Abstract
Aims
Related transcription enhancer factor-1 (RTEF-1) has previously been demonstrated to play an important role in both endothelial cells and cardiomyocytes. However, the function of RTEF-1 in the communication between these two adjacent cell types has not been elucidated.
Methods and results
We have found that endothelium-specific RTEF-1 transgenic mice (VE-Cad/RTEF-1) developed significant cardiac hypertrophy after transverse aortic constriction surgery, as evidenced by an increased ratio of heart weight to tibia length, enlarged cardiomyocyte size, thickened left ventricular wall and elevated expression of hypertrophic gene markers, with up-regulation of vascular endothelial growth factor B (VEGF-B). Additionally, VEGF-B was increased in endothelial cells from VE-Cad/RTEF-1 mice, as well as in endothelial cells with forced RTEF-1 expression (HMEC-1/RTEF-1), and coincidentally decreased when RTEF-1 was deficient in HMEC-1. Using chromatin immunoprecipitation and luciferase assays, we found that RTEF-1 increased VEGF-B promoter activity through a direct interaction. Hypertrophy-associated genes and protein synthesis were up-regulated in cardiomyocytes that were incubated with conditioned medium from HMEC-1/RTEF-1 and the endothelial cells of VE-Cad/RTEF-1 mice. This effect could be abrogated by treating the myocytes with VEGF-B small interfering RNA and extracellular signal-regulated kinase 1/2 inhibitor.
Conclusion
Our data demonstrated that increased RTEF-1 in endothelial cells upregulates VEGF-B, which is able to stimulate hypertrophic genes in cardiomyocytes. These results suggest that the RTEF-1-driven increase of VEGF-B plays an important role in communication between the endothelium and myocardium.
Keywords: Transcription factor, Growth factor, Endothelial cell, Cardiomyocyte, Related transcription enhancer factor-1, Vascular endothelial growth factor B, Hypertrophy, Endothelium, Hypoxia
1. Introduction
The anatomically close relationship between capillaries and myocardium allows not only for physiological transportation but also for cell-to-cell signalling between the capillary endothelial cells and cardiomyocytes.1 Recently, it has been argued that signalling between myocardium and vasculature promotes reciprocal growth in a paracrine fashion.2 In addition, physiological or compensatory cardiac hypertrophy is accompanied by normal or increased numbers of myocardial capillaries, suggesting that angiogenesis occurring within the heart may promote hypertrophy of adjacent cardiomyocytes.3 Blocked or disrupted angiogenesis leads to pathological hypertrophy or heart failure,4 a finding that is consistent with the fact that dilated cardiomyopathy is associated with an irregular capillary pattern and a reduction in overall capillary density.5
Related transcription enhancer factor-1 (RTEF-1) has been reported to regulate cardiac hypertrophy6 and angiogenesis.7 As a member of the transcriptional enhancer factor (TEF) family, RTEF-1 is primarily expressed in skeletal muscle,8 smooth muscle,9 and cardiac muscle.8 RTEF-1 regulates gene expression through binding to the muscle-specific cytidine-adenosine-thymidine (MCAT) element in the promoters of muscle-specific genes.8 RTEF-1 can mediate the reactivation of the α1-adrenergic response to induce hypertrophy and reactivate cardiac and skeletal genes, such as β-myosin heavy chain and skeletal α-actin.6 Transgenic mice that over-express RTEF-1 specifically in cardiomyocytes develop progressive atrial arrhythmias.10 Recently, we demonstrated that RTEF-1 increases promoter activity and expression of vascular endothelial growth factor (VEGF)-A in both normoxic and hypoxic conditions.7 Sequential deletion and site-directed mutational analyses of the VEGF-A promoter demonstrated that a GC-rich region containing four special protein 1 (Sp1) response elements was essential for regulation of VEGF-A by RTEF-1. Interestingly, this region does not contain MCAT elements.7,11
There are five members of the VEGF family [VEGF-A, –B, –C, -D, and placental growth factor (PIGF)], which are key regulators of angiogenesis, vasculogenesis, and lymphoangiogenesis.12 VEGF-B is widely expressed in various tissues and is particularly abundant in heart.13 It was reported to promote cell growth and vascular growth in Matrigel and to accelerate recovery from hindlimb ischaemia via activation of Akt- and endothelial nitric oxide synthase-related pathways.14 Endothelium-specific VEGF-B transgenic mice displayed elevated vascular growth in an aortic explant assay.14 However, VEGF-B deficient mice showed no gross abnormalities in the heart, and exhibited normal responses to VEGF- or fibroblast growth factor-(FGF)-induced angiogenesis.15 Moreover, VEGF-B was found to be unnecessary for blood vessel growth in the mouse cornea pocket assay and rabbit hindlimb ischaemia model.16 VEGF-B exerts a pro-survival role on endothelial cells, smooth muscle cells, and pericytes by regulating vascular pro-survival genes.16 Cardiac-specific over-expression of VEGF-B altered lipid metabolism and induced myocardial hypertrophy17 through the activation of cardiac VEGF receptor 1 (VEGFR-1).18 Taken together, these findings indicate that VEGF-B plays a role in the regulation of endothelial cells and of cardiomyocytes. However, the means by which VEGF-B regulation of endothelial cells and of cardiomyocytes are linked has not been determined.
In the present study, we report the molecular mechanisms of transcriptional control of VEGF-B expression in vivo and in vitro. We found that increased RTEF-1 expression by endothelial cells up-regulated VEGF-B, which was able to stimulate hypertrophic genes in cardiomyocytes. These results suggest that an RTEF-1-driven increase in expression of VEGF-B plays an important role in communication between vascular endothelium and myocardium.
2. Methods
2.1. Animal models and transverse aortic constriction surgery
RTEF-1 transgenic mice were generated on the FVB background at the Beth Israel Deaconess Medical Center (BIDMC) Transgenic Core Facility using the VE-cadherin promoter to drive endothelium-specific expression of human RTEF-1. The investigation conforms to the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85-23, 1996) and was approved by the Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center. Pressure overload was produced by transverse aortic constriction (TAC; see Supplementary material online 1) as described.19 Echocardiography was performed at regular intervals. Left ventricular diastolic dimension (LVDd) and left ventricular systolic dimension (LVDs) were measured. The percentage of LV fractional shorting (FS) was calculated as follows: (LVDd − LVDs)/LVDd × 100%.
