Abstract
The most notable change in the metabolic profile of hypertrophied hearts is an increased reliance on glucose with an overall reduced oxidative metabolism, i.e. a reappearance of the foetal metabolic pattern. In animal models, this change is attributed to the down-regulation of the transcriptional cascades promoting gene expression for fatty acid oxidation and mitochondrial oxidative phosphorylation in adult hearts. Impaired myocardial energetics in cardiac hypertrophy also triggers AMP-activated protein kinase (AMPK), leading to increased glucose uptake and glycolysis. Aside from increased reliance on glucose as an energy source, changes in other glucose metabolism pathways, e.g. the pentose phosphate pathway, the glucosamine biosynthesis pathway, and anaplerosis, are also noted in the hypertrophied hearts. Studies using transgenic mouse models and pharmacological compounds to mimic or counter the switch of substrate preference in cardiac hypertrophy have demonstrated that increased glucose metabolism in adult heart is not harmful and can be beneficial when it provides sufficient fuel for oxidative metabolism. However, improvement in the oxidative capacity and efficiency rather than the selection of the substrate is likely the ultimate goal for metabolic therapies.
Keywords: Glycolysis, Metabolic flexibility, Foetal metabolic profile
1. Introduction
Glucose is an important fuel that is used by nearly all organisms through a common set of metabolic pathways. Our knowledge of glucose metabolism dates back to 1860 with the identification of glycolysis by Louis Pasteur and climaxes in 1937 when the complete glycolytic pathway was unearthed through the work of Gustav Embden and Otto Fritz Meyerhof.1 In the same year, building on the work of Albert Szent-Gyorgyi, Hans A. Krebs and William A. Johnson showed that pyruvate could form succinate in animal tissues, thus providing the foundation for the citric acid cycle.2 More than 20 years later, Peter D. Mitchell hypothesized a chemiosmotic mechanism that ultimately led to the elucidation of the electron transport chain and oxidative phosphorylation,3 and thus, completed the pathway for aerobic glucose metabolism for ATP generation.
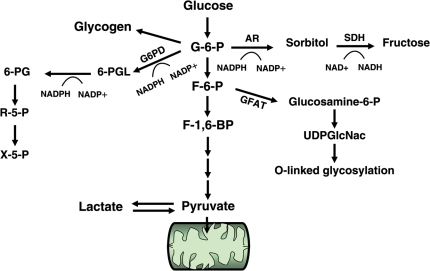
We now know that glucose can be metabolized in multiple pathways providing not only an energy supply, but also many other important metabolites for cell growth and function. Figure 1 provides an overview of the metabolic fates of glucose in a cardiac myocyte. Glucose enters the cardiac myocyte by facilitated diffusion via glucose transporters (GLUT). GLUT1 and GLUT4 are the major isoforms present in the heart; GLUT1 mediates insulin-independent and GLUT4 mediates insulin-sensitive glucose transport. Intracellular glucose is phosphorylated by hexokinase to glucose-6-phosphate (G-6-P) and through glycolysis eventually yields pyruvate, which is oxidized in the mitochondria. Although the amount of ATP derived from glycolysis is rather small (2ATP/glucose), it has been proposed to play a critical role for the maintenance of ion pump function due to the proximity of glycolytic enzymes and the ATPases.4,5 However, G-6-P can also be processed in several other pathways such as glycogen synthesis, the pentose phosphate pathway (PPP), or the aldose reductase (AR)/polyol pathway. In addition, the formation of fructose-6-phosphate (F-6-P) can feed through the hexosamine biosynthetic pathway (HBP). Although glycolysis and pyruvate oxidation are the preferred fates of glucose, the accessory pathways play important roles in regulating biological functions of the cell.
Figure 1.
Glucose metabolism pathways. 6-PG, 6-phosphogluconate; 6-PGL, 6-phosphoglucono-δ-lactone; AR, aldose reductase; F-6-P, fructose-6-phosphate; F-1,6,-BP, fructose-1,6-bisphosphate; G-6-P, glucose-6-phosphate; GFAT, glutamine fructose-6-phosphate amidotransferase; G6PD: glucose-6-phosphate dehydrogenase; NAD+, nicotinamide adenine dinucleotide; NADH, reduced nicotinamide adenine dinucleotide; NADP+, nicotinamide adenine dinucleotide phosphate; NADPH, reduced nicotinamide adenine dinucleotide phosphate; R-5-P, ribose-5-phosphate; SDH, sorbitol dehydrogenase; UDPGlcNAc, uridine diphosphate-N-acetylglucosamine; X-5-P, xylose-5-phosphate.
2. Glucose metabolism is increased in cardiac hypertrophy
In the normal, adult heart, oxidation of fatty acids contributes the majority of carbon substrates to ATP generation.6 However, the heart possesses tremendous metabolic flexibility highlighted by its ability to utilize glucose, lactate, ketones, and amino acids. Preference in substrate utilization can change in response to altered substrate availability or altered regulation of metabolic pathways. For example, it is well documented that fuel preference switches from glucose and lactate (collectively referred to as carbohydrates) in the foetal heart to predominantly lipids in the adult heart.7,8 It has also been observed that the substrate metabolism in animal models of cardiac hypertrophy recapitulates the ‘foetal metabolic profile’ with an increased preference for carbohydrate sources.9–11 This observation was consistent with the reappearance of the foetal gene expression in cardiac hypertrophy and was thus considered integral to the pathological remodelling of the heart. Although there are multiple cell types in the heart, this paper reviews the changes of myocardial energy metabolism in the beating heart which primarily originates from cardiomyocytes as they are responsible for the majority of oxygen consumption during contraction.12
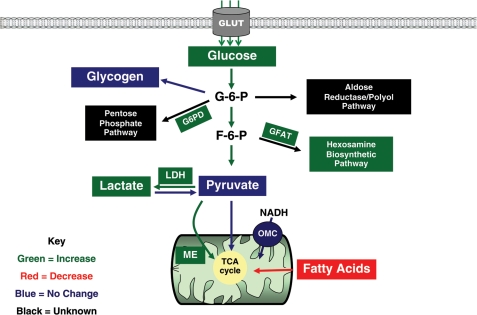
Major changes of glucose metabolism in cardiac hypertrophy are summarized in Figure 2. The hallmark of increased glucose metabolism in the hypertrophied heart is accelerated glycolysis, a finding supported by studies using carbon isotope labelling techniques in isolated perfused hearts.13–16 Consistent with this observation, several studies showed a higher rate of glucose uptake in animal models of cardiac hypertrophy.16–19 Interestingly, alterations in expression or capacity of glycolytic enzymes do not consistently coincide with increased glycolysis16,18,20 suggesting that the altered glycolytic flux is attributable to altered regulation rather than the expression of the glycolytic enzymes. Under aerobic conditions, NADH produced from glycolysis is delivered to the mitochondria through the malate–aspartate shuttle.21 Therefore, with an accelerated glycolytic rate, the potential for adaptations in the hypertrophied heart to increased quantities of NADH may be present. In the foetal heart, NADH shuttling is accomplished through the activity of the malate–aspartate shuttle and the α-glycerophosphate shuttle, with the malate–aspartate shuttle being predominant in the adult heart.21–23 It has been shown, in accordance with the high production of glycolytic NADH in the foetal heart, that the activity of the malate–aspartate shuttle and the expression of a key shuttle protein, the oxoglutarate–malate carrier (OMC), are elevated.24 However, two separate studies failed to show a demonstrable increase in the activity or expression of the OMC, especially during the compensatory phase of cardiac hypertrophy,25,26 despite an overall increase in the glycolytic flux.26 It is possible that the inherent capacity of this shuttle is sufficient to deal with elevated NADH levels or, alternatively, other unidentified mechanisms may have responded to the increased cytosolic NADH in the hypertrophied myocardium.
