip repair), with the attending surgeon’s approval, infants were randomized to receive study medication in 2 ml D5W (placebo, 0.5 mg/kg, or 1 mg/kg racemic ketorolac) as a 10-minute IV infusion. For infants staying through postoperative day 1, study medication was given the morning following surgery after randomization. Blood was sampled serially (1 ml at 0, 5 or 10 minutes, 30 or 60 minutes, 2, 4, 8 hours and 2 ml at 12 hours post infusion) up to 7 times over the next 12 hours, either from the in-dwelling IV catheter or arterial catheter. At 12 hours, liver and renal function tests were repeated and urine was sent for analysis.
All infants received morphine sulfate either as IV bolus doses of 0.05 mg/kg, 4 hourly as needed, or by continuous IV infusion at 5–30 mcg/kg/hr with bolus doses (0.05 mg/kg) as needed for pain control. An infant pain scale (MIPS)(16) was assessed every 2 hours to assure consistent pain management. Low scores (less than 12 of 20 possible comfort points) mandated analgesic treatment. Acetaminophen was held for > 6 hours pre-study drug administration and for the following 12 hours.
Safety assessments included the renal (BUN, Creatinine) and hepatic function (AST, ALT, GGT) blood work and urinalysis, obtained pre- and at 12 hours after study drug, as well as continuous pulse oximetry (Masimo Radical SET, Irvine, California). Blood loss from any surgical drains was recorded. Hemoccult testing of stools and any gastric output was also performed.
Ketorolac Assay
Plasma concentrations of the R (+) and S (−) enantiomers of ketorolac were determined by high pressure liquid chromatography (HPLC) on a Varian Pro Star 210 gradient system with UV detection at 313 nm as previously described (12).
Pharmacokinetic Analysis
For the pharmacokinetic analysis, the dose of the R (+) and S (−) ketorolac isomers was considered to be 50% of the racemic dose given, i.e. a 1 mg/kg dose of racemic ketorolac was taken to be composed of 0.5 mg/kg each of R (+) ketorolac and of S (−) ketorolac. Descriptive pharmacokinetic parameters for the R (+) and S (−) ketorolac isomers were estimated by non-compartmental analysis. The terminal elimination rate constant (β) was determined by linear regression of at least three points in the terminal phase. The terminal elimination half-life (T1/2β) was calculated as 0.693/β. The area under the concentration time curve (AUC) was determined by the linear trapezoidal rule. The terminal portion of the AUC was estimated as Cn/β where Cn was the last measurable serum concentration
For the population pharmacokinetic analysis, the R (+) ketorolac and S (−) ketorolac concentration - time data were characterized by a two-compartmental model with first-order elimination from the central compartment. For parameter estimation, the model was parameterized in terms of clearance (CL), central volume of distribution (V1), inter-compartmental clearance (Q) and peripheral volume of distribution (V2). The inter-compartmental clearance, Q, was considered fixed across subjects based on the previous modeling results in the older infants (12). The model’s between-subject variability structure (random effects on all parameters except intercompartmental clearance, and correlation between clearance and volume random effects) was left unchanged with respect to the one established for the older infants. Model parameter estimation was performed using the Nonlinear Mixed Effects Modeling (NONMEM) software (version VI, ADVAN3, TRANS4; NONMEM Project Group, University of California, San Francisco, San Francisco, CA, USA), interfaced with PLT Tools Version 3.0.0 (PLT Soft), Xpose 4.2.2 (17). Following a procedure described by Beal (18), measurements that were below the limit of quantification (LOQ) (0.001 µg/ml) were replaced with a value equal to half the quantification limit prior to the NONMEM analysis. There were 20 measurement points below the LOQ in the S-isomer data set and 1 below LOQ measurement in the R-isomer data set.
Two modeling approaches were used to quantify the pharmacokinetic parameters since both approaches provide insight in this study. A Maximum A Posteriori (MAP) Bayesian analysis integrating population prior and individual data information estimated each subject’s PK parameters (19). In this case the ketorolac pharmacokinetic structural model and population prior information were taken from a previously published study (12). The inter-compartmental clearance, Q, was fixed across subjects at a constant 6.22 ml/min (R (+) isomer) and 98 ml/min (S (−) isomer) based on the previous modeling results in the older infants (12). Individual model parameter estimation was then performed using the population parameters from the Lynn et al. study as priors. Population mean and inter-subject standard deviation estimates were calculated as the sample mean and standard deviation of the individual estimates, respectively.
In the second modeling approach, the new data acquired in this study were pooled with the data from the previously reported study, and estimated model parameters for the pooled data set. The first-order conditional estimation method with random effects interaction was used to estimate the model parameters. Univariate covariate analysis was then performed on model random effects using age, weight and surgical procedure, as previously described. A change in objective function of 3.84 points was considered significant at the 5% level. The analysis was then repeated with adjustment for body size, since this is believed to improve separation of other covariate effects from size alone. Exponents of 0.75 and 1.00 were used for clearances and volumes respectively, as has been previously proposed(20).
Pharmacodynamic Analysis
Morphine bolus and cumulative doses were compared between the 2–6 month infant groups for the 12 hours pre-drug administration (or from recovery unit until study drug administration in infants studied the evening of surgery), as well as for the 12 hours following drug or placebo administration. To determine differences between the placebo and both doses of ketorolac, the combined data obtained for the infants receiving 0.5 mg/kg and 1.0 mg/kg data was evaluated compared to placebo using unpaired t-test. Level of significance was determined at p < 0.05.