2.2. Cell culture and hypoxia
The cell lines used included human dermal microvascular endothelial cells-1 (HMEC-1; Center for Disease Control and Prevention), rat myoblast cell line (H9C2) and human embryonic kidney cell line (HEK293). Mouse endothelial cells were isolated using PECAM-1 antibody (Pharmingen) and Dynabeads (Dynal) and confirmed by PECAM-1 immunostaining. Neonatal rat ventricular myocytes (NRVMs) were isolated from ventricular tissue of 1-day-old Sprague–Dawley rats (see Supplementary material online 2). Hypoxia was induced using a Modular Incubator Chamber (Billumps-Rothenberg) flushed with 5% CO2 and 95% N2. The concentration of oxygen (1–3%) was determined before and after incubation by using an oxygen analyser (Vascular Technology). All cells were starved with serum-free medium for 12 h before normoxia, hypoxia or growth factor treatment.
2.3. Retroviral transduction and small interfering RNA transfection
The coding sequence of human RTEF-1 (Genbank ID: NM_003213.1) was subcloned into pBMN-GFP vector (Orbigen) from PXJ40/RTEF-1 construct (a gift from Dr Alexandre Stewart, University of Ottawa). HEK 293T cells were transfected with pBMN-GFP-RTEF-1 or pBMN-GFP, pMD-VSVG, pJK3, and pCMV-tat using polyethylenimine (PEI; Polysciences). The virus-containing medium was transferred to HMEC-1 and selected with puromycin (250 ng/mL). Small interfering RNA (siRNA) encoding human RTEF-1 or VEGF-B (NM_003377.3; Genpharma Shanghai; Supplementary material online 3) was transfected using Lipofectamine 2000 (Invitrogen) and confirmed by western blotting. Small interfering RNA that is not targeted to any human genes was used as a negative control.
2.4. RNA and protein analyses
Total RNA was extracted from tissues or cultured cells. Gene expression was analysed by quantitative real-time PCR (qPCR). The primers for VEGF-B, RTEF-1, 36b4, atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), α-myosin heavy chain (α-MHC), β-myosin heavy chain (β-MHC), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) are listed in Supplementary material online 4. Cells or tissue samples were lysed in RIPA buffer (Boston BioProducts) and blotted with the following antibodies: VEGF-B (R&D Systems), VEGF-A (Santa Cruz Biotechnology), FGF2 (Upstate BioLab) tumour necrosis factor-α (Sigma), RTEF-1 (Genemed Synthesis Inc.), vinculin (Sigma), β-actin (Santa Cruz Biotechnology), extracellular signal-regulated kinase (ERK), pERK, and pP38 (Cell Signaling; see Supplementary material online 5).
2.5. Promoter activity and chromatin immunoprecipitation
The VEGF-B reporter construct containing the 5′ flanking region (−851 to +156) of human VEGF-B (Genbank ID: NM_003377.3) was purchased from SwitchGear Genomics. HEK293 cells were transfected with VEGF-B reporter and RTEF-1 constructs using Lipofectamine 2000. The amount of control vector PJX40 was used for compensatory total volume of DNA. After 24 h transfection, luciferase activity was determined using the Dual-Luciferase assay system (Promega). Chromatin immunoprecipitation (ChIP) was performed with the ChIP-IT Express Kit (Active Motif) according to the manufacturer's instructions. The primer pairs 5′-CCAGGTGCCCTCTCCTCCAG-3′ and 5′-AAGGAAGCAAAGCGGGAACG-3′ for VEGF-B and 5′-TGCACTGTGCGGCGAAGC-3′ and 5′-TCGAGCCATAAAAGGCAA-3′ for actin as control were amplified. VEGF-B primers were designed to the putative MCAT element binding site for RTEF-1.
2.6. Immunofluorescence analysis
Heart samples were embedded in O.C.T. compound (Sakura Finetek USA Inc.) and frozen at −80°C. Tissue sections and fixed cultured neonatal cardiomyocytes were stained with antibodies against wheat germ agglutinin (Sigma), lectin (Sigma), α-actinin (Sigma) and 4′6-diamidino-2-phenylindole (DAPI) (Invitrogen) according to the manufacturers’ instructions. The cross-sectional area of cardiomyocytes and vessel density were determined using ImageJ software.
2.7. Amino acid incorporation assay
Cardiomyocytes (H9C2, RNCM) were plated in a 24-well dish and subsequently starved for 24 h in DMEM lacking fetal calf serum. 3H-Leucine (3 µCi/mL; Perkin Elmer) was then added to each well containing cells either in the presence or absence of 100 ng/mL VEGF-B, or 100 ng/mL VEGF-B + 10 µM U0126 treatment and cultured for an additional 20 h. Subsequently, cells were washed twice with 1 mL cold phosphate-buffered saline, followed by an incubation for 1 h at 4°C in 1 mL cold 10% trichloracetic acid (TCA). After one wash with TCA solution and one wash with phosphate-buffered saline, proteins were solubilized with 0.2 mL 0.5 M NaOH for 1 h at room temperature and transferred to scintillation tubes containing 3 mL Ultima Gold XR scintillation liquid. The 3H-leucine incorporation was determined using a LS6500 Beckman Coulter scintillation counter.
2.8. Statistical analysis
Results are expressed as the mean values ± SEM. Multiple comparisons among three or more groups were carried out by one-way ANOVA and Fisher's exact test for post hoc analysis. A value of P < 0.05 was considered significant.
3. Results
3.1. Generation of endothelium-specific RTEF-1 transgenic mice
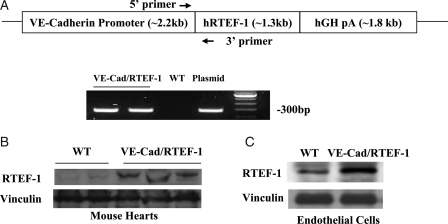
Transgenic mice with endothelium-specific expression of human RTEF-1 were generated using a mouse VE-Cadherin promoter. As expected, genotyping by PCR produced a 300 bp band for the transgenic gene (Figure 1A). Western blot was used to assess the specificity of endothelial expression of RTEF-1. Increased expression of RTEF-1 was found in hearts (Figure 1B) and isolated endothelial cells from VE-Cad/RTEF-1 mouse hearts in comparison with endogenous RTEF-1 expression (Figure 1C).
