Abstract
The multifunctional structural protein 4.1R is required for assembly and maintenance of functional nuclei but its nuclear roles are unidentified. 4.1R localizes within nuclei, at the nuclear envelope, and in cytoplasm. Here we show that 4.1R, the nuclear envelope protein emerin and the intermediate filament protein lamin A/C co-immunoprecipitate, and that 4.1R-specific depletion in human cells by RNA interference produces nuclear dysmorphology and selective mislocalization of proteins from several nuclear subcompartments. Such 4.1R-deficiency causes emerin to partially redistribute into the cytoplasm, whereas lamin A/C is disorganized at nuclear rims and displaced from nucleoplasmic foci. The nuclear envelope protein MAN1, nuclear pore proteins Tpr and Nup62, and nucleoplasmic proteins NuMA and LAP2α also have aberrant distributions, but lamin B and LAP2β have normal localizations. 4.1R-deficient mouse embryonic fibroblasts show a similar phenotype. We determined the functional effects of 4.1R-deficiency that reflect disruption of the association of 4.1R with emerin and A-type lamin: increased nucleus–centrosome distances, increased β-catenin signaling, and relocalization of β-catenin from the plasma membrane to the nucleus. Furthermore, emerin- and lamin-A/C-null cells have decreased nuclear 4.1R. Our data provide evidence that 4.1R has important functional interactions with emerin and A-type lamin that impact upon nuclear architecture, centrosome–nuclear envelope association and the regulation of β-catenin transcriptional co-activator activity that is dependent on β-catenin nuclear export.
Key words: 4.1R, Nuclear envelope, Actin, Emerin, Lamin A/C, Centrosome, RNA interference, β-Catenin
Introduction
The nuclear envelope comprises a double lipid membrane, embedded nuclear pores and a filamentous lamin meshwork lying beneath the inner nuclear membrane. Multi-protein complexes traverse the perinuclear space between the two nuclear membranes, linking cytoplasmic skeletal networks to inner nuclear structural networks housing chromatin, nucleoplasmic RNA splicing and DNA replication assemblies, and regulatory complexes (Crisp et al., 2006; Razafsky and Hodzic, 2009; Stewart et al., 2007). Thus, although visually distinct, the nucleus is biochemically and physically integrated with cytoplasmic structures, the plasma membrane and the extracellular matrix. In this current view, nuclear structural proteins are no longer regarded as largely passive architectural elements but are now recognized to also mediate dynamic regulatory processes, such as macromolecular trafficking through nuclear pores, gene expression and signaling vital to cellular remodeling during proliferation and differentiation. The striking discovery that defects in nuclear architectural proteins are linked to pathobiology of diverse human diseases, including ~30 nuclear envelope diseases (for reviews, see Burke and Stewart, 2002; Worman and Courvalin, 2005), is concordant with a revised perspective of the nuclear functions extending well beyond safeguarding the genome.
Protein 4.1R has for decades been regarded mainly as an ~80 kDa cytoskeletal erythrocyte protein crucial for stabilizing interactions within spectrin–actin lattices in the erythrocyte membrane skeleton and linking them to transmembrane proteins in the overlying plasma membrane. Defects in 4.1R linkages alter erythrocyte shape and mechanical stability, producing hereditary elliptocyosis characterized by erythrocyte membrane fragmentation (Benz, 1994; Conboy, 1993; Mohandas and Chasis, 1993). It was subsequently discovered, however, that nucleated cells express multiple 4.1R isoforms at several subcellular locations (De Carcer et al., 1995; Delhommeau et al., 2002; Krauss et al., 1997b; Krauss et al., 1997a). In nucleated cells, protein 4.1R not only organizes plasma-membrane–cytoskeletal microstructure, but, as we have previously shown, it is also integral to mitotic spindle and centrosome assembly and structure (Krauss et al., 2004; Krauss et al., 2008). Furthermore, we found that 4.1R epitopes in mammalian and Xenopus laevis nuclei are partially coincident with RNA splicing and/or transcription factories, DNA replication assemblies, nuclear pores and the nuclear periphery, and are largely coincident with nuclear anti-actin signals in nuclear matrix (Krauss et al., 2003; Krauss et al., 1997a). At the resolution of electron microscopy, we detected 4.1R and actin closely associated on intranuclear filaments in mammalian cells (Krauss et al., 2003). Protein 4.1 and actin epitopes were also identified on filaments linked with nuclear pores in Xenopus oocytes (Kiseleva et al., 2004). Independently, 4.1R was identified by mass spectroscopy analysis of mammalian nuclear envelope proteins (Schirmer et al., 2003).
We have previously shown that 4.1R is functionally significant in the nucleus by using Xenopus egg extracts that recapitulate nuclear assembly in vitro. By 4.1 depletion and add-back experiments, we established that 4.1 is essential for proper assembly of nuclei capable of DNA synthesis and for transitioning into mitosis following nuclear envelope breakdown. Furthermore, we have documented by several different means, including use of 4.1R peptides mutant for actin binding, that the capacity of 4.1R to bind actin is required for nuclear assembly and function (Krauss et al., 2003; Krauss et al., 2002). Interestingly, a 4.1R nuclear localization signal has been identified that partially overlaps with 4.1R spectrin-actin-binding domain sequences (Gascard et al., 1999). Although 4.1R clearly is important for nuclear structure and function, underlying mechanisms are not well understood.
One key strategy for defining mechanisms of 4.1R functions, used successfully in the case of erythrocytes, has been to determine its binding partners (Baines et al., 2009; Calinisan et al., 2006; Conboy, 1999; Salomao et al., 2008; Takakuwa, 2000). The four major domains of prototypical 4.1R in nucleated cells have diverse interactions, indicative of its roles as a multifunctional linker and/or adaptor protein (Krauss et al., 2002; see figure 1 within). In brief, the 4.1R 30 kDa FERM domain contains a microtubule-binding site, as does the C-terminal domain, and also interacts with phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2], calmodulin and membrane proteins (An et al., 2006; Krauss et al., 2004; Nunomura et al., 2000; Perez-Ferreiro et al., 2001). The spectrin - actin-binding domain (Cohen and Foley, 1984; Discher et al., 1993; Gimm et al., 2002; Schischmanoff et al., 1995) additionally binds myosin, an actin-dependent ATPase (Kontrogianni-Konstantopoulos et al., 2000; Pasternack and Racusen, 1989). The C-terminus binds the structural protein ‘nuclear mitotic apparatus protein’ (NuMA), present in the nucleoplasm during interphase, as well as ZO2 and FKB13 (Mattagajasingh et al., 2000; Mattagajasingh et al., 1999; Walensky et al., 1998).
To further delineate the nuclear roles of 4.1R, we are focusing on our previous findings that 4.1R–actin interactions are crucial for assembly and maintenance of nuclear structure and function. We reasoned that 4.1R might act as a linker and/or adaptor in the nucleus to form functionally important actin-containing complexes with other nuclear actin-binding proteins. Actin is involved in variety of important nuclear processes, such as transcription, chromatin remodeling, regulation of architecture, chromatin movement and RNA export (Chuang et al., 2006; Dundr et al., 2007; Hofmann, 2009; Hofmann et al., 2004; Olave et al., 2002), and several major actin-binding nuclear proteins have been identified.
One very attractive candidate for a potential 4.1R-interacting protein is emerin, a 34 kDa protein encoded by the EMD gene located on the X-chromosome (Bione et al., 1994), which is expressed in nearly all tissues and contributes to many vital cellular functions, including nuclear assembly, chromatin segregation and cell cycle progression (Bengtsson and Wilson, 2004; Holaska and Wilson, 2006). Emerin resides at the inner and outer regions of the nuclear envelope and the Wilson laboratory has extensively characterized a number of nuclear architectural and regulatory emerin complexes (Holaska and Wilson, 2007). Notably, emerin caps and stabilizes the pointed end of F-actin in vitro (Holaska et al., 2004) and has important interactions with a chromosome-associated barrier to autointegration factor, with gene regulators and with nesprins (Lee et al., 2001). Members of the nesprin family also bind actin and span the nuclear envelope, as components of LINC complexes, to connect with the cytoskeleton (Haque et al., 2006; Libotte et al., 2005; Mislow et al., 2002; Ostlund et al., 2009; Wheeler et al., 2007). As with 4.1R, emerin binds to microtubules and to myosin (Holaska and Wilson, 2007). More recently other intriguing functions of emerin have been discovered: emerin was shown to couple centrosomes to the nuclear envelope and to regulate β-catenin activity (Markiewicz et al., 2006; Salpingidou et al., 2007).
Another attractive actin-binding nuclear candidate is lamin A, which has two actin-binding sites. Importantly, emerin binds A-type lamins, and the localization of emerin at the nuclear envelope is dependent on lamin A (Vaughan et al., 2001). Mutations in the emerin- and lamin-A-encoding genes can cause Emery–Dreifuss muscular dystrophy (EDMD), a disease characterized by slowly progressive skeletal muscle wasting, joint contractures and cardiomyopathy leading to increased risk of cardiac arrest (Bione et al., 1994; Emery, 2000; Morris, 2001). Defective A-type lamin is also associated with a large group of diseases collectively called laminopathies (Mounkes et al., 2003; Muchir and Worman, 2004).
Here we show that emerin and lamin A/C are co-immunoprecipitated with 4.1R, and that human cells depleted of 4.1R by RNA interference (RNAi) treatment have dysmorphic nuclei, emerin mislocalization, disrupted A-type lamin, increased centrosome–nuclear distances, increased β-catenin transcriptional activity and increased nuclear accumulation of β-catenin. We also studied mouse embryonic fibroblasts (MEFs) from a mouse model in which the first two 4.1R translation initiation sites were deleted (4.1R AUG1,2−/−) (Shi et al., 1999). Although this is not a knockout of the entire 4.1R gene, and expression of residual 4.1R has been reported (Stagg et al., 2008), the mice have significant phenotypes. In addition to a predicted hemolytic anemia, the mice have cardiac and kidney defects, neurobehavioral deficits and smaller litter sizes with low survival (Kang et al., 2009; Rivera et al., 2006; Stagg et al., 2008). Examining MEFs from these mice, we observed a number of nuclear deficits resembling those in human cells with depleted 4.1R, including emerin and A-type lamin malfunctions. Taken together, our results from these two independent systems indicate functional associations of 4.1R with emerin and A-type lamins that impact upon nuclear architecture, association of centrosomes with the nuclear envelope and β-catenin transcriptional regulation.
