Abstract
Unfolded protein responses (UPRs) of the endoplasmic reticulum and mitochondrial matrix have been described. Here, we show that the accumulation of proteins in the inter-membrane space (IMS) of mitochondria in the breast cancer cell line MCF-7 activates a distinct UPR. Upon IMS stress, overproduction of reactive oxygen species (ROS) and phosphorylation of AKT triggers estrogen receptor (ER) activity, which further upregulates the transcription of the mitochondrial regulator NRF1 and the IMS protease OMI (officially known as HTRA2). Moreover, we demonstrate that the IMS stress-induced UPR culminates in increased proteasome activity. Given our previous report on a proteasome- and OMI-dependent checkpoint that limits the import of IMS proteins, the findings presented in this study suggest that this newly discovered UPR acts as a cytoprotective response to overcome IMS stress.
Key words: Mitochondria, Unfolded protein response, Estrogen receptor, Protein quality control, Proteasome
Introduction
The accumulation of misfolded proteins in the lumen of the endoplasmic reticulum activates several signaling cascades collectively known as the unfolded protein response (UPR) (Mori, 2009; Schroder, 2008). The initial outcomes of the UPR are the transcription of endoplasmic reticulum chaperones, such as BiP (GRP-78), and a reduction in protein translation, to diminish the stress in the lumen of the endoplasmic reticulum (Mori, 2009; Schroder, 2008). Likewise, accumulation of misfolded proteins in the matrix of the mitochondria, referred to as matrix stress, activates a mitochondrial UPR (mtUPR) (Biswas et al., 1999; Zhao et al., 2002). The mtUPR involves upregulation of the transcription factor CHOP (DDIT-3) and endonuclease G (EndoG), a nuclease of the inter-membrane space (IMS) of mitochondria (Aldridge et al., 2007; Zhao et al., 2002). In addition, the mitochondrial protease ClpP and the nuclear transcription factor DVE-1 have also been identified as mediators of a mtUPR in Caenorhabditis elegans (Haynes et al., 2007). However, the precise sequence of events that occur in the mtUPR remains to be determined.
Evidence indicates that both the endoplasmic reticulum UPR and the matrix stress mtUPR are activated in parallel with protein quality control mechanisms, which act to eliminate damaged proteins (Brodsky and Wojcikiewicz, 2009; Tatsuta and Langer, 2009). We previously reported a protein quality control mechanism that limits the accumulation of misfolded proteins in the IMS (Radke et al., 2008). Our data revealed that the regulation of IMS proteins occurs at two levels: first, through a proteasome-dependent degradation of IMS proteins that occurs before their import into the IMS; and, second, through an OMI-dependent degradation of excessive IMS proteins that occurs following their import into the IMS (Radke et al., 2008).
As the protein quality control mechanisms for mitochondrial proteins from the matrix and the IMS are different, we initiated this study to determine whether the accumulation of IMS proteins also activates a different UPR using the breast cancer cell line MCF-7. We found that IMS stress activates a distinct UPR from that triggered by matrix stress. Notably, this UPR is mediated by estrogen receptor alpha (ERα) that is phosphorylated on serine 167 in a reactive oxygen species (ROS)- and AKT-dependent manner. In turn, activated ERα upregulates NRF1, a transcription factor required for the expression of several genes of the mitochondrial respiratory chain. In addition, both transcription of OMI (officially known as HTRA2) and the activities of the proteasome are elevated. These results suggest that this newly discovered IMS stress-mediated UPR activates a cytoprotective response aimed at upregulating components of the IMS protein quality control mechanism and genes required to protect the integrity of the mitochondria.
Results and Discussion
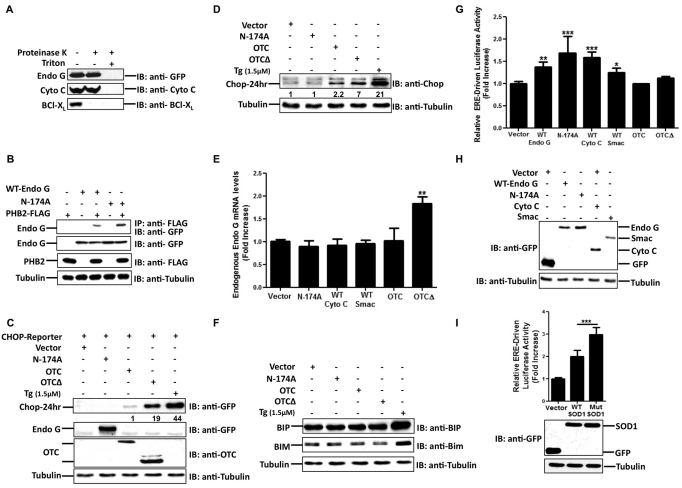
Whereas the accumulation of the misfolded proteins in the mitochondrial matrix has been explored, the consequences of excessive or misfolded proteins in the mitochondrial IMS have not been previously addressed. Therefore, we aimed to examine the effects of stress in the IMS by overexpressing IMS proteins. In a previous study, we reported that overexpression of a mutant form of EndoG (EndoG-N174A) leads to the formation of aggregates in the mitochondrial IMS and, subsequently, to mitochondrial stress (Radke et al., 2008). Similarly, we found that overexpression of EndoG-N174A in the breast adenocarcinoma MCF-7 cells led to the formation of aggregates (data not shown). To determine whether mitochondrial stress is confined within the mitochondrial IMS, we first explored whether overexpression of EndoG led to its accumulation in the IMS or to its association with the mitochondrial outer-membrane owing to saturation of the protein import machinery. A proteinase K digestion assay revealed that although the mitochondrial surface protein BCL-XL was sensitive to proteinase K digestion in intact mitochondria, both transfected EndoG and endogenous cytochrome c were resistant to digestion. By contrast, both of them were degraded upon addition of Triton X-100, suggesting that EndoG accumulates in the IMS (Fig. 1A).
