Posttraumatic Stress Disorder (PTSD)1 is a debilitating disorder that develops in some individuals who have been exposed to psychological trauma. Functional neuroimaging studies have mapped out a functional neuro-circuitry of PTSD 2. Three interconnected brain regions have consistently shown abnormal patterns of activity in this disorder 3: the amygdala, which is involved with processing of emotionally-salient stimuli; the medial prefrontal cortex (mPFC, a functionally heterogeneous region that includes the anterior cingulate cortex, the subcallosal cortex, and the medial frontal gyrus) which plays a role in modulation of affect; and the hippocampus, which is involved with memory encoding and retrieval. Auditory presentation of personalized trauma narratives during functional neuroimaging has proven useful in investigating PTSD-specific abnormalities in neural function (for a review, see 4). A common finding in this line of research is reduced mPFC response to trauma-related versus neutral scripts in individuals with PTSD 5–10.
Selective serotonin reuptake inhibitors (SSRIs), including paroxetine, have been shown to be effective in the treatment of PTSD symptoms 11, 12; however, the neural mechanisms of treatment efficacy in PTSD have not been fully established. A few studies have provided some insights into these mechanisms. Seedat and colleagues (2004) treated 11 adults with PTSD (related to different traumatic events) with citalopram for 8 weeks, conducting SPECT imaging before and after treatment; the authors found significant correlations between increases in activity in regions of the mPFC and symptom reductions, as well as decreased left medial temporal activity with treatment 13. In a case report study of a war torture victim with PTSD, Fernandez and colleagues (2001) presented war-related sounds to the patient during PET imaging; this was done both before and after 6-month treatment with fluoxetine. The authors found increased regional cerebral blood flow in various frontal regions of the brain (including the orbitofrontal cortex, prefrontal cortex, and inferior frontal cortex) during imaging, as well as a 48% decrease in symptoms, following fluoxetine treatment 14.
A third treatment study examined changes in brain function within a group of individuals with different anxiety disorders, including obsessive-compulsive disorder (OCD), PTSD, social phobia, and generalized anxiety disorder, grouped together 15. Interestingly, this study revealed decreased activity in some medial frontal regions following treatment; however, given that the authors studied a heterogeneous group of anxious participants, these findings may have limited applicability to PTSD. Individuals with disorders such as OCD have historically shown increased metabolism in medial prefrontal brain regions, while individuals with PTSD have shown an opposite pattern of activity in the mPFC 16.
In sum, functional neuroimaging studies using script-driven imagery have been useful in identifying atypical patterns of neural processing in PTSD, and SSRIs have proven effective in treating the clinical symptoms of PTSD. However, few studies have combined these methods to examine the effects of psychopharmacological treatment on brain function in PTSD, which would provide valuable insights toward understanding neural mechanisms of treatment in this disorder. Thus, the purpose of this pilot study was to assess the effects of the SSRI paroxetine on neural responses to traumatic memories in patients with PTSD through PET imaging; we hypothesized that paroxetine treatment would be associated with increased function in medial frontal regions of the brain, and that these associations would not be found in placebo-treated individuals.
Method
Participants
All participants were recruited through fliers and public bulletins distributed within the community. This study was approved by the Investigational Review Board of Emory University, and all enrolled subjects provided written informed consent. All participants spoke fluent English and had at least an 8th grade reading ability. Participants were considered ineligible if they had experienced significant head trauma or loss of consciousness for at least 2 minutes, or if they reported significant medical histories, current alcohol or substance abuse or psychotic illness as identified by DSM-IV criteria in the Structured Clinical Interview for DSM-IV (SCID; 17). All participants had been free from psychotropic medication for at least four weeks prior to data collection. A total of 13 individuals met eligibility criteria (7 women, 6 men) and completed all procedures for this study. Participant demographics and clinical characteristics are described in Table 1. Five participants had been exposed to more than one traumatic event. The most frequently reported primary trauma was childhood sexual abuse (7) followed by adult sexual assault (5), adult physical assault (3), motor vehicle accident (2) and combat exposure (1). A total of 11 participants (85%) met criteria for current major depressive episode.
Table 1.