Figure 1.
Generation of endothelium-specific RTEF-1 transgenic mice, and expression of the RTEF-1 transgene. (A) Schematic structure of the VE-cadherin–RTEF-1 transgene construct, in which the human RTEF-1 transgene is driven by a promoter sequence from the VE-Cadherin gene. hGH pA indicates human growth hormone polyadenylation signal. The arrows indicate the primers for genotyping. The lower image shows results of genotyping the transgenic mice by PCR. (B) Western blots showing RTEF-1 expression in the hearts of wild-type (WT) and VE-Cad/RTEF-1 mice. (C) Western blots showing expression of RTEF-1 in endothelial cells isolated from the hearts of neonatal WT and VE-Cad/RTEF-1 mice.
3.2. Exacerbation of cardiac hypertrophy in VE-Cad/RTEF-1 mice after pressure overload
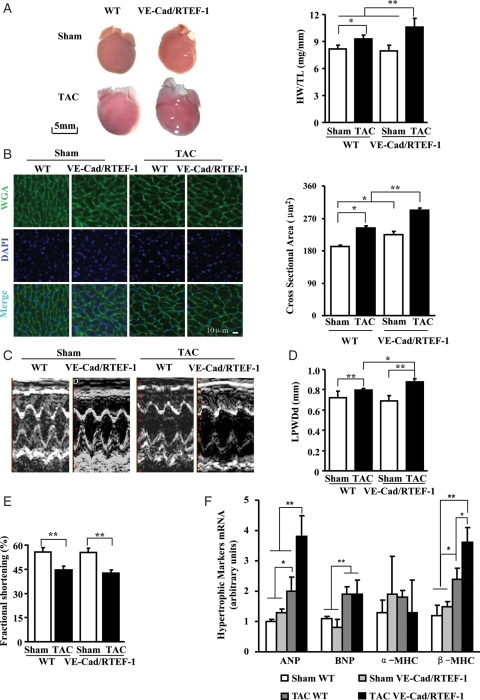
To assess the effect of endothelium-specific increased expression of RTEF-1 on the heart, cardiac parameters of VE-Cad/RTEF-1 mice that underwent TAC surgery were examined. The VE-Cad/RTEF-1 mice showed an increased response to the pressure overload stimulus. Measurements made 8 weeks after TAC revealed that the ratio of heart weight to tibia length (HW/TL) was increased in VE-Cad/RTEF-1 compared with wild-type (WT) mice (10.6 ± 1.0 vs. 9.3 ± 0.5 mg/mm; Figure 2A). Enlarged cross-sectional areas of cardiomyocytes were found in VE-Cad/RTEF-1 compared with WT mice (293.6 ± 4.7 vs. 242.8 ± 5.6 μm2; Figure 2B). Echocardiography revealed a significant increase in wall thickness in VE-Cad/RTEF-1 mice compared with WT mice (0.88 ± 0.03 vs. 0.80 ± 0.01 mm; Figure 2C and D), and a significant decline of heart function in both VE-Cad/RTEF-1 and WT mice (42.5 ± 2.0 vs. 44.6 ± 2.3%) after TAC (Figure 2E). In addition, transcripts of hypertrophic gene markers, ANP and β-MHC, were significantly up-regulated in VE-Cad/RTEF-1 mice compared with WT mice after TAC (Figure 2F). These results indicated that endothelium-specific RTEF-1 transgenic mice developed more significant cardiac hypertrophy after a pathological stimulus resulting from TAC.
Figure 2.
Cardiac hypertrophy in VE-Cad/RTEF-1 mice in response to TAC. (A) TAC surgery was performed on adult WT and VE-Cad/RTEF-1 mice 8 weeks before assessment. Increased heart size (left), and the ratio of heart weight to tibia length (right) were observed in VE-Cad/RTEF-1 mice compared with WT mice after TAC and sham operation (n = 8). (B) Left: sections of mouse hearts were incubated with wheat germ agglutinin (WGA, green) and DAPI (blue). WGA staining indicates the edge of cardiomyocytes. Right: analysis of cardiomyocyte size from WT and VE-Cad/RTEF-1 mice after TAC or sham operation (n = 5). (C–E) Conscious mice underwent echo analysis 8 weeks after TAC surgery. In the VE-Cad/RTEF-1 mice, representative M-mode echocardiographs show increased wall thickness and enlarged chamber size of the left ventricle after TAC surgery (C). Analysis of LPWDd (D) and fractional shortening percentage (E) indicate thickened walls in the VE-Cad/RTEF-1 mice and decreased heart function in both WT and VE-Cad/RTEF-1 mice after TAC (n = 8). (F) RNA samples were extracted from the apex of left ventricles and reverse transcribed. qPCR analysis demonstrates elevated expression of ANP and β-MHC in VE-Cad/RTEF-1 mice after TAC, as well as increased BNP levels in both WT and VE-Cad/RTEF-1 mice after TAC. The data from three independent experiments performed in triplicate were analysed and normalized to 36b4 content (n = 3). *P< 0.05 and **P< 0.01.
3.3. Elevation of VEGF-B in VE-Cad/RTEF-1 mice exacerbates cardiac hypertrophy
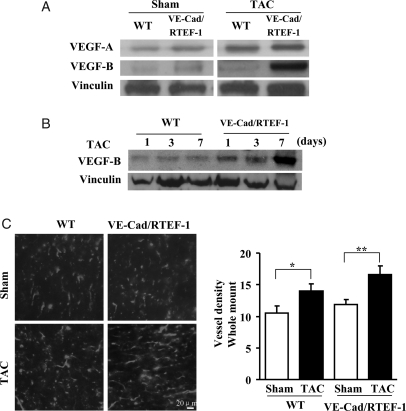
Our previous finding suggested that RTEF-1 is a pro-angiogenic transcription factor as a consequence of its VEGF-A promoter regulatory activity.7,11 We therefore examined the expression of VEGFs in mouse hearts. As shown in Figure 3A, expression of both VEGF-A and VEGF-B was increased in the hearts of VE-Cad/RTEF-1 mice. However, compared with WT mice 8 weeks after TAC, VEGF-B was dramatically elevated in VE-Cad/RTEF-1 mice (Figure 3A). Furthermore, the elevation was detected as early as day 7 after TAC (Figure 3B), indicating that VEGF-B might participate in the development of cardiac hypertrophy in endothelium-specific RTEF-1 transgenic mice. Moreover, lectin staining of cardiac sections showed increased vessel density 8 weeks after TAC (Figure 3C), suggesting that angiogenesis is also involved in cardiac hypertrophy.