Fig. 1.
Matrix and IMS stress trigger distinct UPR responses. (A) Equal amounts of mitochondria isolated from MCF-7 cells transfected with plasmids encoding mutant EndoG-N174A (Endo G) conjugated to GFP for 24 hours were assayed before and after proteinase K treatment in the presence or absence of Triton X-100. Levels of cytochrome c, EndoG-N174A and BCL-XL were detected by immunoblotting (IB) with the indicated antibodies. (B) Lysates of cells transfected with the plasmids encoding the indicated proteins (WT-Endo G, wild-type EndoG–GFP; N-174A, EndoG-N174A–GFP) were subjected to immunoprecipitation (IP) to pull down PHB2 by use of the anti-FLAG antibody. Levels of EndoG in complex with PHB2 or in crude extracts were detected with the anti-GFP antibody. (C,D) Crude extracts from MCF-7 cells transfected with the indicated plasmids (CHOP-Reporter, CHOP promoter driving GFP; Vector, GFP control vector) for 24 hours were subjected to immunoblotting to detect the levels of either GFP (27 kDa) driven by the CHOP promoter (C, top panel) or endogenous CHOP (D) by using anti-GFP or anti-CHOP antibodies, respectively. Levels of EndoG–GFP (57 kDa, Endo G) and tubulin (bottom panel) were detected by using anti-GFP and anti-tubulin antibodies, respectively. Levels of OTC and OTCΔ in the mitochondrial fraction (middle panel) were monitored using an anti-OTC antibody. Thapsigargin (Tg; 1.5 μM) was used as a positive control. The numbers in C represent the fold induction of GFP protein levels normalized to tubulin in cells transfected with EndoG-N174A, OTC and OTCΔ relative to control cells transfected with the CHOP reporter and the control GFP vector. The numbers in D represent the fold induction of CHOP expression levels normalized to tubulin in cells transfected with EndoG-N174A, OTC, OTCΔ and Tg relative to control cells transfected with GFP vector (Vector). (E) Levels of endogenous EndoG mRNA were quantified by qRT-PCR in cells transfected with the indicated plasmids (Vector, control GFP; WT Cyto C, wild-type cytochrome c; WT Smac, wild-type Smac). Data are the means+s.d. of the fold increase relative to control cells overexpressing control GFP from three independent experiments performed in triplicate. (F) Crude extracts from cells overexpressing the indicated plasmids were analyzed by immunoblotting to determine the levels of BiP (BIP) and BIM using anti-BiP and anti-BIM antibodies, respectively. Thapsigargin (1.5 μM) was used as a positive control. (G) Cells maintained in phenol-free medium and transfected with the indicated plasmids were assayed after 24 hours for ER transcription activity by measuring the ERE-driven luciferase activity. Data are means+s.d. of the fold increase relative to the control cells overexpressing control GFP from five independent experiments performed in triplicate. (H) Levels of the indicated IMS proteins were determined in cells overexpressing the respective plasmids by immunoblotting using an anti-GFP antibody. (I) Transiently transfected cells overexpressing either mitochondrially targeted wild-type (WT SOD1) only or mutant SOD1 (Mut SOD) with control GFP were analyzed for ER activity by mean of the ERE-driven luciferase assay. Data are the mean+s.d. of the fold increase relative to the control cells overexpressing GFP from three independent experiments performed in triplicate. Levels of SOD1 and GFP were evaluated by immunoblotting using anti-GFP antibody. Tubulin served as the loading control. *P<0.05; **P<0.01; ***P<0.001 for the elevation in ERE luciferase activity compared with that observed in control vector-treated cells.
The IMS localization of EndoG was further confirmed by immunoprecipitation of wild-type and mutant (N174A) EndoG with PHB2 (also known as REA), which resides in the IMS and acts as a chaperone for newly imported proteins in the IMS. We found that both the wild-type and N174A forms of EndoG associated with the chaperone PHB2 (Fig. 1B), ruling out the possibility that EndoG becomes mislocalized into the matrix upon its overexpression. As the interaction between the chaperone and newly imported protein is usually transient, we interpret the interaction between PHB2 and wild-type EndoG, or the N174A mutant, as an indication of the activation of protein quality control mechanisms.
Accumulation of a misfolded form of the matrix protein ornithine transcarbamylase (OTCΔ; a deletion of amino acids 30–114) has been reported to promote a UPR leading to the production of the IMS protein EndoG and the transcription factor CHOP (Aldridge et al., 2007; Zhao et al., 2002). Consequently, an elevation in the levels of both of these proteins can be used as a marker of matrix stress. We confirmed that expression of OTCΔ in MCF-7 cells led to an elevation in CHOP levels when using either a CHOP-dependent promoter driving the expression of the GFP reporter (Fig. 1C, top panel), or with endogenous CHOP expression (Fig. 1D). Treatment with thapsigargin was used as a positive control, as thapsigargin is known to induce CHOP by causing stress in the endoplasmic reticulum owing to a defect in glycosylation (Schroder, 2008). In parallel, an increase in EndoG mRNA levels was also evident (Fig. 1E). In contrast with OTCΔ, overexpression of EndoG-N174A did not result in either the transcription of EndoG (Fig. 1E) or CHOP (Fig. 1C,D). Expression of other IMS proteins also failed to activate EndoG transcription (Fig. 1E), suggesting that IMS stress and matrix stress do not trigger the same response.