Results
Twenty-five infants, aged 2–6 months, were enrolled in the study. Six infants had heparin-lock IV catheters that did not allow blood sampling, one was withdrawn early at parent request, two infants had elevated liver function tests on screening, one had concerns for excessive postoperative bleeding precluding giving study drug and one had anemia precluding extra blood draws on postoperative day 1. Thus, fourteen infants enrolled and received study infusions, 5 the evening of surgery and 9 on postoperative day 1. Table 1 lists patient ages, weight, height, and surgical procedures for the 3 groups (placebo, 0.5 and 1 mg/kg ketorolac). All infants enrolled had normal renal and hepatic function tests and normal urinalyses at screening except the two infants who were dropped from the study. The ages, weight, height and sex of the three groups were similar (Table 1). Craniectomy for craniosynostosis was the surgical procedure in 9 infants, similar to the older infants, 6–18 months previously reported (12).
Table 1.
Patient Characteristics a
| Placebo | Ketorolac 0.5 mg/kg |
Ketorolac 1.0 mg/kg |
|
|---|---|---|---|
| Age (months) | 2.7 – 4.9 | 3.3, 4.0 | 2.2 – 6 |
| Weight (kg) | 5.4 – 7.6 | 5.6, 6.4 | 5.5 – 7.6 |
| Height (cm)a | 57 – 64 | 64, 58 | 56 – 69 |
| Sex | 5 M 1 F | 1M 1F | 2M 4F |
| Surgical Procedures | |||
| Craniectomy | 4 | 1 | 4 |
| Cheiloplasty | 1 | 1 | 2 |
| Orbit implants | 1 | 0 | 0 |
| Oximetry % time < 90% | 2.1 – 7.3 | 0.4, nd | 0.1 – 5.1 |
| BUN (mg/dl) | |||
| Pre- | 5 – 10 | 4, 9 | 4 – 10 |
| 12 H Post | <4 – 10 | <4, nd | <4 – 5 |
| Drain Output (ml) | 192 – 528 | 245, nd | 102 – 286 |
data presented as ranges, except for 0.5 mg/kg dose where individual data is provided for the 2 subjects, nd: not done
Population Pharmacokinetic Analysis
MAP Bayesian analysis
Individual model parameter estimates for the R (+) enantiomer and S (−) enantiomers are given in Table 2. The mean and inter-subject standard deviation of the estimates of the R (+) isomer were: central volume of distribution 992 ± 440 ml, peripheral volume of distribution 658 ± 207 ml and clearance from the central compartment 6.5 ± 2.2 ml/min. Those of the S (−) isomer were 1480 ± 920 ml, 341± 342 ml and 30.7 ± 22.8 ml/min, respectively. Plots comparing predicted and observed concentration-time courses for each subject are shown in Figure 1 for the R (+) enantiomer and active S (−) enantiomers.
Table 2.
Individual estimates and standard-two-stage population values for the pharmacokinetic parameters of R (+) and S (−) Ketorolac in 8 infants aged 2.5–6 months.
| R (+) | Ketorolac | S (−) | Ketorolac | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ID | Dose mg/kg |
CL (ml/min) |
CL (ml/min/kg) |
V1 (ml) |
V2 (ml) |
CL (ml/min) |
CL (ml/min/kg) |
V1 (ml) |
V2 (ml) |
| 1 | 1.0 | 10.5 | 1.73 | 1809 | 809 | 82.8 | 13.6 | 3481 | 182 |
| 2 | 1.0 | 3.9 | 0.73 | 738 | 544 | 18.0 | 3.4 | 867 | 609 |
| 3 | 1.0 | 6.7 | 1.05 | 1157 | 708 | 32.7 | 5.1 | 1639 | 201 |
| 4 | 0.5 | 3.7 | 0.63 | 361 | 219 | 5.4 | 0.9 | 348 | 139 |
| 5 | 1.0 | 5.4 | 0.98 | 694 | 803 | 23.7 | 4.3 | 1145 | 171 |
| 6 | 0.5 | 7.1 | 1.27 | 867 | 612 | 30.7 | 5.5 | 1214 | 118 |
| 7 | 1.0 | 8.1 | 1.07 | 1303 | 701 | 29.8 | 3.9 | 1429 | 1093 |
| 8 | 1.0 | 6.6 | 0.88 | 1003 | 869 | 22.3 | 3.0 | 1714 | 214 |
| Mean | 6.5 | 1.04 | 992 | 658 | 30.7 | 5.0 | 1480 | 341 | |
| SDb | 2.2 | 0.34 | 440 | 207 | 22.8 | 3.8 | 920 | 342 | |
| %BSV | 34 | 33 | 44 | 31 | 74 | 76 | 62 | 100 | |
Fixed-effect parameters: clearance (CL), central volume of distribution (V1) and peripheral volume of distribution (V2).
Standard deviation
BSV, between-subject variability
Figure 1.