Demographic and clinical characteristics by treatment group
| Mean (SD) N=13 | ||
|---|---|---|
| Placebo | Paroxetine | |
| Age | 42.5 (12.3) | 37.4 (8.1) |
| Gender (female) | (n=3) 50% | (n=4) 57% |
| Race | (n= 2) 33% Caucasian (n=4) 67% African- American |
(n=5) 71% Caucasian (n=2) 29% African- American |
| Years of Education | 14.7 (1.8) | 15.3 (1.9) |
Treatment
All enrolled participants were randomized (by an outside researcher) to a 12 week double-blind course of treatment with controlled-release paroxetine (paroxetine CR) or placebo, and received a baseline PET scan. In this sample, 7 received paroxetine and 6 received placebo during the double-blind phase. All participants received toxicology screenings prior to their enrollment in the study, and women received monthly urine pregnancy tests. Study medication was started at one capsule (placebo or paroxetine 12.5 mg) per day. The medication was increased by one capsule (12.5 mg) per study visit, as tolerated by the participant, up to five capsules a day (i.e., 62.5 mg of paroxetine CR). During medication adjustment (i.e., from weeks 0 to 8), subjects were seen biweekly by a study physician (blind to treatment status), who performed side effect and symptom checks. Patients were asked to return unused medication as a way to ensure compliance.
Following the double-blind period, participants underwent a second PET scan while still on study medication. At the end of treatment, subjects were notified of their treatment status, and were offered several treatment options: being treated with three months of open label paroxetine; obtaining a referral for continuing treatment by an outside provider; or gradually tapering off medication while being monitored by a board-certified study physician.
Measures
Diagnostic and Symptom Severity Measures
The Clinical Administered PTSD Scale (CAPS 18) was administered by a study researcher to evaluate current PTSD symptom severity at baseline and at the end of double-blind treatment. The CAPS is a psychometrically sound measure that assesses presence and severity of PTSD (both lifetime and current PTSD), and can be used to assess changes in PTSD symptoms with treatment 18. The CAPS includes indices measuring re-experiencing, avoidance and numbing, and hyperarousal symptoms.
Scripts
Participants were presented with neutral and trauma-related scripts during each of four separate PET scans, completed in a single day; these procedures were conducted both before the start of treatment and on the last day of double-blind treatment. With the assistance of a study researcher, personalized scripts of traumatic events were prepared for auditory presentation during PET imaging using previously-described methods 5. Participants were asked to provide a written description of their traumatic event (individuals exposed to multiple events were asked to write about the event that was the most distressing), which was then separated into two parts. Each part was 30 seconds in length, and was recorded in a neutral tone of voice by a study researcher, to be played back during scanning.
Two different neutral scripts were presented before the trauma scripts in two separate scans. The instructions for these scripts were designed to control for the effects of attention, auditory perception, and comprehension of a verbal narrative. Participants were asked to attend to two different neutral narratives; before the start of each narrative, they were asked to count the number of times they heard the letter “D.” Following the presentation of these scripts, participants underwent two more scans during which they were presented with two trauma-related scripts. All scripts were presented at a uniform auditory level.
PET Procedures
PET scanning was conducted according to previously published procedures 5. Briefly, participants received instructions for each script, which was followed by script presentation. As the script reading began, subjects received a bolus injection of 30 mCi of [15O]H2O, followed 10 seconds later by a PET scan acquisition that was 80 seconds in length. The onset of the PET scan acquisition was timed to correspond to the point of maximum rate of increase in uptake of tracer into the brain. PET imaging was performed with a Siemens HRRT camera (Siemens, Inc., Knoxville TN), and images were reconstructed and analyzed on a SunSparc workstation equipped with Statistical Parametric Mapping software (SPM2). Images were realigned to the first scan of the study session. The mean concentration of radioactivity in each scan was obtained as an area-weighted sum of the concentration of each slice and was adjusted to a nominal value of 50 ml/minute per 100 g. The data underwent transformation into a common anatomical space and were smoothed with a three-dimensional Gaussian filter to 16-mm FWHM.
Regional blood flow was compared for traumatic minus neutral script conditions; these analyses were performed for pre- and post-treatment scans for both paroxetine CR and placebo groups. Statistical analyses yielded image data sets in which the values assigned to individual voxels correspond to the t statistic. Statistical images were displayed with values of z score units. A threshold z score of 2.67 (p < .005, uncorrected) was used to examine areas of activation within hypothesized areas. Location of areas of activation was identified as the distance from the anterior commissure in millimeters, with x, y, and z coordinates; a standard stereotaxic (Talairach) atlas was used 19. Pre-treatment and post-double-blind treatment CAPS scores were compared for paroxetine CR- and placebo-treated participants through use of repeated measures analysis of variance, with time as the repeated measure, to examine time by group interactions.