Figure 3.
VEGF-B expression in VE-Cad/RTEF-1 mice after TAC. (A) Western blots of mouse heart lysates showing expression of VEGF-A and VEGF-B in VE-Cad/RTEF-1 or WT mice after TAC or sham treatment. (B) Western blots of VEGF-B in mouse heart lysates from VE-Cad/RTEF-1 mice or WT mice after TAC or sham treatment. (C) Left: sections of mouse heart from VE-Cad/RTEF-1 mice or WT mice after TAC or sham treatment were incubated with lectin antibody to show vessel density. Right: analysis of vessel densities shown in left panels (n = 3). *P< 0.05 and **P< 0.01.
3.4. Induction of VEGF-B expression by RTEF-1 in hypoxic endothelial cells
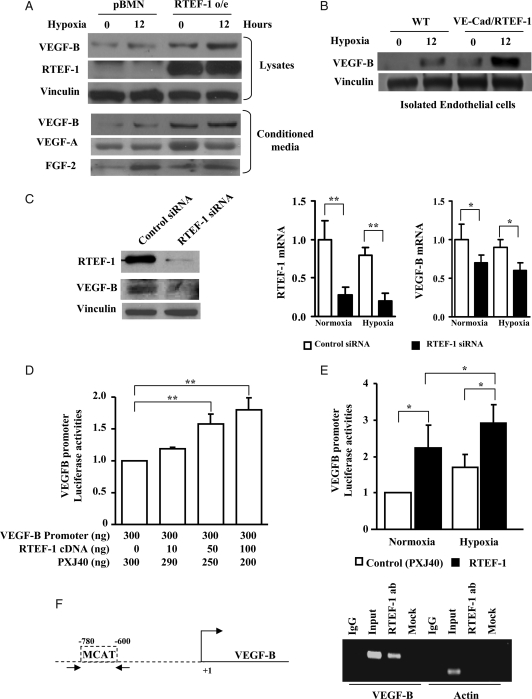
In considering how endothelial expression of RTEF-1 stimulates cardiac signalling, the target genes of RTEF-1 were examined by a microarray analysis in endothelial cells with RTEF-1 siRNA (data not shown). Among the down-regulated genes, secreted proteins characterized by signal sequences without any transmembrane domains were determined using amino acid sequence screening with Signal IP and SOSUI software.20 VEGF-B as a secreted protein was found to be significantly induced by RTEF-1 in endothelial cells. Considering that there is a mismatch of capillaries and cardiac growth, leading to myocardial hypoxia during the development of cardiac hypertrophy,21 secreted VEGF-B in hypoxia-conditioned medium was examined. VEGF-B was found to be significantly increased in HMEC-1/RTEF-1 compared with HMEC-1/pBMN (vector only for control), as well as in isolated endothelial cells from hearts of VE-Cad/RTEF-1 mice in both normoxia and hypoxia (Figure 4A and B). Increased VEGF-B secretion could also be detected in conditioned medium from HMEC-1/RTEF-1 exposed to normoxia or hypoxia for 12 h (Figure 4A). Meanwhile, VEGF-A and FGF2 but not tumour necrosis factor-α were found in the conditioned medium and could be induced by hypoxia, whereas the expression of FGF2 did not change in RTEF-1/HMEC cells (Figure 4A). Furthermore, knockdown of RTEF-1 by siRNA was associated with decreased expression of VEGF-B in endothelial cells in both normoxia and hypoxia (Figure 4C).
Figure 4.
Up-regulation of VEGF-B mediated by RTEF-1. (A) Western blots showed an increase in the protein level of RTEF-1 in HMEC-1 over-expressing RTEF-1 (upper panel). Cells were starved and then treated with normoxia or hypoxia for 12 h. VEGF-B was increased in both cell lysates and conditioned medium from HMEC-1/RTEF-1 (lower panel). VEGF-A and FGF2 were also detected in the conditioned medium. Vinculin was used as loading control. HMEC-1 transfected with pBMN vector was used as negative control. (B) Isolated endothelial cells from hearts of WT and VE-Cad/RTEF-1 mice were starved and then treated with normoxia or hypoxia for 12 h. VEGF-B was increased and further elevated by hypoxia in the lysates of VE-Cad/RTEF-1 compared with WT endothelial cells. (C) Endothelial cells were transfected with RTEF-1 siRNA. Western blot (left) and qPCR (right) indicated a significant knock down of RTEF-1 expression, accompanied by a significant reduction of VEGF-B expression in both normoxic and hypoxic conditions. qPCR data were from three independent experiments performed in triplicate and normalized to 36b4 content (n = 3). (D) VEGF-B promoter construct was co-transfected with various combinations of control vector (PXJ40) and RTEF-1 into HEK293 cells. Note that VEGF-B promoter activity was increased as the amount of RTEF-1 increased. The data are expressed as the means ± SEM of three independent experiments. (E) HEK293 cells were transfected for 24 h with PXJ40 or an equal amount of RTEF-1 and VEGF promoter constructs. Luciferase activity was determined after 6 h incubation of transfected cells in normoxic or hypoxic conditions. The data are expressed as the means ± SEM of three independent experiments. (F) Chromosomal immunoprecipitation assay showing co-immunoprecipitation of VEGF-B promoter with RTEF-1 antibody. The arrows in the schematic representation indicate the primers on the VEGF-B promoter sequence. Primers for actin promoter were used as controls. *P < 0.05 and **P < 0.01.
