Abstract
Aims
Doxorubicin (DOX) is a highly effective chemotherapeutic agent; however, cumulative dose-dependent cardiotoxicity is a significant side effect of this therapy. Because DOX is a polyaromatic hydrocarbon, we hypothesized that it will be metabolized by the activation of the aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor that is involved in the metabolism of numerous xenobiotic agents. These studies were performed to determine whether DOX activates AhR and whether this activation modulates the toxicity of DOX in cardiomyocytes.
Methods and results
Treatment with DOX induced AhR migration to the nucleus, increased AhR binding with its co-factor, aryl hydrocarbon receptor nuclear translocator-1 (ARNT1), and increased the expression of AhR-regulated phase I (CYP1A1) and phase II (GSTA1) drug-metabolizing enzymes in both cardiomyocytes and in the intact heart. Knockdown of AhR in H9C2 cells abolished DOX-induced increases in CYP1A1 and GSTA1 expression. Similar results were obtained by treating adult rat ventricular myocytes with the AhR antagonist, CH-223191. Taken together, these findings indicate that DOX-induced upregulation of CYP1A1 and GSTA1 expression is AhR dependent. AhR null mice treated with 10 mg/kg DOX did not show any activation of CYP1A1 or GSTA1 expression. Moreover, lack of AhR in vivo resulted in a significant decrease in left ventricular function compared with wild-type animals, and increased p53 activation and apoptosis in the heart after treatment with DOX.
Conclusions
These findings indicate that AhR plays an important role in DOX metabolism by the heart and further demonstrate that AhR is cardioprotective against DOX-induced cardiotoxicity.
Keywords: Aryl hydrocarbon receptor, Doxorubicin cardiotoxicity, Reactive oxygen species, Apoptosis, Cardioprotection
1. Introduction
The anthracycline chemotherapeutic agent, doxorubicin (DOX), has been shown to be effective in treating malignancies by interfering with DNA replication and causing DNA cross-linking. The efficacy of DOX is related to its cumulative dose. Unfortunately, use of DOX is limited by cardiotoxic effects, which are also dose dependent.1–3 The reactive oxygen species (ROS) produced by DOX metabolism in cardiomyocytes cause cell death through the activation of apoptotic pathways.4–6 Specifically, DOX treatment of cardiomyocytes causes p53,7–9 caspase 3 and caspase 9 activation9,10 and opening of the mitochondrial permeability transition pore with subsequent release of cytochrome c.11–13 While ROS generation and the activation of apoptotic pathways in the heart in response to DOX have been well characterized, little is known about other aspects of the cellular response to DOX that may modulate its toxicity in cardiomyocytes.
Given the polycyclic aromatic structure of DOX, which is shared with other xenobiotic agents including dioxin (2,3,7,8-tetrachlorodibenzodioxin, TCDD, Supplementary material online, Figure S1), we hypothesized that DOX may activate the aryl hydrocarbon receptor (AhR). AhR is a basic helix-loop-helix (bHLH) transcription factor that resides in the cytoplasm in association with Hsp90, AhR interacting protein (XAP2) and p23. In response to TCDD, AhR dissociates from p23 and translocates to the nucleus through a mechanism that is dependent on a nuclear localization signal in the amino terminal region of the protein.14 Once in the nucleus, AhR dissociates from Hsp90, and heterodimerizes with the AhR nuclear translocator (ARNT). The AhR/ARNT complex then binds to xenobiotic response elements (XRE) in genes involved in xenobiotic metabolism, cytochrome P450 isoforms (CYP1A1, CYP1A2, CYP1B1), NAD(P)H-quinone oxidoreductase, aldehyde dehydrogenase 3, UDP glucuronosyltransferase, and glutathione transferase (GSTA1).15 In the case of TCDD, activation of AhR is responsible for its toxic effects,16,17 although the activation of AhR by other drugs results in the metabolism of those drugs to inactive compounds.
To test the hypothesis that cardiac AhR is activated by DOX, we examined whether DOX treatment of cardiomyocytes results in AhR translocation to the nucleus with subsequent increased expression of AhR responsive genes. Next, we examined whether loss of AhR expression alters DOX-induced cardiotoxicity in vivo.
2. Methods
An expanded Methods section is available in the Supplementary online material, Materials and Methods.
2.1. Animals
Eight-week-old AhR−/− mice (provided by Frank Gonzalez, PhD, National Cancer Institute, Bethesda, MD, USA) and C57Bl/6 control mice (Jackson Labs, Bar harbor, ME, USA), weighing 20–23 g, were housed under controlled conditions (12 h light–dark cycle, 22°C, 60% humidity). They were fed a standard rodent chow ad libitum and had free access to water. All studies were approved by the Institutional Animal Care and Use Committee of Yale University and conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.2. Antibodies
Antibodies against the following proteins were used in this study: actin (Santa Cruz Biotechnology, I-19, catalog #sc1616), AhR (Santa Cruz Biotechnology, H-211, catalog #sc5579, and N-19, catalog #sc8088), ARNT1 (Cell Signaling Technology, catalog #3718), β-tubulin (Santa Cruz Biotechnology, H-235, catalog #sc9104), CYP1A1 (Santa Cruz Biotechnology, G-18, catalog #sc9828), GSTA1 (Abcam, catalog #ab53940), HSP90 (Cell Signaling Technology, catalog #4877), phosphoserine 46-p53 (Abcam, catalog #ab76242), and TATA-binding protein (TBP, Abcam, catalog #ab62125).
2.3. Isolated adult rat ventricular myocytes
Primary adult rat cardiomyocytes were isolated using a collagenase perfusion method with stepwise increases in the Ca2+ concentration. Briefly, adult male Sprague Dawley rats, 150–220 g (Charles River Laboratory, Raleigh, NC, USA) were anaesthetized with pentobarbital (100 mg/kg) and heparinized, the heart was quickly removed and immediately perfused at 37°C with the following media: medium A (6 mM KCl, 1 mM NaH2PO4, 1.4 mM MgSO4, 128 mM NaCl, 10 mM NaHEPES, 5.5 mM glucose, 2 mM pyruvate, 5 min), medium B [Medium A plus 0.7% bovine serum albumin (BSA) fraction V, 1.1 mg/mL collagenase, 15 mM 2,4-butanedione monoxime, 15 min]. Calcium chloride (10 mM) was added serially over a 10-min period to a final concentration of 100 µM. The heart was placed in a culture dish containing 25 mL of medium B and 25 mL of medium C (Medium A plus 100 µM CaCl2, 2% BSA fraction V), carefully torn open and transferred to a 250 mL flask at 37°C. Heart tissue was shaken for 20 min in 95% O2/5% CO2. The calcium concentration was increased stepwise up to a final concentration of 1 mM. The heart tissue was filtered through a nylon net (mesh size: 200 × 200 µm) and centrifuged. The pellet containing cardiomyocytes was resuspended in resting medium [Medium 199, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum (FBS)] and plated on 100 mm tissue culture plates (5 × 105 cells/plate) precoated with mouse laminin (1.42 μg/cm2). After 2–3 h, the resting medium was carefully replaced with similar medium containing 1% FBS overnight. Cells were then used to determine the effects of 2.5 µM DOX on the expression of AhR-responsive genes as well as the effect of the AhR antagonist, CH-223191, on the response to DOX.
