Abstract
In the advanced stages of heart failure, many key enzymes involved in myocardial energy substrate metabolism display various degrees of down-regulation. The net effect of the altered metabolic phenotype consists of reduced cardiac fatty oxidation, increased glycolysis and glucose oxidation, and rigidity of the metabolic response to changes in workload. Is this metabolic shift an adaptive mechanism that protects the heart or a maladaptive process that accelerates structural and functional derangement? The question remains open; however, the metabolic remodelling of the failing heart has induced a number of investigators to test the hypothesis that pharmacological modulation of myocardial substrate utilization might prove therapeutically advantageous. The present review addresses the effects of indirect and direct modulators of fatty acid (FA) oxidation, which are the best pharmacological agents available to date for ‘metabolic therapy’ of failing hearts. Evidence for the efficacy of therapeutic strategies based on modulators of FA metabolism is mixed, pointing to the possibility that the molecular/biochemical alterations induced by these pharmacological agents are more complex than originally thought. Much remains to be understood; however, the beneficial effects of molecules such as perhexiline and trimetazidine in small clinical trials indicate that this promising therapeutic strategy is worthy of further pursuit.
Keywords: Heart failure, β-Oxidation, Fatty acid, Modulators, Therapy
1. Introduction
A long-known feature of the failing heart phenotype is the profoundly altered energy metabolism.1 ATP concentration is ∼30% lower in failing compared with normal human myocardium2 and its flux through creatine kinase is also reduced.3 Such depletion of chemical energy stores is associated with marked changes in substrate utilization.4 Cardiac muscle satisfies its high-energy requirements by oxidizing fatty acids (FAs) and carbohydrates and, to a lesser extent, amino acids. Despite some inconsistent reports, there is growing consensus that the failing heart looses its metabolic flexibility and relies more on glucose as its preferential substrate.4 Is this metabolic shift an adaptive mechanism that protects the heart or a maladaptive process that accelerates cardiac pathology? The question remains open; however, the metabolic remodelling of the failing heart has induced a number of investigators to test the hypothesis that pharmacological modulation of myocardial substrate utilization might prove therapeutically advantageous. The present review will go over data that do or do not support the efficacy of therapeutic strategies for heart failure (HF) based on modulators of FA metabolism. For more detailed descriptions of the cardiac metabolic pathways and of their fine regulation, we refer the reader to the numerous reviews on this topic.4–9
2. Metabolic changes in the failing myocardium
2.1. Regulation of myocardial FA oxidation
The healthy cardiac muscle can oxidize various energy substrates, although under post-absorptive conditions, it preferentially utilizes FA10 (Figure 1). Myocardial FA oxidation (FAO) is a complex process that provides almost 70% of cardiac ATP in the fasting state, whereas the remaining portion yields mostly from the oxidation of the competing substrates lactate, glucose, and pyruvate.4,10 FAO is less energy efficient than glucose oxidation, theoretically requiring 11–12% more oxygen for a given amount of ATP produced.4 The amount of ATP synthesized per mole of oxygen consumed is dependent on the coupling of oxygen consumption to ATP production in the mitochondria. Classical studies in humans and dogs show that very high plasma levels of free FA have a profound oxygen wasting effect on the myocardium, resulting in a fall in the rate of left ventricular (LV) power by up to 30% relative to the rate of myocardial oxygen consumption.4,11,12 The mechanism for this effect is not clear, but may involve mitochondrial uncoupling and stimulation of futile substrate cycles that waste ATP.4 The normo-perfused, healthy heart readily extracts and oxidizes circulating FA in proportion to their arterial concentration.4,5,13 Once in the cytosol, FA are first converted into long-chain acyl-CoA esters by fatty acyl-CoA synthase: 75% of them are transferred into mitochondria through the carnitine palmitoyltransferase type 1 (CPT)-1/carnitine system and immediately oxidized,5,13 whereas the remaining are stored into the triglyceride pool for later oxidation. FAO requires the conversion of acyl-CoA into acyl-carnitine14 by CPT-1, a key limiting step enzyme whose activity is inhibited by malonyl-CoA. This latter is formed by acetyl-CoA carboxylase (ACC) and degraded by malonyl-CoA decarboxylase (MCD).15 The activities of MCD and ACC, which are highly expressed in the myocardium, determine the cytosolic levels of malonyl-CoA and play a pivotal role in regulating FAO during physiological and pathological conditions, such as fasting, diabetes, and ischaemia.5
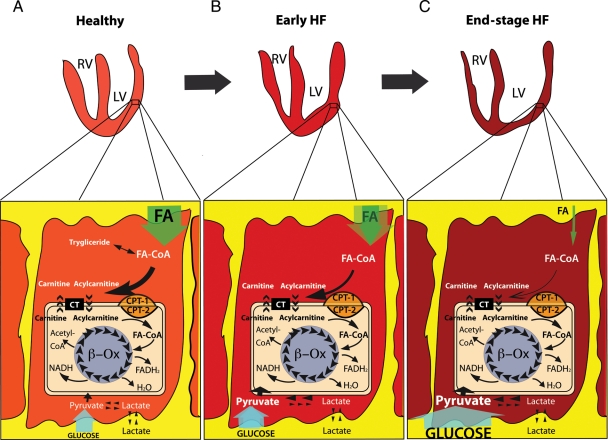
Figure 1.
Cardiomyocyte substrates utilization. (A) Healthy cardiomyocyte. Cardiomyocyte mainly uses FAs that enter into the cell and are converted in the mitochondria through the carnitine palmitoyltransferase type 1 and type 2 (CPT-1 and CPT-2), and the carnitine acylcarnitine translocase (CT) before being used by β-oxidation (β-Ox) to produce FADH2, H2O, NADH, and acetyl-CoA. Glucose and lactate enter into the cells and are transformed into pyruvate by glycolysis and lactate dehydrogenase, respectively. (B) Early HF cardiomyocyte. In early HF, FA utilization is blunted, mitochondrial FA β-Ox decreases, while uptake and metabolism of the competing substrate glucose increase. (C) End-stage HF cardiomyocyte. In overt HF, FA uptake and metabolism are significantly depressed; although, the glucose uptake and metabolism is significantly enhanced. RV, right ventricle; LV, left ventricle; FA-CoA, fatty acyl-coenzyme A; FADH2, reduced form of flavine adenine dinucleotide; NADH, reduced form of nicotinamide adenine dinucleotide.