To determine whether RTEF-1 stimulates VEGF-B at a transcriptional level, the activity of a luciferase construct under the control of a VEGF-B promoter was measured. Co-transfection of VEGF-B/luciferase with increasing amounts of RTEF-1 cDNA produced a dose-dependent increase in luciferase activity (Figure 4D). Moreover, RTEF-1 stimulated VEGF-B promoter activity in both normoxic and hypoxic conditions, although significantly greater stimulation occurred in hypoxic conditions (Figure 4E). Sequence analysis indicated that there are MCAT-like elements and Sp1 binding sequences located in the promoter region of VEGF-B. To confirm the interaction between RTEF-1 and VEGF-B, ChIP assay revealed a direct interaction between RTEF-1 and the VEGF-B promoter via binding to MCAT-like element (Figure 4F), and Sp1 sequences were not detected to have any binding activity (data not shown). These results imply that RTEF-1 induces VEGF-B expression and secretion in endothelial cells, and this induction was enhanced in hypoxic conditions.
3.5. Pro-hypertrophic role of RTEF-1-induced VEGF-B on cardiomyocytes
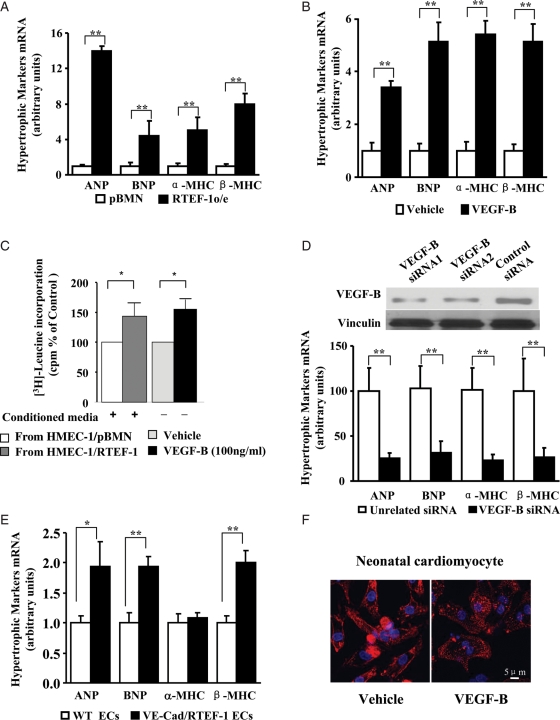
To further investigate the effect on cardiomyocytes of RTEF-1 expressed by endothelial cells, H9C2 were incubated with conditioned medium from hypoxia-treated HMEC-1/RTEF-1 or HMEC-1/pBMN. qPCR showed that mRNA levels of the hypertrophic gene markers ANP, BNP, α-MHC, and β-MHC were significantly increased after incubation with conditioned medium from HMEC-1/RTEF-1 compared with incubation with conditioned medium from HMEC-1 control cells (Figure 5A). Similar results were found in H9C2 cells after incubation with recombinant human VEGF-B 167 (rhVEGF-B167; Figure 5B). The effects of hypoxia-conditioned medium from HMEC-1/RTEF-1 and VEGF-B on protein synthesis were analysed by measurement of 3H-Leucine incorporation into H9C2 as shown in Figure 5C. To further confirm that VEGF-B is a key molecule in RTEF-1-driven endothelial cell communication with cardiomyocytes, we used two siRNAs targeted at VEGF-B to knock down VEGF-B expression in HMEC-1/RTEF-1 (Figure 5D). VEGF-B siRNA significantly inhibited the pro-hypertrophic effect of the conditioned medium (Figure 5D). To confirm these results, experiments were repeated using isolated endothelial cells from hearts of VE-Cad/RTEF-1 mice and neonatal rat ventricular myocytes (NRVMs). As observed for H9C2 cells, the expression of ANP, BNP, and β-MHC was induced in NRVMs after incubation with conditioned medium from hypoxia-treated endothelial cells of VE-Cad/RTEF-1 but not after incubation with conditioned medium from WT mice (Figure 5E). Moreover, NRVMs incubated with rhVEGF-B167 exhibited enlarged cardiomyocytes (Figure 5F). These data suggested that increased RTEF-1 in endothelial cells stimulated production and secretion of VEGF-B, thereby triggering expression of hypertrophic genes in cardiomyocytes.
Figure 5.
Pro-hypertrophic effect of VEGF-B induced by RTEF-1. (A) qPCR reveals significant increases of ANP, BNP, α-MHC, and β-MHC in H9C2 cells starved and then incubated for 12 h with medium from hypoxia-treated HMEC-1/RTEF-1 compared with results in H9C2 cells incubated with conditioned medium from HMEC-1/pBMN. (B) qPCR analysis for ANP, BNP, α-MHC, and β-MHC expression in H9C2 cells starved and then incubated with 100 ng of rhVEGF-B167 or vehicle control for 12 h. (C) 3H-Leucine incorporation indicates the level of protein synthesis in H9C2 cultures after incubation with hypoxia-conditioned media and VEGF-B. Bars represent radioactivity of incorporated 3H-Leucine (cpm); means ± SEM. (D) Western blot showing VEGF-B expression by HMEC-1/RTEF-1 transfected with VEGF-B siRNA or control siRNA (upper panel). qPCR showing expression of ANP, BNP, α-MHC and β-MHC by H9C2 cells incubated with conditioned media from VEGF-B siRNA-transfected and hypoxia-treated HMEC-1/RTEF-1. (E) qPCR showing expression of ANP, BNP, α-MHC, and β-MHC in neonatal rat ventricular myocytes after 12 h incubation with conditioned media from hypoxia-treated endothelial cells isolated from hearts of WT and VE-Cad/RTEF-1 mice. (F) NRVMs incubated with or without 100 ng/mL of recombinant hrVEGF-B167 for 48 h and stained by α-actinin (red). The data are expressed as the means ± SEM of three independent experiments. *P < 0.05 and **P < 0.01.