2.4. DOX and AhR antagonist treatments
Mice were injected with a single intraperitoneal dose of DOX (10 mg/kg) or saline (control group) and sacrificed after 4, 24 h, or 2 weeks. Mice were anaesthetized with pentobarbital (100 mg/kg) and hearts were removed and freeze clamped for subsequent analyses. In addition, a subset of mice underwent echocardiographic assessment of left ventricular function as described below.
For H9C2 embryonic rat cardiac myoblast cells (ATCC, Manassas, VA, USA) and adult rat ventricular myocyte (ARVM), DOX was added at a concentration of 2.5 μM and incubated for 4, 6, or 24 h. CH-223191 was dissolved in dimethyl sulfoxide according to the manufacture's instruction and single experiment aliquots were prepared and frozen at −20°. The AhR antagonist was added to the ARVM at a concentration of 10 μM. After the experiment, cells were harvested on ice using Cellytic-M solution, and the lysate was passed at least five times through a 25-gauge needle and centrifuged at 800 g for 15 min to pellet the nuclei-containing fraction. To isolate DNA or RNA, cells were trypsinized and harvested, and nucleic acids were isolated according to Qiagen protocols.
2.5. Echocardiographic assessment of left ventricular function
Mice injected with saline or DOX were lightly anaesthetized by inhalation of 1–2% isoflurane and underwent echocardiographic assessment using a 40 MHz probe and a Vevo 770 Utrasound system. Two-dimensionally guided M-mode images of the left ventricle were acquired along the long and short axes to assess left ventricular fractional shortening.
2.6. siRNA knockdown of AhR in H9C2 cells
To determine the effect of loss of AhR on the response to DOX, H9C2 cells were transfected with scrambled or AhR siRNA using FuGene and based on the manufacturer's instructions and then treated with 2.5 µM DOX. The level of expression of the AhR-responsive genes, CYP1A1, and GSTA1, on the mRNA level and on the protein level by quantitative PCR and immunoblot analysis, respectively.
2.7. Electrophoretic mobility shift assay
A LightShift Chemiluminescent electrophoretic mobility shift assay (EMSA) Kit was used to perform EMSA based on a 100 bp fragment of the CYP1A1 promoter containing an XRE-binding site18 that was amplified by PCR from rat genomic DNA (for primer structures see Supplementary material online, Table S1). The labelled DNA fragment was incubated with nuclear extract isolated from control H9C2 cells and cells incubated with 2.5 µM DOX for 4 h. Detection was performed using the Chemiluminescent Nucleic Acid Detection Module kit according to the manufacturer's protocol.
2.8. Oxidative status and cell viability
Lucigenin-derived chemiluminescence (LDCL) was used to determine ROS generation using homogenates from myocytes incubated either with saline or 2.5 µM DOX. Cell viability following treatment with 2.5 µM DOX was determined using a cytotoxicity detection kit based on the measurement of lactate dehydrogenase (LDH) activity released from the cytosol of damaged cells into the supernatant.
2.9. Image processing and statistical analyses
Immunoblot and DNA laddering data were analysed using ImageJ software. Data are expressed as the mean ± SD. Statistical comparisons between two groups were done using an unpaired Student's t-test. For comparisons of more than two groups, analysis of variance was performed. Post hoc comparisons were performed using Tukey's multiple comparison analysis. All statistical analyses were performed using Prism 4.0 (GraphPad Software).
3. Results
3.1. DOX treatment activates AhR
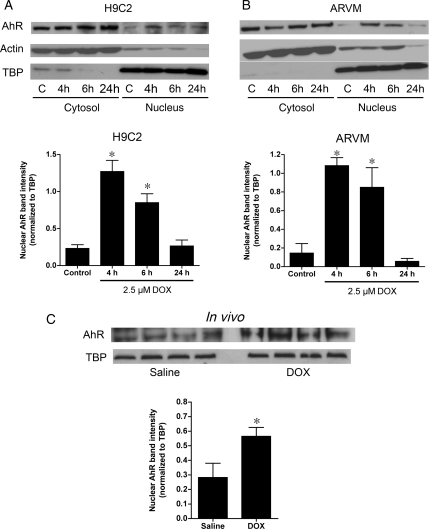
To determine whether DOX activates AhR, the translocation of AhR from the cytoplasm to the nucleus of H9C2 cells in response to DOX was assessed in vitro and in vivo. Significant nuclear AhR localization was observed following 4 h of incubation with 2.5 or 5 μM DOX, similar to the effect seen with the known AhR activator, TCDD (Supplementary material online, Figure S2). Based on these results, a DOX concentration of 2.5 μM was used for all the following in vitro experiments. Of note, this value is similar to the plasma DOX concentration in patients receiving chemotherapy.19 After 4 h of incubation with DOX, the nuclear AhR content increased in H9C2 cells (Figure 1A). After 24 h, the nuclear content of AhR returned to baseline values. Similar changes were observed in ARVM treated with DOX (Figure 1B). Translocation of AhR was also seen in response to DOX treatment in vivo. Specifically, hearts from wild-type mice had a significantly higher nuclear AhR content 4 h after receiving a single 10 mg/kg injection of DOX compared with saline injection (Figure 1C).
Figure 1.