Results
Either treatment resulted in a significant improvement in PTSD symptoms (see Table 2; F1, 11=36.3, p <.001). Treatment with paroxetine resulted in a 69% reduction in CAPS score (Cohen’s d=3) versus a 61% reduction in CAPS score (Cohen’s d=1.55), a difference that was not statistically significant (i.e. no significant treatment group by time interaction; F1, 11=3.4, p =.091). There were also no significant treatment group by time interactions in CAPS subscales, including re-experiencing symptoms (F1, 11=.85, p >.05), avoidance and numbing symptoms (F1, 11 =4.6, p =.055), or hyperarousal symptoms (F1, 11=1.65, p<.05).
Table 2.
CAPS score at baseline and following double-blind treatment
| CAPS score | Baseline Mean (SD) | Following Double-blind treatment Mean (SD) | ||
|---|---|---|---|---|
| Placebo | Paroxetine | Placebo | Paroxetine | |
| Re-experiencing | 15.16 (8.23) | 24.14 (5.79) | 2.5 (4.81) | 7.57 (8.1) |
| Avoidance/Numbing | 18.67 (6.31) | 35.57 (8.34) | 8.83 (8.2) | 8.86 (11.58) |
| Hyperarousal | 17.33 (7.39) | 25.14 (4.49) | 8.83 (6.55) | 9.86 (11.05) |
| Total score | 51.17 (19.8) | 84.86 (8.15) | 20.17 (20.17) | 26.29 (26.29) |
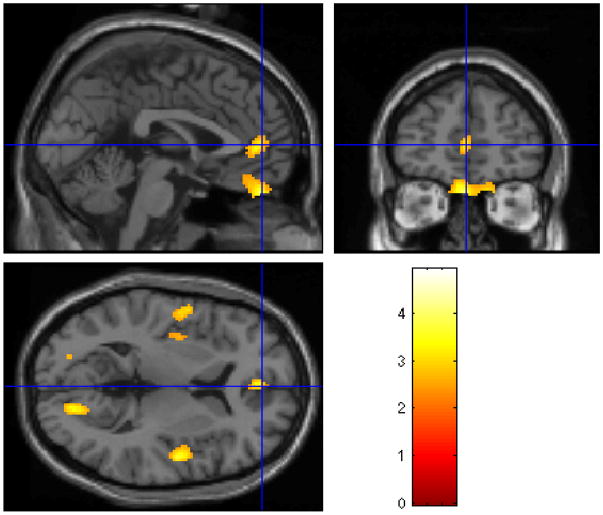
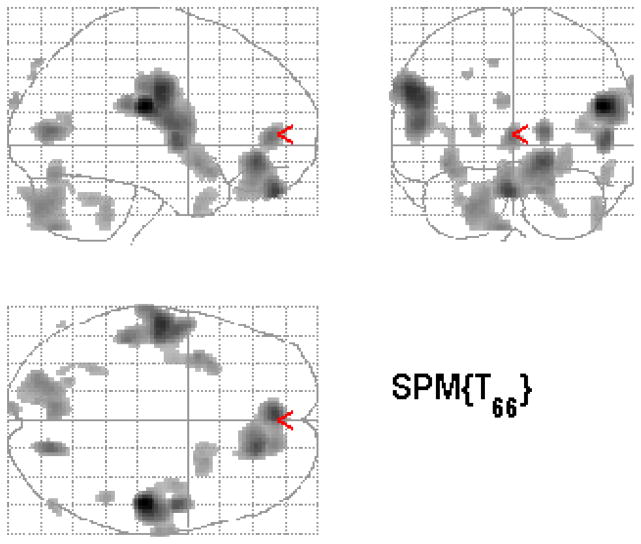
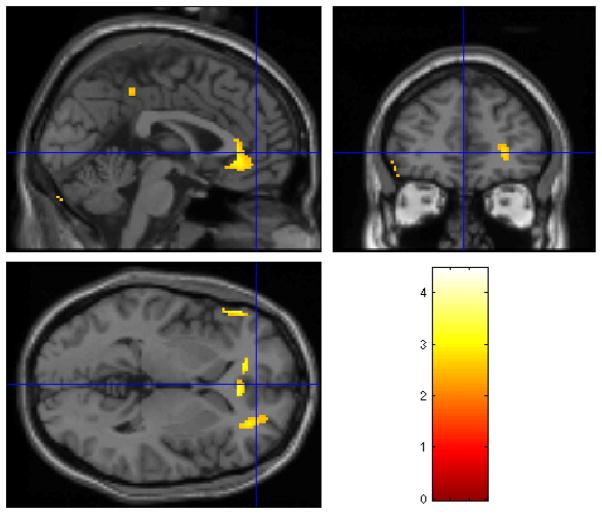
Individuals treated with paroxetine CR demonstrated increased blood flow in response to traumatic scripts in the orbitofrontal cortex, superior temporal gyrus, inferior frontal gyrus, anterior cingulate, paracentral lobe, cerebellum, and occipital cortex (see Table 3 and Figure 1). Placebo treatment was associated with increased blood flow in response to traumatic scripts in the right superior temporal gyrus, left inferior frontal gyrus, anterior cingulate, paracentral lobule, and left occipital cortex (see Table 3 and Figure 2).
