Abstract
Metabolic remodelling is an integral part of the pathogenesis of heart failure. Although much progress has been made in our current understanding of the metabolic impairment involving carbohydrates and fatty acids in failing hearts, relatively little is known about the changes and potential impact of amino acid metabolism in the onset of heart diseases. Although most amino acid catabolic activities are found in the liver, branched-chain amino acid (BCAA) catabolism requires activity in several non-hepatic tissues, including cardiac muscle, diaphragm, brain and kidney. In this review, the new insights into the regulation of cardiac BCAA catabolism and functional impact on cardiac development and physiology will be discussed along with the potential contribution of impairment in BCAA catabolism to heart diseases. A particular focus will be the new information obtained from recently developed genetic models with BCAA catabolic defects and metabolomic studies in human and animal models. These studies have revealed the potential role of BCAA catabolism in cardiac pathophysiology and have helped to distinguish BCAA metabolic defects as an under-appreciated culprit in cardiac diseases rather than an epiphenomenon associated with metabolic remodelling in the failing heart.
Keywords: Branched-chain amino acid, PP2Cm, Heart failure
1. Metabolic regulation in heart failure
The onset of heart failure is associated with a major alteration of metabolism in cardiomyocytes.1–3 The well-studied shift from a fatty acid-dominant bioenergetic state into a more glycolytic state in stressed myocardium is generally viewed as a compensatory response with the initial purpose of enhancing oxygen/fuel utilization efficiency.4 This metabolic switch to a ‘foetal’-like pattern parallels similar changes in the gene expression profile. However, impairments in glucose utilization and suppression of fatty acid oxidation in myocytes in the pathological state ultimately lead to a deficiency in energy supply to overloaded myocardium.2,5 Bioenergenic defects are recognized as a critical contributor to the disease progression of heart failure.2,4,5 Therefore, a great deal of effort has been devoted to investigating the regulatory mechanisms underlying the metabolic defects seen in failing hearts. Indeed, encouraging evidence has emerged that manipulating the fuel supply and substrate consumption can have a significant impact on the disease progression of heart failure.3,5 However, much of our current investigations is focused on fatty acids and carbohydrates; in contrast, protein and amino acid metabolism is underexplored, except in the rare cases of hereditary metabolic cardiomyopathies.6 The relevance of amino acid metabolism in the general population of heart diseases remains poorly understood.
Most metabolic and catabolic activities of amino acids occur in the liver. However, a subgroup of essential amino acids, the branched-chain amino acids (BCAAs), including leucine, isoleucine, and valine, are catabolized in non-hepatic tissues, mostly cardiac muscle, neuron, and kidney.7 In this review, we will focus on some of the recent studies that have revealed a surprising role of BCAA catabolic activity in cardiac regulation, and we will examine the potential implication of these findings in clinical management and therapy of heart failure.
2. Branched-chain amino acids: catabolism and regulation
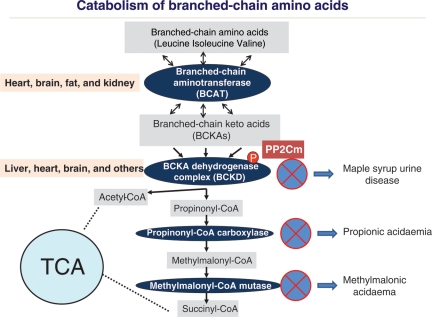
Leucine, isoleucine, and valine are collectively referred to as BCAAs due to their shared structural features in side-chain and a common catabolic pathway. BCAAs are essential amino acids in animals and must be acquired from external food. In addition to their role as key building blocks for peptide synthesis, BCAAs are also significant sources for biosynthesis of sterol, keto bodies, and glucose.8 The molecular basis and the regulation of BCAA uptake remain poorly studied; however, l-type amino acid transporters9 and bidirectional transporter for l-glutamine and l-leucine/EAA appear to play a major role in BCAA entry and activation of downstream signalling.10 The final catabolic products of BCAA are acetyl-CoA and succinyl-CoA, which are consumed in mitochondria through tri-carboxylic acid cycle for the production of reduced nicotinamide adenine dinucleotide for respiration. Other than these metabolic roles, BCAAs, particularly leucine, have potent nutrient signalling activity in cells to promote protein synthesis, cellular metabolism, and cell growth in a mammalian target of rapamycin (mTOR)-dependent manner.11–13 Therefore, BCAAs are essential for normal growth and function at cellular and organism levels. However, an excess amount of free BCAA or their catabolic products can also be cytotoxic. Maple syrup urine disease, methylmalonic acidaemia, and propionic acidaemia are known genetic disorders resulting from defects in BCAA catabolic pathway (Figure 1). The clinical manifestations of these genetic disorders are mainly in the central nervous system, including seizure and mental retardation.14 However, propionic acidaemia and methylmalonic acidaemia are highly associated with dilated and hypertrophic cardiomyopathies.15–20 In essence, maintaining BCAA homeostasis is critical to normal physiology, including heart muscle function.