Results
Co-immunoprecipitation and partial colocalization of 4.1R with emerin
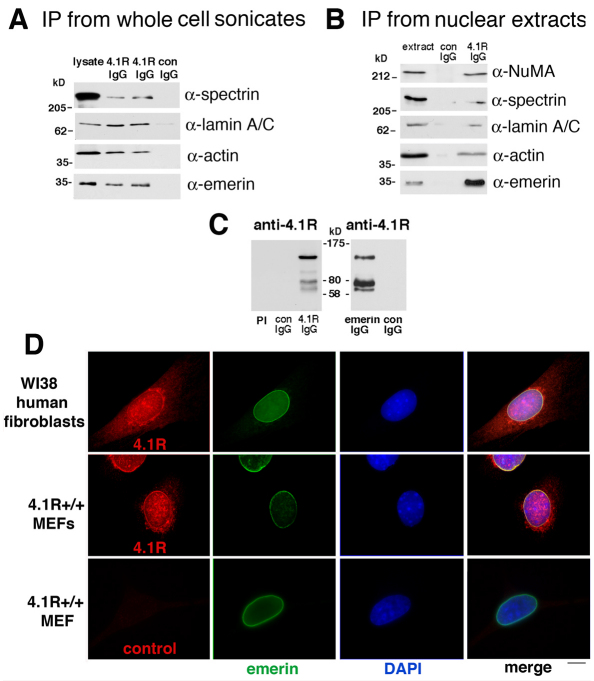
To initially test for any evidence of a 4.1R association with emerin, we probed 4.1R immunoprecipitates of HeLa whole-cell sonicates using several different detergents to increase stringency. We readily detected a protein migrating at ~34 kDa that reacted with specifically with anti-emerin antibody in immune, but not non-immune, complexes separated by SDS-PAGE (Fig. 1A). We also confirmed that both actin and αII-spectrin were co-immunoprecipitated, as would be predicted. As emerin binds lamin A/C, but lamins can be difficult to solubilize, we checked that the whole-cell sonicates contained lamin A/C and then probed the 4.1R immunoprecipitates and detected a lamin A/C band (Fig. 1A). To test further association of 4.1R and emerin in a less-complex cell fraction, we prepared HeLa nuclear extracts and immunoprecipitated 4.1R-associated proteins (Fig. 1B). We again detected robust emerin co-immunoprecipitation with 4.1R. As positive controls, we probed and detected the 4.1R-binding partners actin, αII-spectrin and NuMA (Krauss et al., 2002; Mattagajasingh et al., 1999). Lamin A/C was also present in 4.1R immunoprecipitates in HeLa nuclear extract under these conditions. To confirm that 4.1R itself was co-precipitated, immunoprecipitated proteins were probed with anti-4.1R antibody. Bands at ~135 and 80 kDa, consistent with full-length products generated from the 4.1R AUG1 and AUG2 translation initiation sites, were present (Fig. 1C). Other bands detected at ~105 and ~64 kDa are likely to be alternatively spliced 4.1R isoforms (Anderson et al., 1988; Conboy et al., 1991; Conboy et al., 1988), although the lower band could also be the product generated from 4.1R AUG3 (Gascard et al., 1998; Luque and Correas, 2000). Parallel samples with control IgG or pre-immune serum did not contain anti-4.1R-antibody-reactive bands. As further evidence of a 4.1R–emerin complex, reciprocal emerin immunoprecipitates were probed with anti-4.1R antibody and found to have 4.1R bands at ~135, 80 and 64 kDa (Fig. 1C).
Fig. 1.
4.1R and emerin co-immunoprecipitate and partially colocalize in human and murine fibroblasts. (A) Immunoprecipitation (IP) from HeLa whole cell sonicates. Clarified whole cell sonicates were prepared, immunoprecipitated with anti-4.1R antibody, and eluted proteins separated by SDS-PAGE were subjected to western blotting as described in the Materials and Methods. Emerin, actin, lamin A/C and αII-spectrin were detected using the antibodies indicated in lysate and immune duplicate samples (4.1R IgG) but not in a non-immune sample (con IgG). (B) Analysis of immunoprecipitates from HeLa nuclear extract. HeLa nuclear extract was incubated with antibodies, immunoprecipitated with anti-4.1R antibody, and eluted proteins separated by SDS-PAGE were subjected to western blotting using the antibodies indicated on the right. In 4.1R IgG immunoprecipitates (right-hand lane) a strong 34 kDa emerin band was detected. 4.1R immunoprecipitates also contained actin, lamin A/C, αII-spectrin and NuMA. These proteins were not detected in control IgG precipitates (center lane). (C) Western blot probed with anti-4.1R antibody showing 4.1R bands migrating at ~135, 105, 80 and 62 kDa detected specifically in 4.1R IgG immunoprecipitates but not in the pre-immune (PI) or control (con) lanes. The right-hand panel shows a western blot demonstrating that 4.1R bands at ~135, 80, and 62 kDa were present in anti-emerin antibody immunoprecipitates but not in those with control IgG. (D) Partial colocalization of 4.1R epitopes (red) with emerin epitopes (green) in human and murine fibroblasts. Fixed WI38 fibroblasts and wild-type MEFs were probed by double-label indirect immunofluorescence. The nuclear envelope protein emerin was detected at the periphery of the nucleus stained by DAPI. 4.1R epitopes were detected in the nucleus, cytoplasm and in the region of the nuclear envelope, partially coincident with emerin signals (yellow coloration in the ‘merge’ image). Signals for an equal amount of control anti-rabbit IgG antibody staining (bottom left panel) were not above background levels of other controls in which primary rabbit antibody was omitted but murine anti-emerin and both fluorescent secondaries were included. Scale bar: 6 μm.
To additionally document a potential 4.1R–emerin association in situ, we examined human and murine fibroblasts by double-label indirect immunofluorescence. As has been reported by several investigators, in nucleated mammalian cells 4.1R epitopes are distributed in the cytoplasm, at intranuclear sites, and at centrosomes. At a cytoplasmic focal plane in cells double-labeled with emerin, 4.1R staining shows an enrichment at the nuclear periphery coincident with emerin in overlaid images (Fig. 1D). Although this nuclear peripheral localization was apparent previously (Krauss et al., 1997a) it was not sufficiently noted or appreciated in light of efforts to establish 4.1 at nucleoplasmic ‘speckles’ (Correas, 1991; Krauss et al., 1997a).
4.1R depletion induces nuclear dysmorphology with altered emerin and A-type lamin distribution
Emerin-deficient MEFs, as well as cells from EDMD patients, often have irregularly shaped nuclei and blebbing (Lammerding et al., 2005). If 4.1R has a functional association with emerin, 4.1R depletion might also alter nuclear morphology. To analyze the effects of 4.1R depletion on nuclear morphology in intact mammalian cells, we examined DAPI-stained cells after 4.1R downregulation by RNAi (Krauss et al., 2008) and MEFs from 4.1R AUG1,2−/− mice relative to matched MEFs from wild-type mice. We found that 4.1R-deficient cells have an ~sixfold increase in perturbed nuclei (those that are markedly irregular with blebbing or are at least twofold larger or smaller relative to smooth ellipsoid control nuclei) (Fig. 2A; Fig. 3A). Telomerase-immortalized human diploid cells (RPE1) and HeLa cells also have increased nuclear dysmorphology after 4.1R RNAi treatment (S.W.K., data not shown).
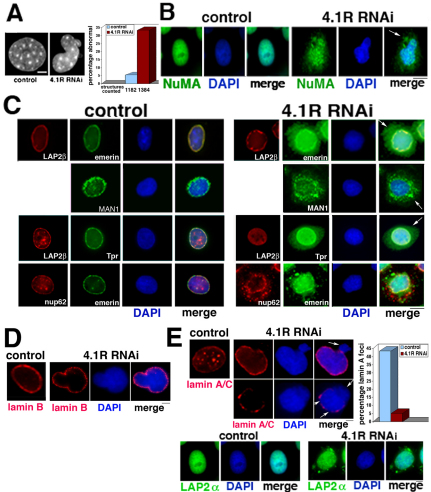
Fig. 2.
Nuclear morphology is perturbed and selective nuclear proteins are redistributed in 4.1R-deficient human cells. (A) The left-hand panels show examples of nuclear morphology of control (noncoding RNAi) and aberrant CaSki nuclei (4.1R RNAi) stained with DAPI. The right-hand graph shows a quantification of the number of perturbed nuclei in control compared with 4.1R-RNAi-treated cells. Numbers of nuclei examined from four independent experiments are shown below. Nuclei were scored as normal if spheroidal or ellipsoid, and abnormal if blebbed, lobulated or at least twice as large or small as those in controls. (B) Indirect immunofluorescent staining of nucleoplasmic protein NuMA in control and 4.1R-RNAi-treated cells. In cells with normal 4.1R, NuMA is entirely within the nuclear area stained by DAPI, whereas in 4.1R-deficient cells, NuMA is detected in the cytoplasm, sometimes in apparent aggregates (arrow). (C) Localization of nuclear envelope proteins by indirect immunofluorescence. The top row on the right-hand side shows that emerin is partially redistributed from the nuclear envelope into the cytoplasm of cells when 4.1R is depleted (arrow, top row on the right-hand side). However, the nuclear envelope protein LAP2β remains in the nuclear envelope. The LEM domain protein MAN1 redistributes from the nuclear envelope/perinuclear region to further out in the cytoplasm when 4.1R is deficient (arrow, second row on the right-hand side). Localizations of two nuclear pore proteins are also affected when 4.1R is deficient. In the examples shown in the two bottom rows, there is significant redistribution of the nuclear pore inner basket protein Tpr in a 4.1R-deficient cell although the localization of LAP2β in the nuclear envelope remains unchanged in the same cell. Distribution of the central nuclear pore channel protein Nup62 is also altered in 4.1R-deficient cells. A cell is shown in which emerin, as well as Nup62, is partially redistributed from the nuclear envelope but does not overlap with Nup62 in cytoplasmic regions. (D) Localization of lamin B. Lamin B distribution, normally at the nuclear periphery (control) is not perturbed when 4.1R is deficient, even at the large bleb in the nucleus shown. (E) Localization of lamin A/C and LAP2α. Control cell nuclei have continuous lamin A/C rims with multiple nucleoplasmic lamin A/C foci. However, 4.1R-RNAi-treated cells have nuclei without lamin A/C staining surrounding nuclear blebs (upper arrows in the top and bottom panel examples) and can be discontinuous, as shown in bottom panels (lower arrows in bottom panel). In 4.1R-depleted cells internal lamin A/C foci are absent or severely reduced in number. The graph shows a quantification of cells with more than six lamin A/C foci in control and RNAi-treated cells (n=250 each in two independent experiments). In the images below, the lamin-A-associated protein LAP2α localizes strictly within the boundaries of nuclei (DAPI staining) in control cells, but is also detected in the cytoplasm in 4.1R-RNAi-treated cells, often in aggregates. Scale bars: 7 μm (B,C); 5 μm (A,D,E).