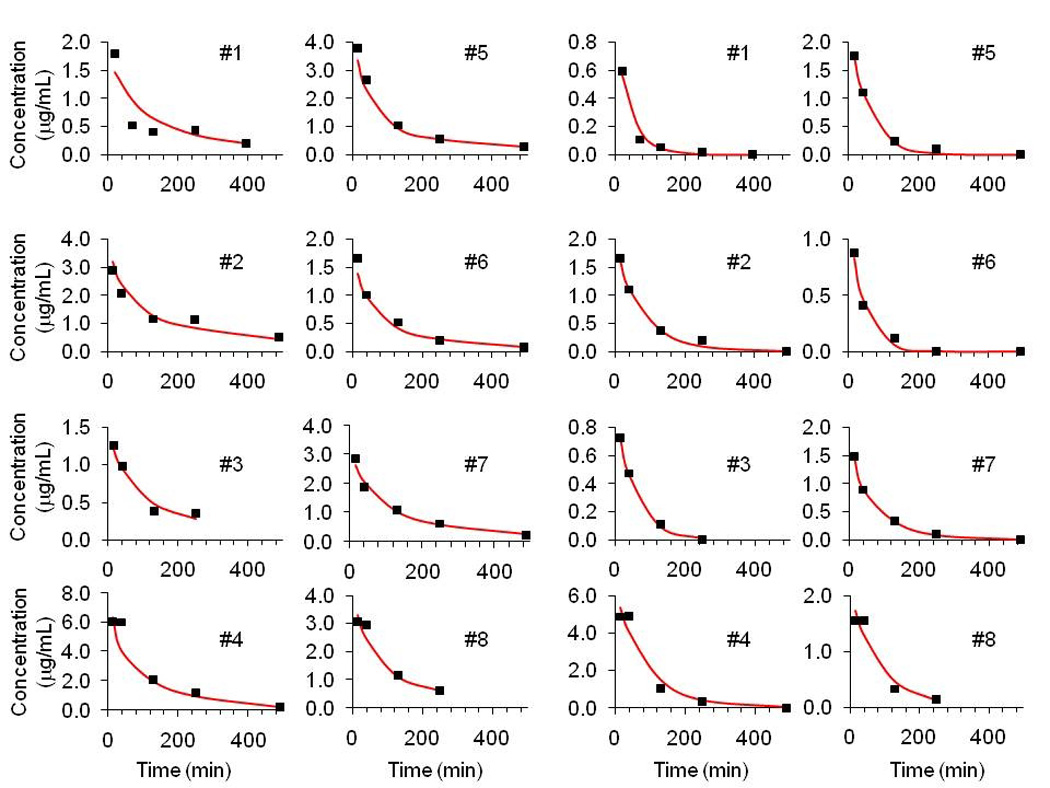
MAP Bayesian individual predictions compared with patient data for R (+) Ketorolac (left) and S (−) Ketorolac (right) plasma concentrations. Note: Patients 4 and 6 received 0.5 mg/kg; Patients 1, 2, 3, 5,7 and 8 received 1.0 mg/kg See Table 2 for individual pharmacokinetic parameters.
Pooled population analysis
Population model parameter estimates for the R (+) enantiomer and active S (−) enantiomers are given in Table 3 for the combined cohort of 2–6 and 6–18 month old infants. The pharmacokinetic parameter values compare favorably with those in our previous study and also with the Bayesian analysis reported here, with variability either decreasing or remaining unchanged, with the exception of unexplained residual variability for the S(−) ketorolac, which increases by approximately two-fold. The increase in the S(−) isomer unexplained residual variability (approximately two-fold) suggests that the data in the younger subjects are noisier than the data from the original, older, subject set, and also could result from the small sample size. According to the base model, the population compartmental analysis distribution half-life for the R(+) enantiomer was 35 min (average of individual estimates 35 min), compared with 190 min (average 199 min) for the elimination half-life. Distribution and elimination half-lives were 2 min (average 2 min) and 40 min (average 48 min) respectively for the (S−) enantiomer. Plots comparing population and individualized predicted and observed concentration-time courses are shown in Figure 2 for the R (+) enantiomer and active S (−) enantiomer. A univariate covariate search with additive, multiplicative and exponential models produced strongest results (objective function test, −337.764 vs. −333.939, slightly below the statistical significance threshold) only for age on clearance of the (S−) enantiomer (the covariate model was 40.3*[1+(AGE-10)*(1.04-1)], where 10 months is median age, with inter-subject variability decreasing to 0.380 vs. 0.462; see Table 3). A plot of all individual covariates against pharmacokinetic parameter values is shown in Figure 3 for the R (+) and active S (−) enantiomers. The analysis was repeated after body size adjustment and parameters; these results are reported in Table 4. While the objective function improved, goodness of fit plots changed only slightly (data not shown). Model-based estimated distribution and elimination half-lives for the R(+) enantiomer changed slightly to 22 min (average 37 min) and 115 min (average 204 min) and for the (S−) enantiomer to 1.3 min (average 2.6 min) and 24 min (average 48 min) respectively. Interestingly, no covariates (including age) were found to be statistically significant after body size adjustment was applied.
Table 3.
