Abstract
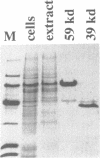
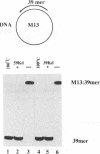
Adenovirus DNA binding protein is a multifunctional protein essential for viral DNA replication. To investigate the role of the DNA binding protein in this process its interaction with partial DNA duplexes was examined. Duplex regions of DNA, created when a short DNA strand is annealed to its complementary sequence present in the single stranded form of M13 phage DNA, were efficiently unwound by DNA binding protein in a reaction that required neither ATP nor MgCl2. The unwinding activity of DNA binding protein was reduced by conditions which increased the stability of DNA duplexes. DNA unwinding by DNA binding protein was highly co-operative and required the single stranded DNA to be completely coated with the protein. Completely double stranded DNA could also be unwound by DNA binding protein but this reaction was sensitive to the G+C content of the DNA and could only be observed with relatively short DNA duplexes up to 45 base pairs in length. When these short double stranded DNA molecules contained binding sites for the transcription factors NFI and NFIII addition of the cognate factor blocked DNA binding protein mediated unwinding of the particular DNA duplex. Cleavage of DNA binding protein with chymotrypsin and isolation of the 39,000 molecular weight C-terminal fragment indicated that the unwinding activity was located in this domain of the protein. In support of this contention a monoclonal antibody, which had previously been mapped to this region, specifically inhibited the DNA unwinding activity. These activities of DNA binding protein are likely to be involved in DNA replication, where the destabilisation of DNA duplexes could be important both during initiation and elongation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boehmer P. E., Lehman I. R. Herpes simplex virus type 1 ICP8: helix-destabilizing properties. J Virol. 1993 Feb;67(2):711–715. doi: 10.1128/jvi.67.2.711-715.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher J., Robinson E. C., Hay R. T. Interactions between the adenovirus type 2 DNA polymerase and the DNA binding domain of nuclear factor I. New Biol. 1990 Dec;2(12):1083–1090. [PubMed] [Google Scholar]
- Chen M., Mermod N., Horwitz M. S. Protein-protein interactions between adenovirus DNA polymerase and nuclear factor I mediate formation of the DNA replication preinitiation complex. J Biol Chem. 1990 Oct 25;265(30):18634–18642. [PubMed] [Google Scholar]
- Clark L., Matthews J. R., Hay R. T. Interaction of enhancer-binding protein EBP1 (NF-kappa B) with the human immunodeficiency virus type 1 enhancer. J Virol. 1990 Mar;64(3):1335–1344. doi: 10.1128/jvi.64.3.1335-1344.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleat P. H., Hay R. T. Co-operative interactions between NFI and the adenovirus DNA binding protein at the adenovirus origin of replication. EMBO J. 1989 Jun;8(6):1841–1848. doi: 10.1002/j.1460-2075.1989.tb03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J., Gronostajski R. M., Hurwitz J. Properties of the adenovirus DNA polymerase. J Biol Chem. 1984 Aug 10;259(15):9487–9495. [PubMed] [Google Scholar]
- Georgaki A., Hübscher U. DNA unwinding by replication protein A is a property of the 70 kDa subunit and is facilitated by phosphorylation of the 32 kDa subunit. Nucleic Acids Res. 1993 Aug 11;21(16):3659–3665. doi: 10.1093/nar/21.16.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R. T., Russell W. C. Recognition mechanisms in the synthesis of animal virus DNA. Biochem J. 1989 Feb 15;258(1):3–16. doi: 10.1042/bj2580003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R. T. The origin of adenovirus DNA replication: minimal DNA sequence requirement in vivo. EMBO J. 1985 Feb;4(2):421–426. doi: 10.1002/j.1460-2075.1985.tb03645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitts P. A., Ayres M. D., Possee R. D. Linearization of baculovirus DNA enhances the recovery of recombinant virus expression vectors. Nucleic Acids Res. 1990 Oct 11;18(19):5667–5672. doi: 10.1093/nar/18.19.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbaum J. O., Field J., Hurwitz J. The adenovirus DNA binding protein and adenovirus DNA polymerase interact to catalyze elongation of primed DNA templates. J Biol Chem. 1986 Aug 5;261(22):10218–10227. [PubMed] [Google Scholar]