Long-chain acyl-CoA metabolism in the mitochondrial matrix occurs via the β-oxidation pathway involving the activity of acyl-CoA dehydrogenase, enoyl-CoA hydratase, l-3-hydroxyacyl-CoA dehydrogenase, and 3-ketoacyl-coenzyme A thiolase (3-KAT).5 Each cycle of FAO results in the production of acetyl-CoA, FADH2, and NADH, which can also modulate the activity of the above enzymes through an inhibitory feedback. An acute increase in workload of healthy hearts, for instance during exercise or β-adrenergic stimulation, increases myocardial FAO.16 Interestingly, the increase in FAO following an acute increase in workload does not depend on the activity of MCD-malonyl-CoA pathway.17,18
The transcriptional control of genes encoding for FAO enzymes is in large part mediated by peroxisome proliferator-activated receptor (PPAR)-α, -β, -δ, and -γ, the retinoid X receptor-α (RXRα) and the PPAR co-activator γ (PGC-1α).5,19 PPARs, RXRα, and PGC-1α form heterotrimers binding to responsive elements of various promoters, thus activating the transcription process. The rate of FAO is partially dependent on the expression level of PPAR-regulated genes that encode FAO enzymes.5
2.2. Myocardial FAO in the failing heart
A number of clinical studies have clearly documented a reduced utilization of FA and an increased utilization of glucose as energy substrates by the failing heart.20–23 When FAO was directly measured using radiolabelled tracers and normalized to myocardial oxygen consumption, it was found reduced by 70% in patients with dilated cardiomyopathy compared with control subjects, even during pacing stress.22 These results are consistent with previous ones showing a significant reduction in FAO in a dog model of severe non-ischaemic dilated cardiomyopathy.24–26 Various mechanisms have been proposed to explain the switch in substrate preference, from FA to carbohydrate, observed in the failing heart.4,27 One hypothesis is that the altered fuel selection reflects a reversal from the adult to the foetal metabolic phenotype, which may protect the failing heart from further irreversible structural and functional impairment.27 The foetal heart, endowed with fewer, immature mitochondria compared with the adult heart,28 has a limited ability to oxidize long-chain FA29,30; therefore lactate and glucose oxidation constitute the major sources of energy for ATP synthesis.31 During the transition from foetal to neonatal life, the heart is exposed to an increased haemodynamic load and oxygen tension which, in turn, drive its metabolic transformation. A reverse change would occur during advanced HF as a direct consequence of reduced expression of the FA-handling enzymes in response to changes in haemodynamic load, myocardial blood flow, and metabolic milieu. The exact sequence of events involved in this phenomenon is not yet well defined.
Other studies failed to observe abnormalities in cardiac FA uptake and utilization in HF.32,33 For instance, HF patients with elevated plasma FA displayed even an increased uptake of this substrate.34 Another study based on indirect measures of the rate of FAO from FA extraction and transmyocardial respiratory quotient showed an elevated arterial FA concentration and a higher oxidation rate in HF patients compared with controls, despite no differences in coronary blood flow or cardiac energy expenditure.33 In this regard, it is important to consider that HF patients have approximately a 20–50% increase in the circulating levels of FA.35,36 Moreover, dogs with moderately/severe HF showed normal myocardial FAO,37 which supports the hypothesis that FAO is not down-regulated during early HF, but it is significantly depressed in the more advanced stages (Figure 1).
Several studies documented a decreased expression and activity of enzymes involved in mitochondrial FAO in the failing heart. Myocardial expression of enzymes of the first and third step of FA β-oxidation was first found down-regulated in a study on patients with terminal HF and undergoing cardiac transplantation.38 A reduced expression and activity of the key enzyme CPT-1 was found in failing hearts of dogs25 and patients.39 Similar to patients, the expression of medium-chain acyl-CoA dehydrogenase, one of the enzymes of the FA β-oxidation, is down-regulated in the dog model of pacing-induced HF.25 In the light of data collected from several experimental models, it has become clear that the HF-induced mRNA down-regulation of enzymes involved in FAO is far more pronounced than the actual changes in enzymatic activity or protein levels.4,5,40,41
The mechanisms responsible for the down-regulation of FAO in the failing myocardium are not well defined, but appear to be in part the result of reduced transcriptional activation of genes regulated by PPARα/RXRα/PGC-1α heterotrimer. In fact, the transcriptional activation complex, when activated by long-chain FA, is able to bind specific responsive elements that regulate the expression of genes that encode enzymes involved in FAO.42 PPARα protein levels were found to be significantly decreased in the myocardium of human end-stage HF compared with control donor hearts,43 but unchanged in the myocardium of dogs with pacing-induced HF, despite a significant decrease in RXRα protein levels.25 PPARα protein levels were also unchanged in rodent models of HF.40,44,45 Although it is conceivable that impaired formation of the nuclear heterotrimer or the peroxisome responsive element's affinity for PPARα contributes to downregulate essential enzymes for FAO in the failing myocardium, this has not been clearly demonstrated. It is also noteworthy that the down-regulation of FAO genes in the failing human heart is consistent with lower levels of oestrogen-related receptor-α and PGC-1α.46,47 On the other hand, PGC-1α expression was found not significantly different from control in our canine model of HF.48
The decrease in FAO is accompanied by enhancement of glucose uptake and glycolysis in the failing heart, which can have both beneficial and toxic effects.49 Genetic strategies have been used to test whether enhancing glucose utilization can render hearts more tolerant to chronic injury. Transgenic mice with cardiac specific overexpression of glucose transporter type 1 (GLUT-1), which increases basal myocardial glucose uptake, are more resistant to HF progression compared with wild-type.50 This suggests that accelerating glucose metabolism in HF above what is induced by the normal switch in metabolism is beneficial. However, it has been noted that accelerated glucose uptake and metabolism can result in ‘cardiac glucotoxicity’ which is further exacerbated by elevated circulating FA.51 Studies in experimental models and in patients suggest that glucose utilization is enhanced in the failing heart mostly due to the down-regulation of the competing FAO rather than to an up-regulation of the oxidative glycolytic pathway.22,41 Enzymes of both carbohydrate and FA metabolism are down-regulated in the severely failing heart.51 In fact, during pacing stress, the failing heart produces more lactate compared with control, even in the absence of coronary pathology.22 Moreover, we found that the increase in myocardial glucose uptake in HF is associated with higher NADPH levels due to up-regulated glucose-6-phosphate dehydrogenase, a key enzyme of the oxidative pentose phosphate pathway, which can fuel myocardial superoxide generation by NADPH oxidase. We interpret these phenomena as the consequence of a limited capacity of the failing heart to channel the higher amount of extracted glucose into the glycolytic/oxidative pathway, with consequent re-direction towards the pentose phosphate pathway which, in turn, would worsen oxidative stress.52,53
Finally, in dogs with pacing-induced HF, we have tested the hypothesis that the recovery of cardiac substrate oxidation capacity might match with functional recovery, but we found that the basal FA and glucose oxidation is normalized at an early stage of post-failure recovery, when reverse morphological remodelling is not complete and contractile function is still partially impaired.26
3. Pharmacological modulation of myocardial FA metabolism
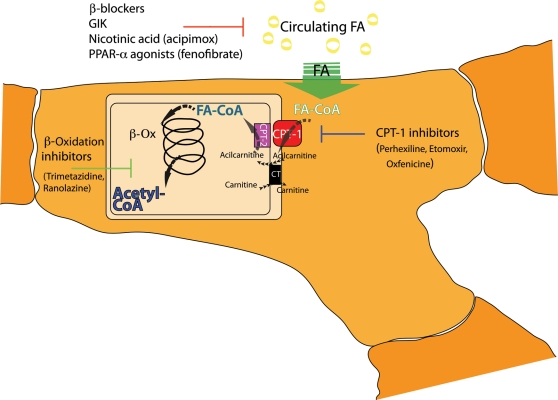
The results of the above-cited studies indicate that the primary goal of metabolic therapies for HF should be the maintenance of myocardial capacity for oxidative metabolism and the flexibility in substrate use. Several studies investigated the possibility of improving the function of the failing myocardium without affecting oxygen consumption and haemodynamics, using agents aimed at enhancing myocardial energy efficiency. Shifting energy substrate preference away from the use of FA towards glucose as an oxidative fuel is a promising therapeutic approach to better preserve or improve the mechanical function and limit the progression of HF.54 Broadly speaking, myocardial FA metabolism can be modulated by indirect and direct approaches. Indirect approaches are aimed at decreasing circulating FA levels, such as by the administration of glucose–insulin–potassium solutions,55 nicotinic acid or related analogues,56 or β-adrenergic blocking drugs.57 Direct approaches include the activation of PPARs, the inhibition of FA mitochondrial uptake via suppression of CPT-1, or the inhibition of 3-KAT, the last enzyme involved in β-oxidation58 (Figure 2). To date, the most effective metabolic modulation of β-oxidation in the failing myocardium is seen with pharmacological agents such as trimetazidine and perhexiline, which directly inhibit FAO and improve regional and global myocardial function.
