Abstract
Extracellular proteolysis is an indispensable requirement for the formation of new blood vessels during neovascularization and is implicated in the generation of several angiogenic regulatory molecules. Anti-proteolytic agents have become attractive therapeutic strategies in diseases associated with excessive neovascularization. Annexin A2 (AnxA2) is an endothelial cell-surface receptor for the generation of active proteolytic factors, such as plasmin. Here, we show that AnxA2 is abundantly expressed in the neovascular tufts in a murine model of neovascularization. Exposure to hypoxic conditions results in elevation of AnxA2 and tissue plasminogen activator (tPA) in human retinal microvascular endothelial cells (RMVECs). We show that the hexapeptide competitive inhibitor LCKLSL, which targets the N-terminal tPA-binding site of AnxA2, binds efficiently to cell-surface AnxA2 compared with binding of the control peptide LGKLSL. Treatment with the competitive peptide inhibits the generation of plasmin and suppresses the VEGF-induced activity of tPA under hypoxic conditions. Application of the competitive peptide in two in vivo models of angiogenesis demonstrated suppression of the angiogenic responses, which was also associated with significant changes in the vascular sprouting. These results suggest that AnxA2-mediated plasmin generation is an important event in angiogenesis and is inhibited by a specific competitive peptide that inhibits the binding of tPA to AnxA2.
Key words: Annexin A2, Tissue plasminogen activator, Retinal neovascularization, Plasmin, Angiogenesis
Introduction
Neovascularization, the sprouting of new blood capillaries from pre-existing blood vessels is a multistep process that occurs in a series of coordinated steps (Milkiewicz et al., 2006). Neovascularization involves the degradation of extracellular matrix (ECM) surrounding the basement membrane, formation of a lesion in the basement membrane, migration of the endothelial cells to form a new capillary sprout and further migration and proliferation of endothelial cells to elongate the sprout (Milkiewicz et al., 2006). Proteolysis of the ECM is an important prerequisite for the endothelial cells to migrate, proliferate and form a new blood vessel and requires an array of extracellular proteolytic events (Pepper, 2001; van Hinsbergh et al., 2006). The extracellular proteolytic system comprises serine proteases and their activators, including tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA), and their physiological inhibitors, plasminogen activator inhibitor (PAI)-1 and PAI-2 (Lijnen, 2001). Plasmin, a central component of this system, is a broad-spectrum trypsin-like serine protease that degrades several components of the ECM-like laminin and fibrin, activates inactive pro-matrix metalloproteinases (MMPs) and also increases the bioavailability of isoform 165 and 189 of the angiogenic growth factor vascular endothelial growth factor (VEGF) (Hajjar and Deora, 2000; Houck et al., 1992; Lijnen, 2001). The generation of the plasmin-induced angiogenic response is regulated by the balance of plasminogen activators tPA and uPA (Castellino and Ploplis, 2005; Fukao et al., 1997). Recent evidence indicates a correlation between elevated levels of VEGF, tPA and PAI-1 in patients with proliferative diabetic retinopathy (Simpson et al., 1999). In addition, elevated levels of plasminogen activators have been observed in type 1 diabetes patients with retinopathy (Almér et al., 1975; Grant and Guay, 1991; Hattenbach et al., 1999).
Spatial restriction of proteolysis has been identified as a common mechanism for not only limiting the proteolysis to the pericellular environment but also amplifying the extracellular proteolytic cascade (Pepper, 2001). Annexin A2 (AnxA2), a member of a family of Ca2+-dependent phospholipid-binding proteins, is a cell-surface coreceptor for tPA and plasminogen (Kim and Hajjar, 2002). By binding to both plasminogen and tPA, AnxA2 brings the two enzymes in close proximity and increases the catalytic efficiency of plasmin generation by ~60-fold (Hajjar and Menell, 1997). The result is a highly efficient plasmin-mediated proteolytic cascade, which promotes neovascularization by increasing the efficiency of endothelial cell invasion across the ECM (Hajjar and Acharya, 2000).