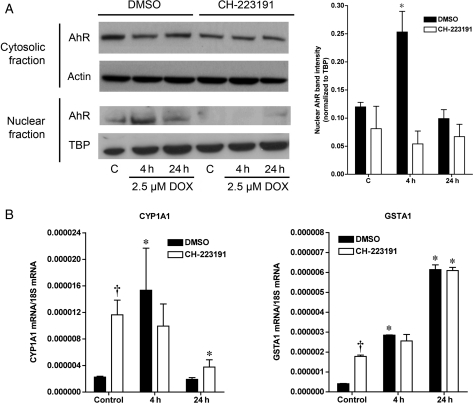
Nuclear translocation of aryl hydrocarbon receptor (AhR) in response to doxorubicin (DOX). (A) Cytosolic and nuclear content of AhR in H9C2 cells under control, C, conditions or after treatment with 2.5 µM DOX for 4, 6, or 24 h (n = 4 for all groups). Actin and TATA-binding protein (TBP) were used as markers for cytosolic and nuclear enrichment, respectively. (B) Cytosolic and nuclear content of AhR in adult rat ventricular myocytes (ARVM, n = 4 for all groups) following treatment with 2.5 µM DOX. AhR band intensity for the nuclear fractions is normalized for the TBP expression is quantified in the graphs below the representative immunoblots. (C) Nuclear content of AhR in hearts from mice treated with either saline or 10 mg/kg of DOX demonstrating a two-fold increase in the nuclear content of AhR (n = 4 for both groups). *P < 0.05 compared with control/saline treatment.
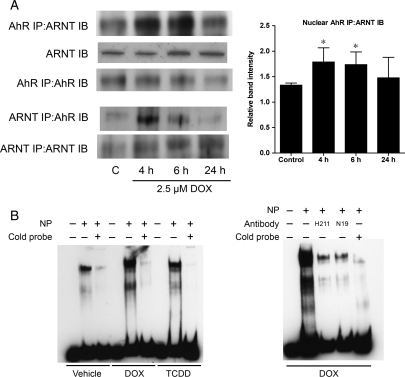
Activation of AhR causes it to translocate into the nucleus where it dimerizes with ARNT1 and activates transcription of AhR-responsive genes. To determine whether DOX-mediated AhR activation is accompanied by association with nuclear ARNT1, AhR-containing protein complexes were immunoprecipitated from the nuclear fraction of ARVM treated with DOX. Following 4 h of treatment with DOX, there was increased ARNT1 binding with AhR in the nucleus that decreased by 24 h (Figure 2A). Complementary experiments were performed in which ARNT-containing protein complexes were immunoprecipitated from the nuclear fraction and confirmed increased association between AhR and ARNT with DOX treatment (Figure 2A).
Figure 2.
Doxorubicin increases AhR association with aryl hydrocarbon receptor nuclear translocator (ARNT) in the nucleus and increases association of AhR with AhR-responsive elements. (A) ARNT association with AhR immunoprecipitated from nuclear protein from myocytes treated with 2.5 µM DOX (n = 4 for all groups). AhR association with ARNT immunoprecipitated from nuclear protein similarly demonstrated increased association between the two proteins following DOX treatment. (B) Alterations in the electrophoretic mobility patterns for DNA fragments containing the xenobiotic response elements (XRE) consensus sequence by the addition of nuclear protein from H9C2 cells treated with DOX or 50 nM TCDD, a known activator of AhR. (C) Changes in the electrophoretic mobility pattern for the XRE when nuclear extracts from cells treated with DOX were preincubated with two distinct anti-AhR antibodies (H211 and N19, Santa Cruz). NP, nuclear protein; *P < 0.05 compared with control treatment.
To show that DOX-induced activation of AhR results in AhR binding to XRE, EMSA were performed using a CYP1A1 promoter fragment containing an XRE-binding site. Incubation of the labelled CYP1A1 promoter fragment with nuclear extracts from cardiomyocytes under control conditions resulted in formation of at least two complexes (Figure 2B). The specificity of binding to this fragment was demonstrated by competition with unlabelled probe. There was a significant increase in the amount of both complexes detected in nuclear extracts after DOX or TCDD treatment. To demonstrate that AhR is involved in these complexes, two AhR antibodies directed against different epitopes within AhR were added to the incubations. The addition of these antibodies significantly decreased formation of both complexes (Figure 2B), suggesting that DOX treatment enhances AhR binding to the CYP1A1 promoter.
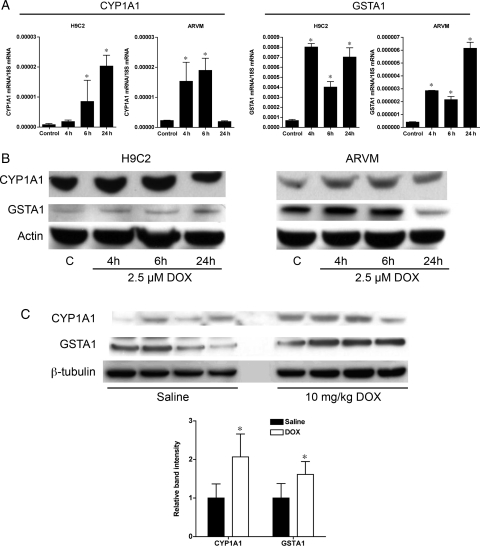
To further demonstrate that DOX induces AhR activation, the transcriptional activation of two representative AhR responsive genes, CYP1A1 (phase I drug metabolism), and GSTA1 (phase 2 drug metabolism) was determined. Expression of both CYP1A1 and GSTA1 increased six to eight-fold following DOX treatment in H9C2 cells as well as ARVM (Figure 3A). Of note, the expression of CYP1A1 RNA returned to basal levels in ARVM by 24 h of DOX treatment, which may be due to differences between immortalized rat atrial H9C2 myocytes and primary adult ventricular cardiomyocytes. CYP1A1 and GSTA1 protein levels also increased in both cell types in response to DOX treatment (Figure 3B). CYP1A1 and GSTA1 protein expression increased similarly in the hearts of mice 4 h after injection of 10 mg/kg of DOX (Figure 3C).
Figure 3.
Doxorubicin treatment induces increased expression of AhR-regulated phase I (CYP1A1) and phase II (GSTA1) drug-metabolizing enzymes. (A) Quantitative PCR analysis of CYP1A1 and GSTA1 total RNA expression in H9C2 and ARVM cells treated with 2.5 µM DOX for 4, 6, or 24 h (n = 4 for all groups). (B) Representative immunoblots for CYP1A1 and GSTA1 from H9C2 and ARVM cells treated with 2.5 µM DOX (n = 4 for all groups). (C) CYP1A1 and GSTA1 expression in the heart of mice 4 h after injection of 10 mg/kg of DOX (n = 4 for all groups). *P < 0.05 compared with control or saline treatment.