Figure 1.
Regulation of the branched-chain amino acid catabolic pathway. A schematic illustration of major steps and enzyme complexes involved in the BCAA catabolic pathway and targeted organs as well as genetic disorders associated with the specific steps. TCA, tricarboxylic acid cycle (also known as the Krebs cycle).
As BCAAs are essential amino acids and can be obtained only from external food sources, the homeostasis of free BCAA must be maintained at their catabolic steps. Unlike much of the other amino acid metabolic/catabolic activities that take place in liver, the first step of BCAA catabolism occurs in brain, muscle, and many non-hepatic tissues to convert BCAA into branched-chain α-keto-acids (BCKA) by branched-chain amino-transferase (BCAT).21 The conversion of BCAA into BCKA can be affected by glutamate concentration and glutamate dehydrogenase activity since 2-oxoglutarate is the main acceptor of the amino group in this reaction.7 BCKA are then oxidized by the branched-chain α-keto acid dehydrogenase (BCKD) complexes and eventually degraded into acetyl-CoA or succinyl-CoA (Figure 1). The BCKD-mediated reaction is the rate-limiting step in the BCAA catabolic pathway and its activity determines the overall level of BCAA/BCKA. Therefore, BCKD expression and activity is subjected to tight regulation to maintain BCAA homeostasis in response to external nutrient and growth signals.7,21 The BCKD complex genetically parallels pyruvate dehydrogenase complex (PDH), with similar subunit composition and regulatory mechanisms. Like PDH, BCKD holoenzyme activity is determined by the phosphorylation status of its regulatory subunit E1α. When the BCAA level is low, E1α is hyper-phosphorylated by a BCKD kinase, leading to inhibition of BCKD activity and preservation of free BCAA. When the BCAA level is high, E1α is dephosphorylated by a BCKD phophatase, leading to BCKD activation and reducing total BCAA. Therefore, BCKD dephosphorylation is a critical step in its enzyme activation and BCKD phosphatase likely has a major role in BCAA homeostasis.21
3. Regulation of BCAA catabolism and functional impact on heart
A mitochondrial-targeted 2C-type ser/thr protein phosphatase named PP2C in mitochondria (PP2Cm) was discovered based on genome scanning.22 By proteomic and biochemical analysis, it has been established that PP2Cm is the endogenous BCKD phosphatase responsible for BCAA-induced dephosphorylation and activation of BCKD.23 As expected, PP2Cm-deficient mice have impaired regulation of BCKD activity, leading to significantly higher plasma levels of BCAA and BCKA either at basal or following ingestion of a high dose of BCAA.23
In both zebrafish embryos and adult mice, PP2Cm is highly expressed in cardiac muscle cells as well as in the central nervous system,22 which correlates well with BCKD activities observed in these tissues.7,22 The expression of PP2Cm in heart is also dynamically regulated by stress, as measured at both mRNA and protein levels, with significantly reduced expression in hypertrophic and failing hearts.22 This raises questions about the potential role of BCAA and their catabolic regulation in cardiac muscle cell physiology and pathology. However, it is not clear whether the loss of PP2Cm expression and the resulting BCAA catabolic defects are merely epiphenomena associated with the global molecular and metabolic remodelling in stressed myocardium, or if they play an active role in overall pathology in the heart. By using morpholinos specifically targeted to the zPP2Cm (zebrafish homologue of mammalian PP2Cm) translation initiation codon (ATG-MO), PP2Cm inactivation was achieved, leading to a dose-dependent loss of cardiac contractility and premature death.22 Induced apoptosis in PP2Cm-deficient zebrafish embryos or isolated rat ventricular myocytes was also observed.22 This study provides strong evidence that BCAA catabolism is essential for normal cardiac physiology and cellular viability, and that PP2Cm-mediated BCAA catabolic regulation can be a potentially significant contributor to cardiac pathophysiology and disease progression. Although the results from the zebrafish study are supportive of this new concept, more in vivo evidence from diseased human hearts and other models of heart failure is needed.