Figure 2.
Targets for modulation of myocardial FA metabolism. Indirect modulators of FAO: β-blockers, glucose–insulin–potassium solution (GIK), nicotinic acid or related analogues (acipimox). Direct modulators of FAO: PPARα agonists, carnitine palmitoyltransferase type 1 (CPT-1) inhibitors, β-oxidation (β-Ox) inhibitors. FA-CoA, fatty acyl-coenzyme A; CPT-2, carnitine palmitoyltransferase type 2; CT, carnitine acylcarnitine translocase.
3.1. Indirect FAO modulators
The excessive mobilization of FA from adipose tissue and chronic elevation of their circulating levels in HF,36 at least in part induced by β-adrenergic overdrive, inhibits myocardial uptake of glucose, promotes the onset of insulin resistance,59 and can be pro-arrhythmic.57,60 Moreover, the excess of plasma FA can cause abnormalities of myocardial function, including the formation of reactive oxygen species61 and oxygen wastage.4,5 It has been suggested that a decreased capacity for FAO may result in the accumulation of long-chain fatty acyl intermediates and triglyceride, which would lead to the generation of toxic lipid metabolites and accelerate the progression of HF.62 Interestingly, the pathological role of triglyceride accumulation in cardiomyocytes has been recently challenged by a clinical study showing that myocardial lipid accumulation is not related to the severity of cardiac dysfunction in patients with HF of various etiologies.63 On the other hand, the toxic effects of long-chain fatty acyl metabolites are supported by more solid evidence.64 Therefore, an indirect therapeutic approach to modulate FAO in failing heart is to reduce the circulating levels of FA. The best-known pharmacological agents utilized for this strategy are nicotinic acid and its derivatives, which can decrease myocardial FAO through a progressive decrease in plasma levels of FA.56 Acute treatment with acipimox, a nicotinic acid derivative with profound anti-lipolytic effects, caused a decrease in myocardial FAO and enhanced glucose uptake in patients with dilated cardiomyopathy.65 Surprisingly, however, these metabolic effects were associated with a significant fall in cardiac work and efficiency. One possible explanation is the fact that although FAO is reduced in the failing heart, it still represents a critical source of energy and its further inhibition by an aggressive pharmacological treatment would necessarily cause a functional derangement. Unfortunately, this study did not include a control group or placebo employed. A very recent study in patients with ischaemic HF treated with either acipimox or placebo for 28 days demonstrated no beneficial effect on cardiac function, despite a significant decrease in plasma FA levels.66 Taken together, the available evidence suggests that FA lowering by suppression of lipolysis in adipose tissue does not improve cardiac function in HF.
Long-term therapy with β-adrenergic receptor antagonists (metoprolol and carvedilol) is known to improve cardiac performance and survival in patients with HF through an energy-sparing effect, in part due to a switch in myocardial substrate preference away from FAO towards carbohydrate oxidation.67–69 Little is known about the effects of β-adrenergic receptor activation in HF on myocardial substrate metabolism. Studies in the canine tachypacing model of HF found that β2-adrenergic receptor stimulation shifts substrate preference towards free fatty acids (FFA) oxidation associated with greater and deleterious myocardial oxygen requirement.70 Several studies showed that long-term therapy with β-adrenergic receptor antagonists decrease oxidative metabolism and improve myocardial performance in patients with HF, which is associated with a switch in myocardial substrate preference away from FAO towards carbohydrate oxidation.68,69,71,72 There are clear differences in the pharmacological effects and clinical efficacy among various β-adrenergic receptor antagonists, as seen in clinical studies showing that chronic administration of carvedilol rather than metoprolol increased the survival in HF patients.73 Studies in a canine HF model found a more pronounced shift in substrate preference from FFA to glucose, increased plasma insulin levels, and suppressed glucagon levels, leading to increased myocardial glucose uptake with carvedilol than metoprolol.74 At present, the precise effects of antagonism of β1- and β2-adrenergic receptors on substrate metabolism of failing cardiomyocytes in vivo are not clear, as the pharmacological effect of these drugs can profoundly alter heart rate, preload, afterload, and circulating substrates, and hormones.
3.2. Direct FAO modulators
3.2.1. PPAR agonists
A key question that remains open is whether the reduced FAO is an adaptive or a maladaptive process in the failing heart. If it is adaptive, then enhancing FAO should accelerate the progression of failure towards decompensation. We found that chronic pharmacological activation of PPARα with the agonist fenofibrate upregulates medium-chain acyl-CoA activity and expression and prevented the HF-induced reduction in myocardial FAO, but did not without affecting LV function or chamber volume in dogs with pacing-induced HF.75 These data are in agreement with another study performed by our group in rats with infarct-induced HF.44 Conversely, other authors tested fenofibrate treatment in pigs with pacing-induced HF and found increased expression of PPARα-regulated genes, prevention of LV hypertrophy, and delayed development of LV dilation and dysfunction; however, the effects on FAO were not assessed.76 Taken together, these findings support the important regulatory role of the PPAR/RXRα/PGC-1α heterodimeric complex on FAO, but leave open the question on whether the selective pharmacological modulation of FAO at the transcriptional level has a positive impact on the progression of HF.
3.2.2. CPT-1 inhibitors
Several findings suggest that direct inhibition of mitochondrial FA uptake is a helpful approach to increase glucose oxidation at the expense of myocardial FAO and to limit the progression of HF. Three CPT-1 inhibitors have been evaluated for this purpose: oxfenicine, etomoxir, and perhexiline. Oxfenicine is an effective inhibitor of cardiac CPT-1 and FAO that was initially developed for the treatment of chronic stable angina.77 Oxfenicine is not available for human use; however, we evaluated it in the canine tachypacing model of HF, comparing normal dogs with untreated and oxfenicine-treated dogs with HF.48 Oxfenicine extended the development of terminal failure and attenuated haemodynamic alterations and LV chamber dilation. Interestingly, oxfenicine also prevented the HF-induced transcriptional down-regulation of metabolic enzymes [CPT-1, medium-chain acyl-CoA dehydrogenase (MCAD), GAPDH, and citrate synthase]. These results were the first to show in a large animal experimental model that CPT-1 inhibition might be effective for slowing the progression of clinical HF.