Figure 2.
Altered glucose metabolism in cardiac hypertrophy. Key changes in the metabolic pathway have been colour coded. Green: increased; Red: decreased; Blue: no change; Black: unknown. F-6-P, fructose-6-phosphate; G-6-P, glucose-6-phosphate; G6PD, glucose-6-phosphate dehydrogenase; GFAT, glutamine fructose-6-phosphate amidotransferase; GLUT, glucose transporter; LDH, lactate dehydrogenase; ME, malic enzyme; NADH, reduced nicotinamide adenine dinucleotide; OMC, oxoglutarate-malate carrier; TCA, tricarboxylic acid.
Despite increased glycolysis many studies have found either no change or a decrease in the glucose oxidation, suggesting an ‘uncoupling’ of glycolysis and glucose oxidation in cardiac hypertrophy.13,14,27–29 As consistently shown in experimental models, increased activity of lactate dehydrogenase (LDH), the enzyme responsible for the conversion of pyruvate to lactate, has been documented as well as the elevated efflux of lactate from the myocardium.30,31 Although the production and efflux of lactate are increased in hypertrophied hearts, the overall rate of lactate oxidation is similar to non-hypertrophied hearts.13,32,33 Furthermore, the fatty acid oxidation (FAO) rate and the relative contribution of fatty acids to the overall substrate oxidation are significantly decreased in hypertrophied hearts.13,34
Pyruvate has the ability to participate in accessory pathways that supply carbon-based substrates to the tricarboxylic acid (TCA) cycle, a process termed ‘anaplerosis’.35 In particular, pyruvate can be carboxylated by pyruvate carboxylase yielding oxaloacetate or carboxylated by malic enzyme (ME) yielding malate. In hypertrophied rat hearts, an 80–90% increase in anaplerotic flux was observed and supported with increased tissue content of malate.36,37 A significant increase in the gene expression of ME without a change in pyruvate carboxlyase37 supported the idea of increased anaplerosis through the pyruvate–malate pathway. Although the increased anaplerotic flux in hypertrophied myocardium is sufficient to maintain overall TCA cycle flux, it reduces the efficiency of ATP production from pyruvate. To date, there is limited research on the contribution of anaplerosis in cardiac hypertrophy and its progression to heart failure. Further investigation in this direction is clearly warranted.
3. Potential mechanisms for increased glucose reliance
A large number of studies in the past two decades have demonstrated that the transcriptional regulation of genes involved in mitochondrial oxidative metabolism has changed significantly during the development of pathological hypertrophy and heart failure.38 Down-regulation of peroxisome proliferator-activated receptor alpha (PPARα) and peroxisome proliferator-activated receptor gamma co-activator-1 (PGC-1), master regulators of genes involved in fatty acid oxidation and mitochondrial biogenesis, has been observed in rodent models of cardiac hypertrophy and failure.32,39–42 The PPARα target, carnitine-palmitoyl transferase 1 (CPT1), which facilitates fatty acid transport into the mitochondria, is also down-regulated.18,37,41,43 Likewise, medium chain acyl-CoA dehydrogenase, an enzyme important for beta-oxidation, is also reduced.18,32,41 Moreover, it has been shown that the liver isoform of CPT1, which is highly expressed in the foetal heart, increases in pressure-overload hypertrophy.37 In addition, decreases in plasma membrane-bound fatty acid transporters44,45 and carnitine, which is necessary for transport of fatty acyl CoAs into the mitchondria,13,46,47 have been noted. Therefore, an overall reduction in the supply of cytosolic and mitochondrial fatty acids may be responsible for hampered fatty acid metabolism in cardiac hypertrophy. Deletion of PPARα in mice results in decreased FAO and increased glucose oxidation in the heart, whereas deletion of PGC-1α and/or β results in decreased expression of proteins in oxidative phosphorylation, increased expression of foetal metabolic genes, and defective ATP supply.48,49 Therefore, it has been proposed that increased reliance on glucose is likely a counter to the down-regulation of FAO and overall oxidative metabolism in cardiac hypertrophy.
As described above, increased glycolytic flux in cardiac hypertrophy is associated with a higher rate of insulin-independent glucose uptake with no significant changes in the expression of the glucose transporter proteins or glycolytic enzymes.11,16,20,50 One of the mechanisms proposed for these findings is the activation of an intracellular energy-sensor, 5′-AMP-activated protein kinase (AMPK), triggered by impaired myocardial energetics.16,18,51 Increased AMPK activity promotes the translocation of the glucose transporters onto the plasma membrane and enhances glucose uptake.16 In addition, AMPK stimulates glycolysis by phosphorylation and activation of phosphofructokinase 2 (PFK2), which generates fructose-2,6-diphosphate that acts as a potent allosteric stimulant of the rate-limiting enzyme, PFK1.52,53 The AMPK mechanism is consistent with the observation that increased glucose uptake and glycolysis is insulin-independent in cardiac hypertrophy. However, evidence from loss-of-function studies is still pending to demonstrate the necessity of increased AMPK activity and to determine to what extent AMPK activation contributes to increased glycolysis in cardiac hypertrophy and failure.