Base model population estimates for the pharmacokinetic parameters of R (+) (NONMEM Objective Function Value OFV −206.749) and S (−) (OFV −333.939) Ketorolac in 8 infants aged 2.5–6 months combined with previously studied dataset comprising 25 infants and toddlers (aged 6–18 months). The covariate model for S (−) ketorolac (OFV −337.764) is also reported.
| Parameter Estimates | Between-Subject Variability |
|||
|---|---|---|---|---|
| Valuea (θ) | S.E.(%)b | |||
| BSVc (ω) | S.E (%).b | |||
| R (+) Ketorolac Base Model | ||||
| CL (ml/min) | 7.91 | 9.4 | 0.244 | 29.1 |
| V1 (ml) | 1110 | 9.0 | 0.304 | 23.4 |
| Q (ml/min) | 6.74 | 14.8 | NE | NE |
| V2 (ml) | 670 | 17.3 | 0.373 | 68.9 |
| σ2 | ||||
| Proportional | 0.0305 | 21.8 | ||
| Additive | 0.00436 | 45.0 | ||
| S (−) Ketorolac Base Model | ||||
| CL (ml/min) | 39.5 | 13.0 | 0.462 | 33.3 |
| V1 (ml) | 1930 | 15.2 | 0.471 | 23.6 |
| Q (ml/min) | 95.1 | 3.1 | NE | NE |
| V2 (ml) | 319 | 58.3 | 1.76 | 45.1 |
| σ2 | 0.0176 | 59.7 | ||
| S (−) Ketorolac Covariate Model | ||||
| Intercept on CL (ml/min) | 40.3 | 10.3 | 0.380 | 30.0 |
| Normalized slope on CL | 1.04 | 2.16 | ||
| (1/month) | 2060 | 11.8 | 0.447 | 22.2 |
| V1 (ml) | 94.2 | 3.96 | NE | NE |
| Q (ml/min) | 205 | 54.6 | 2.71 | 39.1 |
| V2 (ml) | 0.0179 | 59.2 | ||
| σ2 | ||||
Fixed-effect parameters: clearance (CL), central volume of distribution (V1), inter-compartmental clearance (Q) and peripheral volume of distribution (V2). Residual error parameters: variance of the exponential error model for R (+) ketorolac and variance of the additive error model for S (−) ketorolac. NE, not estimated.
Standard error expressed as percent coefficient of variation.
BSV, between-subject variability expressed in variance units (approximately coefficient of variation squared).
Figure 2.
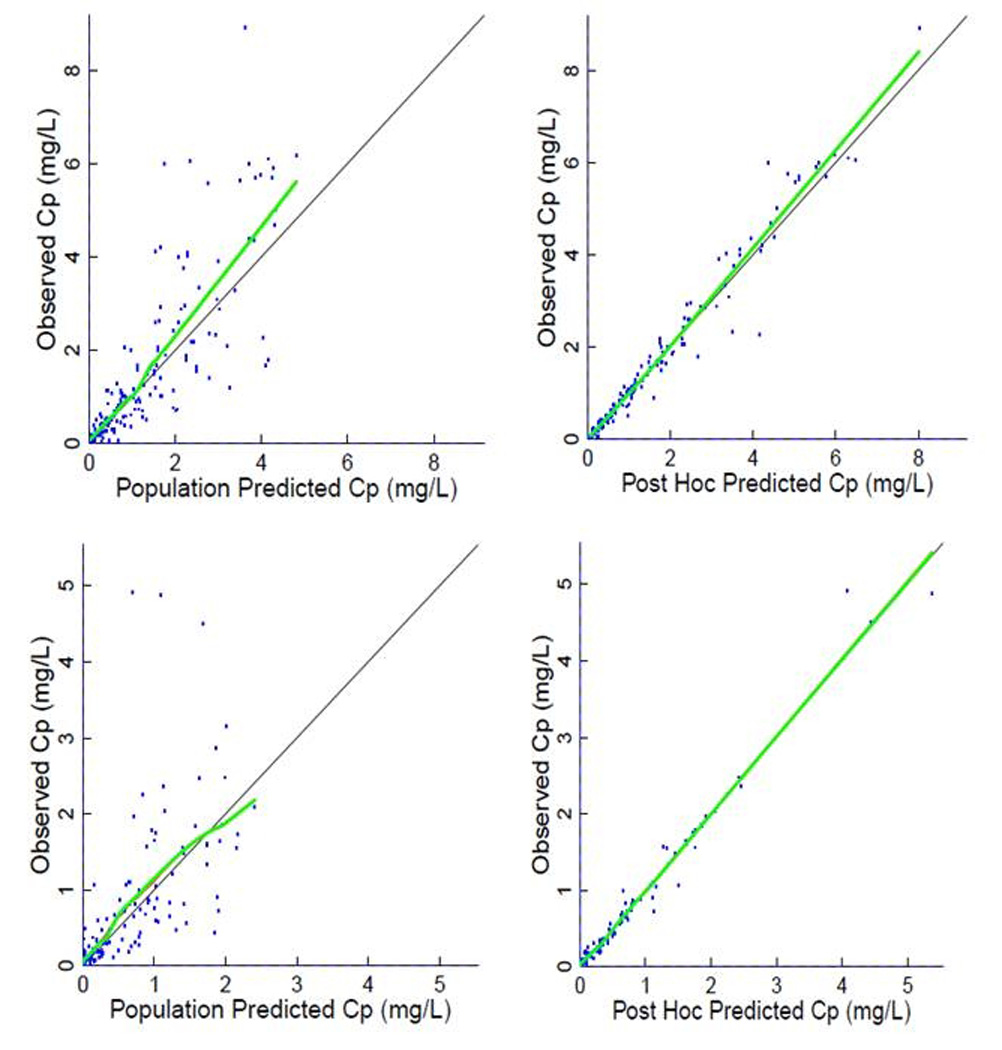
Population predicted Cp (plasma concentration) (left) and post hoc predicted (right) values compared with observed concentrations for R (+) Ketorolac (top) and S (−) Ketorolac (bottom) plasma concentrations. See Table 2 for individual pharmacokinetic parameters. Thick line is a smoothing line, thin line is the identity line.