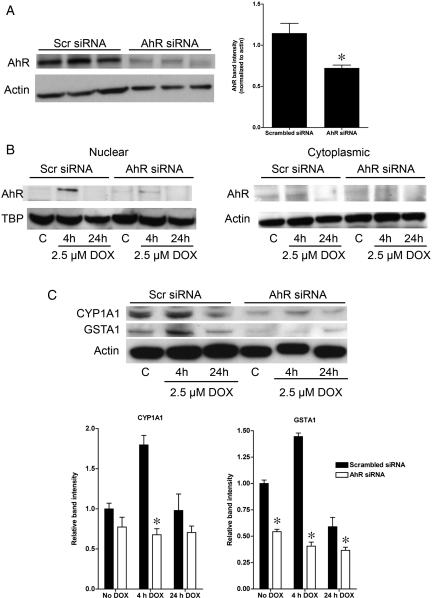
To determine whether AhR is directly involved in downstream target gene activation following DOX treatment, AhR expression was knocked down in H9C2 cells using AhR siRNA. AhR protein levels in cells expressing AhR siRNA were 37% lower than in cells expressing scrambled siRNA (Figure 4A), with decreased expression observed in both the nuclear and cytoplasmic fractions (Figure 4B). This was associated with attenuated accumulation of AhR in the nucleus in response to DOX (Figure 4B). Knockdown of AhR resulted in a decrease in the expression of CYP1A1 and GSTA1 under basal conditions. Furthermore, the inhibition of AhR expression and translocation blocked DOX-induced upregulation of CYP1A1 and GSTA1 RNA (Supplementary material online, Figure S3) and protein expression (Figure 4C). Similarly, treatment of ARVM with CH-223191 abolished DOX-induced nuclear translocation of AhR (Figure 5A). Interestingly, treatment with CH-223191 increased the basal expression of CYP1A1 and GSTA1, an effect that has been previously described in glioblastoma cells20 and may be due to ligand-dependent effects of CH-223191.21 However, despite the increase in the basal expression of CYP1A1 and GSTA1, addition of CH-223191 inhibited the increase in the expression of both CYP1A1 and GSTA1 in response to DOX treatment (Figure 5B).
Figure 4.
Knockdown of AhR in H9C2 cells decreases AhR expression and inhibits the upregulation of CYP1A1 and GSTA1 in response to DOX. (A) Effects of scrambled (Scr) and anti-AhR siRNA on whole cell expression of AhR. (B) Effect of scrambled (Scr) and anti-AhR siRNA on the expression of AhR both in the cytosol and nucleus and the response to 4 or 24 h of treatment with 2.5 µM DOX (n = 4 for all groups). (C) Changes in CYP1A1 and GSTA1 expression in H9C2 cells following knockdown of AhR expression and treatment with 2.5 µM DOX for 4 or 24 h (n = 4 for all groups). *P < 0.05 compared with scrambled siRNA.
Figure 5.
Pharmacological inhibition of AhR in ARVM blocks both the nuclear translocation of AhR and the increase in CYP1A1 and GSTA1 in response to DOX. (A) Effects of CH-223191 on the translocation of AhR to the nucleus following treatment with 2.5 µM DOX for 4 or 24 h (n = 4 for all groups). (B) Quantitative PCR analysis of the effects of AhR inhibition by CH-223191 on induction of CYP1A1 and GSTA1 expression by treatment with 2.5 µM DOX for 4 or 24 h (B, n = 4 for all groups). *P < 0.05 compared with control value, †P < 0.05 compared with dimethyl sulfoxide group.
Loss of AhR in vivo altered basal and DOX-induced changes in the expression of AhR-responsive genes. Specifically, in AhR−/− mice, CYP1A1 could not be detected on either the RNA or protein level under control conditions (Supplementary material online, Figure S4A), which was in keeping with the effects of AhR knockdown in H9C2 cells with AhR siRNA. Furthermore, in contrast to wild-type animals, treatment with 10 mg/kg of DOX did not increase the expression of CYP1A1 to a detectable level (data not shown). While GSTA1 expression was detectable in AhR null hearts, there was no change in the level of expression of GSTA1 following treatment with DOX (Supplementary material online, Figure S4B).
3.2. Effects of loss of AhR on the cardiac response to DOX
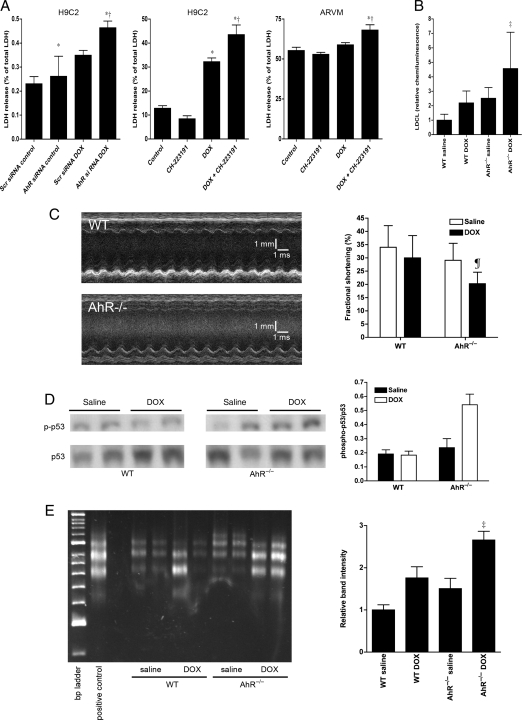
We determined whether AhR modulates the cardiotoxicity caused by DOX. Knockdown of AhR with AhR siRNA increased LDH release in H9C2 cells following DOX treatment (Figure 6A). H9C2 and ARVM were then pretreated with vehicle or CH-223191 and then exposed to 2.5 µM DOX. Under basal conditions, there was greater release of LDH from the ARVM compared with H9C2 cells that is most likely related to the relative fragility of isolated primary ventricular myocyte cultures. Pretreatment with CH-223191 decreased cell viability after 24 h of incubation with DOX (Figure 6A). There was no difference in cell survival between CH-223191- and vehicle-treated cells incubated without DOX. These findings suggest that AhR is important in protecting against DOX-induced cardiotoxicity.
Figure 6.
Loss of AhR function increases DOX toxicity in vitro and in vivo. (A) Effects of AhR knockdown in H9C2 cells and AhR inhibition by CH-223191 on DOX-induced cell necrosis in H9C2 and ARVM cells based on lactate dehydrogenase (LDH) release (n = 4 for all groups). (B) Effects of in vivo treatment with 10 mg/kg DOX on myocyte oxidant stress based on lucigenin-derived chemiluminescence (LDCL) in wild-type (WT) and AhR−/− mice (n = 5 for all groups). (C) Representative m-mode echocardiograms from WT and AhR−/− mice 2 weeks after treatment with 10 mg/kg DOX and changes in left ventricular function 2 weeks after treatment with 10 mg/kg DOX as assessed by the fractional shortening (n = 5 for all groups). (D) Representative blot (left) and analysis (right) of phosphorylation of serine 46 of p53 in WT and AhR−/− mice 2 weeks after treatment with DOX (n = 5 for all groups). (E) Representative DNA laddering assay and analysis of band intensity for WT and AhR−/− mice 2 weeks after treatment with either saline or DOX (n = 5 for all groups). *P < 0.05 compared with control treatment conditions, †P < 0.05 compared with DOX without AhR inhibition, ‡P < 0.01 compared with saline, ¶P < 0.05 compared with saline.