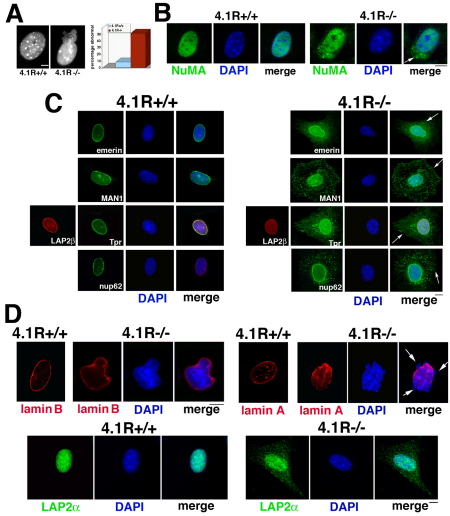
Fig. 3.
Nuclear morphology is perturbed and selective nuclear proteins are redistributed in 4.1R AUG1,2−/− MEFs. (A) The left-hand panels show examples of nuclear morphology of wild-type and 4.1R AUG1,2−/− MEFs (4.1R−/−) stained with DAPI. The right-hand graph shows a quantification of the number of perturbed nuclei in wild-type (4.1R+/+) compared with 4.1R−/− MEFs at equal passage in three independent experiments in which 500 nuclei of each type were scored as described in Fig. 2. (B) Indirect immunofluorescent staining of nucleoplasmic protein NuMA in 4.1R+/+ and 4.1−/− MEFs. In cells with normal 4.1R, NuMA is within the nuclear area stained by DAPI, whereas in 4.1R−/− cells, NuMA is detected in the cytoplasm, sometimes in apparent aggregates (arrow). (C) Localization of nuclear envelope proteins by indirect immunofluorescence. The top row on the right-hand side shows that emerin is partially redistributed from the nuclear envelope into the cytoplasm in 4.1R−/−MEFs (arrow). MAN1 also redistributes from the nuclear envelope/perinuclear region to further out in the cytoplasm in 4.1R−/− MEFs (arrow). Additionally, localization of two nuclear pore proteins is affected when 4.1R is depleted. In the examples shown in the two bottom panels, there is a major redistribution of the nuclear pore inner basket protein Tpr in 4.1R−/− MEFs, as well as the central nuclear pore protein Nup62 (arrows). However, LAP2β remains in the nuclear envelope of 4.1R−/− MEFs. (D) Localization of lamin B, lamin A/C and LAP2α. The upper left-hand panels show that wild-type cell nuclei have lamin B rims at the nuclear periphery, as do 4.1R−/− MEFs, where lamin B is continuous even at blebs and irregular contours. The upper right-hand panels show that wild-type cell nuclei have lamin A/C rims with multiple nucleoplasmic lamin A/C foci. However, a 4.1R−/− nucleus has discontinuous lamin A/C staining at nuclear blebs (arrows). The lower panels show that the lamin-A-associated protein LAP2α localizes within the boundaries of the nuclei (DAPI staining) in 4.1R+/+ MEFs but is detected in the cytoplasm of 4.1−/− MEFs. Scale bars: 5 μm.
To investigate whether the absence of 4.1R affects the distribution of major nuclear proteins, notably emerin and A-type lamin, we first analyzed whether 4.1R downregulation affected the nuclear localization of a known binding partner of 4.1R in the nucleus. NuMA binds directly to the C-terminus of 4.1R (Krauss et al., 2002; Mattagajasingh et al., 1999) and both are components of the nuclear matrix of interphase nuclei. We imaged 4.1R-RNAi-treated cells and 4.1R AUG1,2−/− MEFs and detected NuMA in cytoplasmic aggregates rather than being localized exclusively in the nucleoplasm, as in control RNAi-treated cells or wild-type MEFs (Fig. 2B; Fig. 3B). NuMA mislocalization could reflect a failure of proper anchorage of NuMA by 4.1R at intranuclear sites due to 4.1R downregulation and might also contribute to nuclear dysmorphology (Krauss et al., 2002; Krauss et al., 1997a; Merdes and Cleveland, 1998).
We then examined the distribution of emerin in 4.1R-depleted human and murine cells. There was a substantial mislocalization of a portion of emerin into the cytoplasmic area relative to its normal position at the nuclear envelope, as demarcated by another nuclear envelope protein LAP2β (Fig. 2C; Fig. 3C). This altered localization was confirmed by western blotting for emerin in HeLa nuclear and cytoplasmic fractions, which showed that there was a threefold increase in the amount of emerin present in the cytoplasm of 4.1R-RNAi-treated cells (average 37.7% in 4.1R-deficient cells compared with 12.9% in controls in three experiments; supplementary material Fig. S1). Both emerin and LAP2β contain an ~40 amino acid LEM domain (for Lamina-associated polypeptide-emerin-MAN1), in common with the inner nuclear envelope protein MAN1 and a group of lamin-associated proteins (LAPs) (Foisner and Gerace, 1993; Holmer and Worman, 2001; Lin et al., 2000; Wagner and Krohne, 2007). As with emerin, MAN1 distribution is altered in cells with depleted 4.1R (Fig. 2C, Fig. 3C). However, the fact that LAP2β is retained at the nuclear envelope when 4.1R is deficient probably reflects the fact that although these proteins each have LEM domains, their repertoire of interactions differs. For example, LAP2β binds to B-type lamins (Foisner and Gerace, 1993), whereas LAP2α preferentially binds A-type lamins (Dechat et al., 2000). Indeed, when we tested lamin B distribution in 4.1R-deficient cells, we found that lamin B forms a continuous rim, including around nuclear blebs (Fig. 2D, Fig. 3D), consistent with our finding that LAP2β is not perturbed.
Nuclear pores are embedded in the nuclear envelope, traversing both the inner and outer nuclear membranes and the perinuclear space. Because several major nuclear envelope proteins are displaced when 4.1R is deficient, we were curious as to whether nuclear pore proteins were also affected and, hence, examined proteins from two different regions of the pore structure. We found that both a nuclear pore inner nuclear basket protein (Tpr) and a nuclear pore central channel protein (Nup62) were detected in the perinuclear region of 4.1R-deficient cells in addition to their nuclear envelope distribution (Fig. 2C, Fig. 3C). In the examples shown, Tpr distribution was massively altered, although LAP2β was not, as previously noted. When 4.1R was depleted by RNAi, Nup62 and emerin are localized in the cytoplasmic region independently, again emphasizing that it is probable that 4.1R is a pivotal linker or adaptor anchoring components of independent, as well as interdependent, networks (Fig. 2C).
As emerin is anchored at the nuclear membrane through its interactions with lamin A (Vaughan et al., 2001), and emerin localization is affected by 4.1R downregulation, it would be predicted that lamin A/C might be perturbed. When we examined lamin A/C staining in 4.1R-deficient mammalian cells, we found that blebbed or multi-lobulated mammalian nuclei had discontinuous peripheral lamin A/C at the nuclear envelope (Fig. 2E; Fig. 3D). Furthermore, we observed that 4.1R-RNAi-treated cells, with normal or abnormal nuclear morphology, had an absence of nucleoplasmic lamin A/C foci: fewer than 5% of the cells had six or more internal lamin A/C foci (Fig. 2E, graph; Fig. 3D). Additionally, a substantial amount of LAP2α, a nucleoplasmic lamin-A/C-binding partner, was detected beyond the nuclear periphery (Fig. 2E; Fig. 3D). These data suggest that 4.1R is required for A-type lamin integrity and not for B-type lamin integrity. A- and B-type lamins form separate networks of intermediate filaments beneath the nuclear envelope. The A-type lamin-related findings are potentially significant because nuclear dysmorphology is a hallmark of cells from laminopathic patients and mouse models of laminopathies (Capell and Collins, 2006; Mounkes et al., 2003; Muchir et al., 2004; Worman and Bonne, 2007) and internal lamin A/C foci are associated with the tumor suppressor retinoblastoma protein (pRb) (Markiewicz et al., 2002).
Taken together, our data show that downregulation of 4.1R expression causes nuclear dysmorphology with selective, but not global, changes within multiple nuclear subcompartments: mislocalization of a nuclear skeletal protein (NuMA), as well as another nucleoplasmic protein (LAP2α); altered distribution of several but not all inner nuclear membrane and pore proteins (emerin, MAN1 and two nuclear pore proteins but not LAP2β); and major disruption of one member of the nuclear lamina (lamin A/C) but not lamin B. Interestingly, 4.1R AUG1,2−/− MEFs, although not entirely devoid of 4.1R (Stagg et al., 2008) (S.W.K., unpublished), have a similar cellular phenotype.
4.1R distribution is altered in cells lacking emerin or lamin A/C
In view of the data presented above, we reasoned that at least some fraction of emerin and A-type lamin is associated with 4.1R such that their subcellular localization is 4.1R-dependent. We next decided to test whether this was also the case for 4.1R or whether its localization was entirely independent of emerin or lamin A. Therefore, we first analyzed patterns of 4.1R immunostaining in emerin−/Y MEFs which do not contain any emerin (Lammerding et al., 2005). In wild-type (emerin+/Y) MEFs, the preponderance of 4.1R epitopes localized in the nuclear area, visualized by DAPI staining, with lesser amounts in the cytoplasm. However, in emerin−/Y MEFs, an increased proportion of 4.1R epitopes are cytoplasmic (the ratio of nuclear to cytoplasmic 4.1R signals was 1.97±0.28 in wild-type and 0.54±0.15 in emerin-null cells; n=10 each) (Fig. 4, top left). Next we investigated the effects on 4.1R when emerin was not absent but mislocalized. Emerin mislocalization has been reported in fibroblasts from lamin-A-knockout mice (Lmna−/− MEFs) (Hale et al., 2008; Sullivan et al., 1999). Immunostaining of Lmna−/− MEFs also showed increased 4.1R cytoplasmic staining relative to that in wild-type Lmna+/+ cells (the ratio of nuclear to cytoplasmic 4.1R signals was 1.97±0.17 in Lmna+/+ and 0.86±0.19 in Lmna−/−; n=10 each) (Fig. 4, top right). As lamin A organization is not perturbed in emerin−/Y MEFs (Hale et al., 2008; Lammerding et al., 2005), these results are consistent with the notion that emerin is a determinant for a substantial portion of 4.1R nuclear anchorage. To see whether 4.1R-deficient cells closely resembled cells lacking emerin or lamin A, we stained these cells for NuMA, LAP2α, LAP2β, MAN1, Tpr and Nup62 (Fig. 4). Our results, summarized in Table 1, show that 4.1R-deficient human and murine cells have a unique phenotype.