tPA binds to amino acid residues at position 7–12 in the N-terminus of AnxA2, corresponding to the site LCKLSL (Cesarman et al., 1994; Roda et al., 2003). The presence of a cysteine residue is crucial for tPA binding, and the interaction is inhibited in the presence of homocysteine (Hajjar et al., 1998). Sequential mutations in the N-terminus of AnxA2 have indicated that the cysteine residue at position 8 is crucial for the binding of tPA to AnxA2, and mutation of this residue significantly reduces the efficiency of tPA binding to AnxA2 (Roda et al., 2003). In a previous study, an array of synthetic peptides containing cysteine residues and spanning the N-terminus of AnxA2 had been generated and tested for their ability to bind to the tPA-binding site of AnxA2 (Roda et al., 2003). Among these peptides, the hexapeptide LCKLSL, which is identical in sequence to the tPA-binding site of AnxA2 and contains a cysteine residue at position 8, had been shown to be >95% efficient in competitively inhibiting tPA binding to AnxA2 (Roda et al., 2003).
Here, we tested the anti-angiogenic activity of this well-characterized competitive peptide inhibitor (i.e. the N-terminal LCKLSL hexapeptide that prevents the binding of tPA to AnxA2) using both in vitro and in vivo angiogenesis assays. We show that hypoxia-induced angiogenic responses result in an increase in the endothelial cell-surface binding of tPA and AnxA2 followed by a concomitant increase in the generation of plasmin. Furthermore, we demonstrate that treatment of hypoxic human retinal microvascular endothelial cells (RMVECs) with the hexapeptide competitive peptide inhibitor prevents the formation of the AnxA2–tPA complex at the cell surface, along with causing a reduction in the plasmin-generating activity of tPA. Utilizing the chicken chorioallantoic membrane (CAM) and murine Matrigel plug models, we demonstrate that the hexapeptide is able to specifically inhibit VEGF-induced neovascularization, suggesting its potential involvement in the VEGF pathway. Taken together, this study elucidates the mechanisms by which excessive extracellular proteolysis can be inhibited by modulating the ability of tPA to bind to AnxA2, its cell-surface receptor.
Results
Isolation of a homogenous population of human RMVECs and characterization for the expression of AnxA2
Endothelial cells are known to express AnxA2 (Hajjar et al., 1994), hence we used RMVECs as a model to study the involvement of AnxA2 in hypoxia-induced angiogenic events. RMVECs grew to confluence in 8 or 9 days, forming contact-inhibited monolayers and with endothelial-cell-specific cobblestone morphology (supplementary material Fig. S1A). We also tested the cells for their ability to form capillary-tube-like structures on Matrigel basement membrane; supplementary material Fig. S1B is a phase-contrast image of the capillary-like tubular structures that formed within 3 hours after seeding the cells on a Matrigel basement membrane. In order to confirm that the isolated cells were indeed RMVECs, we immunostained these cells with von Willebrand factor (vWF), followed by confocal microscopy, which showed that nearly all of the cells were positive for vWF (supplementary material Fig. S1C).
RMVECs were tested for the intracellular and cell-surface expression of AnxA2. Immunostaining for AnxA2 followed by confocal microscopy revealed the intracellular expression of AnxA2 (supplementary material Fig. S1C) and no colocalization of vWF and AnxA2 was observed. Total internal reflection (TIRF) microscopy showed extracellular AnxA2 as dense and punctate spots on the cell surface (supplementary material Fig. S1D).