4. Potential mechanisms in BCAA-dependent cardiac regulation
The underlying mechanisms for the adverse effects of BCAA catabolic defects in the heart remain to be established. BCAA are not only an important nutrient source, but are also potent signalling molecules. BCAAs, especially l-leucine, are highly effective activators of mTOR signalling.24 In the heart, mTOR activity is directly implicated in cardiac hypertrophy in a key pathway for protein synthesis regulation.25–27 In addition, BCAA-induced mTOR activation suppresses autophagy.10 Autophagic activity is a known player in cardiac pathology, although whether it is protective or detrimental to the heart remains controversial.28–33 Finally, mTOR activation also triggers metabolic changes in muscle, liver, and other tissues by altering insulin sensitivity.11–13,34–36 It is also known that BCAA/BCKA can inhibit pyruvate and fatty acid transport and utilization.13,37 Therefore, it is plausible that impaired BCAA catabolic activity in the stressed heart and the ensuing elevation of local BCAA concentration can lead to chronic induction of cardiac mTOR activity, which in turn promotes cardiac hypertrophy, suppressing cardioprotective autophagy, and impairing bioenergetic regulation in the heart. However, a recent report from D'Antona et al.38 suggests that BCAA dietary supplement can promote skeletal and cardiac muscle survival and prolongs life span in middle-aged mice. The underlying mechanisms appear to be related to mTOR-mediated insulin signalling. Therefore, the culprit responsible for the BCAA catabolic defect-induced cardiac phenotype cannot be simply attributed to the mTOR pathway induced by BCAA.
In addition to mTOR, BCAA and/or their catabolic intermediate products, such as BCKAs, can have a direct impact on mitochondrial function and cellular viability. In propionic aciduria or methylmalonic aciduria patients, dilated cardiomyopathy is a frequent complication that is associated with defects in the mitochondrial respiratory chain.16 Romano et al.15 reported recently that liver transplant in propionic aciduria patients could reverse the clinical symptoms of cardiomyopathy along with other signs of metabolic stress, supporting the hypothesis that systemic induction of catabolic intermediates of BCAA may be the culprit that triggers cardiac dysfunction. However, the direct impact of BCAA and their catabolic intermediate products on mitochondrial performance and contractile function in the heart has never been investigated.
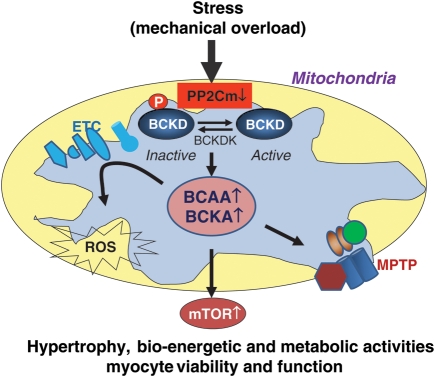
Associated with loss of cellular viability, reactive oxygen species (ROS) levels are also significantly elevated in PP2Cm-deficient cells and tissues, and PP2Cm-deficient mitochondria are more susceptible to calcium-induced permeability transition pore opening in the absence of external BCKA/BCAA challenge.22 These data suggest that PP2Cm as a mitochondrial matrix phosphatase may have other direct or indirect downstream targets to regulate myocyte function and viability via modulating ROS and permeability transition pore (Figure 2).
Figure 2.
Potential role of BCAA/BCKA catabolism in heart failure. Illustration of the potential impact of reduced expression of PP2Cm in stressed heart in BCAA catabolism and cardiac remodelling. BCKD, branched-chain α-keto acid dehydrogenase; BCKDK, BCKD kinase; ETC, electron transfer chain; ROS, reactive oxygen species; BCAA, branched-chain amino acids; BCKA, branched-chain keto acids; MPTP, mitochondrial permeability transition pore.
5. Perspectives
It is interesting to observe that PP2Cm mRNA is enriched in the brain, heart muscle, and diaphragm muscle in adult mice, but not in skeletal muscle and other tissues.22 It implies that BCAA catabolic activities are higher in organs of vital importance that have a high sensitivity to energy supply. It can be assumed that if BCAAs are used as a source of bioenergetic fuel, tissues like the heart, brain, and diaphragm would have priority access. This may be particularly important under nutrient-starved conditions when chronic malnutrition can lead to skeletal muscle waste while preserving cardiac or diaphragm muscle function and integrity. This hypothesis can now be tested in PP2Cm null mice to determine whether BCAA regulation in the brain, heart, and diaphragm is related to tolerance for nutrient deficit.