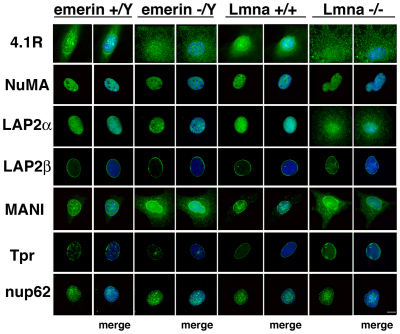
Fig. 4.
Distribution of 4.1R and nuclear proteins in MEFs lacking either emerin or lamin A/C relative to that in cognate wild-type MEFs. Fixed cells were stained with antibodies against the proteins listed at the left for immunofluorescent microscopy (green) and nuclei were stained by DAPI (blue in ‘merge’ images). Whereas in wild-type MEFs, 4.1R epitopes are predominant in the nuclear area, emerin−/Y and Lmna−/− MEFs show increased cytoplasmic 4.1R staining relative to that in wild-type cells. Of the other proteins tested, emerin−/Y MEFs have increased cytoplasmic MAN1 epitopes and Lmna−/− MEFs have mislocalized LAP2α and MAN1 (Ostlund et al., 2006). NuMA, LAP2β, Tpr and Nup62 localizations are normal in the null MEFs. See Table 1 for a summary of these results and from 4.1R-deficient human and murine cells (Figs 2, 3). Scale bar: 4 μm.
Table 1.
Distribution of nuclear proteins in MEFs deficient for 4.1R, emerin or lamin A/C
Increased centrosome–nuclear envelope distance after 4.1R depletion
The Hutchinson laboratory recently discovered a new emerin function: emerin is essential for centrosome linkage to the outer nuclear membrane, which, in addition to the inner nuclear membrane, has a significant fraction of emerin (Salpingidou et al., 2007). They demonstrated that emerin interacts with microtubules to anchor centrosomes to the outer nuclear membrane. In addition to emerin-null fibroblasts and emerin-RNAi-treated cells, lamin-A/C-null fibroblasts with mislocalized emerin also have centrosomes detached from the nuclear envelope (Hale et al., 2008; Salpingidou et al., 2007). Recently, it was shown that nesprin-2-deficient cells have an increased centrosome–nuclear distance (Schneider et al., 2010; Zhang et al., 2009) and it has been suggested that LINC complex disruption also has this effect (Razafsky and Hodzic, 2009).
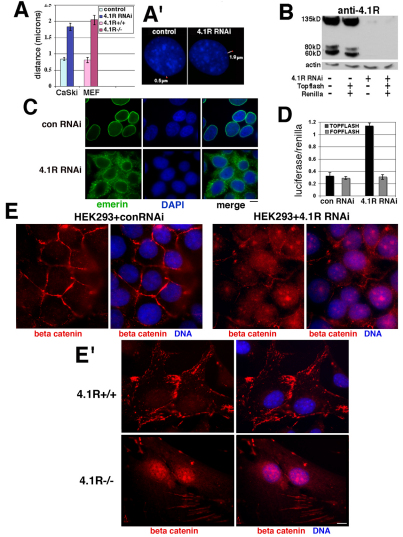
We previously reported that 4.1R depletion causes microtubule disorganization in vitro and in intact cells (Krauss et al., 2002; Krauss et al., 2004; Krauss et al., 2008). We reasoned that 4.1R could have significant interactions at the nucleo-cytoskeletal interface affecting centrosome tethering and microtubules, particularly if 4.1R were functionally associated with emerin and lamin A. Therefore, we used antibodies against several centrosomal markers and DAPI staining to determine centrosome position in cells treated with 4.1R RNAi. In control cells, centrosomes are located juxtaposed or within 0.8 μm of the nucleus. By contrast, in cells depleted of 4.1R, with mislocalized emerin, centrosomes are an average of 1.8 μm from the nucleus (Fig. 5A,A′). This is similar to the fold increase in centrosome distance reported for emerin-null and lamin-A/C-null human diploid fibroblast lines relative to normal human diploid fibroblasts (Salpingidou et al., 2007). To independently test whether 4.1R depletion affects centrosome attachment, we also measured centrosome distances in 4.1R AUG1,2−/− MEFs stained with centrosome markers and DAPI because these cells also have disorganized microtubules (S.W.K., unpublished). However, we noted increased levels of centrosome amplification in the 4.1R AUG1,2−/− MEFs relative to matched wild-type MEFs (supplementary material Fig. S2). Therefore, we selected only those 4.1R AUG1,2−/− MEFs with normal centrosome numbers for our measurements. The results show that, again, there was an approximate twofold increase in the nucleus–centrosome distance in murine cells with depleted 4.1R (Fig. 5A). Emerin-null MEFs were reported to show an ~2.5-fold increase in distance (Hale et al., 2008). Thus, decreased expression of 4.1R in MEFs phenocopied the detached centrosomes characteristic of emerin-null MEFs. Our new data demonstrate that the relative absence of 4.1R in both human and murine cells interferes with the linkage of centrosomes to the outer nuclear membrane that depends at least in part on a microtubule–emerin pathway.
Fig. 5.
Functional evidence implicating a 4.1R–emerin association. (A,A′) Nuclear–centrosome distances are increased in 4.1R-deficient human and murine cells. Cells were stained with DAPI to image nuclei (blue) and anti-γ-tubulin antibody (red) to localize centrosomes. Distances were measured in overlaid images using Image J. The graph shows data from duplicate experiments using the cells indicated (n=200 cells; mean±s.e.). An example of centrosome position in a control and a 4.1R-RNAi-treated cell is shown in A′. The white line measures the nuclear–centrosome distance indicated. (B) Downregulation of 4.1R expression in HEK-293 cells. Western blot of 4.1R protein in HEK-293 cell extracts untransfected or transfected as indicated below the image. In cells exposed to control RNAi, strong 4.1R bands were detected at ~135, 80, and 60 kDa in extracts regardless of whether or not they had been co-transfected with Topflash and Renilla luciferase (left side). In 4.1R-RNAi-treated HEK-293 cells, 4.1R bands were absent or barely detectable (right side). The actin blot below shows that similar amounts of extracts were probed. (C) Emerin mislocalization in 4.1R-depleted HEK-293 cells. Indirect immunofluorescence of HEK-293 cells treated with control RNAi (top) or with 4.1R RNAi (bottom) showing that in 4.1R-depleted HEK-293 cells, as in other cells tested (Figs 2, 3), emerin is partially mislocalized to the cytoplasm. (D) Increased β-catenin activation in 4.1R-depleted cells. HEK-293 cells were transfected with either TOPFLASH (a TCF-activated reporter) or FOPFLASH (a reporter with mutated TCF-binding sites; control) plasmids and Renilla luciferase. Luciferase activity was corrected to Renilla luciferase signals in the same sample to normalize transfection efficiency (four experiments, mean±s.e.). (E,E′) Nuclear β-catenin accumulation in 4.1R-RNAi-treated HEK-293 cells and in 4.1R AUG1,2−/− (4.1R−/−) MEFs. Cells were fixed and stained with DAPI and with anti-β-catenin for immunofluorescent imaging. HEK-293 cells with control RNAi (E) and 4.1R wild-type (4.1R+/+) MEFs (E′) show predominant staining at peripheral cell adhesion plaques, whereas 4.1R-RNAi-treated cells (E) and 4.1R AUG1,2−/− MEFs (E′) have a nuclear accumulation of β-catenin with loss of β-catenin at the cell periphery. Scale bar: 5 μm (C); 15 μm (E′).
Increased β-catenin transcriptional activity and β-catenin nuclear accumulation when 4.1R is deficient
To substantiate that a potential 4.1R association with emerin has further biological significance, we tested the prediction that 4.1R downregulation, with concomitant displacement of emerin from the nuclear envelope in intact cells, compromises a second emerin function. Markiewicz and co-workers have shown that emerin binds to β-catenin through an adenomatous polyposis coli homology domain (Markiewicz et al., 2006). β-Catenin is a transcriptional coactivator and the downstream target of the canonical Wnt signaling pathway, participating in many developmental processes. Emerin was shown to regulate the flux of β-catenin out of the nucleus in a manner that was dependent on its correct localization at the inner nuclear envelope. When an emerin mutant lacking β-catenin-binding capacity is expressed, and in emerin-null fibroblasts, there is enhanced nuclear accumulation and activation of β-catenin (Markiewicz et al., 2006). On that basis, we hypothesized that 4.1R depletion might lead to increased β-catenin activity and nuclear accumulation due to mislocalization of a portion of emerin into the cytoplasm (Fig. 2C, Fig. 3C), thereby compromising the capacity of emerin to export β-catenin from the nucleus.
To directly test whether 4.1R depletion affects emerin-dependent β-catenin activity, we measured β-catenin-induced TCF-activated transcription in human embryonic kidney (HEK)-293 cells. HEK-293 cells exposed for 72 hours to 4.1R RNAi had a 93% depletion of ~135 kDa, ~80 kDa, and ~60 kDa 4.1R bands, relative to the levels in controls, when normalized to actin (Fig. 5B). We confirmed that emerin is mislocalized in these cells (Fig. 5C), as in other 4.1R-deficient cells (Fig. 2C, Fig. 3C). The HEK-293 cells were simultaneously transfected with a luciferase reporter plasmid in which luciferase expression is controlled by a promoter containing multiple β-catenin-TCF-binding sites (TOPGLOW). A plasmid with a mutant promoter (FOPGLOW) was used as a control and co-transfection with Renilla luciferase was used to monitor transfection efficiency. Using this assay, cells with depleted 4.1R showed an ~3.5-fold increase in β-catenin activity relative to control cells expressing 4.1R (Fig. 5D). As β-catenin activity is regulated by its nuclear accumulation, we investigated expression of β-catenin in the nucleus. In control cells, the predominant β-catenin staining was at the cell adhesion junctions with very little nuclear staining (Fig. 5E,E′). By contrast, 4.1R-RNAi-treated HEK-293 cells and 4.1R AUG1,2−/− MEFs had significantly higher levels of nuclear β-catenin epitopes, with decreased staining at the cell periphery (Fig. 5E,E′). Thus 4.1R depletion alters emerin function in exporting β-catenin from the nucleus, resulting in both increased transcriptional activity and nuclear accumulation of β-catenin.
Discussion
Here we report for the first time that 4.1R, emerin and lamin A/C co-immunoprecipitate, along with actin and other known 4.1R-binding partners, and that 4.1R loss impacts upon the organization and functions of emerin and the A-type lamin network. Depletion of 4.1R, either by 4.1R RNAi or genetic deletion, affects nuclear structure and function in multiple ways: emerin is mislocalized and peripheral and nucleoplasmic lamin A is perturbed; a emerin- and lamin-binding partner MAN1 (Mansharamani and Wilson, 2005) and the lamin-A/C-binding partner LAP2α (Dechat et al., 2000; Naetar and Foisner, 2009) distribute aberrantly; distances of centrosomes from nuclei increase; the emerin-binding partner β-catenin relocalizes from the plasma membrane to the nucleus; and β-catenin signaling activity increases. Another effect of 4.1R, emerin and A-type lamin disruption is that in emerin- and lamin-A-null MEFs the amount of nuclear 4.1R is decreased. Because the nuclear proteins that we found to be affected by 4.1R depletion are linked to human disease, it is important and timely to examine potential contributions of 4.1R. On the basis of our data, we hypothesize that 4.1R acts as a linker or adaptor at nodes vital for interconnections between nucleoplasmic substructures, nuclear envelope components and the nucleo-cytoskeletal interface.