Figure 3.
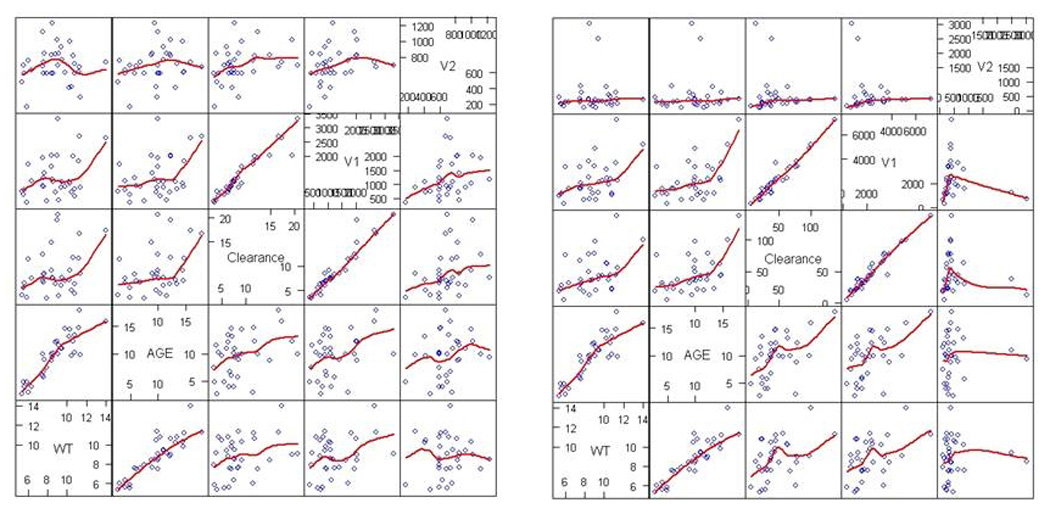
Scatterplot of R (+) Ketorolac (left) and S (−) Ketorolac (right) pharmacokinetic parameters vs. covariates. See text for details.
Table 4.
Body size-adjusted model population pharmacokinetic parameters of R (+) (OFV −207.867) and S (−) (OFV −339.784) Ketorolac in 8 infants aged 2.5–6 months combined with previously studied dataset comprising 25 infants and toddlers (aged 6–18 months).
| Parameter Estimates | Between-Subject Variability | |||
|---|---|---|---|---|
| Valuea (θ) | S.E.(%)b | BSVc (ω) | S.E (%).b | |
| R (+) Ketorolac | ||||
| CL (ml/min/kg) | 1.56 | 8.72 | 0.208 | 32.7 |
| V1 (ml/kg) | 131 | 9.08 | 0.287 | 20.0 |
| Q (ml/min/kg) | 1.29 | 14.19 | NE | NE |
| V2 (ml/kg) | 79.5 | 16.6 | 0.322 | 59.9 |
| σ2 | ||||
| Proportional | 0.0312 | 23.9 | ||
| Additive | 0.00430 | 47.9 | ||
| S (−) Ketorolac | ||||
| CL (ml/min) | 7.8 | 11.71 | 0.378 | 30.9 |
| V1 (ml) | 218 | 12.66 | 0.381 | 21.3 |
| Q (ml/min) | 19.2 | 3.82 | NE | NE |
| V2 (ml) | 44.9 | 25.17 | 1.56 | 30.6 |
| σ2 | 0.0176 | 59.7 | ||
Fixed-effect parameters: clearance (CL), central volume of distribution (V1), inter-compartmental clearance (Q) and peripheral volume of distribution (V2). Residual error parameters: variance of the exponential error model for R (+) ketorolac and variance of the additive error model for S (−) ketorolac. NE, not estimated.
Standard error expressed as percent coefficient of variation.
BSV, between-subject variability expressed in variance units (approximately coefficient of variation squared).
Non-compartmental Pharmacokinetic Analysis
Cmax at the end of infusion was approximately two times higher for the inactive R (+) enantiomer compared to the active S (−) enantiomer, for the 0.5 mg/kg (R= 1.3, 1.7µg/ml and S= 0.7, 0.9) and 1.0 mg/kg racemic doses (R = 3.4 ± 0.8 µg/ml and S = 2.0 ± 0.7 µg/ml) in the 2–6 month old infants. Pharmacokinetic data from the previously published study in infants ages 6 to 18 months was combined with the eight infants who received ketorolac in this study due to the lack of the effect of age in the analysis above. As shown in Table 5, there were significant differences between the S (−) and the R (+) enantiomer for all of the pharmacokinetic parameters. The T½ β at the end of infusion were approximately three times longer for the analgesically inactive R (+) enantiomer compared to the active S (−) enantiomer, 197 ± 82 min and 67 ± 33 min, respectively. A linear increase of AUC with dose was observed in the combined analysis (Table 5)
TABLE 5.