Much of our understanding of the metabolic adaptations that occur during the transition of cardiac hypertrophy to failure has been garnered from studying animal models. In this regard, the translation from animal studies to the human population remains a serious challenge. For example, down-regulation of PPARα is not evident in human heart failure and the expression level of PGC-1 in human failing hearts is variable, with the most recent reports showing a down-regulation of oestrogen-related receptor α rather than PGC-1.54,55 Although evidence that the human failing heart is energy ‘starved’ suggests a strong link to mitochondrial dysfunction,56 mechanisms contributing to altered substrate metabolism in human failure are much more complex than in animal models. The only available method in assessing myocardial glucose and fatty acid uptake and utilization is by positron emission tomography (PET). The PET study is not routinely used clinically and its results are influenced by myocardial perfusion, plasma fatty acid levels, and insulin sensitivity. A number of conflicting results have been reported in heart failure patients depending on aetiology and co-morbidity.57–61 Therefore, further experimentation elucidating the differences in animal and human models are warranted.
4. Pentose phosphate pathway in the hypertrophied heart
The PPP allows for an alternative fate of glycolytic intermediates. The pathway has been identified in the cytosol of all cells and is divided into two branches (Figure 1): oxidative PPP and non-oxidative PPP.62 The primary function of the oxidative PPP is to form NADPH, which is important in combating reactive oxygen species by maintaining reduced glutathione levels. The oxidative PPP utilizes G-6-P created from the initial reaction of glycolysis as a substrate through the action of glucose-6-phosphate dehydrogenase (G6PD). In the non-oxidative PPP, formation of ribose-5-phosphate and/or xylulose-5-phosphate is important in nucleotide or nucleic acid synthesis or as a possible transcriptional signalling molecule, respectively.63,64 Although this pathway is present in all cells, its overall importance in normal cardiac metabolism is believed to be minor.15,65,66
Early studies reported an up-regulation of the PPP in cardiac hypertrophy.67,68 Activity of the regulatory enzyme, G6PD, has been shown to be elevated in animal models of pressure overload66,69 and a canine model of heart failure,70 whereas no changes in the flux or enzymes involved in oxidative or non-oxidative PPP in hypertrophied hearts have been found.15,71 Of note, mice deficient in G6PD had increased ischaemia-reperfusion injury, alluding to the importance of the PPP in protection against oxidative injury.72 However, recent studies suggest that excessive NADPH derived from the oxidative PPP contributes to cardiomyopathy and heart failure.70,73 Thus, whether the observed increases in G6PD are beneficial or detrimental in the development of pathological cardiac hypertrophy and failure requires further investigation.
5. Other pathways for glucose metabolism in the hypertrophied heart
Glycogen metabolism has been shown to be an active process that contributes to glycolysis especially during periods of increased work and ischaemia.65,74–76 Both normal and pressure-overloaded hearts possess similar glycogen content at baseline and have equal rates of glycogen contribution to glycolysis, especially during low-flow ischaemia.27,77 However, hypertrophied hearts preferentially oxidize glucose from glycogen stores as opposed to exogenous glucose,27 suggesting an enhanced glycogen turnover rate, specifically detected during an interval of severe ischaemia.28 Despite this, there does not appear to be an appreciable difference in the contribution of glycogen metabolism to the metabolic profile in the hypertrophied heart.
Increased glucose entry affords an opportunity for the recruitment of the HBP (Figure 1). This pathway converts F-6-P to the principal end product uridine diphosphate-N-acetylglucosamine (UDPGlcNAc).78 UDPGlcNAc can subsequently be used for the O-linked glycosylation of a variety of proteins through the actions of transferases.79 To this end, a recent study showed that one of these proteins, O-linked β-N-acetylglucosamine transferase, was elevated in a mouse model of heart failure.80 Additionally, increased amounts of UDPGlcNAc and increased gene expression of the rate-limiting enzyme, glutamine fructose-6-phosphate amidotransferase (GFAT), were identified in a mouse model of pressure overload hypertrophy71 (Figure 2). Increased HBP flux may promote protein O-linked β-N-acetylglucosamine glycosylation (O-GlcNAcylation). It has been shown that increased O-GlcNAcylation resulted in cardiomyocyte dysfunction associated with decreased sarcoplasmic reticulum Ca2+-ATPase expression and abnormal calcium transients in cardiomyocytes exposed to hyperglycaemia or from diabetic rats.81,82 In addition, increased glycosylation has been linked to increased apoptosis.83
Recent studies have also suggested that glucose metabolism regulates protein acetylation in many cell types 84,85. Multiple enzymes involved in glycolysis, β-oxidation, and the TCA cycle were acetylated in liver tissue in response to glucose or fatty acids.85 More recently, acetylation of myosin heavy chain isoforms was found in cardiac hypertrophy which resulted in increased affinity between actin and myosin with a higher sliding velocity, suggesting a possible mechanism for enhanced contractile performance of the hypertrophied myocardium.86 Overall, post-translational modifications via glycosylation and acetylation in cardiac hypertrophy is unexplored. As these modifications are dependent on cell metabolism, they potentially are important mechanisms connecting altered cardiac metabolism to the pathogenesis of heart failure, hence, an attractive future direction of research.
A surplus of G-6-P may also be converted to sorbitol via the enzyme AR in the polyol pathway (Figure 1). Increased flux through this pathway is linked to abnormal glucose metabolism in diabetes. In human studies, an approximate two-fold increase in gene expression of AR was noted87 while AR inhibition was associated with increased cardiac function in diabetic patients.88 In perfused rodent hearts and cardiomyocytes, cardiac dysfunction and abnormal calcium transients under high-glucose conditions were abolished with an AR inhibitor.89 Using mouse models to study this pathway is problematic as the levels of AR expression and activity are much lower in mice than in humans; however, overexpression of the human form of AR is associated with impaired functional recovery after ischaemia.90 The exact role that the polyol pathway plays in the development of cardiac hypertrophy is yet to be elucidated.
6. The functional consequence of altered glucose metabolism in cardiac hypertrophy
Because of its association with the ‘foetal profiles’, increased glucose utilization in cardiac hypertrophy was initially considered maladaptive. However, results from bioengineered mouse models with enhanced or reduced glucose utilization have demonstrated that glucose reliance in the adult heart is not harmful while reduced ability to utilize glucose is detrimental in cardiac hypertrophy and failure. For example, mice overexpressing the insulin-independent glucose transporter GLUT1 in the heart have increased glucose uptake, a high glycolytic flux partially uncoupled with glucose oxidation, and a concomitant decrease in FAO.91–94 These mice lived a normal life span with unaltered cardiac function despite demonstrating a foetal-like metabolic profile.93 When subjected to pressure overload by aortic constriction, they were protected against the development of cardiac dysfunction and left ventricular dilation.91 Conversely, deletion of the insulin-sensitive glucose transporter GLUT4 or the insulin receptor in the heart results in cardiac dysfunction and poor outcome in response to hypertrophic stimuli.95,96 Furthermore, pharmacological compounds that improve insulin signalling, such as glucagon-like peptide, decreased circulating fatty acid levels and increased myocardial glucose uptake, which were beneficial in the short-term treatment of heart failure in both animal experiments and clinical studies.28,97–100 It is also important to recognize that these approaches have changed not only the relative contribution, but also the capacity of glucose utilization. The resultant benefit could be attributed to the improved oxidative ATP production.