Our discovery of the 4.1R cellular phenotype is particularly relevant to several previous findings. We previously reported partial coincidence of 4.1R with nuclear pore epitopes (Krauss et al., 1997a), and 4.1R epitopes were detected by electron microscopy in Xenopus oocytes on nuclear pore-linked filaments thought to be related to Tpr (Kiseleva et al., 2004). Tpr is proposed to be a component of nuclear basket filaments projecting into the nuclear interior that specifically function in mRNA transport (Cordes et al., 1998; Frosst et al., 2002; Huve et al., 2008; Krull et al., 2004). As Tpr is substantially mislocalized into the cytoplasm when 4.1R is depleted, as is emerin, this suggests specific mechanisms to account for how 4.1R depletion compromises emerin-dependent β-catenin nuclear export through nuclear pores.
We also previously reported that, in confocal optical sections of nuclei, 4.1R is detected at the periphery of areas stained by the SC35 splicing factor and is partially coincident with PCNA, a DNA polymerase accessory protein (Krauss et al., 1997a). Independently the Correas laboratory reported interrelationships between 4.1R and pre-mRNA splicing factors (Lallena et al., 1998). Intranuclear lamin A/C foci are also associated with splicing assemblies (Jagatheesan et al., 1999) and DNA replication centers (Kennedy et al., 2000). As 4.1R depletion decreases intranuclear lamin A/C speckles, 4.1R–lamin-A/C complexes might have important functions at these sites involving SC35 and PCNA, which binds lamin C (Shumaker et al., 2008). Moreover, it has been proposed that there is a nucleoplasmic lamin A fibrillar network with molecular links to various proteins, including NuMA (Barboro et al., 2003; Bridger et al., 2007; Broers et al., 1999; Dechat et al., 2008; Naetar and Foisner, 2009; Vlcek et al., 2001). However, NuMA was not displaced in Lmna−/− cells, although a substantial portion was mislocalized outside the nucleus in 4.1R-deficient cells. As Lmna−/− cells are not devoid of nuclear 4.1R, this suggests a predominant requirement for 4.1R in NuMA nuclear anchorage. Defective mitotic spindles with mislocalized NuMA were also observed in 4.1R-deficient cells (Krauss et al., 2008). As 4.1R depletion affects the distribution of MAN1, it could have downstream affects on transforming growth factor (TGF)-β and bone morphogenic protein (BMP) signaling (Cohen et al., 2007; Lin et al., 2005; Pan et al., 2005). Clearly there remain many tantalizing questions to resolve regarding specific roles of 4.1R in nuclear pore structural integrity and function, DNA replication, mRNA processing and signaling.
Several possible molecular models are consistent with our results: 4.1R could associate with emerin-specific, A-type lamin-specific and/or emerin–lamin-A/C complexes. Formation of these complexes could be mediated through interactions with actin, αII-spectrin or microtubules that bind 4.1R; αII-spectrin has been detected in several emerin complexes purified from HeLa cells (Holaska and Wilson, 2007) and emerin has been identified in αII-spectrin complexes (Sridharan et al., 2006). These are particularly attractive models, with precedent in erythrocytes, where 4.1R forms multiprotein complexes ultimately crucial for mature erythrocyte morphology and mechanical properties. Future experiments could test binary or multimeric interactions in vitro by comparing recombinant 4.1R isoforms (particularly those detected in immunoprecipitates) with 4.1R recombinants lacking spectrin-actin-binding domains or microtubule-binding domains, or with mutations in those domains (Gimm et al., 2002; Krauss et al., 2004). Additional multimeric interactions might even be more likely if nuclear networks with actin–spectrin or actin–nesprin (a spectrin-repeat protein) were to be proven. In fact, in addition to emerin, we find that the spectrin-repeat protein nesprin, and the actin-binding chromatin remodeling protein BRG1 also co-immunoprecipitate with 4.1R (S.W.K., unpublished), suggesting future avenues of investigation into nucleoplasmic and nucleo-cytoplasmic networks and micromechanics. Within these networks that are beginning to be deciphered, 4.1R may link components in series or may modulate interactions between proteins that bind directly to each other.
Characterizing functional interactions between 4.1R, emerin and lamin A/C has important implications for deciphering molecular etiologies for a wide spectrum of human diseases. Multiple diseases are associated with aberrant emerin and A-type lamins (laminopathies) (Capell and Collins, 2006; Worman and Bonne, 2007). Analysis of 4.1R interactions with emerin and lamin A/C mutants linked to human diseases such as EDMD and cardiomyopathy might contribute to a more detailed understanding of their biogenesis. However, when emerin or lamin A genes are not mutated (~60% of EDMD cases for example), mutant 4.1R or dysregulated 4.1R expression could alter emerin and A-type lamin distribution in architectural or regulatory complexes with specific pathologic consequences. Thus 4.1R should be considered as a candidate disease gene for laminopathy. Although there are no known human 4.1R ‘null’ patients, 4.1R deficiency is linked to progressive heart failure in humans and 4.1R AUG1,2−/− mice have disturbances in cardiac electrophysiology (Birks et al., 2005; Stagg et al., 2008). In these instances accompanying pathologic emerin and A-type lamin alterations in cardiac tissue merit examination. Furthermore, 4.1R deficiencies are associated with meningiomas and myeloid malignancies (Alanio-Brechot et al., 2008), ependymomas (Rajaram et al., 2005) and neuroancanthocytosis (Orlacchio et al., 2007). During disease progression, formation of crucial protein complexes could be dynamic and dependent on 4.1R, emerin and A-type lamin partnering that may fluctuate under different physiological conditions. An additional overlay is that 4.1R has tissue and developmental-specific isoform expression, for example, it is highly expressed in cardiac muscle and detected in specific subregions of kidney, lung and brain (Ramez et al., 2003; Shi et al., 1999). Therefore, altered 4.1R expression or mutation might impact upon tissues differently and be an unappreciated contributor to tissue degeneration and aging-related functional decrements, as well as to diverse human diseases.
Materials and Methods
Cells and media
HeLa and CaSki cells were obtained from the American Type Culture Collection, and HEK-293 cells and MDCKdN90-A cells were the gifts from Angela I. Barth, Stanford University, Palo Alto, CA. MEFs were harvested from embryos from 12–14 day wild-type and 4.1R-null pregnant mice (Shi et al., 1999), by standard procedures (Hogan et al., 1994), and used at early passages before spontaneous transformation. All mouse experiments were conducted in accordance with IACUC approved protocols at Lawrence Berkeley National Laboratory. Lamin A/C and emerin-null MEFs were the kind gift of Colin Stewart and Teresa Sullivan (NCI, Frederick, MD). HeLa, HEK-293 cells and MEFs were cultured in Dulbecco's modified Eagle's medium H21 (Gibco BRL) and CaSki cell were cultured in RPMI 1640 (Cell Gro), both supplemented with 10% fetal calf serum and penicillin-streptomycin at 37°C and under 5% CO2, as described previously (Krauss et al., 1997a).
Immunofluorescence
Cells were grown on coverslips and fixed for 10 minutes at −20°C in methanol or with 2% paraformaldehyde and 0.2% Triton X-100, with further 1% Triton X-100 permeabilization, as described previously (Krauss et al., 2008). Alternatively, paraformaldehyde or Triton fixation was followed addition of cold methanol. Commercial antibodies were the monoclonal GTU-88 against γ-tubulin (Sigma), rabbit anti-pericentrin antibody (Covance), mAb 414 against nuclear pore protein p62 (Davis and Blobel, 1986) (Babco), mouse anti-NuMA antibody (Oncogene), mouse anti-β-catenin antibody (BD Transduction Labs), goat anti-(lamin B) antibody (Santa Cruz Biotechnology), anti-(spectrin αII) monoclonal antibody 1622 (Chemicon) and mouse NCL anti-emerin antibody (Leica). The following antibodies were gifts from colleagues: anti-centrin antibody 20H5 (Jeffery Salisbury, Mayo Clinic Foundation, Rochester, MN), anti-Tpr antibody (Volker Cordes, Max Planck Institute, Gottingen, Germany), anti-(lamin A/C) monoclonal antibody XB10 (Kyle Roux and Brian Burke, University of Florida, Gainesville, FL), anti-MANI antibody (Kunxin Luo, University of California-Berkeley, Berkeley, CA), and anti-LAP2α and anti-LAP2β antibodies (Roland Foisner, University of Vienna, Vienna, Austria). Anti-4.1R Berkeley was generated in rabbits immunized with a peptide derived from exon 19 of 4.1R (AAQTDDNSGDLDPGVC) that had no cross-reactivity with 4.1B, 4.1G, or 4.1N. Secondary antibodies with minimal species cross-reactivity were from Molecular Probes or Jackson ImmunoResearch. Parallel samples probed with equal amounts of control non-immune IgG or without primary antibody showed no fluorescent patterns under conditions used for experimental samples. DNA was stained with 4,6-diamidino-2-phenylindole (Vectorshield, VectoLabs). Images were acquired with a Nikon Eclipse 2000 microscope using a 60× 1.4 NA objective equipped with a Retiga Ex camera and ImagePro software. Images were processed using Adobe Photoshop. Measurements of distances between fluorescence signals were made using Image J. Signals, within a linear range, were quantified in cell areas using Image Pro. Nuclear signals were measured as the pixels within the area stained by DAPI in overlay images. Cytoplasmic measurements were determined by subtracting the nuclear pixels from the total pixels in the cell. Nuclear and cell boundaries were visually confirmed by phase-contrast microscopy.