Although we observed a significant change of PP2Cm expression in the pathologically stressed heart, the underlying significance remains to be established. It can be expected that reduced expression of PP2Cm will lead to increased concentration of free BCAA (as demonstrated in PP2Cm null animals), which may provide necessary building material as well as additional nutrient signals to promote myocyte hypertrophy and other remodelling processes. In such a case, reduced BCAA catabolism can be viewed as part of a well-orchestrated metabolic remodelling programme in stressed heart to meet the shift in energetic demand as well as growth demand. However, since PP2Cm deficiency is sufficient to cause cardiac pathology, this would imply that reduced BCAA catabolic activity in diseased heart may not be entirely beneficial or compensatory in nature, but rather that it has a potential contributing role to the overall pathogenesis of the disease. Chronic activation of the mTOR pathway, suppressing cardioprotective autophagy activity and shift in bioenergetic activities, as well as mis-regulation of other intra-mitochondrial activities, such as ROS and mPTP, may be the unintended ‘side-effects’. A recent study based on metabolic profiling of peripheral blood has demonstrated a link between abnormal BCAA metabolism and coronary diseases,39 while other studies showed a strong link between BCAA catabolic defects and the onset of metabolic syndrome, such as insulin resistance and obesity.12,36 In the whole landscape of metabolic cardiomyopathy, the specific role of BCAA catabolic regulation may have been under-appreciated. Future studies in this area will be greatly facilitated by the availability of a number of new genetic models40 that can manipulate BCAA catabolic activity in a tissue and developmental stage-specific manner. The insights from these studies would shed new light on the pathogenesis of metabolic cardiomyopathy as well as other metabolic diseases, such as diabetics, obesity, and central nervous system abnormalities.
Conflict of interest: none declared.
Funding
This work is in part supported by grants from the National Institutes of Health, HL70079, HL103205, HL098954, HL080111 and HL088640, and China Nature Science Foundation Research Grant (30971094, M.Z.). Shanghai Qimingxing Plan (11QA1403700, Y.H.). Y.W. is an Established Investigator of American Heart Association, H.S. is a recipient of a Post-doctoral Fellowship award from the Great Western Affiliate of the American Heart Association.
References
- 1.Bing RJ, Siegel A, Vitale A, Balboni F, Sparks E, Taeschler M, et al. Metabolic studies on the human heart in vivo. I. Studies on carbohydrate metabolism of the human heart. Am J Med. 1953;15:284–296. doi: 10.1016/0002-9343(53)90082-5. doi:10.1016/0002-9343(53)90082-5. [DOI] [PubMed] [Google Scholar]
- 2.Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res. 2009;81:412–419. doi: 10.1093/cvr/cvn301. doi:10.1093/cvr/cvn301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Revenco D, Morgan JP. Metabolic modulation and cellular therapy of cardiac dysfunction and failure. J Cell Mol Med. 2009;13:811–825. doi: 10.1111/j.1582-4934.2009.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Bilsen M, van Nieuwenhoven FA, van der Vusse GJ. Metabolic remodelling of the failing heart: beneficial or detrimental? Cardiovasc Res. 2009;81:420–428. doi: 10.1093/cvr/cvn282. doi:10.1093/cvr/cvn282. [DOI] [PubMed] [Google Scholar]
- 5.Horowitz JD, Chirkov YY, Kennedy JA, Sverdlov AL. Modulation of myocardial metabolism: an emerging therapeutic principle. Curr Opin Cardiol. 2010;25:329–334. doi: 10.1097/HCO.0b013e328339f191. doi:10.1097/HCO.0b013e328339f191. [DOI] [PubMed] [Google Scholar]