Noncompartmental pharmacokinetic parameters for R (+) Ketorolac and S (−) Ketorolac
| R (+) Ketorolac | S (−) Ketorolac | Probability | |
|---|---|---|---|
| Cmax (µg/ml) | |||
| 0.5 mg/kg (n=10) | 2.5 ± 1.4 | 1.1 ± 0.6 | P=0.014 |
| 1.0 mg/kg (n=20) | 4.1 ±1.8 | 2.0 ± 1.1 | P= 0.001 |
| AUC (µg/ml/min) | |||
| 0.5 mg/kg (n=10) | 375 ± 224 | 75 ± 26 | P=0.0024 |
| 1.0 (mg/kg (n=20) | 711 ± 612 | 183 ± 167 | P=0.0013 |
| Cl (ml/min/kg) | 0.95 ± 0.54 | 5.1 ± 4.3 | P< 0.001 |
| Vβ (L/kg) | 0.27 ± 0.17 | 0.45 ± 0.33 | P=0.006 |
| T½ β (min) | 197 ± 82 | 67 ± 33 | P < 0.001 |
Pharmacodynamic Analysis
Renal function testing (BUN, creatinine) showed no deterioration from screening to the 12 hour post drug sample. Individual group data for the 2–6 month olds are given in Table 1. Urinalyses also were unchanged. Continuous oximetry for the first postoperative day showed a mean percent time with oxygen saturations < 90% not different in the 3 groups. For infants post craniectomy, surgical drain amounts were similar in all groups.
As shown in Table 6, ketorolac had no significant effect on the morphine cumulative doses, or the need for additional morphine boluses for the 12 hours following drug or placebo administration. There also were no significant effects when the combined ketorolac data was evaluated in the patients receiving ketorolac compared to placebo.
Table 6.
Effect of Ketorolac on Morphine (MS) Doses (mg/kg)
| Placebo n = 6 |
Ketorolac n = 8 |
P | |
|---|---|---|---|
| Pre-Drug MS Bolus (12 hr total) | 0.067 ± 0.05 | 0.028 ± 0.04 | p = 0.17 |
| Pre-Drug MS Total (12 hr total) | 0.12 ± 0.19 | 0.26 ± 0.59 | p = 0.55 |
| Post Drug MS Bolus (12 hr total) | 0.037 ± 0.04 | 0.189 ± 0.33 | p = 0.27 |
| Post Drug MS Total (12 hr total) | 0.077 ± 0.12 | 0.123 ± 0.28 | p = 0.68 |
| Pre-Drug - Post-Drug difference | 0.052 ± 0.12 | −0.004 ± 0.50 | p = 0.77 |
Discussion
The stereo-isomer specific pharmacokinetics of ketorolac in infants 2 to 6 months of age showed a linear response to dose as shown with the AUC value twice as high at 1 mg/kg than at 0.5 mg/kg for both the R (+) and S (−) isomers. The ideal concentration for ketorolac isomers in infants, children or adults remains undefined.
The MAP Bayesian analysis is conditional on the assumption of inter-compartmental clearance being the same. Given this assumption, the mean population parameters for the infants, aged 2.5 – 6 months, exhibit two similar trends to those observed in the older infant population. First, S (−) ketorolac clearance was observed to be 4–5 times faster than that of the R (+) ketorolac. Second, the central compartment volume, V1, was observed to be higher on average for the S (−) isomer results compared with those for the R (+) isomer. In the case of the R (+) isomer data, the average (or typical) parameter values (CL, V1 and V2) for this new population were lower than those observed in the original study. This trend also was observed in the S (−) isomer data, with the exception that the peripheral compartment volume, V2, was higher in the S (−) isomer data for the new population (341 versus 224 ml). However, it is noted that high variability was observed in V2 in this small subject set as was also found in our older infants (12).
The trends identified in the Bayesian analysis also were observed in the pooled population analysis. Here again, the S (−) ketorolac clearance was observed to be 4–5 times faster than that of the R (+) ketorolac, while the central compartment volume, V1, was again observed to be higher on average for the S (−) isomer results compared with those for the R (+) isomer.
The pooled analysis without body size adjustment does suggest possibly nonlinear trends relating pharmacokinetic parameters with covariates (Fig. 3). However, the only statistically significant inclusion of covariates such as age, weight, or allometric and/or maturation functions was on the clearance of the S (−) isomer. It is possible that larger sample sizes would allow detection of more statistically significant trends, as was recently done in Potts et al.(21). However, when including adjustment for body sizes in the model, all between-subject variability estimates decreased and no covariate was significant, suggesting that changes in body size account for most of the variability in ketorolac disposition. Although no relationship with age was detected in our infants aged 2–18 months, the pharmacokinetic parameters showed a pattern seen in adult patients but exaggerated with increased clearance, Vss, and shorter elimination half-life for the S-isomer than for R+ketorolac (22, 23)
In rodent models, the anti-inflammatory and analgesic activity of the S (−) isomer is 57 and 230 times, respectively more potent than the R (+) isomer (24). The relative potency of the isomers in causing renal, hematological or other side effects has not been established. In addition, the significance of the difference in isomeric potency has not been determined in humans. Therefore, the clinical importance of differences in R+ and S− isomer kinetics remain to be established.
Safety assessments showed no adverse effects from a single 0.5 or 1 mg/kg dose of ketorolac in infants 2– 6 months of age. Serum creatinine and BUN remained in the normal range after drug administration. Drug administration did not result in increases in hepatic enzymes in the infants in this study.
Continuous oximetry showed no difference in percent time with room air saturations < 90% in infants given drug versus placebo (Table 1). Surgical drains were used in all craniectomy surgery infants and drainage totals were not different in the treated vs. placebo groups (Table 1).