Etomoxir is an irreversible inhibitor of CPT-1 that efficaciously inhibits myocardial FAO and causes reciprocal activation of pyruvate dehydrogenase and glucose oxidation.78,79 Rupp et al. showed that etomoxir prevented the initiation of pathological gene expression and development of HF in rats with pressure overload-induced cardiac hypertrophy.79 On the other hand, treatment with etomoxir failed to reverse contractile dysfunction in rats with established HF induced by chronic pressure overload.78 The initial open-label clinical trial in HF patients with etomoxir showed promising results80,81; however, the subsequent placebo-controlled trial was stopped due to hepatotoxicity.82 Nevertheless, this latter trial showed trends for improved cardiac function in treated patients82 and suggested that CPT-1 inhibition could be exerting a positive effect on the myocardium.
Perhexiline is a drug for the treatment of chronic stable angina that is used in Australia and some parts of Asia, but is not clinically available in the USA or Europe.77 It is more effective at inhibiting the cardiac isoform of CPT-1 than the liver isoform.83 Small placebo-controlled clinical studies report that perhexiline enhances the quality of life and increases the LV ejection fraction in patients with contractile dysfunction.84,85 Abozguia et al.85 demonstrated in symptomatic patients with hypertrophic cardiomyopathy that perhexiline increased the myocardial ratio of phosphocreatine to ATP as measured by nuclear magnetic resonance, consistent with a metabolic mechanism of action. Although data from these small clinical studies suggest that perhexiline should improve clinical outcome in HF patients, a large-scale pivotal trial has not be conducted in patients with HF or hypertrophic cardiomyopathy.
3.2.3. Inhibitors of FA β-oxidation
Direct partial inhibition of mitochondrial FA β-oxidation has been shown to be effective anti-ischaemic agents and has shown clinical efficacy in patients with chronic stable angina, as seen in improved exercise time to onset of symptoms and a decrease in the frequency in episodes of angina.77,5 Trimetazidine and ranolazine are anti-anginal drugs that have been shown to inhibit myocardial FAO in vitro and result in reciprocal activation of glucose oxidation,86–88 and both compounds inhibit myocardial FA uptake in humans.65,89 Trimetazidine is a partial inhibitor of the terminal enzyme in β-oxidation long-chain 3-ketoacyl thiolase.90 Few studies have investigated the effects of trimetazidine in animal models of HF. Studies in the rat infarct model of HF showed no benefit on LV function or chamber remodelling,91,92 but it prolonged survival in cardiomyopathic hamsters.93
Trimetazidine is widely used in Europe and Asia for treating chronic stable angina, where it improves exercise tolerance and decreases the frequency of anginal episodes with lowering blood pressure or heart rate like traditional drugs for this indication.94 Several small studies in HF patients evaluated the effects of trimetazidine on LV function and clinical indices of HF severity. In general, these studies have shown that trimetazidine is well tolerated in HF patients and has no direct effect on heart rate or blood pressure.65,95–104 The duration of treatment in these studies has been relatively short (3–6 months), and endpoints have focused on LV function. Results generally support either a trend or significant improvement in LV ejection fraction concomitant with a reduction in systolic and diastolic volumes. Exercise performance and LV wall motion during dobutamine stress has been found to improve relative to placebo treatment.95 In addition, plasma markers of HF severity improve with treatment (brain natriuretic peptide and inflammatory cytokines).99,103,104 All together, evidence from these short-term studies with surrogate endpoints of HF outcome suggest that long-term treatment with trimetazidine could improve hard clinical endpoints (i.e. survival and hospitalization). As with perhexiline, large controlled trials have not been conducted, at this point, there is no evidence that trimetazidine improved clinical outcomes; thus, it is not approved by regulatory agencies for the treatment of HF.
Ranolazine is an anti-anginal drug with a clinical pharmacology similar to trimetazidine.105 It is approved in the USA and some European countries for the treatment of chronic stable angina in patients who have not achieved an adequate response with other anti-anginal drugs.106 The mechanism responsible for the anti-anginal actions of ranolazine are not known; however, in vitro studies found that it inhibits FAO in skeletal muscle and increases pyruvate dehydrogenase activity and glucose oxidation in the myocardium. There have been few reports on the effects of ranolazine on human metabolism, although it has been shown to lower haemoglobin A1c in diabetic patients and to decrease cardiac FA uptake in humans. Ranolazine also inhibits the late Na+ current and prevent ventricular repolarization abnormalities.107 There are no reports on the effect of ranolazine on cardiac function or clinical outcome in HF patients. Studies with acute intravenous treatment in dogs with microembolization-induced chronic HF showed rapid improvement in LV function (greater ejection fraction, stroke volume, and cardiac output), despite no increase in myocardial oxygen consumption, and thus improve mechanical efficiency.108 There was no change in net myocardial extraction of glucose, lactate, or free FAs from the blood, suggesting that ranolazine was not acting through effects on myocardial metabolism. Beneficial effects were observed in LV systolic function and chamber size with 3 months of treatment with ranolazine compared with placebo.109 Although the current evidence suggests that ranolazine could be effective for treating HF, clinical studies have not been performed, and its use in HF might be limited because it increases serum digoxin levels and has effects of cardiac depolarization with potential rhythm disturbances. Importantly, the mechanism of action in HF does not appear to be through inhibition of myocardial FAO.108
4. Conclusions
We started the present review with the question on whether the altered energy substrate utilization characterizing the failing heart is an adaptive/protective mechanism or a maladaptive process that accelerates structural and functional derangement. After reviewing a number of studies on the pharmacological modulators of FAO, a new question arises, namely whether metabolic therapies should be really considered a valid option for the treatment of HF. It is too early to draw conclusions; however, it is undeniable that pharmacological agents such as perhexiline and trimetazidine proved surprisingly efficacious in small clinical trials. The real problem is perhaps that our interpretative paradigm based on the equation FAO inhibition = enhanced carbohydrate oxidation is too simplistic. Depending on the site of inhibition in the FAO pathway, different inhibitors may induce different metabolic changes, ranging from reduced accumulation of toxic intermediates of FA metabolism to re-channelling of glucose in the glycolytic oxidative pathway. Moreover, since cardiac metabolic alterations depend on the severity of HF, a fine modulation of β-oxidation should be finely matched to the stage of the disease. Finally, a new chapter might be opened in the next future: the potential use of metabolic modulators for the treatment of the diastolic HF with preserved systolic function, diagnosed in a growing portion of HF patients, particularly among the elderly and in women.110,111
Conflict of interest: none declared.
Funding
This work was supported by the NIH grant P01-HL-74237 (F.A.R. and W.C.S.). F.A.R. is an Established Investigator of the AHA.