Table 3.
Regions of Significant Increase in Brain Function During Traumatic Minus Neutral Scripts in Participants Treated with Paroxetine CR
| Z Score* | Voxel Number | Talairach Coordinates |
||||
|---|---|---|---|---|---|---|
| x | y | z | Brain Region | Brodman's area | ||
| 4.53 | 1001 | 50 | −22 | 21 | R. Postcentral gyrus | 40 |
| 3.53 | 51 | −6 | 4 | R. Superior temporal gyrus | 22 | |
| 2.77 | 65 | −14 | 34 | R. Precentral gyrus | 6 | |
| 3.96 | 1545 | −55 | −12 | 30 | L. Precentral gyrus | 6 |
| 3.50 | −54 | −5 | 11 | L. Precentral gyrus | 6 | |
| 3.20 | −48 | −32 | 24 | L. Postcentral gyrus | 40 | |
| 3.73 | 1078 | −2 | 47 | −28 | L. Orbitofrontal cortex | 11 |
| 3.63 | 16 | 36 | −11 | R. Anterior cingulate | 32 | |
| 3.00 | 16 | 45 | −28 | R. Orbitofrontal cortex | 11 | |
| 3.33 | 149 | 18 | −75 | 11 | R. Visual cortex | 17 |
| 3.21 | 137 | −2 | 45 | 1 | L. Anterior cingulate | 32 |
| 3.04 | 828 | −22 | −81 | −29 | L. Cerebellum | |
| 3.02 | −8 | −74 | −42 | L. Cerebellum | ||
| 2.93 | −4 | −73 | −13 | L. Cerebellum | ||
| 3.03 | 212 | −38 | 5 | −11 | L. Superior temporal gyrus | 38 |
| 2.94 | −34 | 11 | −15 | L. Inferior frontal gyrus | 47 | |
| 2.81 | 115 | 24 | 9 | −14 | R. Inferior frontal gyrus | 38 |
| 2.73 | 20 | −4 | −92 | 29 | L. Visual associative cortex | 19 |
| 2.69 | 87 | 46 | −44 | −33 | R. Cerebellum | |
Z score > 2.68, p < .005
Figure 1.
Statistical parametric map (below) overlaid on a MRI template (above) showing areas of increased brain blood flow to traumatic scripts in participants treated with paroxetine.
Figure 2.