Immunoprecipitation
For total cell sonicates, HeLa cells were trypsinized, rinsed twice with PBS at 37°C, (2.7 mM KCl, 1.5 mM KH2PO4, 1 mM MgCl2, 1 mM EGTA, 137 mM NaCl and 8.1 mM NaHPO4, pH 7.4) containing protease inhibitors (Mini-Complete Cocktail; Roche), and collected by centrifugation. Pelleted cells were suspended in buffer and sonicated four times at 15 seconds at 50–60% output using a Branson Sonifier 250 with cooling on ice between sonications. Nuclear extracts were prepared from HeLa nuclei isolated following hypotonic shock, douncing, and pelleting through a sucrose cushion, as described previously (Krauss et al., 1997a). Nuclei were extracted with 0.2–0.25 M KCl, centrifuged and the supernatant dialyzed (Mayeda and Krainer, 1999). Nuclear extract was diluted with immunoprecipitation buffer (IP buffer), incubated with 10 μg of IgG (immune or control), rotated overnight at 4°C, then incubated at 4°C for 2 hours with 50 μl of protein-G-coupled magnetic beads (Dynal). The beads were collected magnetically, washed four times with IP buffer, and the immunoprecipitated proteins were eluted and analyzed by western blotting, as described previously (Krauss et al., 1997a). Recovery of proteins was determined by densitometry analysis of western blots using an Alpha Imager 2200 and software. Several IP buffers were compared: RIPA (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% Na-deoxycholate, 0.1% SDS and protease inhibitors) and buffers containing 25 mM HEPES, 150 mM NaCl, 1 mM MnCl2 or MgCl2, protease inhibitors and either 0.5% CHAPS or 1% Brij97. The highest amounts of 4.1R were recovered in immunoprecipitates using 25 mM HEPES, 150 mM NaCl, 1 mM MgCl2, protease inhibitors and 0.5% CHAPS for both whole-cell sonicates (88%) and nuclear extracts (92%) (Fig. 1A,B).
4.1 RNAi
Small interfering RNA duplexes (Dharmacon) were used under conditions described previously (Krauss et al., 2008) including controls for non-specific targeting. We also used RNAi duplexes containing DNA residues (Integrated DNA Technology) with the following sequence: 5′-rGrArArArGrUrCrUrGrUrGrUrArGrArArCrArUrCrArCrACG-3′; 5′-rCrGrUrGrUrGrArUrGrUrUrCrUrArCrArCrArGrArCrUrUrUrCrCrA-3′. This duplex was tested for 4.1R downregulation over a concentration range of 0–100 nM during a timecourse of 24–96 hours. For experiments reported here it was used at 30 nM complexed with Lipofectamine 2000 for transfection of 2×105 cells. Downregulation was assessed by western blotting normalized to actin.
β-Catenin activity measurements
HEK-293 cells (1.5×105) were seeded in 35-mm culture dishes and the next day transfected with 4.R RNAi, control RNAi, or Lipofectamine alone. Samples were co-transfected with 20 ng of Renilla luciferase (to estimate transfection efficiency) and 1 μg of either pFOPFLASH or pTOPFLASH. After 72 hours, the levels of luciferase and Renilla luciferase were determined on a Turner Designs Model TD-20/20 luminometer. Luciferase activity was corrected for differences in transfection efficiencies determined by the Renilla luciferase in the same sample. As a positive control, MDCK cells expressing stabilized β-catenin under doxycycline repression were grown with or without 20 ng/ml doxycycline and tested for β-catenin activity. Samples without doxycycline had 30–50-fold increased β-catenin activity relative to Renilla luciferase activity.
Supplementary Material
Acknowledgments
We would like to thank A. I. Barth, J. G. Conboy, W. J. Holaska, J. Lammerding, W. J. Nelson, K. Roux, C. Shanahan, V. Cordes, K. Wilson and Q. Zhang for valuable discussions. We are particularly grateful to J. Salisbury, R. Foisner, K. Roux, B. Burke, J. Lammerding, T. Sullivan, V. Cordes, K. Luo and A. I. Barth for sharing valuable reagents, methods, and cells. We thank M. Parra and S. Gee for technical advice. This work was supported by National Institutes of Health (grant number DK059079 to S.W.K.). The authors declare no conflicts of interest. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/124/9/1433/DC1
References
- Alanio-Brechot C., Schischmanoff P. O., Feneant-Thibault M., Cynober T., Tchernia G., Delaunay J., Garcon L. (2008). Association between myeloid malignancies and acquired deficit in protein 4.1R: a retrospective analysis of six patients. Am. J. Hematol. 83, 275-278 [DOI] [PubMed] [Google Scholar]
- An X., Zhang X., Debnath G., Baines A. J., Mohandas N. (2006). Phosphatidylinositol-4,5-biphosphate (PIP2) differentially regulates the interaction of human erythrocyte protein 4.1 (4.1R) with membrane proteins. Biochemistry 45, 5725-5732 [DOI] [PubMed] [Google Scholar]
- Anderson R. A., Correas I., Mazzucco C., Castle J. D., Marchesi V. T. (1988). Tissue-specific analogues of erythrocyte protein 4.1 retain functional domains. J. Cell. Biochem. 37, 269-284 [DOI] [PubMed] [Google Scholar]
- Baines A. J., Bennett P. M., Carter E. W., Terracciano C. (2009). Protein 4.1 and the control of ion channels. Blood Cells Mol. Dis. 42, 211-215 [DOI] [PubMed] [Google Scholar]
- Barboro P., D'Arrigo C., Mormino M., Coradeghini R., Parodi S., Patrone E., Balbi C. (2003). An intranuclear frame for chromatin compartmentalization and higher-order folding. J. Cell. Biochem. 88, 113-120 [DOI] [PubMed] [Google Scholar]
- Bengtsson L., Wilson K. L. (2004). Multiple and surprising new functions for emerin, a nuclear membrane protein. Curr. Opin. Cell Biol. 16, 73-79 [DOI] [PubMed] [Google Scholar]
- Benz E. J., Jr (1994). The erythrocyte membrane and cytoskeleton: structure, function, and disorders. In The Molecular Basis of Blood Diseases, 2nd edn (ed. Stamatoyannopoulos G., Neinhuis A. W., Majerus P., Varmus H.), pp. 257-292 Philadelphia: WB Saunders; [Google Scholar]
- Bione S., Maestrini E., Rivella S., Mancini M., Regis S., Romeo G., Toniolo D. (1994). Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat. Genet. 8, 323-327 [DOI] [PubMed] [Google Scholar]
- Birks E. J., Felkin L., Taylor-Harris P., Maggs A., Franklin R., Barton P. J., Baines A., Pinder J. C. (2005). Cytoskeletal protein 4.1 Isoforms in the myocardium of deteriorating patients requiring LVAD support and in myocardial recovery. Circulation 112, U460-U461 [Google Scholar]
- Bridger J. M., Foeger N., Kill I. R., Herrmann H. (2007). The nuclear lamina. Both a structural framework and a platform for genome organization. FEBS J. 274, 1354-1361 [DOI] [PubMed] [Google Scholar]
- Broers J. L., Machiels B. M., van Eys G. J., Kuijpers H. J., Manders E. M., van Driel R., Ramaekers F. C. (1999). Dynamics of the nuclear lamina as monitored by GFP-tagged A-type lamins. J. Cell Sci. 112, 3463-3475 [DOI] [PubMed] [Google Scholar]
- Burke B., Stewart C. L. (2002). Life at the edge: the nuclear envelope and human disease. Nat. Rev. Mol. Cell Biol. 3, 575-585 [DOI] [PubMed] [Google Scholar]
- Calinisan V., Gravem D., Chen R. P., Brittin S., Mohandas N., Lecomte M. C., Gascard P. (2006). New insights into potential functions for the protein 4.1 superfamily of proteins in kidney epithelium. Front. Biosci. 11, 1646-1666 [DOI] [PubMed] [Google Scholar]
- Capell B. C., Collins F. S. (2006). Human laminopathies: nuclei gone genetically awry. Nat. Rev. Genet. 7, 940-952 [DOI] [PubMed] [Google Scholar]
- Chuang C. H., Carpenter A. E., Fuchsova B., Johnson T., de Lanerolle P., Belmont A. S. (2006). Long-range directional movement of an interphase chromosome site. Curr. Biol. 16, 825-831 [DOI] [PubMed] [Google Scholar]
- Cohen C., Foley S. (1984). Biochemical characterization of complex formation by human erythrocyte spectrin, protein 4.1, and actin. Biochemistry 23, 6091-6098 [DOI] [PubMed] [Google Scholar]
- Cohen T. V., Kosti O., Stewart C. L. (2007). The nuclear envelope protein MAN1 regulates TGFbeta signaling and vasculogenesis in the embryonic yolk sac. Development 134, 1385-1395 [DOI] [PubMed] [Google Scholar]
- Conboy J. (1999). The role of alternative pre-mRNA splicing in regulating the structure and function of skeletal protein 4.1. Proc. Soc. Exp. Biol. Med. 220, 73-78 [DOI] [PubMed] [Google Scholar]
- Conboy J. G. (1993). Structure, function and molecular genetics of erythroid membrane skeletal protein 4.1 in normal and abnormal red blood cells. Semin. Hematol. 30, 58-73 [PubMed] [Google Scholar]
- Conboy J. G., Chan J., Mohandas N., Kan Y. W. (1988). Multiple protein isoforms produced by alternative splicing in human erythroid cells. Proc. Natl. Acad. Sci. USA 85, 9062-9065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy J. G., Chan J., Chasis J. A., Kan Y. W., Mohandas N. (1991). Tissue- and development-specific alternative RNA splicing regulates expression of multiple isoforms of erythroid membrane protein 4.1. J. Biol. Chem. 266, 8273-8280 [PubMed] [Google Scholar]
- Cordes V. C., Hase M. E., Muller L. (1998). Molecular segments of protein Tpr that confer nuclear targeting and association with the nuclear pore complex. Exp. Cell Res. 245, 43-56 [DOI] [PubMed] [Google Scholar]
- Correas I. (1991). Characterization of isoforms of protein 4.1 present in the nucleus. Biochem. J. 279, 581-585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp M., Liu Q., Roux K., Rattner J. B., Shanahan C., Burke B., Stahl P. D., Hodzic D. (2006). Coupling of the nucleus and cytoplasm: role of the LINC complex. J. Cell Biol. 172, 41-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. I., Blobel G. (1986). Identification and characterization of a nuclear pore complex protein. Cell 45, 699-709 [DOI] [PubMed] [Google Scholar]
- De Carcer G., Lallena M. J., Correas I. (1995). Protein 4.1 is a component of the nuclear matrix of mammalian cells. Biochem. J. 312, 871-877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechat T., Korbei B., Vaughan O. A., Vlcek S., Hutchison C. J., Foisner R. (2000). Lamina-associated polypeptide 2alpha binds intranuclear A-type lamins. J. Cell Sci. 113, 3473-3484 [DOI] [PubMed] [Google Scholar]