To determine whether AhR is important to in vivo cardiac responses to DOX, wild-type and AhR−/− mice were injected with either 10 mg/kg of DOX or saline. This dose of DOX has previously been shown to have no effect on left ventricular systolic function in wild-type mice, but causes a decrease in fractional shortening in transgenic mice strains that are susceptible to cardiac injury.22 Treatment of AhR−/− mice with DOX increased the production of ROS in the heart compared with saline treatment (Figure 6B). At baseline, wild-type and AhR−/− mice had similar echocardiographic measurements (Supplementary material online, Table S2). As expected, this dose DOX treatment had no effect on fractional shortening in wild-type mice. In contrast, AhR−/− mice had a significant decrease in fractional shortening 2 weeks after treatment with DOX (Figure 6C). These data indicate that loss of AhR enhances the sensitivity of the heart to DOX toxicity.
The left ventricular dysfunction observed in AhR−/− mice 2 weeks after treatment with DOX was associated with increased phosphorylation of p53 at serine 46, a known signalling event in DOX-induced cardiomyocyte apoptosis (Figure 6D).7–9,23,24 Treatment of AhR−/− mice with DOX also resulted in caspase 3 cleavage to the activated form (Supplementary material online, Figure S5). Furthermore, there was increased DNA fragmentation in DOX-treated AhR−/− hearts (Figure 6E). Taken together, these findings indicate that loss of AhR is associated with enhanced cardiac myocyte apoptosis following DOX treatment.
4. Discussion
This is the first study to demonstrate that DOX activates AhR, resulting in nuclear translocation of AhR, dimerization with ARNT1 and increased expression of AhR-regulated genes. Furthermore, we demonstrate that decreasing AhR activity increases the cardiotoxicity of DOX. This toxicity is related to free radical formation caused by DOX metabolism. Specifically, reduction in DOX by NADH dehydrogenase in mitochondrial respiratory complex I forms a semiquinone radical that reacts with molecular oxygen to form superoxide radical.25 Subsequently, redox cycling produces hydrogen peroxide and the hydroxyl radical.26 In addition, formation of DOX–iron complexes catalyse a Fenton reaction resulting in ROS generation.27,28 Prior studies have demonstrated that interventions that decrease ROS production attenuate the detrimental effects of DOX in the heart.29–31 In the present study, we found that AhR−/− hearts had increased production of ROS following treatment with DOX.
Based on the polycyclic aromatic structure of DOX, which is found in other known activators of AhR, we hypothesized that DOX can activate AhR. AhR is a bHLH transcription factor involved in the metabolism of xenobiotic agents. While the ability of other members of the bHLH family to modulate transcriptional activity is regulated by their degree of expression or degradation (e.g. HIF-1α), AhR is the only bHLH transcriptional factor that is activated by a ligand.32 AhR is activated by a variety of ligands, including chemical agents (TCDD, benzo-a-pyrene, omeprazole, and thiabendazole), naturally occurring dietary substances (indole-3-carbinole and curcumin) and endogenous ligands (bilirubin and biliverdin) that have varying affinities to the receptor and are generally characterized by the presence of two or more aromatic rings.33,34 DOX appears to be a much less potent activator of AhR than TCDD, although maximal translocation of AhR occurred at concentrations of DOX similar to the serum concentrations found in patients receiving chemotherapy.19
The activation of AhR results in the upregulation of expression of both phase I (e.g. CYP1A1, CYP1A2, and CYP1B1) and phase II (e.g. GSTA1 and NQO1) drug-metabolizing genes. The former gene products are involved in substrate oxidation and generally increase ROS production while the latter are responsible for metabolite conjugation and generally exhibit antioxidant properties. Furthermore, the relative degree of AhR-mediated induction of phase I vs. phase II drug-metabolizing genes varies in different tissues.35
The effect of AhR-mediated upregulation of phase I and phase II drug-metabolizing enzymes in response to xenobiotic agents can either result in the inactivation of those compounds or the conversion into carcinogenic or teratogenic compounds. Loss of AhR in mice blocks the toxic effects of TCDD.16 Furthermore, treatment with CH-223191 prevents TCDD-induced liver toxicity.36 In contrast, expression of a constitutively active form of AhR causes histological lesions in the liver, kidney, lung, heart, and thymus that are characteristically seen with TCDD exposure.37 In addition to the translocation of AhR to the nucleus, binding to ARNT1 is required for gene induction and the ensuing target organ toxicities.17 The metabolism of TCDD and benzo[a]pyrene produces derivatives that covalently bind to DNA and proteins and increase the risk of cancer.38 In contrast to the detrimental effects of the metabolism of these agents by AhR, the present study demonstrates that AhR plays a cardioprotective role against DOX-induced toxicity, which was associated with reduced oxidative stress. Interestingly, another AhR ligand, curcumin, has been shown to attenuate the cardiotoxic effects of DOX,39 which supports a cardioprotective function for AhR.