Infants aged 2–6 months should have active cyclo-oxygenase systems allowing NSAID agents such as ketorolac to exert analgesic effects. Lieh-Lai et al. (25) reported the analgesic effects of ketorolac after a single dose (0.6 mg/kg) postoperatively in children in intensive care compared to IV morphine (0.1 mg/kg). In both groups, pain relief was achieved in 68% (ketorolac) or 58% (morphine). Most children required remedication for pain within 4 hours; 58% for ketorolac and 63% for morphine. Papacci et al. (26) reported significant analgesic efficacy within 0.5 h and up to 6 h post dose in an observational unblinded study of 18 neonates and premature babies given 1 mg/kg IV ketorolac for postoperative or procedure-related pain. The lack of difference in concomitant morphine usage between treated and placebo patients in our study was surprising. This finding might suggest that ketorolac is not analgesic in postoperative infants, contrary to Papacci’s or Lieh-Lai’s reports. An alternative and, we believe, more likely hypothesis is that concomitant morphine administration may not have been a good reflection of analgesic efficacy of ketorolac in this study. Continuous morphine infusions are the standard for postoperative care at our institution after major surgery. Although infusions could be weaned by the bedside nurse after study drug administration under this protocol, they proved unlikely to be immediately discontinued, if the infant was receiving a satisfactory pain score and had no limiting side effects. As we found in our older infants (12), we hypothesize that this obscured measurement of an analgesic effect from ketorolac as measured by decrease in total morphine dose. The inter-patient variability in morphine usage was large as has been previously reported (27) and could further obscure any difference in treated vs. placebo infants. On average, the analgesic S (−) isomer had a shorter elimination half-life in infants compared with children (7); this may also play a role in a shorter duration of analgesia. While we found an associative trend between age (and body weight) and clearance and central compartment volume of the two isomers, this did not reach statistical significance in the analysis.
Recently the population kinetics of 12 infants given ketorolac 0.5 mg/kg IV during anesthesia were published (28). Stereo-specific isomers were not analyzed; the composite levels fit a 2 compartment model with clearance of 2.8 mL/min/kg. This value is between the values we found for the R (+) and S (−) isomers, as reported in Table 2. Safety and efficacy data were not included. As Zuppa et al report in their infants, we found clearance values higher than previous reports in children. We agree that pharmacodynamic studies to identify therapeutic concentrations would be extremely useful. Studies of repeated dosing would help assess any effects of accumulation of the R(+) isomer.. The pharmacokinetics of the R+ and S− ketorolac isomers are markedly different in infants. As the pharmacologic properties of the two isomers are different, we believe characterization of the stereo-specific pharmacokinetics may aid in characterizing the safety and efficacy in future studies looking at multiple dosing.
We found the stereo-specific pharmacokinetics of ketorolac in infants aged 2 to 6 months showed differences in handling of the R (+) and S (−) isomers, with more rapid elimination of the analgesic (S−) isomer. No adverse effects on renal or hepatic function tests were seen. In the 14 infants studied, no difference in morphine usage was seen between treated and placebo groups, but this may be explained by the pre-existing institutional morphine infusion protocol or our small sample size
Acknowledgements
The authors thank the surgeons and nursing staff for allowing their families to consider study participation. We also are grateful to the families who allowed their infants to participate.
Supported by FDA Orphan Product Development Grant FD-R-001815. The population analysis was partially supported by the University of Washington Applied Physics Laboratory and NIH grant P41 EB001975, "Resource Facility for Population Kinetics." NIH grant M01-RR-00037 partially supported safety assessments.
References
- 1.Splinter WM, Reid CW, Roberts DJ, et al. Reducing pain after inguinal hernia repair in children: caudal anesthesia versus ketorolac tromethamine. Anesthesiology. 1997;87:542–546. doi: 10.1097/00000542-199709000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Munro HM, Walton SR, Malviya S, et al. Low-dose ketorolac improves analgesia and reduces morphine requirements following posterior spinal fusion in adolescents. Can J Anaesth. 2002;49:461–466. doi: 10.1007/BF03017921. [DOI] [PubMed] [Google Scholar]
- 3.Krotz F, Schiele TM, Klauss V, et al. Selective COX-2 inhibitors and risk of myocardial infarction. J Vasc Res. 2005;42:312–324. doi: 10.1159/000086459. [DOI] [PubMed] [Google Scholar]
- 4.de Lima J, Lloyd-Thomas AR, Howard RF, et al. Infant and neonatal pain: anaesthetists' perceptions and prescribing patterns. Bmj. 1996;313:787. doi: 10.1136/bmj.313.7060.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burd RS, Tobias JD. Ketorolac for pain management after abdominal surgical procedures in infants. South Med J. 2002;95:331–333. [PubMed] [Google Scholar]
