Abstract
There is universal agreement that the emergence of antibiotic-resistant bacteria is a significant health problem, leading to preventable morbidity and mortality. Kaiser Permanente (KP) has made great strides in improving the antibiotic-prescribing behavior of its physicians, thereby limiting the emergence of antibiotic resistance in the clinical setting. This, however, is only a beginning. Greater than 70% of the antibiotics used in the United States are for nontherapeutic purposes in animal feed. The resulting emergence of resistant bacteria that cause human disease is described. I propose a campaign throughout KP to broaden our prevention efforts by phasing out meat, dairy, poultry, and fish products raised using antibiotic feed additives.
A Successful First Step
If we want to preserve antibiotics as a valuable therapeutic tool, we must seriously address the crisis of antibiotic resistance. Toward this end, the Chiefs of Infectious Diseases of The Permanente Medical Group in Northern California have enlisted the support of primary care physicians in a campaign to eliminate the unnecessary use of antibiotics. Our prescribing patterns are scrutinized and we are coached to prescribe antibiotics only when they are clearly needed. This campaign has been extremely successful in altering the prescribing behavior of physicians treating upper respiratory tract infections (Figure 1).
Figure 1.
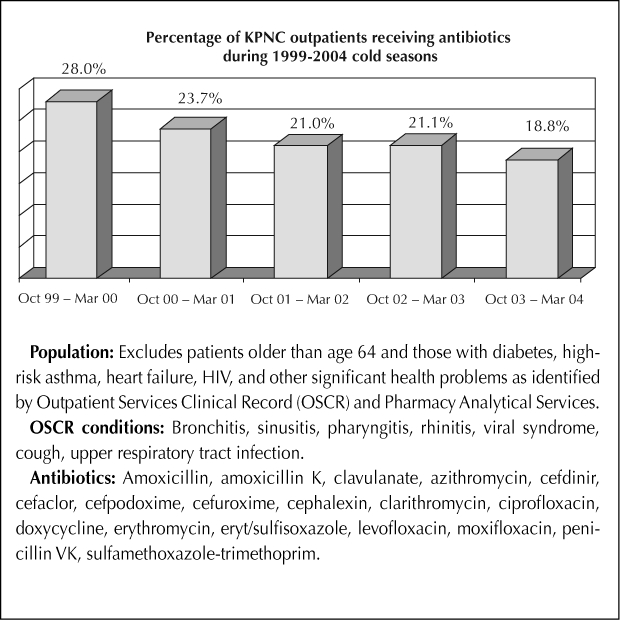
Antibiotic use for bronchitis, sinusitis, pharyngitis, rhinitis, cough, viral syndrome, and upper respiratory tract infection for Kaiser Permanente Northern California.
The Larger Problem
As remarkable as this achievement is in improving our prescribing behavior, it alone will have limited success in preventing the emergence of antibiotic resistant bacteria1 (Figure 2). The reason is quite simple: Most antibiotics are used not in people but as feed additives in the meat production industry. The Union of Concerned Scientists estimates, for example, that 70% of all US antibiotics are given in this way to beef cattle, swine, and poultry2 (Figure 3). Antibiotics are mixed with animal feed, typically not for any therapeutic purpose but to promote growth or to compensate for the inevitable infections in animals raised indoors under stressful, crowded conditions. As we would expect, the widespread use of antibiotics selects for resistance. Bacteria are nature's champions in sharing their genetic information with one another.3 Once resistance emerges, it may spread widely. More than half of the antibiotics added to animal feed belong to classes of antibiotics used in human medicine, including penicillins, tetracyclines, macrolides, and streptogramins.2 The development of resistance to the drug used in animals often confers resistance to the antibiotic used in humans.
Figure 2.

Microbial threats to health, as reported in March 2003 by National Academy of Sciences (NAS), Institute of Medicine (IOM).1
Figure 3.