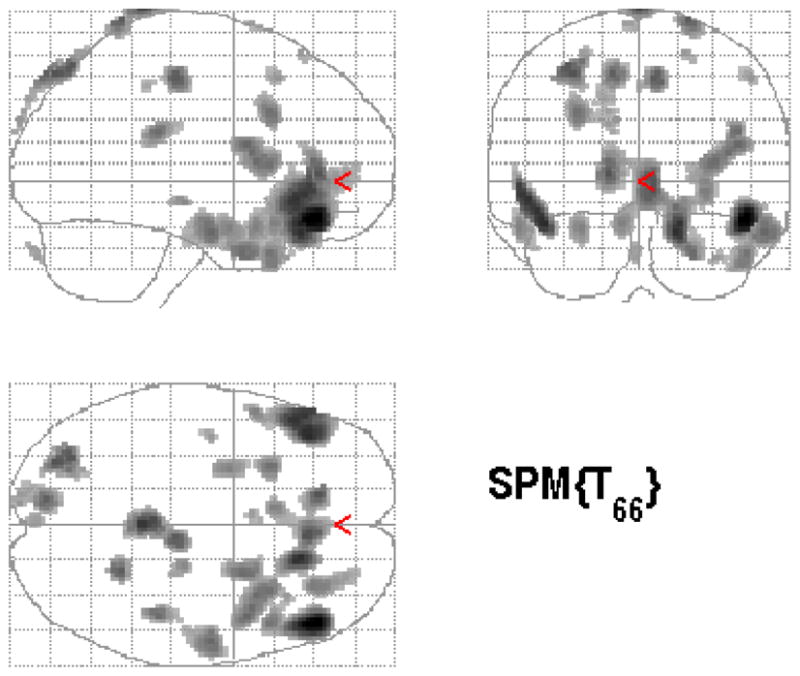
Statistical parametric map (below) overlaid on a MRI template (above) showing areas of increased brain blood flow to traumatic scripts in participants treated with placebo
Discussion
In this sample of individuals with PTSD, treatment with either placebo or paroxetine was associated with increased function in a region of the mPFC (the ACC) in response to traumatic scripts. However, paroxetine was associated with an additional increase in function in the OFC. Although paroxetine treatment was associated with a pattern of greater treatment response, this pattern was not statistically significant in comparison with the placebo-treated group.
Our findings suggest that treatment with either placebo or paroxetine is associated with increased function within a brain region implicated in the pathophysiology of PTSD, namely, the anterior cingulate cortex (ACC) region within the mPFC. The ACC has connections to limbic areas that mediate threat response, and is hypothesized to play a role in extinguishing exaggerated fear responses that are characteristic of PTSD 20. A number of symptom provocation studies have either shown attentuated or no ACC activation in PTSD patients during presentation of trauma stimuli, 5, 6, 9, 21–23 implying that ACC hypoactivation may be a biological marker of this disorder. Our study findings indicate that both placebo or paroxetine CR treatments were associated with increased neural activity in the ACC region of the mPFC; given that some recent placebo-controlled antidepressant studies have observed rather high treatment responses to placebo 24, it is possible that the increased ACC function found in both groups represents a non-specific response to treatment in general, and potentially improved ability to inhibit a fear-driven response.
However, increased function in another mPFC region, the OFC, was specifically found in paroxetine-treated individuals in this study. The OFC plays a role in emotion regulation and is part of a network of brain regions that have been implicated in PTSD. Several neuroimaging studies have shown altered OFC function in PTSD; although some of these studies showed increased OFC response to trauma-related scripts in patients with PTSD 9, 21, a larger number of studies showed decreased response during presentation of traumatic scripts 5, trauma-related word recall 25, and memory tasks 10. Decreased neural activation in the OFC has also been found in patients with borderline personality disorder (many of whom had comorbid PTSD) during script-driven imagery, as compared to non-psychiatric controls 26, 27; decreased OFC function has also been found in patients with major depressive disorder, which is frequently comorbid with PTSD 28, 29.
The increase in OFC function to trauma scripts observed in paroxetine-treated individuals in this study is consistent with the findings of a previous case report study 14, and provides some prelimary evidence for specificity of SSRI treatment effects in PTSD. While a majority of our participants experienced improvements in PTSD symptoms regardless of which treatment they received, paroxetine-treated participants demonstrated a greater reduction in PTSD symptoms than those treated with placebo. Our findings present the possibility that successful SSRI treatment of posttraumatic symptoms is mediated by orbitofrontal mechanisms, a hypothesis that warrants investigation in large-scale studies with a similar research design.
To our knowledge, this is the first empirical study to examine changes in brain function during script-driven imagery in participants with PTSD treated with an SSRI. Overall, there is a profound lack of studies examining functional brain changes in individuals with PTSD treated with SSRIs. One study found increased function in frontal cortical regions in PTSD patients after treatment with citalopram 13 while a case report of fluoxetine treatment in a PTSD patient showed similar results 14. A third study of a mixed group of anxious individuals revealed decreased activity in the ACC in response to citalopram treatment 15. The evidence from our pilot study suggests that short-term treatment with paroxetine is related to increased neural activation in the OFC, a region of the brain that has shown altered function in neuroimaging studies of PTSD. These findings have implications for the mechanisms of SSRI treatment in individuals with post-traumatic psychopathology; SSRIs, such as paroxetine, may act more specifically on orbitofrontal circuits, which serve to modulate exaggerated emotional responses that are typical in PTSD.