While the primary role of AhR activation is to increase the expression of genes involved in xenobiotic metabolism, little is known about the specific role of AhR in the heart. Acute and chronic treatment with TCDD causes contractile impairment and overt cardiomyopathy.40,41 Interestingly, the left ventricular expression of AhR increases in response to heart failure,42 although it is not known if this is an adaptive or maladaptive response. Furthermore, several studies have demonstrated that endothelial AhR is activated in response to hyperglycaemia and changes in shear stress,43,44 and loss of AhR decreases endothelial vascular endothelial growth factor expression and impairs angiogenesis.45
With respect to the downstream targets of AhR activation, prior studies have demonstrated increased cardiac expression of both phase I and phase II drug-metabolizing genes in response to TCDD and other activators of AhR.46 While DOX has been shown to induce numerous cytochrome P450 isotypes in the heart47 as well as GSTA1,48 these prior studies did not determine the role of AhR in the upregulation of these genes. In the present studies, we were able to demonstrate nuclear translocation of AhR, association of AhR with ARNT1, AhR binding an XRE-response element, and increased expression of the XRE-containing genes, CYP1A1 and GSTA1, in response to DOX. Overexpression of GST decreases oxidative stress and apoptosis in H69 small cell lung cancer cells and H9C2 cells exposed to DOX.49 Furthermore, pharmacological upregulation of GSTA1 expression is associated with decreased cardiomyocyte death.50 Blocking DOX-mediated AhR activation by CH-223191 inhibited the increase in CYP1A1 and GSTA1 in vitro. Furthermore, loss of AhR in vivo also inhibited the increase in CYP1A1 and GSTA1 expression induced by DOX. Interestingly, we detected little to no basal CYP1A1 expression either in H9C2 cells treated with AhR siRNA or in the hearts of AhR null mice. This has also been described for mammary glands and liver of AhR null mice.51,52
The present studies demonstrate a direct effect of DOX on the upregulation of AhR-regulated genes, which mediates, at least in part, cardioprotection against DOX toxicity. Other transcriptional factors, including STAT3, ATF3, and GATA4, have been shown to impact the toxicity of DOX in cardiomyocytes.22,53,54 While the exact mechanisms responsible for the cardioprotection afforded by these transcriptional factors is not known, it has been suggested that decreased apoptosis due to reduced p53 expression may be responsible.53
In conclusion, this is the first demonstration that AhR is directly involved in the response of cardiomyocytes to DOX and specifically that AhR is cardioprotective against the effects of DOX. Loss of AhR is associated with increased production of ROS, p53 activation, and apoptosis in the heart in response to DOX. This is in contrast to the generally deleterious effects of AhR activation by other xenobiotic agents in the cardiovascular system. Given the presence of polymorphisms in the AhR gene that have been shown to impact human diseases,55,56 it is important to further our understanding of the role of AhR in modulating the toxicity of DOX therapy.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by a grant from the National Heart, Blood and Lung Institute at the National Institutes of Health to RRR (R01 HL077310).
References
- 1.Von Hoff DD, Layard MW, Basa P, Davis HL, Jr, Von Hoff AL, Rozencweig M, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Khalaf MM, Juneja V, Chung GG, DiGiovanna MP, Sipples R, McGurk M, et al. Long-term assessment of cardiac function after dose-dense and -intense sequential doxorubicin (A), paclitaxel (T), and cyclophosphamide (C) as adjuvant therapy for high risk breast cancer. Breast Cancer Res Treat. 2007;104:341–349. doi: 10.1007/s10549-006-9413-7. doi:10.1007/s10549-006-9413-7. [DOI] [PubMed] [Google Scholar]
- 3.Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. doi:10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 4.Dowd NP, Scully M, Adderley SR, Cunningham AJ, Fitzgerald DJ. Inhibition of cyclooxygenase-2 aggravates doxorubicin-mediated cardiac injury in vivo. J Clin Invest. 2001;108:585–590. doi: 10.1172/JCI11334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotamraju S, Kalivendi SV, Konorev E, Chitambar CR, Joseph J, Kalyanaraman B. Oxidant-induced iron signaling in Doxorubicin-mediated apoptosis. Methods Enzymol. 2004;378:362–382. doi: 10.1016/S0076-6879(04)78026-X. doi:10.1016/S0076-6879(04)78026-X. [DOI] [PubMed] [Google Scholar]
- 6.Kotamraju S, Konorev EA, Joseph J, Kalyanaraman B. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen. Role of reactive oxygen and nitrogen species. J Biol Chem. 2000;275:33585–33592. doi: 10.1074/jbc.M003890200. doi:10.1074/jbc.M003890200. [DOI] [PubMed] [Google Scholar]
- 7.Shizukuda Y, Matoba S, Mian OY, Nguyen T, Hwang PM. Targeted disruption of p53 attenuates doxorubicin-induced cardiac toxicity in mice. Mol Cell Biochem. 2005;273:25–32. doi: 10.1007/s11010-005-5905-8. doi:10.1007/s11010-005-5905-8. [DOI] [PubMed] [Google Scholar]
- 8.Yeh PY, Chuang SE, Yeh KH, Song YC, Chang LL, Cheng AL. Phosphorylation of p53 on Thr55 by ERK2 is necessary for doxorubicin-induced p53 activation and cell death. Oncogene. 2004;23:3580–3588. doi: 10.1038/sj.onc.1207426. doi:10.1038/sj.onc.1207426. [DOI] [PubMed] [Google Scholar]
- 9.Chua CC, Liu X, Gao J, Hamdy RC, Chua BH. Multiple actions of pifithrin-alpha on doxorubicin-induced apoptosis in rat myoblastic H9c2 cells. Am J Physiol Heart Circ Physiol. 2006;290:H2606–2613. doi: 10.1152/ajpheart.01138.2005. doi:10.1152/ajpheart.01138.2005. [DOI] [PubMed] [Google Scholar]
- 10.Wu W, Lee WL, Wu YY, Chen D, Liu TJ, Jang A, et al. Expression of constitutively active phosphatidylinositol 3-kinase inhibits activation of caspase 3 and apoptosis of cardiac muscle cells. J Biol Chem. 2000;275:40113–40119. doi: 10.1074/jbc.M004108200. doi:10.1074/jbc.M004108200. [DOI] [PubMed] [Google Scholar]
- 11.Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. 2002;62:4592–4598. [PubMed] [Google Scholar]