Pharmacological inhibition of CPT1, a key regulator of mitochondrial fatty acid uptake, has been shown to partially reduce FAO and, subsequently, enhance glucose oxidation. Treatment with these compounds has demonstrated positive outcomes in animal models of heart failure. The use of oxfenicine in a canine model of heart failure resulted in an attenuation of LV dilation and wall thinning while maintaining gene expression of key enzymes involved in cardiac energy metabolism.101 In rodent models of heart failure, etomoxir enhanced myocardial performance through partial normalization of myosin isozymes102 and improvement of sarcoplasmic reticulum calcium uptake.103 Recently, short-term treatment with perhexiline, in conjunction with standard therapeutic interventions, in patients with chronic heart failure was sufficient to improve cardiac function and peak exercise oxygen consumption. Another partial FAO inhibitor, trimetazidine, led to improved LV systolic and diastolic function in elderly patients with ischaemic cardiomyopathy.104
Although the preponderance of evidence is in favour of enhancing glucose metabolism in the failing myocardium, a recent clinical study provided contradictory evidence. Administration of an inhibitor of lipolyis in patients with dilated cardiomyopathy resulted in a significant decrease in myocardial fatty acid uptake, which was associated with decreased cardiac work and myocardial efficiency.105 Since FAO is responsible for the majority of energy supply in the normal heart, strategies focusing on preventing the deficiency of FAO in the failing heart seem reasonable. However, enhancing FAO by targeting PPARα has not provided convincing conclusions. Overexpression of PPARα resulted in contractile dysfunction while reactivation of PPARα with an agonist in a model of pressure overload hypertrophy resulted in impaired response to myocardial ischaemia.34,106 Chronic activation of PPARα with fenofibrate in rats post-MI or in dogs with pacing-induced heart failure maintained the FAO gene profile but had modest benefits on the development of heart failure.107,108 Recently, several studies suggested that high-fat diet protected against the development of heart failure in rat models.69,109,110 Of note, this coincides with the clinical observation of the “Obesity Paradox”, in which patients with a high body mass index (BMI) have reduced morality from heart failure.111,112 Although the mechanisms underlying the benefits of a high-fat diet in rats and/or obesity in heart failure patients are unknown, the observations clearly challenge the concept that fatty acids are detrimental to the failing heart.
Recently, several studies have begun to manipulate relative substrate oxidation at the point where they enter the mitochondria using engineered mouse models with cardiac-specific overexpression or deletion of pyruvate dehydrogenase kinase or acetyl-CoA carboxylase 2.113–115 These models should yield valuable information specific to the changes in substrate oxidation in cardiac hypertrophy. However, it is also important to bear in mind the limitations of these models. For example, the baseline metabolic phenotype present at birth could trigger compensatory mechanisms that confound the response to a pathological stimulus. Additionally, the biased use of one particular substrate prior to the induction of pathological hypertrophy could confine the ability to detect therapeutic strategies. Therefore, it is worthwhile to investigate cases in which the metabolic perturbation occurs after cardiac hypertrophy is present.
7. Summary and future perspectives
Research using animal models in the past several decades has consistently demonstrated that the hypertrophied heart possesses an altered metabolic profile that is similar to the foetal heart. Several plausible mechanisms have been proposed that have substantially enhanced our understanding of the metabolic regulation during chronic stress. The challenge we now face is the translation from animal studies to patients to identify therapeutic opportunities. Here, it is important to recognize that human diseases differ from the animal models not only by the fundamental biology, but also by the co-morbidity and environmental inputs such as diet and medications. Most of the work in the past decade has focused on the relative oxidation of fatty acids vs. glucose in the heart. These efforts have collectively shown that there is no single good or bad substrate for the heart, and that maintaining a metabolic flexibility is critical for normal cardiac function. Therefore, the future work should be directed to improve the overall capacity for ATP generation while maintaining a balance of substrate supply and utilization. Furthermore, the changes in the non-energy producing pathways of substrate metabolism may play equally important roles in the disease mechanisms and deserve equal attention in our future research.
Conflict of interest: none declared.
Funding
This work was supported by grants from the National Institutes of Health fund R01 HL059246, R01 HL067970, R01 HL088634 (to R.T.) and F32 HL096284 (to S.C.K.).
References
- 1.Kresge N, Simoni RD, Hill RL. Otto Fritz Meyerhof and the elucidation of the glycolytic pathway. J Biol Chem. 2005;280:e3. [PubMed] [Google Scholar]
- 2.Kornberg H. Krebs and his trinity of cycles. Nat Rev Mol Cell Biol. 2000;1:225–228. doi: 10.1038/35043073. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 4.Hasin Y, Barry WH. Myocardial metabolic inhibition and membrane potential, contraction, and potassium uptake. Am J Physiol. 1984;247:H322–H329. doi: 10.1152/ajpheart.1984.247.2.H322. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura K, Kusuoka H, Ambrosio G, Becker LC. Glycolysis is necessary to preserve myocardial Ca2+ homeostasis during beta-adrenergic stimulation. Am J Physiol. 1993;264:H670–H678. doi: 10.1152/ajpheart.1993.264.3.H670. [DOI] [PubMed] [Google Scholar]
- 6.Lopaschuk GD, Belke DD, Gamble J, Itoi T, Schonekess BO. Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim Biophys Acta. 1994;1213:263–276. doi: 10.1016/0005-2760(94)00082-4. [DOI] [PubMed] [Google Scholar]