The chaperone BiP and the pro-apoptotic protein BIM are two markers of activation of the endoplasmic reticulum UPR (Schroder, 2008). We therefore examined the effect of IMS and matrix stress on these markers of the endoplasmic reticulum UPR. Our data shows that neither of the markers was affected by EndoG-N174A, OTC or OTCΔ (Fig. 1F), suggesting that matrix and IMS stress does not mimic the effect of stress in the endoplasmic reticulum.
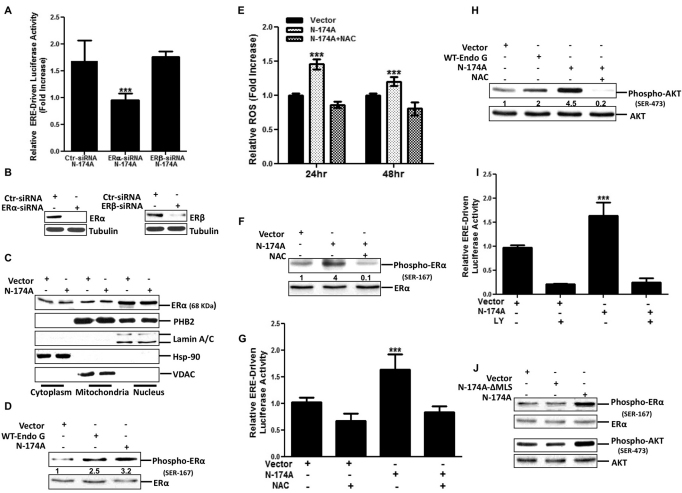
We next aimed to test whether IMS stress activates a distinct response from the one triggered by stress in the mitochondrial matrix or endoplasmatic reticulum. Estrogen receptors (ERs), which have recently been shown to localize in the mitochondria are implicated in the regulation of mitochondrial functions (Pedram et al., 2006). Therefore, we assessed whether ER activation is affected upon IMS stress by transfecting MCF7 cells with a luciferase reporter driven by a promoter containing three ER response elements (EREs). We found that expression of all the IMS proteins tested led to activation of the ERE reporter, whereas wild-type OTC, mutant OTCΔ and the control vector, which expresses GFP only and is not fused to any mitochondrial protein and therefore does not accumulate in the mitochondria, did not (Fig. 1G). As GFP was more abundant than any other IMS proteins in these experiments, the activation of ER was not due to the excessive expression of IMS proteins in itself (Fig. 1H). Although evidence has implicated mutations in the IMS-localized superoxide dismutase 1 (SOD1) enzyme as a causative factor in familial amyotrophic lateral sclerosis (Kawamata and Manfredi, 2010; Takeuchi et al., 2002), its overexpression has also been reported in breast cancer (Punnonen et al., 1994). Hence, we also tested the effect of SOD1 constructs, which localize exclusively to the IMS. Our results indicate that wild-type SOD1, and to a greater extent mutant SOD1 (G93A), where glycine 93 is replaced with an alanine residue, a mutation that has been implicated in the natural development of ALS, stimulated the activity of the ERE reporter (Fig. 1I, top panel). As observed with IMS proteins (Fig. 1H), the increased activation of the ERE reporter was not due to excessive expression of SOD1 as the GFP control protein was expressed at much higher levels than the SOD1 constructs (Fig. 1I, bottom panel). In addition, we found that, upon IMS stress, small interfering RNAs (siRNAs) against ERα, but not ERβ, abolished the activation of the ERE reporter (Fig. 2A), despite the fact that the siRNAs successfully reduced the expression of ERα and ERβ, respectively (Fig. 2B). Overall, we conclude that accumulation of a variety of different IMS proteins promotes the activity of the ERα.
Fig. 2.