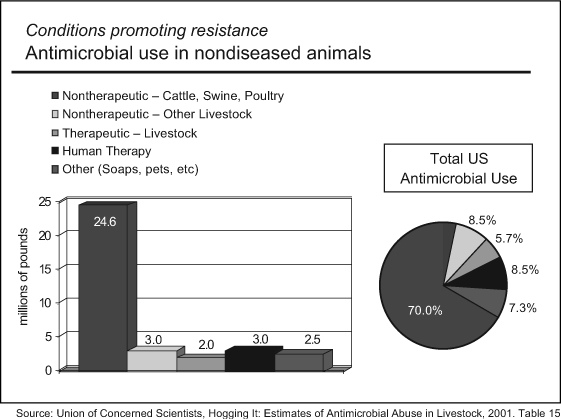
Conditions promoting resistance: antimicrobial use in nondiseased animals.2
Once resistant bacteria emerge in the gastrointestinal tracts of animals, there are a variety of ways for them to enter into the human population and cause illness. First, we ingest the bacteria in undercooked meat products or on foods contaminated by raw meat juices. Multiple studies have now shown that meat and poultry obtained from supermarket shelves routinely carry antibiotic-resistant bacteria. A study done in Washington, DC found that 20% of ground meat obtained in supermarkets was contaminated with Salmonella and that 84% of the isolates were resistant to at least one antibiotic.4 Similar results have been found in poultry contaminated with Campylobacter jejuni resistant to fluoroquinolones.5 The rise in fluoroquinolone resistance occurred after their introduction for use in poultry operations. In mid-2005, the US Food and Drug Administration banned such use because it exacerbates fluoroquinolone resistance in Campylobacter. This was the first time that agency had ever withdrawn approval for use of an agricultural antibiotic because of concerns about antibiotic resistance.6 Another study found that 17% of chickens from supermarkets in four states were contaminated with Enterococcus faecium that was resistant to the streptogramin antibiotic quinupristin-dalfopristin (Synercid).7 The study's authors attribute this resistance to the use of virginiamycin, a related streptogramin antibiotic, in chicken feed.
Another pathway of entry for resistant bacteria is through direct human contact with the animals. This occurs most often in those who work with animals harboring the bacteria. The well-documented case of a child who acquired a strain of ceftriaxone-resistant Salmonella that was identical to one isolated from the cattle on his family's ranch is a very likely example of such transmission.8 In addition, these antibiotic-resistant organisms frequently contaminate local ground water, rivers, and streams9–11 and the air in and around meat production facilities.12,13 The health effects of this water and air pollution are as yet unmeasured.
Of larger concern than food-borne illness is the spreading of resistant bacterial infections among humans.
The Human Cost
The most clearly documented human illnesses resulting from the routine use of antibiotics in animal feed are food-borne illnesses. The Centers for Disease Control and Prevention reported more than 300,000 hospitalizations and 5000 deaths yearly due to food-borne illness. One third of these deaths can be traced to consumption of tainted meat.14 Many of these are caused by resistant organisms. Resistant food-borne pathogens also tend to be more virulent than susceptible ones are.15
Of larger concern than food-borne illness is the spreading of resistant bacterial infections among humans. Although the health and economic cost of community-acquired resistant infections is as yet unmeasured, we do have data on hospital-acquired resistant infections. The National Institute of Allergy and Infectious Diseases reported that there are two million hospital-acquired infections in the United States each year, more than 70% of which are due to resistant bacteria, resulting in 90,000 deaths yearly.16 The US Department of Health and Human Services reported that the hospital cost for just six common kinds of resistant bacterial infections is at least $1.3 billion per year.17
It is difficult to determine the true number of resistant bacterial infections attributable to the agricultural use of antibiotics. The critical variable determining the incidence of both hospital-acquired and community-acquired resistant bacterial infections is the rate of asymptomatic carriage of resistant bacteria in the local population.18,19 It is the human-to-human transmission between these asymptomatic carriers that causes outbreaks of antibiotic-resistant illness. We know that agricultural antibiotic use increases the human carriage of resistant organisms and that phasing out this use results in a markedly decreased incidence of human carriage.20 Several experts in infectious diseases and epidemiology have suggested that agricultural antibiotic use may be more important than hospital antibiotic use in generating the asymptomatic carrier state18 because we all eat and therefore are exposed to a daily small risk of ingesting antibiotic-resistant bacteria. Relatively few people are admitted to the hospital. A large number exposed to a small risk may well result in more cases than would a small number of people exposed to a greater risk.
The Solution
There is a tested, effective approach to the problem of antibiotic resistance: simply phase out the use of antibiotics as routine animal feed additives. Invoking the precautionary principle, our European neighbors have shown that such a phase-out can make a significant difference. For example, Denmark began phasing out additives in the early 1990s. Between 1994 and 2001, antibiotic use in the Danish meat production industry decreased 54%.21 During the same period, vancomycin-resistant Enterococcus was virtually eliminated from the Danish poultry industry with no change in the price of meat (Figure 4). Avoparcin, a vancomycin analogue, was one of the antibiotics phased out and was the presumed source of the vancomycin resistance.18,20 Effective January 1, 2006, the European Union banned the use of all remaining classes of antibiotics as growth promoters.22
Figure 4.