References
- 1.Herrmann G, Decherd GM. The chemical nature of heart failure. Ann Intern Med. 1939;12:1233–1244. [Google Scholar]
- 2.Neubauer S. The failing heart—an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 3.Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci USA. 2005;102:808–813. doi: 10.1073/pnas.0408962102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 5.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 6.Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation. 2007;116:434–448. doi: 10.1161/CIRCULATIONAHA.107.702795. [DOI] [PubMed] [Google Scholar]
- 7.Madrazo JA, Kelly DP. The PPAR trio: regulators of myocardial energy metabolism in health and disease. J Mol Cell Cardiol. 2008;44:968–975. doi: 10.1016/j.yjmcc.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab. 2009;297:E578–E591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res. 2009;81:412–419. doi: 10.1093/cvr/cvn301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bing R. The metabolism of the heart. Harvey Lect. 1955;50:27–70. [PubMed] [Google Scholar]
- 11.Mjos OD. Effect of free fatty acids on myocardial function and oxygen consumption in intact dogs. J Clin Invest. 1971;50:1386–1389. doi: 10.1172/JCI106621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonsen S, Kjekshus JK. The effect of free fatty acids on myocardial oxygen consumption during atrial pacing and catecholamine infusion in man. Circulation. 1978;58:484–491. doi: 10.1161/01.cir.58.3.484. [DOI] [PubMed] [Google Scholar]
- 13.Wisneski JA, Gertz EW, Neese RA, Mayr M. Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. J Clin Invest. 1987;79:359–366. doi: 10.1172/JCI112820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 15.Dyck JR, Barr AJ, Barr RL, Kolattukudy PE, Lopaschuk GD. Characterization of cardiac malonyl-CoA decarboxylase and its putative role in regulating fatty acid oxidation. Am J Physiol Heart Circ Physiol. 1998;275:H2122–H2129. doi: 10.1152/ajpheart.1998.275.6.H2122. [DOI] [PubMed] [Google Scholar]
- 16.Zhou L, Huang H, Yuan CL, Keung W, Lopaschuk GD, Stanley WC. Metabolic response to an acute jump in cardiac workload: effects on malonyl-CoA, mechanical efficiency, and fatty acid oxidation. Am J Physiol Heart Circ Physiol. 2008;294:H954–H960. doi: 10.1152/ajpheart.00557.2007. [DOI] [PubMed] [Google Scholar]
- 17.Hall JL, Lopaschuk GD, Barr A, Bringas J, Pizzurro RD, Stanley WC. Increased cardiac fatty acid uptake with dobutamine infusion in swine is accompanied by a decrease in malonyl CoA levels. Cardiovasc Res. 1996;32:879–885. [PubMed] [Google Scholar]
- 18.King KL, Okere IC, Sharma N, Dyck JR, Reszko AE, Mc-Elfresh TA, et al. Regulation of cardiac malonyl-CoA content and fatty acid oxidation during increased cardiac power. Am J Physiol Heart Circ Physiol. 2005;289:H1033–H1037. doi: 10.1152/ajpheart.00210.2005. [DOI] [PubMed] [Google Scholar]
- 19.Finck BN, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation. 2007;115:2540–2548. doi: 10.1161/CIRCULATIONAHA.107.670588. [DOI] [PubMed] [Google Scholar]
- 20.Davila-Roman VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, et al. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:271–277. doi: 10.1016/s0735-1097(02)01967-8. [DOI] [PubMed] [Google Scholar]
- 21.Sochor H, Schelbert HR, Schwaiger M, Henze E, Phelps ME. Studies of fatty acid metabolism with positron emission tomography in patients with cardiomyopathy. Eur J Nucl Med. 1986;12:S66–S69. doi: 10.1007/BF00258110. [DOI] [PubMed] [Google Scholar]
- 22.Neglia D, De Caterina A, Marraccini P, Natali A, Ciardetti M, Vecoli C, et al. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2007;293:H3270–H3278. doi: 10.1152/ajpheart.00887.2007. [DOI] [PubMed] [Google Scholar]
- 23.Yazaki Y, Isobe M, Takahashi W, Kitabayashi H, Nishiyama O, Sekiguchi M, et al. Assessment of myocardial fatty acid metabolic abnormalities in patients with idiopathic dilated cardiomyopathy using 123I BMIPP SPECT: correlation with clinico-pathological findings and clinical course. Heart. 1999;81:153–159. doi: 10.1136/hrt.81.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikolaidis LA, Sturzu A, Stolarski C, Elahi D, Shen YT, Shannon RP. The development of myocardial insulin resistance in conscious dogs with advanced dilated cardiomyopathy. Cardiovasc Res. 2004;61:297–306. doi: 10.1016/j.cardiores.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 25.Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, et al. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation. 2002;106:606–612. doi: 10.1161/01.cir.0000023531.22727.c1. [DOI] [PubMed] [Google Scholar]
- 26.Qanud K, Mamdani M, Pepe M, Khairallah RJ, Gravel J, Lei B, et al. Reverse changes in cardiac substrate oxidation in dogs recovering from heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H2098–H2105. doi: 10.1152/ajpheart.00471.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taegtmeyer H, Sen S, Vela D. Return to the fetal gene program: a suggested metabolic link to gene expression in the heart. Ann N Y Acad Sci. 2010;1188:191–198. doi: 10.1111/j.1749-6632.2009.05100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1α. Cardiovasc Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 29.Bartelds B, Gratama JW, Knoester H, Takens J, Smid GB, Aarnoudse JG, et al. Perinatal changes in myocardial supply and flux of fatty acids, carbohydrates, and ketone bodies in lambs. Am J Physiol. 1998;274:H1962–H1969. doi: 10.1152/ajpheart.1998.274.6.H1962. [DOI] [PubMed] [Google Scholar]
- 30.Fisher D, Heymann M, Rudolph A. Myocardial consumption of oxygen and carbohydrates in newborn sheep. Pediatr Res. 1981;15:843–846. doi: 10.1203/00006450-198105000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Goodwin CW, Mela L, Deutsch C, Forster RE, Miller LD, Kelivoria-Papadopoulos M. Development and adaptation of heart mitochondrial respiratory chain function in fetus and in newborn. Adv Exp Med Biol. 1976;75:13–19. doi: 10.1007/978-1-4684-3273-2_83. [DOI] [PubMed] [Google Scholar]
- 32.Funada J, Betts TR, Hodson L, Humphreys SM, Timperley J, Frayn KN, et al. Substrate utilization by the failing human heart by direct quantification using arterio-venous blood sampling. PLoS One. 2009;4:e7533. doi: 10.1371/journal.pone.0007533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paolisso G, Gambardella A, Galzerano D, D'Amore A, Rubino P, Verza M, et al. Total-body and myocardial substrate oxidation in congestive heart failure. Metabolism. 1994;43:174–179. doi: 10.1016/0026-0495(94)90241-0. [DOI] [PubMed] [Google Scholar]