ROS-mediated AKT activation is required to induce ERα activity. (A) Cells transfected with either scrambled siRNA (Ctr), or siRNA against ERα or ERβ (10 nM), were incubated in phenol-free medium for 24 hours followed by co-transfection with plasmids encoding mutant EndoG-N174A–GFP (N-174A), the ERE reporter and PRL, which encodes a constitutive luciferase reporter emitting a different wavelength and is used as a loading control. A luciferase assay was performed 24 hours later. Data are the means+s.d. of the fold increase relative to control cells overexpressing control GFP from three independent experiments performed in triplicate. ***P<0.001. (B) Lysates of cells transfected with scrambled siRNA, or siRNA against ERα or ERβ, were collected 5 days after transfection and analyzed by western blotting to evaluate the efficiency of ERα– or ERβ–knockdown using an anti-ERα or anti-ERβ antibody. (C) Subcellular fractionation of cells overexpressing either GFP (Vector) or N-174A were prepared as described in the Materials and Methods section and analyzed by western blotting using the anti-ERα antibody. Lamin A/C, Hsp90 and VDAC represent nuclear, cytoplasmic and mitochondrial protein loading controls, respectively. (D) Crude extracts of the cells transfected with the indicated plasmids (WT-Endo G, wild-type EndoG–GFP) were analyzed by western blotting using an antibody that detects phosphorylated serine 167 in ERα. (E) ROS levels were detected in live cells using the RedoxSensor Red CC-1 fluorescent dye at 24 and 48 hours post-transfection with either GFP (Vector) or N-174A in the presence or absence of NAC (5 mM). Data are the means±s.d. of the fold increase in ROS (units) relative to the GFP control cells at each timepoint. ***P<0.001 for the ROS generation in cells overexpressing mutant EndoG-N174A at 48 hours compared with at 24 hours. (F) Phosphorylated ERα (serine 167) and total ERα levels were analyzed by western blotting in cells overexpressing either GFP (Vector) or N-174A in the presence or absence of NAC (5 mM). (G) Cells treated as described in E were tested for ER transcription activity as described in A. Data are the means+s.d. of the fold increase relative to control cells overexpressing GFP (Vector) from three independent experiments performed in triplicate. ***P<0.001 for the elevation in ERE luciferase activity compared with that in cells expressing the GFP control vector. (H) Western blotting analysis of total and phosphorylated AKT was performed in cells overexpressing control GFP (Vector), wild-type EndoG–GFP or mutant EndoG-N174A–GFP treated for 24 hours with or without NAC (5 mM). The numbers in D represent the fold induction of the phosphorylated levels of ERα normalized to total levels of ERα in cells transfected with either WT-EndoG or EndoG-N174A relative to control cells transfected with the vector (GFP). The numbers in F represent the fold induction of the phosphorylated levels of ERα normalized to total levels of ERα in cells transfected with EndoG-N174A in the presence or absence of NAC relative to control cells transfected with the vector (GFP). The numbers in H represent the fold induction of the phosphorylated levels of ERα normalized to total levels of ERα in cells transfected with either EndoG-N174A-ΔMLS or EndoG-N174A relative to control cells transfected with the vector (GFP). (I) ER transcription activity was measured by ERE-driven luciferase assay in cells overexpressing the indicated plasmids in the presence or absence of LY (10 μM). Data are the mean+s.d. of the fold increase relative to the control cells overexpressing GFP from three independent experiments performed in triplicate. ***P<0.001 for the elevation in ERE luciferase activity compared with that in cells expressing the GFP control vector. (J) Lysates from cells overexpressing either GFP (Vector), EndoG-N174A or EndoG-N174A that lacks the mitochondrial localization signal (N174A-ΔMLS) were analyzed by western blotting to detect levels of phosphorylated ERα, total ERα, phosphorylated AKT and total AKT using antibodies against phosphorylated ERα (serine 167), ERα, phosphorylated AKT (serine 473) and AKT, respectively.
Among IMS proteins, the chaperones PHB1 (also known as PHB) and PHB2 (Nijtmans et al., 2000) have been shown to repress the activity of ERα (He et al., 2008; Montano et al., 1999). Given that IMS stress induces ERα activation (Fig. 1G) and that the PHB2 chaperone binds to EndoG (Fig. 1B), we hypothesized that accumulation of EndoG might titrate PHB1 and/or PHB2 away from ERα, resulting in its release from mitochondria and subsequently in its activation. However, we did not find any changes in the localization of either ERα or PHB2 (Fig. 2C). Similar results were obtained for PHB1 (data not shown), implying that other mechanisms are involved. In line with this possibility, we observed an increase in the phosphorylation of the ERα on serine 167 (Fig. 2D), which is reported to induce a ligand-independent activation of ERα (Lannigan, 2003).
To explore the mechanism leading to the phosphorylation of ERα following IMS stress, we assessed whether IMS stress resulted in ROS overproduction, and this possibility was confirmed (Fig. 2E). In addition, treatment with the antioxidant N-acetylcysteine (NAC), which is reported to prevent glutathione depletion and therefore ROS overproduction (Aruoma et al., 1989; Han et al. 2010; Zafarullah et al., 2003), was efficient at decreasing ROS levels upon IMS stress (Fig. 2E). Furthermore, treatment with NAC abolished the phosphorylation of ERα upon IMS stress (Fig. 2F) and prevented the activation of the ERE reporter (Fig. 2G). Given that ROS overproduction activates AKT, and that AKT phosphorylates ER (Campbell et al., 2001; Sun et al., 2001; Vilgelm et al., 2006), we monitored the phosphorylation of AKT following IMS stress in presence or absence of NAC. We found that although IMS stress was a potent activator of AKT (Fig. 2H), NAC addition abrogated this activation (Fig. 2H). Accordingly, we tested whether AKT inhibition, by using the AKT inhibitor LY, affects the activation of the ERE reporter. Our data shows that inhibition of AKT completely abolished the ability of IMS stress to induce the activity of the ERE reporter (Fig. 2I). We also found that deletion of the mitochondrial localization signal (MLS) of EndoG-N174A abolished the ability of EndoG to promote ERα and AKT phosphorylation, indicating that localization to the IMS is essential to trigger this response (Fig. 2J). We conclude that IMS stress promotes ROS overproduction and AKT activation to induce further the ligand-independent activation of ERα.