3.6. Induction of VEGF-B in cardiac hypertrophy via phosphorylation of ERK1/2
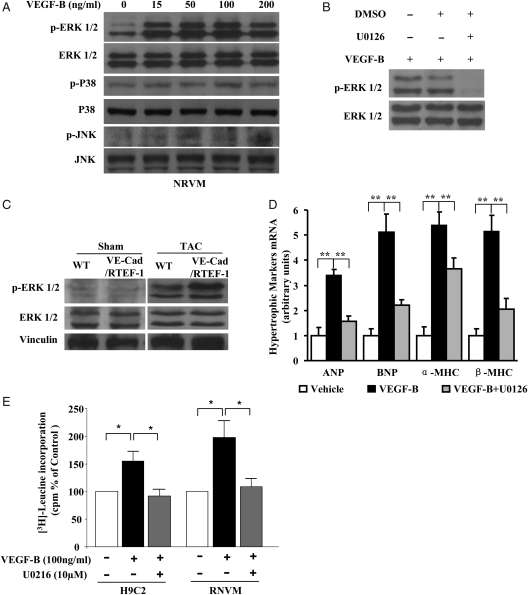
To examine the downstream signalling pathway of VEGF-B, NRVMs were treated with rhVEGF-B167 at different doses for various time periods. ERK1/2, but not P38 or JNK, was significantly phosphorylated by VEGF-B (Figure 6A). The rhVEGF-B167-induced phosphorylation of ERK1/2 could be abolished by treatment with the ERK1/2 specific blocker, U0126 (Figure 6B). Phosphorylation of ERK1/2 was also confirmed in heart samples from VE-Cad/RTEF-1 mice after TAC (Figure 6C). Furthermore, VEGF-B-induced expression of hypertrophic gene markers in H9C2 could be partly blocked by U0126 (Figure 6D). In addition, following rhVEGF-B167 treatment an increase in protein synthesis was observed in H9C2 and RNVM by the amino acid incorporation assay. Furthermore, ERK1/2 inhibitor significantly attenuated VEGF-B-induced protein synthesis in cardiomyocytes (Figure 6E).
Figure 6.
VEGF-B induced cardiac hypertrophy via phosphorylation of ERK1/2. (A) Western blots of ERK1/2, p38, JNK, and their phosphorylated forms in NRVMs incubated with 0, 15, 50, 100, or 200 ng/mL of recombinant hrVEGF-B167 for 15 min. (B) Western blots of ERK1/2 and phosphorylated ERK1/2 in NRVMs incubated with 100 ng/mL of rhVEGF-B167, rhVEGF-B167 with DMSO, or rhVEGF-B167 plus 10 μM U0126 for 15 min. (C) Western blots of ERK1/2 and phosphorylated ERK1/2 in heart samples from VE-Cad/RTEF-1 mice or WT mice after sham treatment or after TAC. (D) qPCR showing expression of ANP, BNP, α-MHC, and β-MHC by H9C2 cells treated with 100 ng/mL of rhVEGF-B167 or rhVEGF-B167 plus 10 μM U0126. (E) 3H-Leucine incorporation assay indicates the level of protein synthesis in H9C2 and RNCM cultures after incubation of VEGF-B with and without ERK1/2 inhibitor U0216. Bars represent radioactivity of incorporated 3H-leucine (cpm). The data are expressed as the means ± SEM of three independent experiments. *P < 0.05 and **P < 0.01.
4. Discussion
It is known that RTEF-1 plays a key role in the regulation of transcription in muscular cells.8 However, the target genes of RTEF-1 in non-muscle cells have not been fully investigated. Endothelium-specific RTEF-1 transgenic mice may provide an efficient model to dissect the function of RTEF-1 in endothelium. In this study, we demonstrated that the mechanism of exacerbated cardiac hypertrophy in endothelium-specific RTEF-1 transgenic mice is correlated to RTEF-1 transcriptional up-regulation of VEGF-B by the following lines of evidences. First, VE-Cad/RTEF-1 mice had elevated VEGF-B expression and developed more significant cardiac hypertrophy after the pressure overload stimulus. Secondly, RTEF-1 stimulated VEGF-B promoter activities via binding to MCAT element and regulated VEGF-B expression in endothelial cells. Thirdly, conditioned media from endothelial cells over-expressing RTEF-1 acted as a cardiac pro-hypertrophic stimulator, which could be blocked by VEGF-B knock-down. Finally, rhVEGF-B167 could induce a similar pro-hypertrophic effect on cardiomyocytes via phosphorylation of the ERK1/2 signalling pathway.
Capillary endothelial cells lie in close proximity to cardiomyocytes in the heart. The communication between these two cell types involves reciprocal gene regulation, signal transduction and energy supply.22 Cardiomyocytes induce endothelial expression of endogenous von Willebrand factor as well as von Willebrand factor promoter activity in both in vitro and in vivo conditions.23 Hypoxic cardiomyocytes induce endothelial expression of cyclo-oxygenase-2 in a VEGF-dependent pathway.24 In contrast, endothelial cells within the heart may release a number of substances, including endothelin25 and angiotensin-converting enzyme,26 to modulate myocardial function. RTEF-1, as a hypoxia-induced transcriptional factor,7,11 may be involved in this communication by targeting several genes in which some have signalling domains. Hypoxia occurs during development of hypertrophy in pressure overload.27 The endothelium-driven RTEF-1 inducing VEGF-B to target cardiomyocytes demonstrates that transcription factors can lead to a cross-talk between two cell types to impact cardiac functions. The changes in endothelium–myocyte cross-talk induced by RTEF-1 may contribute to and/or arise from cardiac pathologies.
RTEF-1 has been reported to regulate gene expression transcriptionally by binding to the MCAT elements and Sp1 binding sequences of promoters.7,8,11 Sequence analyses have indicated that the VEGF-B promoter region contains Sp1 binding sequences and MCAT elements, either of which could be the potential binding site for RTEF-1. The evidence presented in this report indicates that RTEF-1 regulates VEGF-B in endothelial cells through a direct interaction with the MCAT-like elements on the VEGF-B promoter. It has been reported that the VEGF-B transcript was stable when cultured fibroblast cells were treated with hypoxia, serum, growth factors or hormones.28 It is possible that VEGF-B is regulated by different mechanisms in various cell types. Cell-specific co-factors necessary for RTEF-1-mediated expression might be available in endothelial cells but not in other types of cells.