- Dechat T., Pfleghaar K., Sengupta K., Shimi T., Shumaker D. K., Solimando L., Goldman R. D. (2008). Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 22, 832-853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhommeau F., Vasseur-Godbillon C., Leclerc P., Schischmanoff P. O., Croisille L., Rince P., Moriniere M., Benz E. J., Jr, Tchernia G., Tamagnini G., et al. (2002). A splicing alteration of 4.1R pre-mRNA generates 2 protein isoforms with distinct assembly to spindle poles in mitotic cells. Blood 100, 2629-2636 [DOI] [PubMed] [Google Scholar]
- Discher D. E., Parra M., Conboy J. G., Mohandas N. (1993). Mechanochemistry of the alternatively spliced spectrin-actin binding domain in membrane skeletal protein 4.1. J. Biol. Chem. 268, 7186-7195 [PubMed] [Google Scholar]
- Dundr M., Ospina J. K., Sung M. H., John S., Upender M., Ried T., Hager G. L., Matera A. G. (2007). Actin-dependent intranuclear repositioning of an active gene locus in vivo. J. Cell Biol. 179, 1095-1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery A. E. (2000). Emery-Dreifuss muscular dystrophy-a 40 year retrospective. Neuromuscul. Disord. 10, 228-232 [DOI] [PubMed] [Google Scholar]
- Foisner R., Gerace L. (1993). Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell 73, 1267-1279 [DOI] [PubMed] [Google Scholar]
- Frosst P., Guan T., Subauste C., Hahn K., Gerace L. (2002). Tpr is localized within the nuclear basket of the pore complex and has a role in nuclear protein export. J. Cell Biol. 156, 617-630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascard P., Lee G., Coulombel L., Auffray I., Lum M., Parra M., Conboy J. G., Mohandas N., Chasis J. A. (1998). Characterization of multiple isoforms of protein 4.1R expressed during erythroid terminal differentiation. Blood 92, 4404-4414 [PubMed] [Google Scholar]
- Gascard P., Nunomura W., Lee G., Walensky L., Krauss S., Chasis J., Mohandas N., Conboy J. (1999). Deciphering the nuclear import pathway for the cytoskeletal protein 4.1R. Mol. Biol. Cell 10, 1783-1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimm J. A., An X., Nunomura W., Mohandas N. (2002). Functional characterization of spectrin-actin-binding domains in 4.1 family of proteins. Biochemistry 41, 7275-7282 [DOI] [PubMed] [Google Scholar]
- Hale C. M., Shrestha A. L., Khatau S. B., Stewart-Hutchinson P. J., Hernandez L., Stewart C. L., Hodzic D., Wirtz D. (2008). Dysfunctional connections between the nucleus and the actin and microtubule networks in laminopathic models. Biophys. J. 95, 5462-5475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque F., Lloyd D. J., Smallwood D. T., Dent C. L., Shanahan C. M., Fry A. M., Trembath R. C., Shackleton S. (2006). SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol. Cell. Biol. 26, 3738-3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W. A. (2009). Cell and molecular biology of nuclear actin. Int. Rev. Cell Mol. Biol. 273, 219-263 [DOI] [PubMed] [Google Scholar]
- Hofmann W. A., Stojiljkovic L., Fuchsova B., Vargas G. M., Mavrommatis E., Philimonenko V., Kysela K., Goodrich J. A., Lessard J. L., Hope T. J., et al. (2004). Actin is part of pre-initiation complexes and is necessary for transcription by RNA polymerase II. Nat. Cell Biol. 6, 1094-1101 [DOI] [PubMed] [Google Scholar]
- Hogan B., Bettington R., Costini F., Lacy E. (1994). Manipulating the Mouse Embryo: A Laboratory Manual. New York: Cold Spring Harbor, NY; [Google Scholar]
- Holaska J. M., Wilson K. L. (2006). Multiple roles for emerin: implications for Emery-Dreifuss muscular dystrophy. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 288, 676-680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaska J. M., Wilson K. L. (2007). An emerin “proteome”: purification of distinct emerin-containing complexes from HeLa cells suggests molecular basis for diverse roles including gene regulation, mRNA splicing, signaling, mechanosensing, and nuclear architecture. Biochemistry 46, 8897-8908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaska J. M., Kowalski A. K., Wilson K. L. (2004). Emerin caps the pointed end of actin filaments: evidence for an actin cortical network at the nuclear inner membrane. PLoS Biol. 2, E231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmer L., Worman H. J. (2001). Inner nuclear membrane proteins: functions and targeting. Cell. Mol. Life Sci. 58, 1741-1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huve J., Wesselmann R., Kahms M., Peters R. (2008). 4Pi microscopy of the nuclear pore complex. Biophys. J. 95, 877-885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagatheesan G., Thanumalayan S., Muralikrishna B., Rangaraj N., Karande A. A., Parnaik V. K. (1999). Colocalization of intranuclear lamin foci with RNA splicing factors. J. Cell Sci. 112, 4651-4661 [DOI] [PubMed] [Google Scholar]
- Kang Q., Yu Y., Pei X., Hughes R., Heck S., Zhang X., Guo X., Halverson G., Mohandas N., An X. (2009). Cytoskeletal protein 4.1R negatively regulates T cell activation by inhibiting the phosphorylation of LAT. Blood 113, 6126-6137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy B. K., Barbie D. A., Classon M., Dyson N., Harlow E. (2000). Nuclear organization of DNA replication in primary mammalian cells. Genes Dev. 14, 2855-2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiseleva E., Drummond S. P., Goldberg M. W., Rutherford S. A., Allen T. D., Wilson K. L. (2004). Actin- and 4.1-containing filaments link nuclear pore complexes to subnuclear organelles in Xenopus oocyte nuclei. J. Cell Sci. 117, 2481-2490 [DOI] [PubMed] [Google Scholar]
- Kontrogianni-Konstantopoulos A., Huang S. C., Benz E. J., Jr (2000). A nonerythroid isoform of protein 4.1R interacts with components of the contractile apparatus in skeletal myofibers. Mol. Biol. Cell 11, 3805-3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S. W., Larabell C. A., Lockett S., Gascard P., Mohandas N., Chasis J. A. (1997a). Structural protein 4.1 in the nucleus of human cells: dynamic rearrangements during cell division. J. Cell Biol. 137, 275-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S. W., Chasis J. A., Rogers C., Mohandas N., Krockmalnic G., Penman S. (1997b). Structural protein 4.1 is located in mammalian centrosomes. Proc. Natl. Acad. Sci. USA 94, 7297-7302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S. W., Heald R., Lee G., Nunomura W., Gimm J. A., Mohandas N., Chasis J. A. (2002). Two distinct domains of protein 4.1 critical for assembly of functional nuclei in vitro. J. Biol. Chem. 277, 44339-44346 [DOI] [PubMed] [Google Scholar]
- Krauss S. W., Chen C., Penman S., Heald R. (2003). Nuclear actin and protein 4.1: essential interactions during nuclear assembly in vitro. Proc. Natl. Acad. Sci. USA 100, 10752-10757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S. W., Lee G., Chasis J. A., Mohandas N., Heald R. (2004). Two protein 4.1 domains essential for mitotic spindle and aster microtubule dynamics and organization in vitro. J. Biol. Chem. 279, 27591-27598 [DOI] [PubMed] [Google Scholar]
- Krauss S. W., Spence J. R., Bahmanyar S., Barth A. I., Go M. M., Czerwinski D., Meyer A. J. (2008). Downregulation of protein 4.1R, a mature centriole protein, disrupts centrosomes, alters cell cycle progression, and perturbs mitotic spindles and anaphase. Mol. Cell. Biol. 28, 2283-2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull S., Thyberg J., Bjorkroth B., Rackwitz H. R., Cordes V. C. (2004). Nucleoporins as components of the nuclear pore complex core structure and Tpr as the architectural element of the nuclear basket. Mol. Biol. Cell 15, 4261-4277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallena M. J., Martinez C., Valcarcel J., Correas I. (1998). Functional association of nuclear protein. 4.1 with pre-mRNA splicing factors. J. Cell Sci. 111, 1963-1971 [DOI] [PubMed] [Google Scholar]
- Lammerding J., Hsiao J., Schulze P. C., Kozlov S., Stewart C. L., Lee R. T. (2005). Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J. Cell Biol. 170, 781-791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. K., Haraguchi T., Lee R. S., Koujin T., Hiraoka Y., Wilson K. L. (2001). Distinct functional domains in emerin bind lamin A and DNA-bridging protein BAF. J. Cell Sci. 114, 4567-4573 [DOI] [PubMed] [Google Scholar]
- Libotte T., Zaim H., Abraham S., Padmakumar V. C., Schneider M., Lu W., Munck M., Hutchison C., Wehnert M., Fahrenkrog B., et al. (2005). Lamin A/C-dependent localization of Nesprin-2, a giant scaffolder at the nuclear envelope. Mol. Biol. Cell 16, 3411-3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Blake D. L., Callebaut I., Skerjanc I. S., Holmer L., McBurney M. W., Paulin-Levasseur M., Worman H. J. (2000). MAN1, an inner nuclear membrane protein that shares the LEM domain with lamina-associated polypeptide 2 and emerin. J. Biol. Chem. 275, 4840-4847 [DOI] [PubMed] [Google Scholar]
- Lin F., Morrison J. M., Wu W., Worman H. J. (2005). MAN1, an integral protein of the inner nuclear membrane, binds Smad2 and Smad3 and antagonizes transforming growth factor-beta signaling. Hum. Mol. Genet. 14, 437-445 [DOI] [PubMed] [Google Scholar]
- Luque C. M., Correas I. (2000). A constitutive region is responsible for nuclear targeting of 4.1R: modulation by alternative sequences results in differential intracellular localization. J. Cell Sci. 113, 2485-2495 [DOI] [PubMed] [Google Scholar]
- Mansharamani M., Wilson K. L. (2005). Direct binding of nuclear membrane protein MAN1 to emerin in vitro and two modes of binding to barrier-to-autointegration factor. J. Biol. Chem. 280, 13863-13870 [DOI] [PubMed] [Google Scholar]
- Markiewicz E., Dechat T., Foisner R., Quinlan R. A., Hutchison C. J. (2002). Lamin A/C binding protein LAP2alpha is required for nuclear anchorage of retinoblastoma protein. Mol. Biol. Cell 13, 4401-4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewicz E., Tilgner K., Barker N., van de Wetering M., Clevers H., Dorobek M., Hausmanowa-Petrusewicz I., Ramaekers F. C., Broers J. L., Blankesteijn W. M., et al. (2006). The inner nuclear membrane protein emerin regulates beta-catenin activity by restricting its accumulation in the nucleus. EMBO J. 25, 3275-3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattagajasingh S. N., Huang S. C., Hartenstein J. S., Snyder M., Marchesi V. T., Benz E. J., Jr (1999). A nonerythroid isoform of protein 4.1R interacts with the nuclear mitotic apparatus (NuMA) protein. J. Cell Biol. 145, 29-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattagajasingh S. N., Huang S. C., Hartenstein J. S., Benz E. J., Jr (2000). Characterization of the interaction between protein 4.1R and ZO-2. A possible link between the tight junction and the actin cytoskeleton. J. Biol. Chem. 275, 30573-30585 [DOI] [PubMed] [Google Scholar]