- 6.Guertl B, Noehammer C, Hoefler G. Metabolic cardiomyopathies. Int J Exp Pathol. 2000;81:349–372. doi: 10.1046/j.1365-2613.2000.00186.x. doi:10.1046/j.1365-2613.2000.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–454. doi: 10.1146/annurev.nu.04.070184.002205. doi:10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- 8.Baquet A, Lavoinne A, Hue L. Comparison of the effects of various amino acids on glycogen synthesis, lipogenesis and ketogenesis in isolated rat hepatocytes. Biochem J. 1991;273(Pt 1):57–62. doi: 10.1042/bj2730057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verrey F. System L: heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch. 2003;445:529–533. doi: 10.1007/s00424-002-0973-z. [DOI] [PubMed] [Google Scholar]
- 10.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. doi:10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chotechuang N, Azzout-Marniche D, Bos C, Chaumontet C, Gausseres N, Steiler T, et al. mTOR, AMPK, and GCN2 coordinate the adaptation of hepatic energy metabolic pathways in response to protein intake in the rat. Am J Physiol Endocrinol Metab. 2009;297:E1313–E1323. doi: 10.1152/ajpendo.91000.2008. doi:10.1152/ajpendo.91000.2008. [DOI] [PubMed] [Google Scholar]
- 12.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. doi:10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saha AK, Xu XJ, Lawson E, Deoliveira R, Brandon AE, Kraegen EW, et al. Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes. 2010;59:2426–2434. doi: 10.2337/db09-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schadewaldt P, Wendel U. Metabolism of branched-chain amino acids in maple syrup urine disease. Eur J Pediatr. 1997;156(Suppl. 1)):S62–S66. doi: 10.1007/pl00014274. doi:10.1007/PL00014274. [DOI] [PubMed] [Google Scholar]
- 15.Romano S, Valayannopoulos V, Touati G, Jais JP, Rabier D, de Keyzer Y, et al. Cardiomyopathies in propionic aciduria are reversible after liver transplantation. J Pediatr. 2010;156:128–134. doi: 10.1016/j.jpeds.2009.07.002. doi:10.1016/j.jpeds.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 16.de Keyzer Y, Valayannopoulos V, Benoist JF, Batteux F, Lacaille F, Hubert L, et al. Multiple OXPHOS deficiency in the liver, kidney, heart, and skeletal muscle of patients with methylmalonic aciduria and propionic aciduria. Pediatr Res. 2009;66:91–95. doi: 10.1203/PDR.0b013e3181a7c270. doi:10.1203/PDR.0b013e3181a7c270. [DOI] [PubMed] [Google Scholar]
- 17.De Bie I, Nizard SD, Mitchell GA. Fetal dilated cardiomyopathy: an unsuspected presentation of methylmalonic aciduria and hyperhomocystinuria, cblC type. Prenat Diagn. 2009;29:266–270. doi: 10.1002/pd.2218. doi:10.1002/pd.2218. [DOI] [PubMed] [Google Scholar]
- 18.Arn P, Funanage VL. 3-Methylglutaconic aciduria disorders: the clinical spectrum increases. J Pediatr Hematol Oncol. 2006;28:62–63. doi: 10.1097/01.mph.0000199602.35010.89. doi:10.1097/01.mph.0000199602.35010.89. [DOI] [PubMed] [Google Scholar]
- 19.Bowles KR, Bowles NE. Genetics of inherited cardiomyopathies. Expert Rev Cardiovasc Ther. 2004;2:683–697. doi: 10.1586/14779072.2.5.683. doi:10.1586/14779072.2.5.683. [DOI] [PubMed] [Google Scholar]
- 20.Draaisma JM, van Kesteren IC, Daniels O, Sengers RC. Dilated cardiomyopathy with 3-methylglutaconic aciduria. Pediatr Cardiol. 1994;15:89–90. doi: 10.1007/BF00817615. [DOI] [PubMed] [Google Scholar]
- 21.Harris RA, Joshi M, Jeoung NH. Mechanisms responsible for regulation of branched-chain amino acid catabolism. Biochem Biophys Res Commun. 2004;313:391–396. doi: 10.1016/j.bbrc.2003.11.007. doi:10.1016/j.bbrc.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Lu G, Ren S, Korge P, Choi J, Dong Y, Weiss J, et al. A novel mitochondrial matrix serine/threonine protein phosphatase regulates the mitochondria permeability transition pore and is essential for cellular survival and development. Genes Dev. 2007;21:784–796. doi: 10.1101/gad.1499107. doi:10.1101/gad.1499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu G, Sun H, She P, Youn JY, Warburton S, Ping P, et al. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J Clin Invest. 2009;119:1678–1687. doi: 10.1172/JCI38151. doi:10.1172/JCI38151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Proud CG. Regulation of mammalian translation factors by nutrients. Eur J Biochem. 2002;269:5338–5349. doi: 10.1046/j.1432-1033.2002.03292.x. doi:10.1046/j.1432-1033.2002.03292.x. [DOI] [PubMed] [Google Scholar]