- 34.Taylor M, Wallhaus TR, Degrado TR, Russell DC, Stanko P, Nickles RJ, et al. An evaluation of myocardial fatty acid and glucose uptake using PET with [18F]fluoro-6-thia-heptadecanoic acid and [18F]FDG in patients with congestive heart failure. J Nucl Med. 2001;42:55–62. [PubMed] [Google Scholar]
- 35.Lommi J, Kupari M, Koskinen P, Näveri H, Leinonen H, Pulkki K, et al. Blood ketone bodies in congestive heart failure. J Am Coll Cardiol. 1996;28:665–672. doi: 10.1016/0735-1097(96)00214-8. [DOI] [PubMed] [Google Scholar]
- 36.Lommi J, Kupari M, Yki-Järvinen H. Free fatty acid kinetics and oxidation in congestive heart failure. Am J Cardiol. 1998;81:45–50. doi: 10.1016/s0002-9149(97)00804-7. [DOI] [PubMed] [Google Scholar]
- 37.Chandler MP, Kerner J, Huang H, Vazquez E, Reszko A, Martini WZ, et al. Moderate severity heart failure does not involve a downregulation of myocardial fatty acid oxidation. Am J Physiol Heart Circ Physiol. 2004;287:H1538–H1543. doi: 10.1152/ajpheart.00281.2004. [DOI] [PubMed] [Google Scholar]
- 38.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 39.Martin MA, Gomez MA, Guillen F, Bornstein B, Campos Y, Rubio JC, et al. Myocardial carnitine and carnitine palmitoyltransferase deficiencies in patients with severe heart failure. Biochim Biophys Acta. 2000;1502:330–336. doi: 10.1016/s0925-4439(00)00061-2. [DOI] [PubMed] [Google Scholar]
- 40.Morgan EE, Chandler MP, Young ME, McElfresh TA, Kung TA, Rennison JH, et al. Dissociation between gene and protein expression of metabolic enzymes in a rodent model of heart failure. Eur J Heart Fail. 2006;8:687–693. doi: 10.1016/j.ejheart.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Lei B, Lionetti V, Young ME, Chandler MP, d'Agostino C, Kang E, et al. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol. 2004;36:567–576. doi: 10.1016/j.yjmcc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res. 2004;95:568–578. doi: 10.1161/01.RES.0000141774.29937.e3. [DOI] [PubMed] [Google Scholar]
- 43.Karbowska J, Kochan Z, Smolenski RT. Peroxisome proliferator-activated receptor alpha is downregulated in the failing human heart. Cell Mol Biol Lett. 2003;8:49–53. [PubMed] [Google Scholar]
- 44.Morgan EE, Rennison JH, Young ME, McElfresh TA, Kung TA, Tserng KY, et al. Effects of chronic activation of peroxisome proliferator-activated receptor alpha or high-fat feeding in a rat infarct model of heart failure. Am J Physiol Heart Circ Physiol. 2006;290:H1899–H1904. doi: 10.1152/ajpheart.01014.2005. [DOI] [PubMed] [Google Scholar]
- 45.Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, et al. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006;48:1116–1123. doi: 10.1161/01.HYP.0000248430.26229.0f. [DOI] [PubMed] [Google Scholar]
- 46.Karamanlidis G, Nascimben L, Couper GS, Shekar PS, del Monte F, Tian R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ Res. 2010;106:1541–1548. doi: 10.1161/CIRCRESAHA.109.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sihag S, Cresci S, Li AY, Sucharov CC, Lehman JJ. PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J Mol Cell Cardiol. 2009;46:201–212. doi: 10.1016/j.yjmcc.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lionetti V, Linke A, Chandler MP, Young ME, Penn MS, Gupte S, et al. Carnitine palmitoyl transferase-I inhibition prevents ventricular remodeling and delays decompensation in pacing-induced heart failure. Cardiovasc Res. 2005;66:454–461. doi: 10.1016/j.cardiores.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Taegtmeyer H, Stanley WC. Too much or not enough of a good thing? Cardiac glucolipotoxicity versus lipoprotection. J Mol Cell Cardiol. 2011;50:2–5. doi: 10.1016/j.yjmcc.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liao R, Jain M, Cui L, D'Agostino J, Aiello F, Luptak I, et al. Cardiac specific overexpression of GLUT1 prevents the development of heart failure due to pressure-overload in mice. Circulation. 2002;106:2125–2131. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
- 51.Razeghi P, Young ME, Ying J, Depre C, Uray IP, Kolesar J, et al. Downregulation of metabolic gene expression in failing human heart before and after mechanical unloading. Cardiology. 2002;97:203–209. doi: 10.1159/000063122. [DOI] [PubMed] [Google Scholar]
- 52.Gupte SA, Levine RJ, Gupte RS, Young ME, Lionetti V, Labinskyy V, et al. Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. J Mol Cell Cardiol. 2006;41:340–349. doi: 10.1016/j.yjmcc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 53.Gupte RS, Vijay V, Marks B, Levine RJ, Sabbah HN, Wolin MS, et al. Upregulation of glucose-6-phosphate dehydrogenase and NAD(P)H oxidase activity increases oxidative stress in failing human heart. J Card Fail. 2007;13:497–506. doi: 10.1016/j.cardfail.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Fragasso G, Salerno A, Spoladore R, Bassanelli G, Arioli F, Margonato A. Metabolic therapy of heart failure. Curr Pharm Des. 2008;14:2582–2591. doi: 10.2174/138161208786071245. [DOI] [PubMed] [Google Scholar]
- 55.Kalay N, Ozdogru I, Gul A, Yucel Y, Cetinkaya Y, Inanc MT, et al. Effects of intermittent and long-term glucose-insulin-potassium infusion in patients with systolic heart failure. Exp Clin Cardiol. 2008;13:85–88. [PMC free article] [PubMed] [Google Scholar]
- 56.Datta S, Das DK, Engelman RM, Otani H, Rousou JA, Breyer RH, et al. Enhanced myocardial preservation by nicotinic acid, an antilipolytic compound: mechanism of action. Basic Res Cardiol. 1989;84:63–76. doi: 10.1007/BF01907004. [DOI] [PubMed] [Google Scholar]
- 57.Opie LH, Knuuti J. The adrenergic-fatty acid load in heart failure. J Am Coll Cardiol. 2009;54:1637–1646. doi: 10.1016/j.jacc.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 58.Fragasso G, Spoladore R, Cuko A, Palloshi A. Modulation of fatty acids oxidation in heart failure by selective pharmacological inhibition of 3-ketoacyl coenzyme-A thiolase. Curr Clin Pharmacol. 2007;2:190–196. doi: 10.2174/157488407781668776. [DOI] [PubMed] [Google Scholar]
- 59.Murray AJ, Lygate CA, Cole MA, Carr CA, Radda GK, Neubauer S, et al. Insulin resistance, abnormal energy metabolism and increased ischemic damage in the chronically infarcted rat heart. Cardiovasc Res. 2006;71:149–157. doi: 10.1016/j.cardiores.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 60.Oliver MF, Opie LH. Effects of glucose and fatty acids on myocardial ischaemia and arrhythmias. Lancet. 1994;343:155–158. doi: 10.1016/s0140-6736(94)90939-3. [DOI] [PubMed] [Google Scholar]
- 61.Stojiljkovic MP, Lopes HF, Zhang D, Morrow JD, Goodfriend TL, Egan BM. Increasing plasma fatty acids elevates F2-isoprostanes in humans: implications for the cardiovascular risk factor cluster. J Hypertens. 2002;20:1215–1221. doi: 10.1097/00004872-200206000-00036. [DOI] [PubMed] [Google Scholar]