- Mautner V., Boursnell M. E. Recombination in adenovirus: DNA sequence analysis of crossover sites in intertypic recombinants. Virology. 1983 Nov;131(1):1–10. doi: 10.1016/0042-6822(83)90527-5. [DOI] [PubMed] [Google Scholar]
- Mul Y. M., Van der Vliet P. C. Nuclear factor I enhances adenovirus DNA replication by increasing the stability of a preinitiation complex. EMBO J. 1992 Feb;11(2):751–760. doi: 10.1002/j.1460-2075.1992.tb05108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mul Y. M., van der Vliet P. C. The adenovirus DNA binding protein effects the kinetics of DNA replication by a mechanism distinct from NFI or Oct-1. Nucleic Acids Res. 1993 Feb 11;21(3):641–647. doi: 10.1093/nar/21.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mul Y. M., van der Vliet P. C. The adenovirus DNA binding protein effects the kinetics of DNA replication by a mechanism distinct from NFI or Oct-1. Nucleic Acids Res. 1993 Feb 11;21(3):641–647. doi: 10.1093/nar/21.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich N. C., Sarnow P., Duprey E., Levine A. J. Monoclonal antibodies which recognize native and denatured forms of the adenovirus DNA-binding protein. Virology. 1983 Jul 30;128(2):480–484. doi: 10.1016/0042-6822(83)90274-x. [DOI] [PubMed] [Google Scholar]
- Salas M. Protein-priming of DNA replication. Annu Rev Biochem. 1991;60:39–71. doi: 10.1146/annurev.bi.60.070191.000351. [DOI] [PubMed] [Google Scholar]
- Santoro C., Mermod N., Andrews P. C., Tjian R. A family of human CCAAT-box-binding proteins active in transcription and DNA replication: cloning and expression of multiple cDNAs. Nature. 1988 Jul 21;334(6179):218–224. doi: 10.1038/334218a0. [DOI] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Stuiver M. H., Bergsma W. G., Arnberg A. C., van Amerongen H., van Grondelle R., van der Vliet P. C. Structural alterations of double-stranded DNA in complex with the adenovirus DNA-binding protein. Implications for its function in DNA replication. J Mol Biol. 1992 Jun 20;225(4):999–1011. doi: 10.1016/0022-2836(92)90100-x. [DOI] [PubMed] [Google Scholar]
- Stuiver M. H., van der Vliet P. C. Adenovirus DNA-binding protein forms a multimeric protein complex with double-stranded DNA and enhances binding of nuclear factor I. J Virol. 1990 Jan;64(1):379–386. doi: 10.1128/jvi.64.1.379-386.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm R. A., Herr W. The POU domain is a bipartite DNA-binding structure. Nature. 1988 Dec 8;336(6199):601–604. doi: 10.1038/336601a0. [DOI] [PubMed] [Google Scholar]
- Temperley S. M., Hay R. T. Recognition of the adenovirus type 2 origin of DNA replication by the virally encoded DNA polymerase and preterminal proteins. EMBO J. 1992 Feb;11(2):761–768. doi: 10.1002/j.1460-2075.1992.tb05109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temperley S. M., Hay R. T. Replication of adenovirus type 4 DNA by a purified fraction from infected cells. Nucleic Acids Res. 1991 Jun 25;19(12):3243–3249. doi: 10.1093/nar/19.12.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsernoglou D., Tsugita A., Tucker A. D., van der Vliet P. C. Characterization of the chymotryptic core of the adenovirus DNA-binding protein. FEBS Lett. 1985 Sep 2;188(2):248–252. doi: 10.1016/0014-5793(85)80381-1. [DOI] [PubMed] [Google Scholar]