- 25.Xu G, Kwon G, Cruz WS, Marshall CA, McDaniel ML. Metabolic regulation by leucine of translation initiation through the mTOR-signaling pathway by pancreatic beta-cells. Diabetes. 2001;50:353–360. doi: 10.2337/diabetes.50.2.353. doi:10.2337/diabetes.50.2.353. [DOI] [PubMed] [Google Scholar]
- 26.Zhang D, Contu R, Latronico MV, Zhang J, Rizzi R, Catalucci D, et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 2010;120:2805–2816. doi: 10.1172/JCI43008. doi:10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katta A, Kundla S, Kakarla SK, Wu M, Fannin J, Paturi S, et al. Impaired overload-induced hypertrophy is associated with diminished mTOR signaling in insulin-resistant skeletal muscle of the obese Zucker rat. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1666–R1675. doi: 10.1152/ajpregu.00229.2010. doi:10.1152/ajpregu.00229.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill JA. Autophagy in cardiac plasticity and disease. Pediatr Cardiol. 2011;32:282–289. doi: 10.1007/s00246-010-9883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aviv Y, Shaw J, Gang H, Kirshenbaum LA. Regulation of autophagy in the heart: ‘You Only Live Twice. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2010.3479. doi:10.1089/ars.2010.3479. Published online ahead of print 7 January 2011. [DOI] [PubMed] [Google Scholar]
- 30.Sciarretta S, Hariharan N, Monden Y, Zablocki D, Sadoshima J. Is Autophagy in response to ischemia and reperfusion protective or detrimental for the heart? Pediatr Cardiol. 2010;32:275–281. doi: 10.1007/s00246-010-9855-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu H, Rothermel BA, Hill JA. Autophagy in load-induced heart disease. Methods Enzymol. 2009;453:343–363. doi: 10.1016/S0076-6879(08)04017-2. doi:10.1016/S0076-6879(08)04017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishida K, Kyoi S, Yamaguchi O, Sadoshima J, Otsu K. The role of autophagy in the heart. Cell Death Differ. 2009;16:31–38. doi: 10.1038/cdd.2008.163. doi:10.1038/cdd.2008.163. [DOI] [PubMed] [Google Scholar]
- 33.Gustafsson AB, Gottlieb RA. Autophagy in ischemic heart disease. Circ Res. 2009;104:150–158. doi: 10.1161/CIRCRESAHA.108.187427. doi:10.1161/CIRCRESAHA.108.187427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meijer AJ, Dubbelhuis PF. Amino acid signalling and the integration of metabolism. Biochem Biophys Res Commun. 2004;313:397–403. doi: 10.1016/j.bbrc.2003.07.012. doi:10.1016/j.bbrc.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Hinault C, Van Obberghen E, Mothe-Satney I. Role of amino acids in insulin signaling in adipocytes and their potential to decrease insulin resistance of adipose tissue. J Nutr Biochem. 2006;17:374–378. doi: 10.1016/j.jnutbio.2006.02.008. doi:10.1016/j.jnutbio.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Xiao F, Huang Z, Li H, Yu J, Wang C, Chen S, et al. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes. 2011;60:746–756. doi: 10.2337/db10-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishimura J, Masaki T, Arakawa M, Seike M, Yoshimatsu H. Isoleucine prevents the accumulation of tissue triglycerides and upregulates the expression of PPARalpha and uncoupling protein in diet-induced obese mice. J Nutr. 2010;140:496–500. doi: 10.3945/jn.109.108977. doi:10.3945/jn.109.108977. [DOI] [PubMed] [Google Scholar]
- 38.D'Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010;12:362–372. doi: 10.1016/j.cmet.2010.08.016. doi:10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. 2010;3:207–214. doi: 10.1161/CIRCGENETICS.109.852814. doi:10.1161/CIRCGENETICS.109.852814. [DOI] [PubMed] [Google Scholar]
- 40.Skvorak KJ. Animal models of maple syrup urine disease. J Inherit Metab Dis. 2009;32:229–246. doi: 10.1007/s10545-009-1086-z. doi:10.1007/s10545-009-1086-z. [DOI] [PubMed] [Google Scholar]