- 62.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, et al. Intramyocardial lipid accumulation in the failing human heart resemble the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 63.Nakae I, Mitsunami K, Yoshino T, Omura T, Tsutamoto T, Matsumoto T, et al. Clinical features of myocardial triglyceride in different types of cardiomyopathy assessed by proton magnetic resonance spectroscopy: comparison with myocardial creatine. J Card Fail. 2010;16:812–822. doi: 10.1016/j.cardfail.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 64.Wende AR, Abel ED. Lipotoxicity in the heart. Biochim Biophys Acta. 2010;1801:311–319. doi: 10.1016/j.bbalip.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tuunanen H, Engblom E, Naum A, Någren K, Hesse B, Airaksinen KE, et al. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation. 2006;114:2130–2137. doi: 10.1161/CIRCULATIONAHA.106.645184. [DOI] [PubMed] [Google Scholar]
- 66.Halbirk M, Nørrelund H, Møller N, Schmitz O, Gøtzsche L, Nielsen R, et al. Suppression of circulating free fatty acids with acipimox in chronic heart failure patients changes whole body metabolism but does not affect cardiac function. Am J Physiol Heart Circ Physiol. 2010;299:H1220–H1225. doi: 10.1152/ajpheart.00475.2010. [DOI] [PubMed] [Google Scholar]
- 67.Beanlands RS, Nahmias C, Gordon E, Coates G, deKemp R, Firnau G, et al. The effects of β1-blockade on oxidative metabolism and the metabolic cost of ventricular work in patients with left ventricular dysfunction. Circulation. 2000;102:2070–2075. doi: 10.1161/01.cir.102.17.2070. [DOI] [PubMed] [Google Scholar]
- 68.Wallhaus TR, Taylor M, DeGrado TR, Russell DC, Stanko P, Nickles RJ, et al. Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation. 2001;103:2441–2446. doi: 10.1161/01.cir.103.20.2441. [DOI] [PubMed] [Google Scholar]
- 69.Eichhorn EJ, Heesch CM, Barnett JH, Alvarez LG, Fass SM, Grayburn PA, et al. Effect of metoprolol on myocardial function and energetics in patients with nonischemic dilated cardiomyopathy: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 1994;24:1310–1320. doi: 10.1016/0735-1097(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 70.Nikolaidis LA, Hentosz T, Doverspike A, Huerbin R, Stolarski C, Shen YT, et al. Catecholamine stimulation is associated with impaired myocardial O2 utilization in heart failure. Cardiovasc Res. 2002;53:392–404. doi: 10.1016/s0008-6363(01)00490-4. [DOI] [PubMed] [Google Scholar]
- 71.Eichhorn EJ, Bedotto JB, Malloy CR, Hatfield BA, Deitchman D, Brown M, et al. Effect of beta adrenergic blockade on myocardial function and energetics in congestive heart failure. Improvements in hemodynamic, contractile, and diastolic performance with bucindolol. Circulation. 1990;82:473–483. doi: 10.1161/01.cir.82.2.473. [DOI] [PubMed] [Google Scholar]
- 72.Beanlands RSB, Nahmias C, Gordon E, Coates G, deKemp R, Firnau G, et al. The effects of beta1-blockade on oxidative metabolism and the metabolic cost of ventricular work in patients with left ventricular dysfunction. Circulation. 2000;102:2070–2075. doi: 10.1161/01.cir.102.17.2070. [DOI] [PubMed] [Google Scholar]
- 73.Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 74.Nikolaidis LA, Poornima I, Parikh P, Magovern M, Shen YT, Shannon RP. The effects of combined versus selective adrenergic blockade on left ventricular and systemic hemodynamics, myocardial substrate preference, and regional perfusion in conscious dogs with dilated cardiomyopathy. J Am Coll Cardiol. 2006;47:1871–1881. doi: 10.1016/j.jacc.2005.11.082. [DOI] [PubMed] [Google Scholar]
- 75.Labinskyy V, Bellomo M, Chandler MP, Young ME, Lionetti V, Qanud K, et al. Chronic activation of peroxisome proliferator-activated receptor alpha with fenofibrate prevents alterations in cardiac metabolic phenotype without changing the onset of decompensation in pacing-induced heart failure. J Pharmacol Exp Ther. 2007;321:165–171. doi: 10.1124/jpet.106.116871. [DOI] [PubMed] [Google Scholar]
- 76.Brigadeau F, Gelé P, Wibaux M, Marquié C, Martin-Nizard F, Torpier G, et al. The PPARalpha activator fenofibrate slows down the progression of the left ventricular dysfunction in porcine tachycardia-induced cardiomyopathy. J Cardiovasc Pharmacol. 2007;49:408–415. doi: 10.1097/FJC.0b013e3180544540. [DOI] [PubMed] [Google Scholar]
- 77.Wolff AA, Rotmensch HH, Stanley WC, Ferrari R. Metabolic approaches to the treatment of ischemic heart disease: the clinicians' perspective. Heart Fail Rev. 2002;7:187–203. doi: 10.1023/a:1015384710373. [DOI] [PubMed] [Google Scholar]
- 78.Schwarzer M, Faerber G, Rueckauer T, Blum D, Pytel G, Mohr FW, et al. The metabolic modulators, etomoxir and NVP-LAB121, fail to reverse pressure overload induced heart failure in vivo. Basic Res Cardiol. 2009;104:547–557. doi: 10.1007/s00395-009-0015-5. [DOI] [PubMed] [Google Scholar]
- 79.Rupp H, Zarain-Herzberg A, Maisch B. The use of partial fatty acid oxidation inhibitors for metabolic therapy of angina pectoris and heart failure. Herz. 2002;27:621–636. doi: 10.1007/s00059-002-2428-x. [DOI] [PubMed] [Google Scholar]
- 80.Schmidt-Schweda S, Holubarsch C. First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin Sci (Lond) 2000;99:27–35. [PubMed] [Google Scholar]
- 81.Bristow M. Etomoxir: a new approach to treatment of chronic heart failure. Lancet. 2000;356:1621–1622. doi: 10.1016/S0140-6736(00)03149-4. [DOI] [PubMed] [Google Scholar]
- 82.Holubarsch CJ, Rohrbach M, Karrasch M, Boehm E, Polonski L, Ponikowski P, et al. A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: the ERGO (etomoxir for the recovery of glucose oxidation) study. Clin Sci (Lond) 2007;113:205–212. doi: 10.1042/CS20060307. [DOI] [PubMed] [Google Scholar]
- 83.Kennedy JA, Unger SA, Horowitz JD. Inhibition of carnitine palmitoyltransferase-1 in rat heart and liver by perhexiline and amiodarone. Biochem Pharmacol. 1996;52:273–280. doi: 10.1016/0006-2952(96)00204-3. [DOI] [PubMed] [Google Scholar]
- 84.Lee L, Campbell R, Scheuermann-Freestone M, Taylor R, Gunaruwan P, Williams L, et al. Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation. 2005;112:3280–3288. doi: 10.1161/CIRCULATIONAHA.105.551457. [DOI] [PubMed] [Google Scholar]
- 85.Abozguia K, Elliott P, McKenna W, Phan TT, Nallur-Shivu G, Ahmed I, et al. Metabolic modulator perhexiline corrects energy deficiency and improves exercise capacity in symptomatic hypertrophic cardiomyopathy. Circulation. 2010;122:1562–1569. doi: 10.1161/CIRCULATIONAHA.109.934059. [DOI] [PubMed] [Google Scholar]