Expression of VEGF-B was significantly elevated in hearts of VE-CAD/RTEF-1 mice after TAC, implying that VEGF-B might play an important role in cross-talk between RTEF-1-expressing endothelial cells and cardiomyocytes. Unlike VEGF-A, VEGF-B has a very limited angiogenic effect.29 In contrast, VEGF-B was reported to be anti-apoptotic in neurons,30 smooth muscle cells, pericytes, and endothelial cells16 by regulating pro-survival genes via both neuropilin-1 (NP-1) and VEGFR-1. Cardiomyocytes incubated with conditioned medium from RTEF-1-over-expressing endothelial cells or incubated with rhVEGF-B167 exhibited significant increases in expression of hypertrophic gene markers and protein synthesis through ERK mitogen-activated protein kinase activation. Furthermore, induction of these markers was blocked by VEGF-B knockdown with siRNA. These findings are consistent with recent observations that cardiac-specific VEGF-B transgenic mice exhibit significant cardiac hypertrophy,17 as well as with the observation that VEGF-B activates VEGFR-1 to elicit a particular hypertrophic response in cultured cardiomyocytes and in infarcted hearts.18 It was also found that ERK mitogen-activated protein kinase signalling plays a key role in cardiac hypertrophy.31 However, whether RTEF-1-induced VEGF-B in this study exerts an anti-apoptotic effect requires further investigation.
RTEF-1 is able to up-regulate the VEGF-A gene in hypoxic conditions in bovine aortic endothelial cells.7 In this study, VEGF-A was also found to be involved in the development of cardiac hypertrophy, as evidenced by elevated VEGF-A expression and increased vessel density in the heart after pressure overload in vivo. It has been reported that cardiac growth and angiogenesis is co-ordinated by angiogenic growth factors in response to hypertrophic stimuli.3 An imbalance between angiogenesis and cardiac growth can lead to heart failure,3 which is consistent with the clinical findings that dilated cardiomyopathy is associated with an irregular capillary pattern and a reduction in overall capillary density.5,32 We found that transcriptional control of VEGF-B by RTEF-1 in endothelial cells plays a direct role in regulation of hypertrophic gene expression in cardiomyocytes. This regulation of VEGF-B by RTEF-1 might be independent of angiogenesis, providing evidence of communication between the two cell types in the heart.
5. Conclusion
We demonstrated that transcriptional regulation by RTEF-1 in endothelial cells is involved in the development of cardiomyocyte hypertrophy. We identified VEGF-B as a target gene of RTEF-1 that appears to play an important role as a bridge between endothelial cells and cardiomyocytes during development of cardiac hypertrophy induced by pressure overload. These results suggest that transcriptional control of the VEGF pathway in the communication between endothelial cells and cardiomyocytes will lead to a better understanding of the precise mechanisms of angiogenesis and cardiac hypertrophy, and ultimately the development of new therapeutic strategies.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by National Institute of Health (HLR01082837 to J.L.) and China Scholarship Council (M.X.).
Acknowledgements
We thank: Drs Anthony Rosenzweig and Peter Kang (BIDMC, Harvard) for technical support of the TAC mouse surgery and cardiomyocyte isolation; and Dr Laura Benjamin (BIDMC, Harvard) for the VE-cadherin promoter construct for VE-Cad/RTEF-1 mice.
Conflict of interest: none declared.
References
- 1.Hsieh PC, Davis ME, Lisowski LK, Lee RT. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu Rev Physiol. 2006;68:51–66. doi: 10.1146/annurev.physiol.68.040104.124629. doi:10.1146/annurev.physiol.68.040104.124629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giordano FJ, Gerber HP, Williams SP, VanBruggen N, Bunting S, Ruiz-Lozano P, et al. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc Natl Acad Sci USA. 2001;98:5780–5785. doi: 10.1073/pnas.091415198. doi:10.1073/pnas.091415198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh K, Shiojima I. Cardiac growth and angiogenesis coordinated by intertissue interactions. J Clin Invest. 2007;117:3176–3179. doi: 10.1172/JCI34126. doi:10.1172/JCI34126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS, Walsh K. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension. 2006;47:887–893. doi: 10.1161/01.HYP.0000215207.54689.31. doi:10.1161/01.HYP.0000215207.54689.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karch R, Neumann F, Ullrich R, Neumuller J, Podesser BK, Neumann M, et al. The spatial pattern of coronary capillaries in patients with dilated, ischemic, or inflammatory cardiomyopathy. Cardiovasc Pathol. 2005;14:135–144. doi: 10.1016/j.carpath.2005.03.003. doi:10.1016/j.carpath.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Ueyama T, Zhu C, Valenzuela YM, Suzow JG, Stewart AF. Identification of the functional domain in the transcription factor RTEF-1 that mediates α1-adrenergic signaling in hypertrophied cardiac myocytes. J Biol Chem. 2000;275:17476–17480. doi: 10.1074/jbc.M001970200. doi:10.1074/jbc.M001970200. [DOI] [PubMed] [Google Scholar]
- 7.Shie JL, Wu G, Wu J, Liu FF, Laham RJ, Oettgen P, et al. RTEF-1, a novel transcriptional stimulator of vascular endothelial growth factor in hypoxic endothelial cells. J Biol Chem. 2004;279:25010–25016. doi: 10.1074/jbc.M403103200. Epub 22004 Apr 25018 doi:10.1074/jbc.M403103200. [DOI] [PubMed] [Google Scholar]
- 8.Farrance IK, Ordahl CP. The role of transcription enhancer factor-1 (TEF-1) related proteins in the formation of M-CAT binding complexes in muscle and non-muscle tissues. J Biol Chem. 1996;271:8266–8274. doi: 10.1074/jbc.271.14.8266. doi:10.1074/jbc.271.14.8266. [DOI] [PubMed] [Google Scholar]
- 9.Gan Q, Yoshida T, Li J, Owens GK. Smooth muscle cells and myofibroblasts use distinct transcriptional mechanisms for smooth muscle α-actin expression. Circ Res. 2007;101:883–892. doi: 10.1161/CIRCRESAHA.107.154831. Epub 2007 Sep 2006 doi:10.1161/CIRCRESAHA.107.154831. [DOI] [PubMed] [Google Scholar]