- 7.Fisher DJ. Oxygenation and metabolism in the developing heart. Semin Perinatol. 1984;8:217–225. [PubMed] [Google Scholar]
- 8.Lopaschuk GD, Spafford MA, Marsh DR. Glycolysis is predominant source of myocardial ATP production immediately after birth. Am J Physiol. 1991;261:H1698–H1705. doi: 10.1152/ajpheart.1991.261.6.H1698. [DOI] [PubMed] [Google Scholar]
- 9.Barger PM, Kelly DP. Fatty acid utilization in the hypertrophied and failing heart: molecular regulatory mechanisms. Am J Med Sci. 1999;318:36–42. doi: 10.1097/00000441-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Doenst T, Pytel G, Schrepper A, Amorim P, Farber G, Shingu Y, et al. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovasc Res. 2010;86:461–470. doi: 10.1093/cvr/cvp414. [DOI] [PubMed] [Google Scholar]
- 11.Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–2931. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- 12.Saupe KW, Spindler M, Tian R, Ingwall JS. Impaired cardiac energetics in mice lacking muscle-specific isoenzymes of creatine kinase. Circ Res. 1998;82:898–907. doi: 10.1161/01.res.82.8.898. [DOI] [PubMed] [Google Scholar]
- 13.Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol. 1994;267:H742–H750. doi: 10.1152/ajpheart.1994.267.2.H742. [DOI] [PubMed] [Google Scholar]
- 14.El Alaoui-Talibi Z, Guendouz A, Moravec M, Moravec J. Control of oxidative metabolism in volume-overloaded rat hearts: effect of propionyl-L-carnitine. Am J Physiol. 1997;272:H1615–H1624. doi: 10.1152/ajpheart.1997.272.4.H1615. [DOI] [PubMed] [Google Scholar]
- 15.Leong HS, Grist M, Parsons H, Wambolt RB, Lopaschuk GD, Brownsey R, et al. Accelerated rates of glycolysis in the hypertrophied heart: are they a methodological artifact? Am J Physiol Endocrinol Metab. 2002;282:E1039–E1045. doi: 10.1152/ajpendo.00507.2001. [DOI] [PubMed] [Google Scholar]
- 16.Nascimben L, Ingwall JS, Lorell BH, Pinz I, Schultz V, Tornheim K, et al. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension. 2004;44:662–667. doi: 10.1161/01.HYP.0000144292.69599.0c. [DOI] [PubMed] [Google Scholar]
- 17.Kagaya Y, Kanno Y, Takeyama D, Ishide N, Maruyama Y, Takahashi T, et al. Effects of long-term pressure overload on regional myocardial glucose and free fatty acid uptake in rats. A quantitative autoradiographic study. Circulation. 1990;81:1353–1361. doi: 10.1161/01.cir.81.4.1353. [DOI] [PubMed] [Google Scholar]
- 18.Tian R, Musi N, D'Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Duncker DJ, Ya X, Zhang Y, Pavek T, Wei H, et al. Effect of left ventricular hypertrophy secondary to chronic pressure overload on transmural myocardial 2-deoxyglucose uptake. A 31P NMR spectroscopic study. Circulation. 1995;92:1274–1283. doi: 10.1161/01.cir.92.5.1274. [DOI] [PubMed] [Google Scholar]
- 20.Allard MF, Wambolt RB, Longnus SL, Grist M, Lydell CP, Parsons HL, et al. Hypertrophied rat hearts are less responsive to the metabolic and functional effects of insulin. Am J Physiol Endocrinol Metab. 2000;279:E487–E493. doi: 10.1152/ajpendo.2000.279.3.E487. [DOI] [PubMed] [Google Scholar]
- 21.Scholz TD, Koppenhafer SL. Reducing equivalent shuttles in developing porcine myocardium: enhanced capacity in the newborn heart. Pediatr Res. 1995;38:221–227. doi: 10.1203/00006450-199508000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Scholz TD, Koppenhafer SL, TenEyck CJ, Schutte BC. Developmental regulation of the alpha-glycerophosphate shuttle in porcine myocardium. J Mol Cell Cardiol. 1997;29:1605–1613. doi: 10.1006/jmcc.1997.0394. [DOI] [PubMed] [Google Scholar]
- 23.Scholz TD, Koppenhafer SL, tenEyck CJ, Schutte BC. Ontogeny of malate-aspartate shuttle capacity and gene expression in cardiac mitochondria. Am J Physiol. 1998;274:C780–C788. doi: 10.1152/ajpcell.1998.274.3.C780. [DOI] [PubMed] [Google Scholar]
- 24.Griffin JL, O'Donnell JM, White LT, Hajjar RJ, Lewandowski ED. Postnatal expression and activity of the mitochondrial 2-oxoglutarate-malate carrier in intact hearts. Am J Physiol Cell Physiol. 2000;279:C1704–C1709. doi: 10.1152/ajpcell.2000.279.6.C1704. [DOI] [PubMed] [Google Scholar]
- 25.Rupert BE, Segar JL, Schutte BC, Scholz TD. Metabolic adaptation of the hypertrophied heart: role of the malate/aspartate and alpha-glycerophosphate shuttles. J Mol Cell Cardiol. 2000;32:2287–2297. doi: 10.1006/jmcc.2000.1257. [DOI] [PubMed] [Google Scholar]
- 26.Lewandowski ED, O'Donnell JM, Scholz TD, Sorokina N, Buttrick PM. Recruitment of NADH shuttling in pressure-overloaded and hypertrophic rat hearts. Am J Physiol Cell Physiol. 2007;292:C1880–C1886. doi: 10.1152/ajpcell.00576.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allard MF, Henning SL, Wambolt RB, Granleese SR, English DR, Lopaschuk GD. Glycogen metabolism in the aerobic hypertrophied rat heart. Circulation. 1997;96:676–682. doi: 10.1161/01.cir.96.2.676. [DOI] [PubMed] [Google Scholar]
- 28.Wambolt RB, Henning SL, English DR, Dyachkova Y, Lopaschuk GD, Allard MF. Glucose utilization and glycogen turnover are accelerated in hypertrophied rat hearts during severe low-flow ischemia. J Mol Cell Cardiol. 1999;31:493–502. doi: 10.1006/jmcc.1998.0804. [DOI] [PubMed] [Google Scholar]
- 29.Wambolt RB, Lopaschuk GD, Brownsey RW, Allard MF. Dichloroacetate improves postischemic function of hypertrophied rat hearts. J Am Coll Cardiol. 2000;36:1378–1385. doi: 10.1016/s0735-1097(00)00856-1. [DOI] [PubMed] [Google Scholar]
- 30.Taegtmeyer H, Overturf ML. Effects of moderate hypertension on cardiac function and metabolism in the rabbit. Hypertension. 1988;11:416–426. doi: 10.1161/01.hyp.11.5.416. [DOI] [PubMed] [Google Scholar]
- 31.Smith SH, Kramer MF, Reis I, Bishop SP, Ingwall JS. Regional changes in creatine kinase and myocyte size in hypertensive and nonhypertensive cardiac hypertrophy. Circ Res. 1990;67:1334–1344. doi: 10.1161/01.res.67.6.1334. [DOI] [PubMed] [Google Scholar]
- 32.Akki A, Smith K, Seymour AM. Compensated cardiac hypertrophy is characterised by a decline in palmitate oxidation. Mol Cell Biochem. 2008;311:215–224. doi: 10.1007/s11010-008-9711-y. [DOI] [PubMed] [Google Scholar]
- 33.Schonekess BO, Allard MF, Lopaschuk GD. Propionyl L-carnitine improvement of hypertrophied heart function is accompanied by an increase in carbohydrate oxidation. Circ Res. 1995;77:726–734. doi: 10.1161/01.res.77.4.726. [DOI] [PubMed] [Google Scholar]