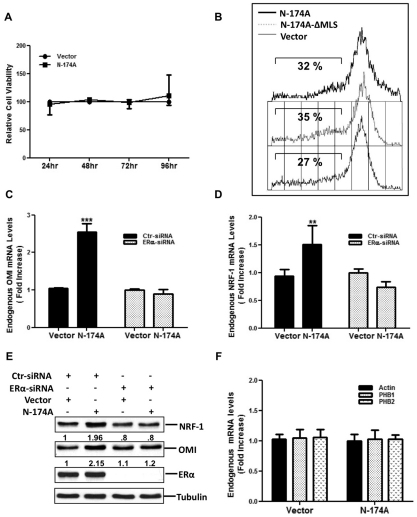
Although IMS stress is associated with ROS overproduction, our data indicated that neither cell viability (Fig. 3A) nor cleavage of PARP1 and caspase-3 (data not shown) are affected by these events. To assess the mitochondrial integrity, we performed fluorescence-activated cell sorting (FACS) analysis in cells stained with the tetramethylrhodamine ethyl ester (TMRE) dye, which only accumulates in actively respiring mitochondria with an intact membrane potential. This analysis revealed no changes in mitochondrial potential in cells undergoing IMS stress when compared with that in unstressed cells, indicating that IMS stress does not lead to the loss of mitochondrial potential (Fig. 3B).
Fig. 3.
IMS stress triggers ER-dependent up-regulation of OMI and NRF1. (A) Cell viability was measured in cells at 24, 48, 72 and 96 hours after transfection with either control GFP (Vector) or mutant EndoG-N174–GFP (N-174A) using an MTT assay as described in the Materials and Methods section. Results are the means±s.d. of the percentage cell viability relative to control GFP overexpressing cells. (B) Mitochondrial potential was evaluated in cells transfected with the indicated plasmids followed by TMRE staining and FACS analysis. The percentage of cells without TMRE staining is shown on each panel. (C) Cells transfected with control scramble siRNA (Ctr) or siRNA against ERα, followed by transfection with either control GFP (Vector) or N-174A were used to determine the levels of endogenous OMI by qRT-PCR. ***P<0.001 for the elevation in OMI mRNA compared with that in cells expressing the GFP control vector. (D) Levels of endogenous NRF1 were assessed by qRT-PCR in cells treated as described in C. Data in C and D are the means+s.d. of the fold increase relative to control cells overexpressing GFP from three independent experiments. **P<0.01 for the elevation in NRF1 mRNA compared with that in cells expressing the GFP control vector. (E) Crude extracts from cells treated as described in C were analyzed by western blotting for levels of NRF1, OMI and ERα. The numbers indicate the quantification of the NRF1:tubulin ratio and OMI:tubulin ratio relative to the baseline level set at 1 in control cells overexpressing GFP. The western blot shown is representative of more than three independent experiments. (F) Actin, PHB1 and PHB2 mRNA levels were measured by qRT-PCR. Data represent the means+s.d. of the fold increase relative to control cells overexpressing GFP from two independent experiments.
Collectively, our data suggest that this newly discovered IMS stress-induced ERα phosphorylation might stimulate a cytoprotective response to maintain the mitochondrial integrity. To gain insight in this cytoprotective response, we postulated that IMS stress affects OMI, a protein that is an integral component of a protein quality control mechanism that limits IMS stress (Radke et al., 2008). We found that there was a significant increase in OMI levels in cells exposed to IMS stress and that this was dependent on the expression of ERα (Fig. 3C). Given that estrogen-mediated ER activation induces NRF1, a major transcriptional regulator of mitochondrial respiratory chain proteins (Mattingly et al., 2008), we also evaluated the effect of IMS-stress on NRF1 levels. In a manner similar to that with OMI, IMS stress triggered an elevation of NRF1 transcription, which was dependent on ERα (Fig. 3D). Both of the effects were confirmed at the protein level (Fig. 3E). In addition, we found no alteration in the transcript levels of PHB1, PHB2 or unrelated mRNA, such as that of actin, which served as a control (Fig. 3F), suggesting that the IMS stress triggers the transcription of selected genes.
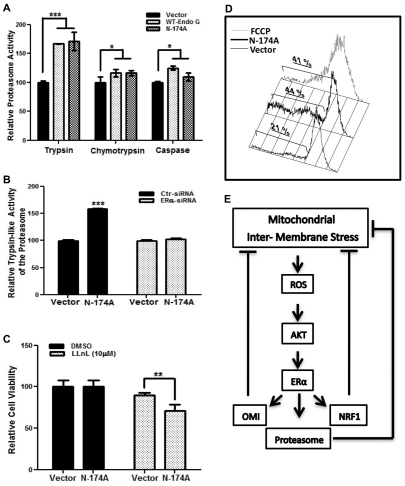
As we reported previously that the activity of the proteasome is also required to limit IMS stress (Radke et al., 2008), we next assessed whether IMS stress induces the activity of the proteasome by using the proteasome substrates succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (Suc-LLVY-AMC), N-acetyl-Gly-Pro-Leu-Ala-AMC (Ac-GPLA-AMC) and Ac-Ala-Leu-Ala-AMC (Ac-ALA-AMC), which monitor the chymotrypsin-, caspase- and trypsin-like activity of the proteasome, respectively. We found a significant increase in the trypsin-like and, to a lesser extent, the chymotrypsin-like and caspase-like activity of the proteasome upon expression of wild-type or mutant (N174A) EndoG (Fig. 4A). Furthermore, the activation of the trypsin-like activity of the proteasome upon IMS stress was abolished by siRNA against ERα (Fig. 4B). In addition, as we have previously shown that the activity of proteasome limits the import of excessive or defective IMS proteins to protect the mitochondria from IMS stress (Radke et al., 2008), we tested whether inhibition of the proteasome affects the viability of cells undergoing IMS stress. These experiments revealed an increased sensitivity of EndoG-N174A overexpressing cells to proteasome inhibition when compared with that of the respective controls (Fig. 4C). Finally, to determine whether ERα is required to maintain the mitochondrial integrity and viability of cells undergoing IMS stress (Fig. 3A,B), TMRE staining and FACS analysis was performed in cells depleted for ERα. In the absence of IMS stress, the lack of ERα had no effect on the mitochondrial potential (Fig. 4D). Likewise, cells transfected with scrambled siRNA failed to show any change in mitochondrial potential, regardless of exposure to IMS stress (data not shown). By contrast, we found that, upon exposure to IMS stress, the absence of ERα led to a twofold increase in the percentage of cells with a loss in mitochondrial potential (Fig. 4D), which was similar to that in the positive control cells treated with carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), an uncoupler of mitochondrial oxidative phosphorylation (Han et al., 2009). This result indicates that upon IMS stress, ERα activation is crucial to prevent a collapse in mitochondrial potential.