- 10.Chen HH, Baty CJ, Maeda T, Brooks S, Baker LC, Ueyama T, et al. Transcription enhancer factor-1-related factor-transgenic mice develop cardiac conduction defects associated with altered connexin phosphorylation. Circulation. 2004;110:2980–2987. doi: 10.1161/01.CIR.0000146902.84099.26. Epub 2004 Nov 2981 doi:10.1161/01.CIR.0000146902.84099.26. [DOI] [PubMed] [Google Scholar]
- 11.Appukuttan B, McFarland TJ, Davies MH, Atchaneeyasakul LO, Zhang Y, Babra B, et al. Identification of novel alternatively spliced isoforms of RTEF-1 within human ocular vascular endothelial cells and murine retina. Invest Ophthalmol Vis Sci. 2007;48:3775–3782. doi: 10.1167/iovs.06-1172. doi:10.1167/iovs.06-1172. [DOI] [PubMed] [Google Scholar]
- 12.Nash AD, Baca M, Wright C, Scotney PD. The biology of vascular endothelial growth factor-B (VEGF-B) Pulm Pharmacol Ther. 2006;19:61–69. doi: 10.1016/j.pupt.2005.02.007. doi:10.1016/j.pupt.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. doi:10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mould AW, Greco SA, Cahill MM, Tonks ID, Bellomo D, Patterson C, et al. Transgenic overexpression of vascular endothelial growth factor-B isoforms by endothelial cells potentiates postnatal vessel growth in vivo and in vitro. Circ Res. 2005;97:e60–e70. doi: 10.1161/01.RES.0000182631.33638.77. doi:10.1161/01.RES.0000182631.33638.77. [DOI] [PubMed] [Google Scholar]
- 15.Aase K, von Euler G, Li X, Ponten A, Thoren P, Cao R, et al. Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation. 2001;104:358–364. doi: 10.1161/01.cir.104.3.358. [DOI] [PubMed] [Google Scholar]
- 16.Zhang F, Tang Z, Hou X, Lennartsson J, Li Y, Koch AW, et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci USA. 2009;106:6152–6157. doi: 10.1073/pnas.0813061106. doi:10.1073/pnas.0813061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karpanen T, Bry M, Ollila HM, Seppanen-Laakso T, Liimatta E, Leskinen H, et al. Overexpression of vascular endothelial growth factor-B in mouse heart alters cardiac lipid metabolism and induces myocardial hypertrophy. Circ Res. 2008;103:1018–1026. doi: 10.1161/CIRCRESAHA.108.178459. doi:10.1161/CIRCRESAHA.108.178459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zentilin L, Puligadda U, Lionetti V, Zacchigna S, Collesi C, Pattarini L, et al. Cardiomyocyte VEGFR-1 activation by VEGF-B induces compensatory hypertrophy and preserves cardiac function after myocardial infarction. FASEB J. 2010;24:1467–1478. doi: 10.1096/fj.09-143180. [DOI] [PubMed] [Google Scholar]
- 19.Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, et al. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci USA. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. doi:10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008;117:3099–3108. doi: 10.1161/CIRCULATIONAHA.108.767673. Epub 2008 Jun 3092 doi:10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimizu I, Minamino T, Toko H, Okada S, Ikeda H, Yasuda N, et al. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. J Clin Invest. 2010;120:1506–1514. doi: 10.1172/JCI40096. doi:10.1172/JCI40096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brutsaert DL. Cardiac endothelial–myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev. 2003;83:59–115. doi: 10.1152/physrev.00017.2002. [DOI] [PubMed] [Google Scholar]
- 23.Aird WC, Edelberg JM, Weiler-Guettler H, Simmons WW, Smith TW, Rosenberg RD. Vascular bed-specific expression of an endothelial cell gene is programmed by the tissue microenvironment. J Cell Biol. 1997;138:1117–1124. doi: 10.1083/jcb.138.5.1117. doi:10.1083/jcb.138.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu G, Mannam AP, Wu J, Kirbis S, Shie JL, Chen C, et al. Hypoxia induces myocyte-dependent COX-2 regulation in endothelial cells: role of VEGF. Am J Physiol Heart Circ Physiol. 2003;285:H2420–H2429. doi: 10.1152/ajpheart.00187.2003. [DOI] [PubMed] [Google Scholar]
- 25.Mohacsi A, Magyar J, Tamas B, Nanasi PP. Effects of endothelins on cardiac and vascular cells: new therapeutic target for the future? Curr Vasc Pharmacol. 2004;2:53–63. doi: 10.2174/1570161043476528. doi:10.2174/1570161043476528. [DOI] [PubMed] [Google Scholar]
- 26.Kuruvilla L, Kartha CC. Molecular mechanisms in endothelial regulation of cardiac function. Mol Cell Biochem. 2003;253:113–123. doi: 10.1023/a:1026061507004. doi:10.1023/A:1026061507004. [DOI] [PubMed] [Google Scholar]
- 27.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. doi:10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 28.Enholm B, Paavonen K, Ristimaki A, Kumar V, Gunji Y, Klefstrom J, et al. Comparison of VEGF, VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene. 1997;14:2475–2483. doi: 10.1038/sj.onc.1201090. doi:10.1038/sj.onc.1201090. [DOI] [PubMed] [Google Scholar]
- 29.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. doi:10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 30.Falk T, Zhang S, Sherman SJ. Vascular endothelial growth factor B (VEGF-B) is up-regulated and exogenous VEGF-B is neuroprotective in a culture model of Parkinson's disease. Mol Neurodegener. 2009;4:49. doi: 10.1186/1750-1326-4-49. doi:10.1186/1750-1326-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris IS, Zhang S, Treskov I, Kovacs A, Weinheimer C, Muslin AJ. Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation. 2004;110:718–723. doi: 10.1161/01.CIR.0000138190.50127.6A. doi:10.1161/01.CIR.0000138190.50127.6A. [DOI] [PubMed] [Google Scholar]
- 32.Abraham D, Hofbauer R, Schafer R, Blumer R, Paulus P, Miksovsky A, et al. Selective downregulation of VEGF-A165, VEGF-R1, and decreased capillary density in patients with dilative but not ischemic cardiomyopathy. Circ Res. 2000;87:644–647. doi: 10.1161/01.res.87.8.644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.