- 34.Young ME, Laws FA, Goodwin GW, Taegtmeyer H. Reactivation of peroxisome proliferator-activated receptor alpha is associated with contractile dysfunction in hypertrophied rat heart. J Biol Chem. 2001;276:44390–44395. doi: 10.1074/jbc.M103826200. [DOI] [PubMed] [Google Scholar]
- 35.Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 36.Pound KM, Sorokina N, Ballal K, Berkich DA, Fasano M, Lanoue KF, et al. Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: attenuating upregulated anaplerosis in hypertrophy. Circ Res. 2009;104:805–812. doi: 10.1161/CIRCRESAHA.108.189951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorokina N, O'Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, et al. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation. 2007;115:2033–2041. doi: 10.1161/CIRCULATIONAHA.106.668665. [DOI] [PubMed] [Google Scholar]
- 38.Tian R. Transcriptional regulation of energy substrate metabolism in normal and hypertrophied heart. Curr Hypertens Rep. 2003;5:454–458. doi: 10.1007/s11906-003-0052-7. [DOI] [PubMed] [Google Scholar]
- 39.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci USA. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehman JJ, Kelly DP. Transcriptional activation of energy metabolic switches in the developing and hypertrophied heart. Clin Exp Pharmacol Physiol. 2002;29:339–345. doi: 10.1046/j.1440-1681.2002.03655.x. [DOI] [PubMed] [Google Scholar]
- 41.Barger PM, Brandt JM, Leone TC, Weinheimer CJ, Kelly DP. Deactivation of peroxisome proliferator-activated receptor-alpha during cardiac hypertrophic growth. J Clin Invest. 2000;105:1723–1730. doi: 10.1172/JCI9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sack MN, Disch DL, Rockman HA, Kelly DP. A role for Sp and nuclear receptor transcription factors in a cardiac hypertrophic growth program. Proc Natl Acad Sci USA. 1997;94:6438–6443. doi: 10.1073/pnas.94.12.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Depre C, Shipley GL, Chen W, Han Q, Doenst T, Moore ML, et al. Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy. Nat Med. 1998;4:1269–1275. doi: 10.1038/3253. [DOI] [PubMed] [Google Scholar]
- 44.Aitman TJ, Glazier AM, Wallace CA, Cooper LD, Norsworthy PJ, Wahid FN, et al. Identification of Cd36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nat Genet. 1999;21:76–83. doi: 10.1038/5013. [DOI] [PubMed] [Google Scholar]
- 45.Vork MM, Trigault N, Snoeckx LH, Glatz JF, van der Vusse GJ. Heterogeneous distribution of fatty acid-binding protein in the hearts of Wistar Kyoto and spontaneously hypertensive rats. J Mol Cell Cardiol. 1992;24:317–321. doi: 10.1016/0022-2828(92)93168-j. [DOI] [PubMed] [Google Scholar]
- 46.Christian B, El Alaoui-Talibi Z, Moravec M, Moravec J. Palmitate oxidation by the mitochondria from volume-overloaded rat hearts. Mol Cell Biochem. 1998;180:117–128. [PubMed] [Google Scholar]
- 47.el Alaoui-Talibi Z, Landormy S, Loireau A, Moravec J. Fatty acid oxidation and mechanical performance of volume-overloaded rat hearts. Am J Physiol. 1992;262:H1068–H1074. doi: 10.1152/ajpheart.1992.262.4.H1068. [DOI] [PubMed] [Google Scholar]
- 48.Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Lai L, Leone TC, Zechner C, Schaeffer PJ, Kelly SM, Flanagan DP, et al. Transcriptional coactivators PGC-1alpha and PGC-lbeta control overlapping programs required for perinatal maturation of the heart. Genes Dev. 2008;22:1948–1961. doi: 10.1101/gad.1661708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paternostro G, Clarke K, Heath J, Seymour AM, Radda GK. Decreased GLUT-4 mRNA content and insulin-sensitive deoxyglucose uptake show insulin resistance in the hypertensive rat heart. Cardiovasc Res. 1995;30:205–211. [PubMed] [Google Scholar]
- 51.Allard MF, Parsons HL, Saeedi R, Wambolt RB, Brownsey R. AMPK and metabolic adaptation by the heart to pressure overload. Am J Physiol Heart Circ Physiol. 2007;292:H140–H148. doi: 10.1152/ajpheart.00424.2006. [DOI] [PubMed] [Google Scholar]
- 52.Depre C, Rider MH, Veitch K, Hue L. Role of fructose 2,6-bisphosphate in the control of heart glycolysis. J Biol Chem. 1993;268:13274–13279. [PubMed] [Google Scholar]
- 53.Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 54.Karamanlidis G, Nascimben L, Couper GS, Shekar PS, del Monte F, Tian R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ Res. 2010;106:1541–1548. doi: 10.1161/CIRCRESAHA.109.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sihag S, Cresci S, Li AY, Sucharov CC, Lehman JJ. PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J Mol Cell Cardiol. 2009;46:201–212. doi: 10.1016/j.yjmcc.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neubauer S. The failing heart—an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 57.Iozzo P. Metabolic toxicity of the heart: insights from molecular imaging. Nutr Metab Cardiovasc Dis. 2010;20:147–156. doi: 10.1016/j.numecd.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 58.Rijzewijk LJ, van der Meer RW, Lamb HJ, de Jong HW, Lubberink M, Romijn JA, et al. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol. 2009;54:1524–1532. doi: 10.1016/j.jacc.2009.04.074. [DOI] [PubMed] [Google Scholar]
- 59.Tuunanen H, Engblom E, Naum A, Nagren K, Scheinin M, Hesse B, et al. Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation. 2008;118:1250–1258. doi: 10.1161/CIRCULATIONAHA.108.778019. [DOI] [PubMed] [Google Scholar]
- 60.Taylor M, Wallhaus TR, Degrado TR, Russell DC, Stanko P, Nickles RJ, et al. An evaluation of myocardial fatty acid and glucose uptake using PET with [18F]fluoro-6-thia-heptadecanoic acid and [18F]FDG in patients with congestive heart failure. J Nucl Med. 2001;42:55–62. [PubMed] [Google Scholar]
- 61.Wallhaus TR, Taylor M, DeGrado TR, Russell DC, Stanko P, Nickles RJ, et al. Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation. 2001;103:2441–2446. doi: 10.1161/01.cir.103.20.2441. [DOI] [PubMed] [Google Scholar]
- 62.Horecker BL, Mehler AH. Carbohydrate metabolism. Annu Rev Biochem. 1955;24:207–274. doi: 10.1146/annurev.bi.24.070155.001231. [DOI] [PubMed] [Google Scholar]