Fig. 4.
ERα is required to accelerate proteasome activity and maintain the mitochondrial potential upon IMS stress. (A) Proteosomal chymotrypsin, trypsin and caspase-like activities were assayed in cells overexpressing control GFP (Vector), wild-type EndoG–GFP (WT-Endo G) and mutant EndoG-N174A–GFP (N-174A) as described in the Materials and Methods section. ***P<0.001; *P<0.05 for the elevation in proteasome activity compared with that in cells expressing the GFP vector control. (B) Cells transfected with either scrambled siRNA (Ctr) or siRNA against ERα followed by transfection with the indicated plasmid were used to measure the trypsin-like activity of the proteasome as described in A. ***P<0.001 for the elevation in the trypsin-like activity of the proteasome compared with that in cells expressing the GFP vector control. (C) Viability of cells treated as described in B and exposed to either DMSO or the proteasome inhibitor LLnL was evaluated by the MTT assay. Data in C are the means+s.d. of the percentage cell viability relative to control cells overexpressing GFP from three independent experiments. **P<0.01 reduction in cell viability compared to cells expressing the GFP vector control. (D) Cells lacking ERα and transfected with the indicated plasmids or treated for 24 hours with FCCP (10 μM) were incubated with TMRE and analyzed by FACS to evaluate the mitochondrial potential. The percentage of cells without TMRE staining is shown on each panel. (E) Model of the IMS-stress-initiated signaling cascade, see text for details.
Taken together, our results indicate that upon IMS stress, both OMI and the proteasome, two components of the IMS protein quality control mechanism, are elevated in an ERα-dependent manner to establish a feedback mechanism (Fig. 4E). Therefore, we propose that following IMS stress, cells activate, in a ligand-independent manner, ERα to overcome mitochondrial dysfunction and maintain cellular integrity. Moreover, this ligand-independent activation of ERα upon IMS stress is due to ROS overproduction and is dependent on both ROS and activation of AKT (Fig. 4E).
Mitochondrial dysfunction is central in carcinogenesis (Plak et al., 2009; Ma, 2006; Pelicano et al., 2009), and although low levels of ROS allow a selective advantage for cancer cells to accumulate mutations, excessive production of ROS leads to cell death. Therefore, the IMS stress-induced UPR might act as a ‘rheostat’ to limit ROS production and allow cell survival. By contrast, in neurodegererative diseases, such as amyotrophic lateral sclerosis, defects in the proteasome pathway and elevated ROS, due to the accumulation of mutant SOD1 in the IMS, have been reported. Therefore, the defect in the IMS-UPR pathway reported here might also play an important role in the ontology of such diseases. Further studies will be needed to address these possibilities.
Materials and Methods
Plasmids
Mitochondrially targeted wild-type SOD1–GFP and mutant SOD1–GFP expression plasmids were gifts from Gen Sobue (Nagoya University, Nagoya, Japan). Wild-type SOD1 and mutant SOD1–GFP vector lead to expression of the IMS mitochondrial SOD1 and SOD1 with the G93A mutation, respectively. The mammalian expression vectors pCAGGS-OTC and pCAGGS-OTCΔ were kindly provided from Nick Hoogenraad (La Trobe University, Melbourne, Australia). Expression of both vectors in the mitochondrial matrix lead to accumulation of OTC and mutant OTC that can not be folded properly owing to a deletion of amino acids 30–114. Wild-type EndoG, cytochrome c, Smac, mutant EndoG-N174A, cloned into the pEGFP-N1 vectors, were kindly provided from Gregor Meiss (Justus-Liebig-University Giessen, Giessen, Germany) and lead to overexpression of each respective protein in the IMS of mitochondria. pEGFP-N1, which expresses GFP alone, was used as a control.
Western blotting
Western blotting was performed as described previously (Radke et al., 2008) using antibodies against the following proteins: ERα (rabbit; G-20, Santa Cruz Biotechnology), GFP (rabbit; Santa Cruz Biotechnology), BiP (mouse; BD Bioscience), phosphorylated AKT (mouse), AKT (rabbit), BIM (rabbit) (all Cell Signaling), phosphorylated ERα (rabbit; Upstate), OMI (rabbit; BioVision), NRF1 (rabbit; Abcam), ERβ (rabbit; Santa Cruz Biotechnology) and OTC (rabbit; Santa Cruz Biotechnology).