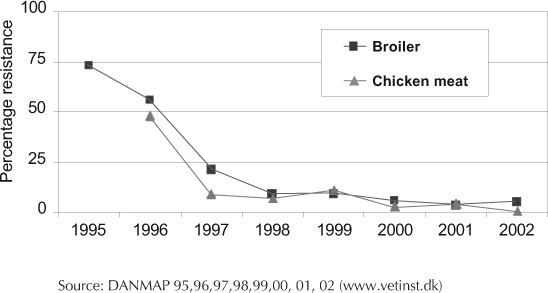
Vancomycin-resistant Enterococcus faecium in Danish broiler chickens and chicken meat.21
A large number of medical professional organizations in the United States, including the American Medical Association, the American Public Health Association, the American Academy of Pediatrics, and the American Academy of Family Physicians, have called for phasing out the routine use of certain antibiotics in meat and poultry production.23 In August 2005, the KP Chiefs of Infectious Diseases for Northern California added their “strong support” to this effort (Figure 5).
Figure 5.

Statement of support made August 2, 2005, by the Chiefs of Infectious Diseases, Kaiser Permanente Northern California, for discontinuation of nontherapeutic antibiotic use in animals.
Our Challenge
The scientific evidence is mounting and the dangers are clear. We at KP are in a position to provide national leadership in this extremely important area. We have made an excellent first step by changing our prescribing behavior. Now it is time for us to take the next step. Once again, we can follow through on a statement of support with a commitment to strong action.
I suggest that we begin a national campaign throughout KP to phase out, in our hospitals and clinics, all meat, poultry, dairy, and fish products raised using antibiotic feed additives.
European health professionals and meat producers have shown us what is possible. Following their example, we can start with our own hospitals, cafeterias, and vending machines. I suggest that we begin a national campaign throughout KP to phase out, in our hospitals and clinics, all meat, poultry, dairy, and fish products raised using antibiotic feed additives. We can then educate our staff and members to do the same in their home kitchens. Our example will encourage others to follow suit. There are more than 8.4 million KP members living in nine states and the District of Columbia.24 By our sheer size, we can help create a large market for food animals raised without antibiotics. By taking this step, we will simultaneously decrease the emergence of resistant bacteria and their adverse health effects and demonstrate our ability to be national leaders in this important effort.
Acknowledgments
The author wishes to thank John Balbus, MD, MPH; Vivien Feyer, EDM, CAS; Karen Florini, JD; and David Wallinga, MD, for their helpful comments, suggestions, and corrections.
Katharine O'Moore-Klopf of KOK Edit provided editorial assistance.
References
- Institute of Medicine, Board on Global Health. Microbial threats to health: emergence, detection, and response [monograph on the Internet] Washington (DC): National Academy of Sciences Press; 2003. [cited 2006 May 31]. Available from: http://fermat.nap.edu/catalog/10636.html#toc. [Google Scholar]
- Mellon M, Benbrook C, Benbrook KL. Hogging it: estimates of antimicrobial abuse in livestock [monograph on the Internet] Cambridge (MA): UCS Publications; 2001. [cited 2006 May 31]. Available from: www.ucsusa.org/food_and_environment/antibiotics_and_food/hogging-it-estimates-of-antimicrobial-abuse-in-livestock.html. [Google Scholar]
- Margulis L, Sagan D. What is life? Berkeley, (CA): University of California Press; 1995. [Google Scholar]
- White DG, Zhao S, Sudler R, et al. The isolation of antibiotic resistant Salmonella from retail ground meats. N Engl J Med. 2001 Oct;345(16):1147–54. doi: 10.1056/NEJMoa010315. [DOI] [PubMed] [Google Scholar]
- Smith KE, Besser JM, Hedberg CW, et al. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992– 1998. N Engl J Med. 1999 May;340(20):1525–32. doi: 10.1056/NEJM199905203402001. [DOI] [PubMed] [Google Scholar]
- US Food & Drug Administration. Enrofloxacin for poultry: withdrawal of approval. Fed Regist. 2005 Aug 1;70:44048–09. [Google Scholar]
- McDonald LC, Rossiter S, Mackinson C, et al. Quinupristin-dalfopristin-resistant Enterococcus faecium on chicken and in human stool specimens. N Engl J Med. 2001 Oct;345(16):1155–60. doi: 10.1056/NEJMoa010805. [DOI] [PubMed] [Google Scholar]
- Fey PD, Safranek TJ, Rupp ME, et al. Ceftriaxone-resistant Salmonella infection acquired by a child from cattle. N Engl J Med. 2000 Apr;342(17):1242–9. doi: 10.1056/NEJM200004273421703. [DOI] [PubMed] [Google Scholar]
- Chee-Sanford JC, Aminov RI, Krapac IJ, Garrigues-Jeanjean N, Mackie RI. Occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities. Appl Environ Microbiol. 2001;67(4):1494–1502. doi: 10.1128/AEM.67.4.1494-1502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ME, Sobsey MD. Detection and occurrence of antibiotic-resistant enteric bacteria in groundwater around swine farms in eastern North Carolina. 2002 May 22. Presented at the 2002 annual general meeting of the American Society for Microbiology, Salt Lake City.