- 63.Doiron B, Cuif MH, Chen R, Kahn A. Transcriptional glucose signaling through the glucose response element is mediated by the pentose phosphate pathway. J Biol Chem. 1996;271:5321–5324. doi: 10.1074/jbc.271.10.5321. [DOI] [PubMed] [Google Scholar]
- 64.Nishimura M, Uyeda K. Purification and characterization of a novel xylulose 5-phosphate-activated protein phosphatase catalyzing dephosphorylation of fructose-6-phosphate,2-kinase:fructose-2,6-bisphosphatase. J Biol Chem. 1995;270:26341–26346. doi: 10.1074/jbc.270.44.26341. [DOI] [PubMed] [Google Scholar]
- 65.Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol. 1974;36:413–459. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- 66.Zimmer HG. Regulation of and intervention into the oxidative pentose phosphate pathway and adenine nucleotide metabolism in the heart. Mol Cell Biochem. 1996;160–161:101–109. doi: 10.1007/BF00240038. [DOI] [PubMed] [Google Scholar]
- 67.Meerson FZ, Spiritchev VB, Pshennikova MG, Djachkova LV. The role of the pentose-phosphate pathway in adjustment of the heart to a high load and the development of myocardial hypertrophy. Experientia. 1967;23:530–532. doi: 10.1007/BF02137950. [DOI] [PubMed] [Google Scholar]
- 68.Zimmer HG, Ibel H, Steinkopff G. Studies on the hexose monophosphate shunt in the myocardium during development of hypertrophy. Adv Myocardiol. 1980;1:487–492. [PubMed] [Google Scholar]
- 69.Chess DJ, Khairallah RJ, O'Shea KM, Xu W, Stanley WC. A high-fat diet increases adiposity but maintains mitochondrial oxidative enzymes without affecting development of heart failure with pressure overload. Am J Physiol Heart Circ Physiol. 2009;297:H1585–H1593. doi: 10.1152/ajpheart.00599.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gupte SA, Levine RJ, Gupte RS, Young ME, Lionetti V, Labinskyy V, et al. Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. J Mol Cell Cardiol. 2006;41:340–349. doi: 10.1016/j.yjmcc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 71.Young ME, Yan J, Razeghi P, Cooksey RC, Guthrie PH, Stepkowski SM, et al. Proposed regulation of gene expression by glucose in rodent heart. Gene Regul Syst Bio. 2007;1:251–262. doi: 10.4137/grsb.s222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jain M, Cui L, Brenner DA, Wang B, Handy DE, Leopold JA, et al. Increased myocardial dysfunction after ischemia-reperfusion in mice lacking glucose-6-phosphate dehydrogenase. Circulation. 2004;109:898–903. doi: 10.1161/01.CIR.0000112605.43318.CA. [DOI] [PubMed] [Google Scholar]
- 73.Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, et al. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130:427–439. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Henning SL, Wambolt RB, Schonekess BO, Lopaschuk GD, Allard MF. Contribution of glycogen to aerobic myocardial glucose utilization. Circulation. 1996;93:1549–1555. doi: 10.1161/01.cir.93.8.1549. [DOI] [PubMed] [Google Scholar]
- 75.Goodwin GW, Taylor CS, Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem. 1998;273:29530–29539. doi: 10.1074/jbc.273.45.29530. [DOI] [PubMed] [Google Scholar]
- 76.Neely JR, Whitfield CF, Morgan HE. Regulation of glycogenolysis in hearts: effects of pressure development, glucose, and FFA. Am J Physiol. 1970;219:1083–1088. doi: 10.1152/ajplegacy.1970.219.4.1083. [DOI] [PubMed] [Google Scholar]
- 77.Schonekess BO, Allard MF, Henning SL, Wambolt RB, Lopaschuk GD. Contribution of glycogen and exogenous glucose to glucose metabolism during ischemia in the hypertrophied rat heart. Circ Res. 1997;81:540–549. doi: 10.1161/01.res.81.4.540. [DOI] [PubMed] [Google Scholar]
- 78.Hebert LF, Jr, Daniels MC, Zhou J, Crook ED, Turner RL, Simmons ST, et al. Overexpression of glutamine: fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance. J Clin Invest. 1996;98:930–936. doi: 10.1172/JCI118876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science. 2001;291:2376–2378. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- 80.Watson LJ, Facundo HT, Ngoh GA, Ameen M, Brainard RE, Lemma KM, et al. O-linked beta-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci USA. 2010;107:17797–17802. doi: 10.1073/pnas.1001907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, et al. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem. 2003;278:44230–44237. doi: 10.1074/jbc.M303810200. [DOI] [PubMed] [Google Scholar]
- 82.Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, et al. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res. 2005;96:1006–1013. doi: 10.1161/01.RES.0000165478.06813.58. [DOI] [PubMed] [Google Scholar]
- 83.Fiordaliso F, Leri A, Cesselli D, Limana F, Safai B, Nadal-Ginard B, et al. Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes. 2001;50:2363–2375. doi: 10.2337/diabetes.50.10.2363. [DOI] [PubMed] [Google Scholar]
- 84.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Samant SA, Courson DS, Sundaresan NR, Pillai VB, Tan M, Zhao Y, et al. HDAC3-dependent reversible lysine acetylation of cardiac myosin heavy chain isoforms modulates their enzymatic and motor activity. J Biol Chem. 2011;286:5567–5577. doi: 10.1074/jbc.M110.163865. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Yang J, Moravec CS, Sussman MA, DiPaola NR, Fu D, Hawthorn L, et al. Decreased SLIM1 expression and increased gelsolin expression in failing human hearts measured by high-density oligonucleotide arrays. Circulation. 2000;102:3046–3052. doi: 10.1161/01.cir.102.25.3046. [DOI] [PubMed] [Google Scholar]
- 88.Johnson BF, Nesto RW, Pfeifer MA, Slater WR, Vinik AI, Chyun DA, et al. Cardiac abnormalities in diabetic patients with neuropathy: effects of aldose reductase inhibitor administration. Diabetes Care. 2004;27:448–454. doi: 10.2337/diacare.27.2.448. [DOI] [PubMed] [Google Scholar]
- 89.Tang WH, Cheng WT, Kravtsov GM, Tong XY, Hou XY, Chung SK, et al. Cardiac contractile dysfunction during acute hyperglycemia due to impairment of SERCA by polyol pathway-mediated oxidative stress. Am J Physiol Cell Physiol. 2010;299:C643–C653. doi: 10.1152/ajpcell.00137.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hwang YC, Kaneko M, Bakr S, Liao H, Lu Y, Lewis ER, et al. Central role for aldose reductase pathway in myocardial ischemic injury. Faseb J. 2004;18:1192–1199. doi: 10.1096/fj.03-1400com. [DOI] [PubMed] [Google Scholar]