- Mayeda A., Krainer A. R. (1999). Preparation of HeLa cell nuclear and cytosolic S100 extracts for in vitro splicing. Methods Mol. Biol. 118, 309-314 [DOI] [PubMed] [Google Scholar]
- Merdes A., Cleveland D. W. (1998). The role of NuMA in the interphase nucleus. J. Cell Sci. 111, 71-79 [DOI] [PubMed] [Google Scholar]
- Mislow J. M., Holaska J. M., Kim M. S., Lee K. K., Segura-Totten M., Wilson K. L., McNally E. M. (2002). Nesprin-1alpha self-associates and binds directly to emerin and lamin A in vitro. FEBS Lett. 525, 135-140 [DOI] [PubMed] [Google Scholar]
- Mohandas N., Chasis J. A. (1993). Red blood cell deformability, membrane material properties and shape: regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin. Hematol. 30, 171-192 [PubMed] [Google Scholar]
- Morris G. E. (2001). The role of the nuclear envelope in Emery-Dreifuss muscular dystrophy. Trends Mol. Med. 7, 572-577 [DOI] [PubMed] [Google Scholar]
- Mounkes L., Kozlov S., Burke B., Stewart C. L. (2003). The laminopathies: nuclear structure meets disease. Curr. Opin. Genet. Dev. 13, 223-230 [DOI] [PubMed] [Google Scholar]
- Muchir A., Worman H. J. (2004). The nuclear envelope and human disease. Physiology (Bethesda) 19, 309-314 [DOI] [PubMed] [Google Scholar]
- Muchir A., Medioni J., Laluc M., Massart C., Arimura T., van der Kooi A. J., Desguerre I., Mayer M., Ferrer X., Briault S., et al. (2004). Nuclear envelope alterations in fibroblasts from patients with muscular dystrophy, cardiomyopathy, and partial lipodystrophy carrying lamin A/C gene mutations. Muscle Nerve 30, 444-450 [DOI] [PubMed] [Google Scholar]
- Naetar N., Foisner R. (2009). Lamin complexes in the nuclear interior control progenitor cell proliferation and tissue homeostasis. Cell Cycle 8, 1488-1493 [DOI] [PubMed] [Google Scholar]
- Nunomura W., Takakuwa Y., Parra M., Conboy J. G., Mohandas N. (2000). Ca(2+)-dependent and Ca(2+)-independent calmodulin binding sites in erythrocyte protein 4.1. Implications for regulation of protein 4.1 interactions with transmembrane proteins. J. Biol. Chem. 275, 6360-6367 [DOI] [PubMed] [Google Scholar]
- Olave I. A., Reck-Peterson S. L., Crabtree G. R. (2002). Nuclear actin and actin-related proteins in chromatin remodeling. Annu. Rev. Biochem. 71, 755-781 [DOI] [PubMed] [Google Scholar]
- Orlacchio A., Calabresi P., Rum A., Tarzia A., Salvati A. M., Kawarai T., Stefani A., Pisani A., Bernardi G., Cianciulli P., et al. (2007). Neuroacanthocytosis associated with a defect of the 4.1R membrane protein. BMC Neurol. 7, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund C., Sullivan T., Stewart C. L., Worman H. J. (2006). Dependence of diffusional mobility of integral inner nuclear membrane proteins on A-type lamins. Biochemistry 45, 1374-1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund C., Folker E. S., Choi J. C., Gomes E. R., Gundersen G. G., Worman H. J. (2009). Dynamics and molecular interactions of linker of nucleoskeleton and cytoskeleton (LINC) complex proteins. J. Cell Sci. 122, 4099-4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D., Estevez-Salmeron L. D., Stroschein S. L., Zhu X., He J., Zhou S., Luo K. (2005). The integral inner nuclear membrane protein MAN1 physically interacts with the R-Smad proteins to repress signaling by the transforming growth factor-β superfamily of cytokines. J. Biol. Chem. 280, 15992-16001 [DOI] [PubMed] [Google Scholar]
- Pasternack G. R., Racusen R. H. (1989). Erythrocycte protein 4.1 binds and regulates myosin. Proc. Natl. Acad. Sci. USA 86, 9712-9716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Ferreiro C. M., Luque C. M., Correas I. (2001). 4.1R proteins associate with interphase microtubules in human T cells: a 4.1R constitutive region is involved in tubulin binding. J. Biol. Chem. 276, 44785-44791 [DOI] [PubMed] [Google Scholar]
- Rajaram V., Gutmann D. H., Prasad S. K., Mansur D. B., Perry A. (2005). Alterations of protein 4.1 family members in ependymomas: a study of 84 cases. Mod. Pathol. 18, 991-997 [DOI] [PubMed] [Google Scholar]
- Ramez M., Blot-Chabaud M., Cluzeaud F., Chanan S., Patterson M., Walensky L. D., Marfatia S., Baines A. J., Chasis J. A., Conboy J. G., et al. (2003). Distinct distribution of specific members of protein 4.1 gene family in the mouse nephron. Kidney Int. 63, 1321-1337 [DOI] [PubMed] [Google Scholar]
- Razafsky D., Hodzic D. (2009). Bringing KASH under the SUN: the many faces of nucleo-cytoskeletal connections. J. Cell Biol. 186, 461-472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera A., De Franceschi L., Peters L. L., Gascard P., Mohandas N., Brugnara C. (2006). Effect of complete protein 4.1R deficiency on ion transport properties of murine erythrocytes. Am. J. Physiol. Cell Physiol. 291, C880-C886 [DOI] [PubMed] [Google Scholar]
- Salomao M., Zhang X., Yang Y., Lee S., Hartwig J. H., Chasis J. A., Mohandas N., An X. (2008). Protein 4.1R-dependent multiprotein complex: new insights into the structural organization of the red blood cell membrane. Proc. Natl. Acad. Sci. USA 105, 8026-8031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpingidou G., Smertenko A., Hausmanowa-Petrucewicz I., Hussey P. J., Hutchison C. J. (2007). A novel role for the nuclear membrane protein emerin in association of the centrosome to the outer nuclear membrane. J. Cell Biol. 178, 897-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer E. C., Florens L., Guan T., Yates J. R., 3rd, Gerace L. (2003). Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science 301, 1380-1382 [DOI] [PubMed] [Google Scholar]
- Schischmanoff P. O., Winardi R., Discher D. E., Parra M. K., Bicknese S. E., Witkowska H. E., Conboy J. G., Mohandas N. (1995). Defining of the minimal domain of protein 4.1 involved in spectrin-actin binding. J. Biol. Chem. 270, 21243-21250 [DOI] [PubMed] [Google Scholar]
- Schneider M., Lu W., Neumann S., Brachner A., Gotzmann J., Noegel A. A., Karakesisoglou I. (2010). Molecular mechanisms of centrosome and cytoskeleton anchorage at the nuclear envelope. Cell. Mol. Life Sci. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z. T., Afzal V., Coller B., Patel D., Chasis J. A., Parra M., Lee G., Paszty C., Stevens M., Walensky L., et al. (1999). Protein 4.1R-deficient mice are viable but have erythroid membrane skeleton abnormalities. J. Clin. Invest. 103, 331-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker D. K., Solimando L., Sengupta K., Shimi T., Adam S. A., Grunwald A., Strelkov S. V., Aebi U., Cardoso M. C., Goldman R. D. (2008). The highly conserved nuclear lamin Ig-fold binds to PCNA: its role in DNA replication. J. Cell Biol. 181, 269-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D. M., McMahon L. W., Lambert M. W. (2006). alphaII-Spectrin interacts with five groups of functionally important proteins in the nucleus. Cell Biol. Int. 30, 866-878 [DOI] [PubMed] [Google Scholar]
- Stagg M. A., Carter E., Sohrabi N., Siedlecka U., Soppa G. K., Mead F., Mohandas N., Taylor-Harris P., Baines A., Bennett P., et al. (2008). Cytoskeletal protein 4.1R affects repolarization and regulates calcium handling in the heart. Circ. Res. 103, 855-863 [DOI] [PubMed] [Google Scholar]
- Stewart C. L., Roux K. J., Burke B. (2007). Blurring the boundary: the nuclear envelope extends its reach. Science 318, 1408-1412 [DOI] [PubMed] [Google Scholar]
- Sullivan T., Escalante-Alcalde D., Bhatt H., Anver M., Bhat N., Nagashima K., Stewart C. L., Burke B. (1999). Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 147, 913-920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakuwa Y. (2000). Protein 4.1, a multifunctional protein of the erythrocyte membrane skeleton: structure and functions in erythrocytes and nonerythroid cells. Int. J. Hematol. 72, 298-309 [PubMed] [Google Scholar]
- Vaughan A., Alvarez-Reyes M., Bridger J. M., Broers J. L., Ramaekers F. C., Wehnert M., Morris G. E., Whitfield W. G. F., Hutchison C. J. (2001). Both emerin and lamin C depend on lamin A for localization at the nuclear envelope. J. Cell Sci. 114, 2577-2590 [DOI] [PubMed] [Google Scholar]
- Vlcek S., Dechat T., Foisner R. (2001). Nuclear envelope and nuclear matrix: interactions and dynamics. Cell. Mol. Life Sci. 58, 1758-1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner N., Krohne G. (2007). LEM-Domain proteins: new insights into lamin-interacting proteins. Int. Rev. Cytol. 261, 1-46 [DOI] [PubMed] [Google Scholar]
- Walensky L. D., Gascard P., Fields M. E., Blackshaw S., Conboy J. G., Mohandas N., Snyder S. H. (1998). The 13-kD FK506 binding protein, FKBP13, interacts with a novel homologue of the erythrocyte membrane cytoskeletal protein 4.1. J. Cell Biol. 141, 143-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M. A., Davies J. D., Zhang Q., Emerson L. J., Hunt J., Shanahan C. M., Ellis J. A. (2007). Distinct functional domains in nesprin-1alpha and nesprin-2beta bind directly to emerin and both interactions are disrupted in X-linked Emery-Dreifuss muscular dystrophy. Exp. Cell Res. 313, 2845-2857 [DOI] [PubMed] [Google Scholar]
- Worman H. J., Bonne G. (2007). ‘Laminopathies’: a wide spectrum of human diseases. Exp. Cell Res. 313, 2121-2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman H. J., Courvalin J. C. (2005). Nuclear envelope, nuclear lamina, and inherited disease. Int. Rev. Cytol. 246, 231-279 [DOI] [PubMed] [Google Scholar]
- Zhang X., Lei K., Yuan X., Wu X., Zhuang Y., Xu T., Xu R., Han M. (2009). SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron 64, 173-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.