Subcellular fractionation
Briefly, subcellular fractionation was performed by resuspending cells in homogenization buffer [300 mM sucrose, 20 mM HEPES-KOH pH 7.5, 1 mM EDTA, 10 mM KCl, 1 mM dithiothreitol (DTT), 1.5 mM MgCl2 and protease inhibitors] and homogenizing them in the Dounce homogenizer. After centrifugation at 800 g for 10 minutes, the crude nuclear fraction (pellet) was separated from mitochondrial and cytoplasmic fraction (supernatant). The pellet was resuspended in lysis buffer (50 mM Tris-HCl pH 7.5, 0.5% NP40, 250 mM NaCl, 5 mM NaF, 0.2 mM Na3VO4, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 μg/ml phenylmethysulfonyl fluoride and 1 mM DTT) to obtain an enriched nuclear fraction. The supernatant from the first fractionation was centrifuged at 10,000 g for 20 minutes to separate mitochondria (pellet) from the cytosol (supernatant). The enriched mitochondrial fraction was further lysed in buffer (Tris-acetate pH 8, 10% NP-40, 5 mM CaCl2, 1 mM DTT and protease inhibitors).
Luciferase assay
The dual luciferase reporter assay (Promega) was performed as described previously (Ishii et al., 2006), using the firefly luciferase reporter plasmid pGL3-(ERE)3 and the Renilla luciferase reporter PRL.
Oxidative stress assay
Intracellular ROS levels were detected using the RedoxSensor Red CC-1 fluorescent dye (Molecular Probes) according to the manufacturer's protocol.
Proteasome activity
Lysates (10 μg) of MCF-7 cells were incubated for 2.5 hours at 37°C in assay buffer (50 mM Tris-HCl pH 7.5) with 10 μM proteasome substrates (Calbiochem). Release of free hydrolyzed 7-amino-4-methylcoumarin (AMC) groups was measured using an ISS Counter with an excitation filter of 380 nm and an emission filter of 460 nm.
siRNA transfection
siRNA transfection was performed with Lipofectamine 2000 (Invitrogen) using control siRNA (scramble; Ambion) or siRNA against ERα (siRNA no. 1, 5′-UCAUCGCAUUCCUUGCAAATT-3′; siRNA no. 2: 5′-AAACAGGAGGAAGAGCTGCCATT-3′) or ERβ (5′-UUUAACUCUCGAAACCUUG-3′) (GeneLink).
RNA isolation and qRT-PCR
Isolation of RNA was performed with the RNeasy Mini Kit (Qiagen) according to the manufacturer's protocol using human EndoG forward (5′-CACGTAAAGTACCAGGTCATC-3′), human NRF1 forward (5′-GGAGTGATGTCCGCACAGAA-3′), human OMI forward (5′-GACCGGCACCCTTTCTTG-3′) (GeneLink). A 100 ng sample of RNA was used for a real-time PCR assay, using the SYBR Green Universal PCR Master Mix (Applied Biosystems). The reaction was performed with the 7500 Real-Time PCR system (Applied Biosystems) under the conditions recommended by the manufacturer. Expression levels were normalized to that of actin.
Proteinase K sensitivity assay
For the proteinase K sensitivity assay, equal amounts of crude mitochondria were resuspended in digestion buffer (20 mM MOPS-KOH pH 7.2, and 250 mM sucrose) and treated for 20 minutes with proteinase K (0.3 mg/ml) in the presence or absence of Triton X-100 (1%). Proteolysis was stopped by the addition of 100 μM PMSF followed by addition of 5× loading buffer and western blotting.
Mitochondrial potential
Mitochondrial membrane potential was assessed by staining cells with 50 nM tetramethylrhodamine ethyl ester (TMRE) dye for 20 minutes at 37°C, followed by washes in 1× PBS. Cells were then subjected to FACS analysis.
Statistical analysis
Statistical significance was assessed by a one-way ANOVA followed by pair-wise contrast (Bonferroni) analysis. Significance was considered to be P<0.05 or less.
Acknowledgments
We thank Matthew O'Connell for his help with the FACS analysis, R. Banmaamar for help with the experiments shown in Fig. 1B and Shachar Shimonovich for critical reading of the manuscript. This work was funded by National Institutes of Health grant RO1 CA109482 (to D.G.). L.P is supported by the National Institutes of Health (National Cancer Institute) training grant T32 CA78207. The authors declare that they have no conflict of interest. Deposited in PMC for release after 12 months.