Several limitations of this study must also be noted. First, as with most pilot studies, the small sample size of this study limits the generalizability of our findings, as well as our ability to detect more subtle changes in other brain regions following treatment. A larger sample would also confer the necessary statistical power to examine differences in brain activation in participants who were more responsive to treatment versus those who were less responsive, and distress rating data following script presentation would be useful to measure variability in subjective response to scripts. Likewise, it is possible that increased power would have allowed us to observe larger differences in treatment response through clinical measures such as the CAPS. Further, as with most studies of PTSD, many individuals in this sample of participants had co-morbid depression, and some participants experienced multiple traumas; thus, comorbidity profiles and unique effects of trauma are potential confounding factors. Overall, further randomized, placebo-controlled neuroimaging studies with larger sample sizes and longer treatment durations are warranted to compare treatment-related effects between groups with greater statistical rigor.
In sum, we found that both placebo and paroxetine CR treatments were associated with increased rCBF in the ACC, a brain region that has previously shown altered function in PTSD. However, only paroxetine-treated participants showed increased OFC function, consistent with one earlier report 14. Clinical implications of these findings are that treatment response is associated with functional changes in a brain region that mediates extinction of fear responses, and that paroxetine may additionally affect another brain area implicated in emotional regulation. Future randomized, placebo-controlled studies with large sample sizes are warranted in order to assess the generalizability of these effects and to further assess their clinical significance.
Table 3.
Regions of Significant Increase in Brain Function During Traumatic Minus Neutral Scripts in Participants Treated with Placebo
| Z Score* | Voxel Number | Talairach Coordinates |
Brodman's area | |||
|---|---|---|---|---|---|---|
| x | y | z | Brain Region | |||
| 4.16 | 417 | 48 | 38 | −16 | R. Middle frontal gyrus | 11 |
| 3.01 | 48 | 16 | −34 | R. Superior temporal gyrus | 38 | |
| 2.77 | 50 | 22 | −24 | R. Superior temporal gyrus | 38 | |
| 3.71 | 563 | −42 | 34 | −20 | L. Middle frontal gyrus | 11 |
| 3.62 | −50 | 28 | −8 | L. Inferior frontal gyrus | ||
| 3.53 | −54 | 34 | −2 | L. Inferior frontal gyrus | ||
| 3.66 | 840 | 16 | 30 | −24 | R. Inferior/middle frontal gyrus | 47 |
| 3.34 | 6 | 34 | −2 | R. Anterior cingulate | ||
| 3.33 | −14 | 38 | 4 | L. Anterior cingulate | ||
| 3.59 | 133 | 0 | −40 | 84 | Paracentral lobule | 7 |
| 2.81 | 8 | −30 | 86 | Paracentral lobule | 7 | |
| 3.32 | 99 | −30 | −80 | 52 | L. Superior parietal lobule | 7 |
| 3.30 | −32 | −72 | 56 | L. Superior parietal lobule | 7 | |
| 2.63 | −26 | −68 | 62 | L. Superior parietal lobule | 7 | |
| 3.27 | 112 | 6 | −26 | 50 | Paracentral lobule | 6 |
| 3.25 | 56 | 22 | −50 | 78 | R. Superior parietal lobule | 7 |
| 3.21 | 103 | −12 | −84 | 52 | Precuneus | 7 |
| 2.71 | −10 | −96 | 30 | L. Occipital cortex | 19 | |
Acknowledgments
This study was supported by an Investigator Initiated Research Grant from GlaxoSmithKline and NIH research grants to JDB R01 HL088726, K24 MH076955, T32 MH067547-01, R01 MH56120, and the clinical interactions network (CIN) of the Atlanta Clinical and Translational Sciences Institute (ACTSI). We wish to acknowledge Sinead Quinn, Delicia Votaw, C.N.M.T. and Margie Jones, C.N.M.T., for their assistance with imaging and analysis procedures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.Bremner JD. Traumatic stress: Effects on the brain. Dialogues in Clinical Neuroscience. 2006;8:445–461. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of New York Academy of Science. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 4.Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Ann N Y Acad Sci. 2006 Jul;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- 5.Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999 Nov;156(11):1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005 Apr 15;57(8):832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 7.Lanius RA, Williamson PC, Hopper J, et al. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol Psychiatry. 