- 12.Wang GW, Klein JB, Kang YJ. Metallothionein inhibits doxorubicin-induced mitochondrial cytochrome c release and caspase-3 activation in cardiomyocytes. J Pharmacol Exp Ther. 2001;298:461–468. [PubMed] [Google Scholar]
- 13.Sardao VA, Oliveira PJ, Holy J, Oliveira CR, Wallace KB. Doxorubicin-induced mitochondrial dysfunction is secondary to nuclear p53 activation in H9c2 cardiomyoblasts. Cancer Chemother Pharmacol. 2009;64:811–827. doi: 10.1007/s00280-009-0932-x. doi:10.1007/s00280-009-0932-x. [DOI] [PubMed] [Google Scholar]
- 14.Ikuta T, Eguchi H, Tachibana T, Yoneda Y, Kawajiri K. Nuclear localization and export signals of the human aryl hydrocarbon receptor. J Biol Chem. 1998;273:2895–2904. doi: 10.1074/jbc.273.5.2895. doi:10.1074/jbc.273.5.2895. [DOI] [PubMed] [Google Scholar]
- 15.Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol. 2000;59:65–85. doi: 10.1016/s0006-2952(99)00310-x. doi:10.1016/S0006-2952(99)00310-X. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140:173–179. doi: 10.1006/taap.1996.0210. doi:10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- 17.Tomita S, Jiang HB, Ueno T, Takagi S, Tohi K, Maekawa S, et al. T cell-specific disruption of arylhydrocarbon receptor nuclear translocator (Arnt) gene causes resistance to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced thymic involution. J Immunol. 2003;171:4113–4120. doi: 10.4049/jimmunol.171.8.4113. [DOI] [PubMed] [Google Scholar]
- 18.Fujii-Kuriyama Y, Imataka H, Sogawa K, Yasumoto K, Kikuchi Y. Regulation of CYP1A1 expression. Faseb J. 1992;6:706–710. doi: 10.1096/fasebj.6.2.1537460. [DOI] [PubMed] [Google Scholar]
- 19.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. doi:10.1016/S0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 20.Gramatzki D, Pantazis G, Schittenhelm J, Tabatabai G, Kohle C, Wick W, et al. Aryl hydrocarbon receptor inhibition downregulates the TGF-beta/Smad pathway in human glioblastoma cells. Oncogene. 2009;28:2593–2605. doi: 10.1038/onc.2009.104. doi:10.1038/onc.2009.104. [DOI] [PubMed] [Google Scholar]
- 21.Zhao B, Degroot DE, Hayashi A, He G, Denison MS. CH223191 is a ligand-selective antagonist of the Ah (Dioxin) receptor. Toxicol Sci. 2010;117:393–403. doi: 10.1093/toxsci/kfq217. doi:10.1093/toxsci/kfq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacoby JJ, Kalinowski A, Liu MG, Zhang SS, Gao Q, Chai GX, et al. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc Natl Acad Sci USA. 2003;100:12929–12934. doi: 10.1073/pnas.2134694100. doi:10.1073/pnas.2134694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Chua CC, Gao J, Chen Z, Landy CL, Hamdy R, et al. Pifithrin-alpha protects against doxorubicin-induced apoptosis and acute cardiotoxicity in mice. Am J Physiol Heart Circ Physiol. 2004;286:H933–939. doi: 10.1152/ajpheart.00759.2003. doi:10.1152/ajpheart.00759.2003. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Mao W, Ding B, Liang C-S. ERKs/p53 signal transduction pathway is involved in doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;295:H1956–1965. doi: 10.1152/ajpheart.00407.2008. doi:10.1152/ajpheart.00407.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies KJ, Doroshow JH. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem. 1986;261:3060–3067. [PubMed] [Google Scholar]
- 26.Doroshow JH, Davies KJ. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem. 1986;261:3068–3074. [PubMed] [Google Scholar]
- 27.Kotamraju S, Chitambar CR, Kalivendi SV, Joseph J, Kalyanaraman B. Transferrin receptor-dependent iron uptake is responsible for doxorubicin-mediated apoptosis in endothelial cells: role of oxidant-induced iron signaling in apoptosis. J Biol Chem. 2002;277:17179–17187. doi: 10.1074/jbc.M111604200. doi:10.1074/jbc.M111604200. [DOI] [PubMed] [Google Scholar]
- 28.Minotti G, Recalcati S, Menna P, Salvatorelli E, Corna G, Cairo G. Doxorubicin cardiotoxicity and the control of iron metabolism: quinone-dependent and independent mechanisms. Methods Enzymol. 2004;378:340–361. doi: 10.1016/S0076-6879(04)78025-8. doi:10.1016/S0076-6879(04)78025-8. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong SC. Anti-oxidants and apoptosis: attenuation of doxorubicin induced cardiomyopathy by carvedilol. J Mol Cell Cardiol. 2004;37:817–821. doi: 10.1016/j.yjmcc.2004.07.001. doi:10.1016/j.yjmcc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Cole MP, Chaiswing L, Oberley TD, Edelmann SE, Piascik MT, Lin SM, et al. The protective roles of nitric oxide and superoxide dismutase in adriamycin-induced cardiotoxicity. Cardiovasc Res. 2006;69:186–197. doi: 10.1016/j.cardiores.2005.07.012. doi:10.1016/j.cardiores.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Neilan TG, Blake SL, Ichinose F, Raher MJ, Buys ES, Jassal DS, et al. Disruption of nitric oxide synthase 3 protects against the cardiac injury, dysfunction, and mortality induced by doxorubicin. Circulation. 2007;116:506–514. doi: 10.1161/CIRCULATIONAHA.106.652339. doi:10.1161/CIRCULATIONAHA.106.652339. [DOI] [PubMed] [Google Scholar]
- 32.Dolwick KM, Swanson HI, Bradfield CA. In vitro analysis of Ah receptor domains involved in ligand-activated DNA recognition. Proc Natl Acad Sci USA. 1993;90:8566–8570. doi: 10.1073/pnas.90.18.8566. doi:10.1073/pnas.90.18.8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casado S, Alonso M, Herradon B, Tarazona JV, Navas J. Activation of the aryl hydrocarbon receptor by carbaryl: Computational evidence of the ability of carbaryl to assume a planar conformation. Environ Toxicol Chem. 2006;25:3141–3147. doi: 10.1897/06-131r.1. doi:10.1897/06-131R.1. [DOI] [PubMed] [Google Scholar]
- 34.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. doi:10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 35.Kanzawa N, Kondo M, Okushima T, Yamaguchi M, Temmei Y, Honda M, et al. Biochemical and molecular biological analysis of different responses to 2,3,7,8-tetrachlorodibenzo-p-dioxin in chick embryo heart and liver. Arch Biochem Biophys. 2004;427:58–67. doi: 10.1016/j.abb.2004.04.021. doi:10.1016/j.abb.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 36.Kim SH, Henry EC, Kim DK, Kim YH, Shin KJ, Han MS, et al. Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol. 2006;69:1871–1878. doi: 10.1124/mol.105.021832. doi:10.1124/mol.105.021832. [DOI] [PubMed] [Google Scholar]