- 86.McCormack JG, Baracos VE, Barr R, Lopaschuk GD. Effects of ranolazine on oxidative substrate preference in epitrochlearis muscle. J Appl Physiol. 1996;81:905–910. doi: 10.1152/jappl.1996.81.2.905. [DOI] [PubMed] [Google Scholar]
- 87.McCormack JG, Barr RL, Wolff AA, Lopaschuk GD. Ranolazine stimulates glucose oxidation in normoxic, ischemic, and reperfused ischemic rat hearts. Circulation. 1996;93:135–142. doi: 10.1161/01.cir.93.1.135. [DOI] [PubMed] [Google Scholar]
- 88.Fantini E, Demaison L, Sentex E, Grynberg A, Athias P. Some biochemical aspects of the protective effect of trimetazidine on rat cardiomyocytes during hypoxia and reoxygenation. J Mol Cell Cardiol. 1994;26:949–958. doi: 10.1006/jmcc.1994.1116. [DOI] [PubMed] [Google Scholar]
- 89.Bagger JP, Bøtker HE, Thomassen A, Nielsen TT. Effects of ranolazine on ischemic threshold, coronary sinus blood flow, and myocardial metabolism in coronary artery disease. Cardiovasc Drugs Ther. 1997;11:479–484. doi: 10.1023/a:1007705707667. [DOI] [PubMed] [Google Scholar]
- 90.Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2000;86:580–588. doi: 10.1161/01.res.86.5.580. [DOI] [PubMed] [Google Scholar]
- 91.Morgan EE, Young ME, McElfresh TA, Kung TA, Hoit BD, Chandler MP, et al. Chronic treatment with trimetazidine reduces the upregulation of atrial natriuretic peptide in heart failure. Fundam Clin Pharmacol. 2006;20:503–505. doi: 10.1111/j.1472-8206.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 92.Mouqueta F, Rousseau D, Domergue-Dupont V, Grynberg A, Liao R. Effects of trimetazidine, a partial inhibitor of fatty acid oxidation, on ventricular function and survival after myocardial infarction and reperfusion in the rat. Fundam Clin Pharmacol. 2010;24:469–476. doi: 10.1111/j.1472-8206.2009.00802.x. [DOI] [PubMed] [Google Scholar]
- 93.D'Hahan N, Taouil K, Dassouli A, Morel JE. Long-term therapy with trimetazidine in cardiomyopathic Syrian hamster BIO 14:6. Eur J Pharmacol. 1997;328:163–174. doi: 10.1016/s0014-2999(97)83042-7. [DOI] [PubMed] [Google Scholar]
- 94.Stanley WC, Marzilli M. Metabolic therapy in the treatment of ischaemic heart disease: the pharmacology of trimetazidine. Fundam Clin Pharmacol. 2003;17:133–145. doi: 10.1046/j.1472-8206.2003.00154.x. [DOI] [PubMed] [Google Scholar]
- 95.Belardinelli R, Purcaro A. Effects of trimetazidine on the contractile response of chronically dysfunctional myocardium to low-dose dobutamine in ischaemic cardiomyopathy. Eur Heart J. 2001;22:2164–2170. doi: 10.1053/euhj.2001.2653. [DOI] [PubMed] [Google Scholar]
- 96.Fragasso G, Piatti Md PM, Monti L, Palloshi A, Setola E, Puccetti P, et al. Short- and long-term beneficial effects of trimetazidine in patients with diabetes and ischemic cardiomyopathy. Am Heart J. 2003;146:E18. doi: 10.1016/S0002-8703(03)00415-0. [DOI] [PubMed] [Google Scholar]
- 97.Rosano GM, Vitale C, Sposato B, Mercuro G, Fini M. Trimetazidine improves left ventricular function in diabetic patients with coronary artery disease: a double-blind placebo-controlled study. Cardiovasc Diabetol. 2003;2:16. doi: 10.1186/1475-2840-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vitale C, Wajngaten M, Sposato B, Gebara O, Rossini P, Fini M, et al. Trimetazidine improves left ventricular function and quality of life in elderly patients with coronary artery disease. Eur Heart J. 2004;25:1814–1821. doi: 10.1016/j.ehj.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 99.Fragasso G, Palloshi A, Puccetti P, Silipigni C, Rossodivita A, Pala M, et al. A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J Am Coll Cardiol. 2006;48:992–998. doi: 10.1016/j.jacc.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 100.Fragasso G, Perseghin G, De CF, Esposito A, Palloshi A, Lattuada G, et al. Effects of metabolic modulation by trimetazidine on left ventricular function and phosphocreatine/adenosine triphosphate ratio in patients with heart failure. Eur Heart J. 2006;27:942–948. doi: 10.1093/eurheartj/ehi816. [DOI] [PubMed] [Google Scholar]
- 101.Sisakian H, Torgomyan A, Barkhudaryan A. The effect of trimetazidine on left ventricular systolic function and physical tolerance in patients with ischaemic cardiomyopathy. Acta Cardiol. 2007;62:493–499. doi: 10.2143/AC.62.5.2023413. [DOI] [PubMed] [Google Scholar]
- 102.Belardinelli R, Cianci G, Gigli M, Mazzanti M, Lacalaprice F. Effects of trimetazidine on myocardial perfusion and left ventricular systolic function in type 2 diabetic patients with ischemic cardiomyopathy. J Cardiovasc Pharmacol. 2008;51:611–615. doi: 10.1097/FJC.0b013e31817bdd66. [DOI] [PubMed] [Google Scholar]
- 103.Di Napoli P, Taccardi AA, Barsotti A. Long term cardioprotective action of trimetazidine and potential effect on the inflammatory process in patients with ischaemic dilated cardiomyopathy. Heart. 2005;91:161–165. doi: 10.1136/hrt.2003.031310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Di Napoli P, Di GP, Gaeta MA, D'Apolito G, Barsotti A. Beneficial effects of trimetazidine treatment on exercise tolerance and B-type natriuretic peptide and troponin T plasma levels in patients with stable ischemic cardiomyopathy. Am Heart J. 2007;154:602–605. doi: 10.1016/j.ahj.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 105.Nash DT, Nash SD. Ranolazine for chronic stable angina. Lancet. 2008;372:1335–1341. doi: 10.1016/S0140-6736(08)61554-8. [DOI] [PubMed] [Google Scholar]
- 106.Vadnais DS, Wenger NK. Emerging clinical role of ranolazine in the management of angina. Ther Clin Risk Manag. 2010;6:517–530. doi: 10.2147/TCRM.S4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hale SL, Shryock JC, Belardinelli L, Sweeney M, Kloner RA. Late sodium current inhibition as a new cardioprotective approach. J Mol Cell Cardiol. 2008;44:954–967. doi: 10.1016/j.yjmcc.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 108.Chandler MP, Stanley WC, Morita H, Suzuki G, Roth BA, Blackburn B, et al. Short-term treatment with ranolazine improves mechanical efficiency in dogs with chronic heart failure. Circ Res. 2002;91:278–280. doi: 10.1161/01.res.0000031151.21145.59. [DOI] [PubMed] [Google Scholar]
- 109.Rastogi S, Sharov VG, Mishra S, Gupta RC, Blackburn B, Belardinelli L, et al. Ranolazine combined with enalapril or metoprolol prevents progressive LV dysfunction and remodeling in dogs with moderate heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H2149–H2155. doi: 10.1152/ajpheart.00728.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kass DA, Bronzwaer JG, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res. 2004;94:1533–1542. doi: 10.1161/01.RES.0000129254.25507.d6. [DOI] [PubMed] [Google Scholar]
- 111.Owan TE, Hodge DO, Herges RM, Jacodbsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]