2003 Feb 1;53(3):204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- 8.Lindauer RJ, Booij J, Habraken JB, et al. Cerebral blood flow changes during script-driven imagery in police officers with posttraumatic stress disorder. Biol Psychiatry. 2004 Dec 1;56(11):853–861. doi: 10.1016/j.biopsych.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Shin LM, McNally RJ, Kosslyn SM, et al. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. Am J Psychiatry. 1999 Apr;156(4):575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 10.Shin LM, Orr SP, Carson MA, et al. Regional cerebral blood flow in amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 11.Marshall RD, Beebe KL, Oldham M, Zaninelli R. Efficacy and safety of paroxetine treatment for chronic PTSD: A fixed-dose, placebo-controlled study. American Journal of Psychiatry. 2001;158:1982–1988. doi: 10.1176/appi.ajp.158.12.1982. [DOI] [PubMed] [Google Scholar]
- 12.Tucker P, Zaninelli R, Yehuda R, Ruggeiro L, Dillingham K, PItts CD. Paroxetine in the treatment of chronic posttraumatic stress disorder: Results of a placebo-controlled, flexible-dosage trial. Journal of Clinical Psychiatry. 2001;62:860–868. doi: 10.4088/jcp.v62n1105. [DOI] [PubMed] [Google Scholar]
- 13.Seedat S, Warwick J, van Heerden B, et al. Single photon emission computed tomography in posttraumatic stress disorder before and after treatment with a selective serotonin reuptake inhibitor. J Affect Disord. 2004 May;80(1):45–53. doi: 10.1016/S0165-0327(03)00047-8. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez M, Pissiota A, Frans O, von Knorring L, Fischer H, Fredrikson M. Brain function in a patient with torture related post-traumatic stress disorder before and after fluoxetine treatment: a positron emission tomography provocation study. Neurosci Lett. 2001 Jan 12;297(2):101–104. doi: 10.1016/s0304-3940(00)01674-8. [DOI] [PubMed] [Google Scholar]
- 15.Carey PD, Warwick J, Niehaus DJ, et al. Single photon emission computed tomography (SPECT) of anxiety disorders before and after treatment with citalopram. BMC Psychiatry. 2004;4:30. doi: 10.1186/1471-244X-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedlander L, Desrocher M. Neuroimaging studies of obsessive-compulsive disorder in adults and children. Clinical Psychology Review. 2006;26:32–49. doi: 10.1016/j.cpr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 17.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV. Washington, D.C: American Psychiatric Press; 1995. [Google Scholar]
- 18.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 19.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: Three-dimensional proportional system. Stuttgart, Germany: Georg Thieme; 1988. [Google Scholar]
- 20.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 21.Rauch SL, Van der Kolk BA, Fisler RW, Alpert NM, Orr SP, Savage CR, et al. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry. 1996;53:380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- 22.Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biological Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanius RA, Williamson PC, Densmore M, et al. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry. 2001 Nov;158(11):1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- 24.Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008 Feb;5(2):e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bremner JD, Vythilingam M, Vermetten E, et al. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biol Psychiatry. 2003 May 15;53(10):879–889. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- 26.Kraus A, Valerius G, Seifritz E, et al. Script-driven imagery of self-injurious behavior in patients with borderline personality disorder: a pilot FMRI study. Acta Psychiatr Scand. Jan;121(1):41–51. doi: 10.1111/j.1600-0447.2009.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmahl CG, Vermetten E, Elzinga BM, Bremner JD. A positron emission tomography study of memories of childhood abuse in borderline personality disorder. Biol Psychiatry. 2004 Apr 1;55(7):759–765. doi: 10.1016/j.biopsych.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Bremner JD, Innis RB, Salomon RM, et al. Positron emission tomography measurement of cerebral metabolic correlates of tryptophan depletion-induced depressive relapse. Arch Gen Psychiatry. 1997 Apr;54(4):364–374. doi: 10.1001/archpsyc.1997.01830160092012. [DOI] [PubMed] [Google Scholar]
- 29.Bremner JD, Vythilingam M, Ng CK, et al. Regional brain metabolic correlates of alpha-methylparatyrosine-induced depressive symptoms: implications for the neural circuitry of depression. JAMA. 2003 Jun 18;289(23):3125–3134. doi: 10.1001/jama.289.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]