- Winokur P, Hall N, Niera N, et al. Spread of multi-drug resistant, cephalosporin-resistant E coli through surface waterways. 2002 Conference on Antibiotic Resistance, abstracts of submitted presentations, S3. Sponsored by National Foundation for Infectious Diseases, June 27– 29, 2002, Bethesda, Maryland.
- Hamscher G, Pawelzick HT, Sczesny S, Nau H, Hartung J. Antibiotics in dust originating from a pig-fattening farm: a new source of health hazard for farmers? Environ Health Perspect. 2003;111(13):1590–4. doi: 10.1289/ehp.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin A, Rule A, Gibson K, Buckley T, Schwab K. Airborne multidrug-resistant bacteria isolated from a concentrated swine feeding operation. Environ Health Perspect. 2005 Feb;113(2):137–42. doi: 10.1289/ehp.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention [part of a homepage on the Internet] Georgia: CDC; 2006. [cited 2006, May 31]. Food Safety Office [about one screen]. Available from: www.cdc.gov/foodsafety/ [Google Scholar]
- Varma JK, Molbak K, Barrett TJ, et al. Antimicrobial-resistant nontyphoidal Salmonella is associated with excess bloodstream infections and hospitalizations. J Infect Dis. 2005 Feb 15;191(4):554–61. doi: 10.1086/427263. [DOI] [PubMed] [Google Scholar]
- National Institute of Allergy & Infectious Diseases. The problem of antibiotic resistance [monograph on the Internet] Washington, (DC): US Department of Health and Human Services; 2006. [cited 2005 Dec 7]. Available from: www.niaid.nih.gov/factsheets/antimicro.htm. [Google Scholar]
- US Department of Health & Human Services. HHS releases action plan to combat antimicrobial resistance [press release on the Internet] Washington, (DC): Department of Health and Human Services; 2001 Jan 18. [cited 2005 Dec 7]. Available from: www.hhs.gov/news/press/2001pres/20010118b.html. [Google Scholar]
- Smith DL, Dushoff J, Morris J. Agricultural Antibiotics and Human Health. Does antibiotic use in agriculture have a greater impact than hospital use? PLoS Med. 2(8):e232. doi: 10.1371/journal.pmed.0020232. [serial on the Internet]. 2005 Aug [cited 2006 Jan 9] Available from: http://medicine.plosjournals.org/perlserv/?request=get-document&doi=10.1371/journal.pmed.0020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Dushoff J, Perencevich EN, Harris AD, Levin SA. Persistent colonization and spread of antibiotic resistance in nosocomial pathogens: resistance is a regional problem. Proc Natl Acad Sci U S A. 2004 March 9;101(10):3709–14. doi: 10.1073/pnas.0400456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarestrup FM, Seyfarth AM, Emborg H-D, Pederson K, Hendriksen RS, Bager F. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob Agents Chemother. 2001 July;45(7):2054–9. doi: 10.1128/AAC.45.7.2054-2059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bager F, Emborg H-D, Heuer OE, et al. DANMAP 2001: Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, foods and humans in Denmark [monograph on the Internet] Copenhagen, Denmark: DANMAP; 2001. [cited 2005 Nov 25]. Available from: www.dfvf.dk/Files/Filer/Zoonosecentret/Publikationer/Danmap/Danmap_2001.pdf. [Google Scholar]
- European Commission. Ban on antibiotics as growth promoters in animal feed enters into effect [press release on the Internet] Brussells: Europa; 2005 Dec 22. [cited 2006 May 31]. Available from: http://europa.eu.int/rapid/pressReleasesAction.do?reference=IP/05/1687&format=HTML&aged=0&language=EN&guiLanguage=en. [Google Scholar]
- Keep Antibiotics Working [homepage on the Internet] Chicago: Keep Antibiotics Working; c2004. [cited 2005 Nov 25]. Endorsements for The Preservation of Antibiotics for Medical Treatment Act; [about three screens]. Available from: www.keepantibioticsworking.org/new/resources_library.cfm?RefID=73271. [Google Scholar]
- Kaiser Permanente. Introduction to Kaiser Permanente and venturing [monograph on the Internet] Oakland: Kaiser Permanente National Venture Development; [cited 2005 Dec 7]. Available from: www.kpventures.net/home.html. [Google Scholar]