- 91.Liao R, Jain M, Cui L, D'Agostino J, Aiello F, Luptak I, et al. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 2002;106:2125–2131. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
- 92.Luptak I, Balschi JA, Xing Y, Leone TC, Kelly DP, Tian R. Decreased contractile and metabolic reserve in peroxisome proliferator-activated receptor-alpha-null hearts can be rescued by increasing glucose transport and utilization. Circulation. 2005;112:2339–2346. doi: 10.1161/CIRCULATIONAHA.105.534594. [DOI] [PubMed] [Google Scholar]
- 93.Luptak I, Yan J, Cui L, Jain M, Liao R, Tian R. Long-term effects of increased glucose entry on mouse hearts during normal aging and ischemic stress. Circulation. 2007;116:901–909. doi: 10.1161/CIRCULATIONAHA.107.691253. [DOI] [PubMed] [Google Scholar]
- 94.Yan J, Young ME, Cui L, Lopaschuk GD, Liao R, Tian R. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation. 2009;119:2818–2828. doi: 10.1161/CIRCULATIONAHA.108.832915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE. Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol. 2003;285:H1261–H1269. doi: 10.1152/ajpheart.00108.2003. [DOI] [PubMed] [Google Scholar]
- 96.Domenighetti AA, Danes VR, Curl CL, Favaloro JM, Proietto J, Delbridge LM. Targeted GLUT-4 deficiency in the heart induces cardiomyocyte hypertrophy and impaired contractility linked with Ca(2+) and proton flux dysregulation. J Mol Cell Cardiol. 2010;48:663–672. doi: 10.1016/j.yjmcc.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 97.Bhashyam S, Fields AV, Patterson B, Testani JM, Chen L, Shen YT, et al. Glucagon-like peptide-1 increases myocardial glucose uptake via p38alpha MAP kinase-mediated, nitric oxide-dependent mechanisms in conscious dogs with dilated cardiomyopathy. Circ Heart Fail. 2010;3:512–521. doi: 10.1161/CIRCHEARTFAILURE.109.900282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.D'Alessio DA, Kahn SE, Leusner CR, Ensinck JW. Glucagon-like peptide 1 enhances glucose tolerance both by stimulation of insulin release and by increasing insulin-independent glucose disposal. J Clin Invest. 1994;93:2263–2266. doi: 10.1172/JCI117225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 100.Poornima I, Brown SB, Bhashyam S, Parikh P, Bolukoglu H, Shannon RP. Chronic glucagon-like peptide-1 infusion sustains left ventricular systolic function and prolongs survival in the spontaneously hypertensive, heart failure-prone rat. Circ Heart Fail. 2008;1:153–160. doi: 10.1161/CIRCHEARTFAILURE.108.766402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lionetti V, Linke A, Chandler MP, Young ME, Penn MS, Gupte S, et al. Carnitine palmitoyl transferase-I inhibition prevents ventricular remodeling and delays decompensation in pacing-induced heart failure. Cardiovasc Res. 2005;66:454–461. doi: 10.1016/j.cardiores.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 102.Turcani M, Rupp H. Etomoxir improves left ventricular performance of pressure-overloaded rat heart. Circulation. 1997;96:3681–3686. doi: 10.1161/01.cir.96.10.3681. [DOI] [PubMed] [Google Scholar]
- 103.Rupp H, Vetter R. Sarcoplasmic reticulum function and carnitine palmitoyltransferase-1 inhibition during progression of heart failure. Br J Pharmacol. 2000;131:1748–1756. doi: 10.1038/sj.bjp.0703741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vitale C, Wajngaten M, Sposato B, Gebara O, Rossini P, Fini M, et al. Trimetazidine improves left ventricular function and quality of life in elderly patients with coronary artery disease. Eur Heart J. 2004;25:1814–1821. doi: 10.1016/j.ehj.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 105.Tuunanen H, Engblom E, Naum A, Nagren K, Hesse B, Airaksinen KE, et al. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation. 2006;114:2130–2137. doi: 10.1161/CIRCULATIONAHA.106.645184. [DOI] [PubMed] [Google Scholar]
- 106.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Morgan EE, Rennison JH, Young ME, McElfresh TA, Kung TA, Tserng KY, et al. Effects of chronic activation of peroxisome proliferator-activated receptor-alpha or high-fat feeding in a rat infarct model of heart failure. Am J Physiol Heart Circ Physiol. 2006;290:H1899–H1904. doi: 10.1152/ajpheart.01014.2005. [DOI] [PubMed] [Google Scholar]
- 108.Labinskyy V, Bellomo M, Chandler MP, Young ME, Lionetti V, Qanud K, et al. Chronic activation of peroxisome proliferator-activated receptor-alpha with fenofibrate prevents alterations in cardiac metabolic phenotype without changing the onset of decompensation in pacing-induced heart failure. J Pharmacol Exp Ther. 2007;321:165–171. doi: 10.1124/jpet.106.116871. [DOI] [PubMed] [Google Scholar]
- 109.Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, et al. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006;48:1116–1123. doi: 10.1161/01.HYP.0000248430.26229.0f. [DOI] [PubMed] [Google Scholar]
- 110.Rennison JH, McElfresh TA, Okere IC, Vazquez EJ, Patel HV, Foster AB, et al. High-fat diet postinfarction enhances mitochondrial function and does not exacerbate left ventricular dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1498–H1506. doi: 10.1152/ajpheart.01021.2006. [DOI] [PubMed] [Google Scholar]
- 111.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43:1439–1444. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 112.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156:13–22. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 113.Wende A, Pires K, Wayment B, Tuinei J, Jeoung N, Litwin S, et al. Pyruvate dehydrogenase kinase 4 (PDK4) deficiency does not impair the cardiac metabolic or contractile response to pressure overload hypertrophy. Circulation. 2009;120:S880. [Google Scholar]
- 114.Kolwicz S, Olson D, Ngoy S, Liao R, Tian R. Cardiac-specific deletion of acetyl-coA carboxylase 2 (ACC2) maintains fatty acid oxidation and left ventricular function during pressure-overload hypertrophy. Circulation. 2009;120:S855. [Google Scholar]
- 115.Zhao G, Jeoung NH, Burgess SC, Rosaaen-Stowe KA, Inagaki T, Latif S, et al. Overexpression of pyruvate dehydrogenase kinase 4 in heart perturbs metabolism and exacerbates calcineurin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;294:H936–H943. doi: 10.1152/ajpheart.00870.2007. [DOI] [PubMed] [Google Scholar]