- 37.Brunnberg S, Andersson P, Lindstam M, Paulson I, Poellinger L, Hanberg A. The constitutively active Ah receptor (CA-Ahr) mouse as a potential model for dioxin exposure–effects in vital organs. Toxicology. 2006;224:191–201. doi: 10.1016/j.tox.2006.04.045. doi:10.1016/j.tox.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 38.Nerurkar PV, Schut HA, Anderson LM, Riggs CW, Fornwald LW, Davis CD, et al. Ahr locus phenotype in congenic mice influences hepatic and pulmonary DNA adduct levels of 2-amino-3-methylimidazo[4,5-f]quinoline in the absence of cytochrome P450 induction. Mol Pharmacol. 1996;49:874–881. [PubMed] [Google Scholar]
- 39.Venkatesan N. Curcumin attenuation of acute adriamycin myocardial toxicity in rats. Br J Pharmacol. 1998;124:425–427. doi: 10.1038/sj.bjp.0701877. doi:10.1038/sj.bjp.0701877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jokinen MP, Walker NJ, Brix AE, Sells DM, Haseman JK, Nyska A. Increase in cardiovascular pathology in female Sprague-Dawley rats following chronic treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin and 3,3′,4,4′,5-pentachlorobiphenyl. Cardiovasc Toxicol. 2003;3:299–310. doi: 10.1385/CT:3:4:299. doi:10.1385/CT:3:4:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brewster DW, Matsumura F, Akera T. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on guinea pig heart muscle. Toxicol Appl Pharmacol. 1987;89:408–417. doi: 10.1016/0041-008x(87)90160-8. doi:10.1016/0041-008X(87)90160-8. [DOI] [PubMed] [Google Scholar]
- 42.Mehrabi MR, Steiner GE, Dellinger C, Kofler A, Schaufler K, Tamaddon F, et al. The arylhydrocarbon receptor (AhR), but not the AhR-nuclear translocator (ARNT), is increased in hearts of patients with cardiomyopathy. Virchows Arch. 2002;441:481–489. doi: 10.1007/s00428-002-0659-0. doi:10.1007/s00428-002-0659-0. [DOI] [PubMed] [Google Scholar]
- 43.Dabir P, Marinic TE, Krukovets I, Stenina OI. Aryl hydrocarbon receptor is activated by glucose and regulates the thrombospondin-1 gene promoter in endothelial cells. Circ Res. 2008;102:1558–1565. doi: 10.1161/CIRCRESAHA.108.176990. doi:10.1161/CIRCRESAHA.108.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conway DE, Sakurai Y, Weiss D, Vega JD, Taylor WR, Jo H, et al. Expression of CYP1A1 and CYP1B1 in human endothelial cells: regulation by fluid shear stress. Cardiovasc Res. 2009;81:669–677. doi: 10.1093/cvr/cvn360. doi:10.1093/cvr/cvn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roman AC, Carvajal-Gonzalez JM, Rico-Leo EM, Fernandez-Salguero PM. Dioxin receptor deficiency impairs angiogenesis by a mechanism involving VEGF-A depletion in the endothelium and transforming growth factor-beta overexpression in the stroma. J Biol Chem. 2009;284:25135–25148. doi: 10.1074/jbc.M109.013292. doi:10.1074/jbc.M109.013292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korashy HM, El-Kadi AO. The role of aryl hydrocarbon receptor in the pathogenesis of cardiovascular diseases. Drug Metab Rev. 2006;38:411–450. doi: 10.1080/03602530600632063. doi:10.1080/03602530600632063. [DOI] [PubMed] [Google Scholar]
- 47.Zordoky BN, Anwar-Mohamed A, Aboutabl ME, El-Kadi AO. Acute doxorubicin cardiotoxicity alters cardiac cytochrome P450 expression and arachidonic acid metabolism in rats. Toxicol Appl Pharmacol. 2010;242:38–46. doi: 10.1016/j.taap.2009.09.012. doi:10.1016/j.taap.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Paranka NS, Dorr RT. Effect of doxorubicin on glutathione and glutathione-dependent enzymes in cultured rat heart cells. Anticancer Res. 1994;14:2047–2052. [PubMed] [Google Scholar]
- 49.L'Ecuyer T, Allebban Z, Thomas R, Vander Heide R. Glutathione S-transferase overexpression protects against anthracycline-induced H9C2 cell death. Am J Physiol Heart Circ Physiol. 2004;286:H2057–H2064. doi: 10.1152/ajpheart.00778.2003. doi:10.1152/ajpheart.00778.2003. [DOI] [PubMed] [Google Scholar]
- 50.Zhu H, Jia Z, Zhou K, Misra HP, Santo A, Gabrielson KL, et al. Cruciferous dithiolethione-mediated coordinated induction of total cellular and mitochondrial antioxidants and phase 2 enzymes in human primary cardiomyocytes: cytoprotection against oxidative/electrophilic stress and doxorubicin toxicity. Exp Biol Med (Maywood) 2009;234:418–429. doi: 10.3181/0811-RM-340. doi:10.3181/0811-RM-340. [DOI] [PubMed] [Google Scholar]
- 51.Zaher H, Yang TJ, Gelboin HV, Fernandez-Salguero P, Gonzalez FJ. Effect of phenobarbital on hepatic CYP1A1 and CYP1A2 in the Ahr-null mouse. Biochem Pharmacol. 1998;55:235–238. doi: 10.1016/s0006-2952(97)00476-0. doi:10.1016/S0006-2952(97)00476-0. [DOI] [PubMed] [Google Scholar]
- 52.Galvan N, Jaskula-Sztul R, MacWilliams PS, Czuprynski CJ, Jefcoate CR. Bone marrow cytotoxicity of benzo[a]pyrene is dependent on CYP1B1 but is diminished by Ah receptor-mediated induction of CYP1A1 in liver. Toxicol Appl Pharmacol. 2003;193:84–96. doi: 10.1016/s0041-008x(03)00338-7. doi:10.1016/S0041-008X(03)00338-7. [DOI] [PubMed] [Google Scholar]
- 53.Nobori K, Ito H, Tamamori-Adachi M, Adachi S, Ono Y, Kawauchi J, et al. ATF3 inhibits doxorubicin-induced apoptosis in cardiac myocytes: a novel cardioprotective role of ATF3. J Mol Cell Cardiol. 2002;34:1387–1397. doi: 10.1006/jmcc.2002.2091. doi:10.1006/jmcc.2002.2091. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi S, Volden P, Timm D, Mao K, Xu X, Liang Q. Transcription factor GATA4 inhibits doxorubicin-induced autophagy and cardiomyocyte death. J Biol Chem. 2010;285:793–804. doi: 10.1074/jbc.M109.070037. doi:10.1074/jbc.M109.070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsuchiya M, Katoh T, Motoyama H, Sasaki H, Tsugane S, Ikenoue T. Analysis of the AhR, ARNT, and AhRR gene polymorphisms: genetic contribution to endometriosis susceptibility and severity. Fertil Steril. 2005;84:454–458. doi: 10.1016/j.fertnstert.2005.01.130. doi:10.1016/j.fertnstert.2005.01.130. [DOI] [PubMed] [Google Scholar]
- 56.Kim JH, Kim H, Lee KY, Kang JW, Lee KH, Park SY, et al. Aryl hydrocarbon receptor gene polymorphisms affect lung cancer risk. Lung Cancer. 2007;56:9–15. doi: 10.1016/j.lungcan.2006.11.010. doi:10.1016/j.lungcan.2006.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.