References
- Aldridge J. E., Horibe T., Hoogenraad N. J. (2007). Discovery of genes activated by the mitochondrial unfolded protein response (mtUPR) and cognate promoter elements. PLoS One 2, e874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B., Hoey B. M., Butler J. (1989). The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 6, 593-597 [DOI] [PubMed] [Google Scholar]
- Biswas G., Adebanjo O. A., Freedman B. D., Anandatheerthavarada H. K., Vijayasarathy C., Zaidi M., Kotlikoff M., Avadhani N. G. (1999). Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: a novel mode of inter-organelle crosstalk. EMBO J. 18, 522-533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky J. L., Wojcikiewicz R. J. (2009). Substrate-specific mediators of ER associated degradation (ERAD). Curr. Opin. Cell Biol. 21, 516-521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. A., Bhat-Nakshatri P., Patel N. M., Constantinidou D., Ali S., Nakshatri H. (2001). Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J. Biol. Chem. 276, 9817-9824 [DOI] [PubMed] [Google Scholar]
- Han Y. H., Moon H. J., You B. R., Kim S. Z., Kim S. H., Park W. H. (2009). Effects of carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone on the growth inhibition in human pulmonary adenocarcinoma Calu-6 cells. Toxicology 265, 101-107 [DOI] [PubMed] [Google Scholar]
- Han Y. H., Yang Y. M., Kim S. Z., Park W. H. (2010). Attenuation of MG132-induced HeLa cell death by N-acetyl cysteine via reducing reactive oxygen species and preventing glutathione depletion. Anticancer Res. 30, 2107-2112 [PubMed] [Google Scholar]
- Haynes C. M., Petrova K., Benedetti C., Yang Y., Ron D. (2007). ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Dev. Cell 13, 467-480 [DOI] [PubMed] [Google Scholar]
- He B., Feng Q., Mukherjee A., Lonard D. M., DeMayo F. J., Katzenellenbogen B. S., Lydon J. P., O'Malley B. W. (2008). A repressive role for prohibitin in estrogen signaling. Mol. Endocrinol. 22, 344-360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y., Pirkmaier A., Alvarez J. V., Frank D. A., Keselman I., Logothetis D., Mandeli J., O'Connell M. J., Waxman S., Germain D. (2006). Cyclin D1 overexpression and response to bortezomib treatment in a breast cancer model. J. Natl. Cancer Inst. 98, 1238-1247 [DOI] [PubMed] [Google Scholar]
- Kawamata H., Manfredi G. (2010). Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space. Antioxid. Redox Signal. 13, 1375-1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannigan D. A. (2003). Estrogen receptor phosphorylation. Steroids 68, 1-9 [DOI] [PubMed] [Google Scholar]
- Ma B.-M. (2006). The role of mitochondria in ageing and carcinogenesis. Clin. Exp. Dermatol. 31, 548-552 [DOI] [PubMed] [Google Scholar]
- Mattingly K. A., Ivanova M. M., Riggs K. A., Wickramasinghe N. S., Barch M. J., Klinge C. M. (2008). Estradiol stimulates transcription of nuclear respiratory factor-1 and increases mitochondrial biogenesis. Mol. Endocrinol. 22, 609-622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano M. M., Ekena K., Delage-Mourroux R., Chang W., Martini P., Katzenellenbogen B. S. (1999). An estrogen receptor-selective coregulator that potentiates the effectiveness of antiestrogens and represses the activity of estrogens. Proc. Natl. Acad. Sci. USA 96, 6947-6952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K. (2009). Signalling pathways in the unfolded protein response: development from yeast to mammals. J. Biochem. 146, 743-750 [DOI] [PubMed] [Google Scholar]
- Nijtmans L. G., de Jong L., Artal Sanz M., Coates P. J., Berden J. A., Back J. W., Muijsers A. O., van der Spek H., Grivell L. A. (2000). Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. EMBO J. 19, 2444-2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A., Razandi M., Wallace D. C., Levin E. R. (2006). Functional estrogen receptors in the mitochondria of breast cancer cells. Mol. Biol. Cell 17, 2125-2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicano H., Lu W., Zhou Y., Zhang W., Chen Z., Hu Y., Huang P. (2009). Mitochondrial dysfunction and reactive oxygen species imbalance promote breast cancer cell motility through a CXCL14-mediated mechanism. Cancer Res. 69, 2375-2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plak K., Czarnecka A. M., Krawczyk T., Golik P., Bartnik E. (2009). Breast cancer as a mitochondrial disorder. Oncol. Rep. 21, 845-851 [DOI] [PubMed] [Google Scholar]
- Punnonen K., Ahotupa M., Asaishi K., Hyoty M., Kudo R., Punnonen R. (1994). Antioxidant enzyme activities and oxidative stress in human breast cancer. J. Cancer Res. Clin. Oncol. 120, 374-377 [DOI] [PubMed] [Google Scholar]
- Radke S., Chander H., Schafer P., Meiss G., Kruger R., Schulz J. B., Germain D. (2008). Mitochondrial protein quality control by the proteasome involves ubiquitination and the protease Omi. J. Biol. Chem. 283, 12681-12685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder M. (2008). Endoplasmic reticulum stress responses. Cell. Mol. Life Sci. 65, 862-894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M., Paciga J. E., Feldman R. I., Yuan Z., Coppola D., Lu Y. Y., Shelley S. A., Nicosia S. V., Cheng J. Q. (2001). Phosphatidylinositol-3-OH Kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERα) via interaction between ERα and PI3K. Cancer Res. 61, 5985-5991 [PubMed] [Google Scholar]
- Takeuchi H., Kobayashi Y., Ishigaki S., Doyu M., Sobue G. (2002). Mitochondrial localization of mutant superoxide dismutase 1 triggers caspase-dependent cell death in a cellular model of familial amyotrophic lateral sclerosis. J. Biol. Chem. 277, 50966-50972 [DOI] [PubMed] [Google Scholar]
- Tatsuta T., Langer T. (2009). AAA proteases in mitochondria: diverse functions of membrane-bound proteolytic machines. Res. Microbiol. 160, 711-717 [DOI] [PubMed] [Google Scholar]
- Vilgelm A., Lian Z., Wang H., Beauparlant S. L., Klein-Szanto A., Ellenson L. H., Di Cristofano A. (2006). Akt-mediated phosphorylation and activation of estrogen receptor alpha is required for endometrial neoplastic transformation in Pten+/− mice. Cancer Res. 66, 3375-3380 [DOI] [PubMed] [Google Scholar]
- Zafarullah M., Li W. Q., Sylvester J., Ahmad M. (2003). Molecular mechanisms of N-acetylcysteine actions. Cell. Mol. Life Sci. 60, 6-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Wang J., Levichkin I. V., Stasinopoulos S., Ryan M. T., Hoogenraad N. J. (2002). A mitochondrial specific stress response in mammalian cells. EMBO J. 21, 4411-4419 [DOI] [PMC free article] [PubMed] [Google Scholar]