- 6.Mandema JW, Stanski DR. Population pharmacodynamic model for ketorolac analgesia. Clin Pharmacol Ther. 1996;60:619–635. doi: 10.1016/S0009-9236(96)90210-6. [DOI] [PubMed] [Google Scholar]
- 7.Kauffman RE, Lieh-Lai MW, Uy HG, et al. Enantiomer-selective pharmacokinetics and metabolism of ketorolac in children. Clin Pharmacol Ther. 1999;65:382–388. doi: 10.1016/S0009-9236(99)70131-1. [DOI] [PubMed] [Google Scholar]
- 8.Hamunen K, Maunuksela EL, Sarvela J, et al. Stereoselective pharmacokinetics of ketorolac in children, adolescents and adults. Acta Anaesthesiol Scand. 1999;43:1041–1046. doi: 10.1034/j.1399-6576.1999.431012.x. [DOI] [PubMed] [Google Scholar]
- 9.Olkkola KT, Maunuksela EL. The pharmacokinetics of postoperative intravenous ketorolac tromethamine in children. Br J Clin Pharmacol. 1991;31:182–184. doi: 10.1111/j.1365-2125.1991.tb05510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dsida RM, Wheeler M, Birmingham PK, et al. Age-stratified pharmacokinetics of ketorolac tromethamine in pediatric surgical patients. Anesth Analg. 2002;94:266–270. doi: 10.1097/00000539-200202000-00007. table of contents. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Martin G, Maggio L, Gonzalez-Sotomayor J, et al. Pharmacokinetics of ketorolac in children after abdominal surgery. Int J Clin Pharmacol Ther. 1997;35:160–163. [PubMed] [Google Scholar]
- 12.Lynn AM, Bradford H, Kantor ED, et al. Postoperative ketorolac tromethamine use in infants aged 6–18 months: the effect on morphine usage, safety assessment, and stereo-specific pharmacokinetics. Anesth Analg. 2007;104:1040–1051. doi: 10.1213/01.ane.0000260320.60867.6c. tables of contents. [DOI] [PubMed] [Google Scholar]
- 13.Lynn AM, Slattery JT. Morphine pharmacokinetics in early infancy. Anesthesiology. 1987;66:136–139. doi: 10.1097/00000542-198702000-00005. [DOI] [PubMed] [Google Scholar]
- 14.McRorie TI, Lynn AM, Nespeca MK, et al. The maturation of morphine clearance and metabolism. Am J Dis Child. 1992;146:972–976. doi: 10.1001/archpedi.1992.02160200094036. [DOI] [PubMed] [Google Scholar]
- 15.Anderson BJ, Merry AF. Data sharing for pharmacokinetic studies. Paediatr Anaesth. 2009;19:1005–1010. doi: 10.1111/j.1460-9592.2009.03051.x. [DOI] [PubMed] [Google Scholar]
- 16.Buchholz M, Karl HW, Pomietto M, et al. Pain scores in infants: a modified infant pain scale versus visual analogue. J Pain Symptom Manage. 1998;15:117–124. [PubMed] [Google Scholar]
- 17.Jonsson EN, Karlsson MO. Xpose--an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Computer Methods and Programs in Biomedicine. 1999;58(1):51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 18.Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28:481–504. doi: 10.1023/a:1012299115260. [DOI] [PubMed] [Google Scholar]
- 19.Sheiner LB, Beal SL. Bayesian individualization of pharmacokinetics: simple implementation and comparison with non-Bayesian methods. Journal of Ppharmaceutical Sci. 1982;71:1344–1348. doi: 10.1002/jps.2600711209. [DOI] [PubMed] [Google Scholar]
- 20.Meibohm B, Laer S, Pancetta JC, Barrett JS. Population pharmacokinetic studies in pediatrics: issues in design and analysis. AAPS J. 2005;7:E475–E487. doi: 10.1208/aapsj070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potts AL, Anderson BJ, Warman G, et al. Dexmedetomidine pharmacokinetics in pediatric intensive care--a pooled analysis. Paediatr Anaesth. 2009;19:1119–1129. doi: 10.1111/j.1460-9592.2009.03133.x. [DOI] [PubMed] [Google Scholar]
- 22.Hamunen K, Maunuksela EL, Sarvela J, Bullingham RES, Olkkola K. Stereoselective pharmacokinetics of ketorolac in children, adolescents, and adults. Acta Anaesthesiol Scand. 1999;43:1041–1046. doi: 10.1034/j.1399-6576.1999.431012.x. [DOI] [PubMed] [Google Scholar]
- 23.Hayball PJ, Wrobel J, Tamblyn JG, Nation RL. The pharmacokinetics of ketorolac enantiomers following intramuscular administration of the racemate. Br J Clin Pharmacol. 1994;37:75–78. doi: 10.1111/j.1365-2125.1994.tb04243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillis JC, Brogden RN. Ketorolac. A reappraisal of its pharmacodynamic and pharmacokinetic properties and therapeutic use in pain management. Drugs. 1997;53:139–188. doi: 10.2165/00003495-199753010-00012. [DOI] [PubMed] [Google Scholar]
- 25.Lieh-Lai MW, Kauffman RE, Uy HG, et al. A randomized comparison of ketorolac tromethamine and morphine for postoperative analgesia in critically ill children. Crit Care Med. 1999;27:2786–2791. doi: 10.1097/00003246-199912000-00030. [DOI] [PubMed] [Google Scholar]
- 26.Papacci P, De Francisci G, Iacobucci T, et al. Use of intravenous ketorolac in the neonate and premature babies. Paediatr Anaesth. 2004;14:487–492. doi: 10.1111/j.1460-9592.2004.01250.x. [DOI] [PubMed] [Google Scholar]
- 27.Lynn AM, Nespeca MK, Bratton SL, et al. Ventilatory effects of morphine infusions in cyanotic versus acyanotic infants after thoracotomy. Paediatr Anaesth. 2003;13:12–17. doi: 10.1046/j.1460-9592.2003.00959.x. [DOI] [PubMed] [Google Scholar]
- 28.Zuppa AF, Mondick JT, Davis L, et al. Population pharmacokinetics of ketorolac in neonates and young infants. American journal of therapeutics. 2009;16:143–146. doi: 10.1097/MJT.0b013e31818071df. [DOI] [PubMed] [Google Scholar]