Induction and evaluation of hypoxia-induced angiogenic events in cultured RMVECs
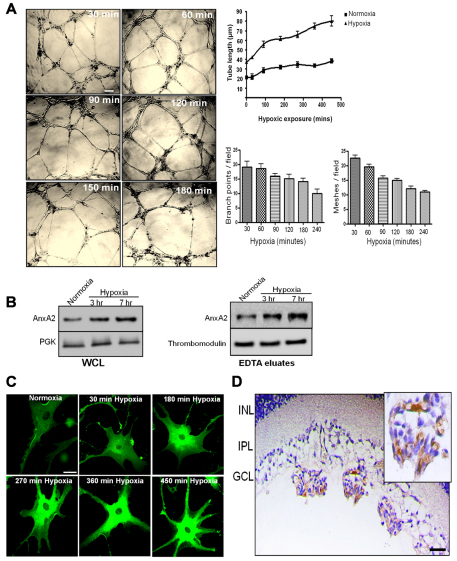
Our preliminary findings indicated that RMVECs exposed to hypoxic conditions (3% O2) for 3–7 hours were morphologically distinguishable from normoxic RMVECs. The hypoxic cells readily formed tube-like endothelial cell networks when seeded on a Matrigel basement membrane. When the cells were incubated under hypoxic conditions (3% O2) for 30 minutes to 4 hours, an increase in the length of the endothelial cell networks, which appeared as tube-like structures, was observed in the presence of growth factors, such as VEGF and fibroblast growth factor (FGF) 2, as visualized in the phase-contrast images (Fig. 1A). Upon quantification of the capillary-tube-like structures using the NIH ImageJ software, tube length following 4 hours of hypoxic exposure was measured to be ~80 μm compared with a tube length of ~25 μm in cells exposed to 30 minutes of hypoxia (Fig. 1A). The endothelial cells seeded on Matrigel formed a meshwork of tube-like structures that contained branching structures with multi-centric junctions. These structures are formed as early as after 30 minutes of exposure to hypoxic conditions. When the cells were exposed to increased periods of hypoxic exposure, there was an increase in the tube length, which was accompanied by a decrease in the topological parameters like the number of branching points and the number of meshes observed per field (Fig. 1A).
Fig. 1.
Enhancement of the length of the capillary-tube-like structures and hypoxia-induced expression of AnxA2 in RMVECs and mouse sections in a mouse model of OIR. (A) Phase-contrast images of RMVECs seeded on Matrigel and exposed to hypoxic conditions for the indicated periods of time. The length of the tube-like structures was quantified in three independent microscopic fields per experimental treatment using the NIH ImageJ software. The data are mean lengths (±s.e.m.). Scale bar: 200 μm. The topological parameters (number of branching points per field and the number of meshes per field) were evaluated and the data are represented as means+s.e.m. (B) RMVECs were exposed to hypoxic conditions for the indicated periods of time. The cells were incubated in EDTA for 20 minutes and later subjected to lysis. The EDTA eluates and the whole cell lysates (WCL) were subjected to western immunoblot analysis with the anti-AnxA2 antibody. PGK and thrombomodulin were used as loading controls for whole cell lysates and EDTA eluates respectively. (C) RMVECs exposed to hypoxic conditions for the indicated periods of time were fixed with 4% paraformaldehyde and immunostained with the anti-AnxA2 antibody. Six representative images are shown, indicating an increase in the expression of AnxA2 in response to hypoxia. Scale bar: 50 μm. (D) Immunohistochemistry of OIR retinal sections stained for AnxA2. Expression of AnxA2 in the vascular tufts penetrating from the inner limiting membrane of the OIR sections. The insert is a magnified image of the vascular tufts with intense staining for AnxA2. Scale bar: 25 μm.
Hypoxia induces an upregulation in the expression of AnxA2 in cultured RMVECs and in the vascular tufts of oxygen-induced retinopathy mouse sections
To test whether the levels of AnxA2 were influenced by hypoxic exposure, RMVECs were subjected to hypoxic conditions. Immunoblotting of whole cell lysates (Fig. 1B, left panel) and EDTA eluates (Fig. 1B, right panel) demonstrated that exposure to hypoxic conditions for 3 and 7 hours resulted in the upregulation of the intracellular (50% increase on hypoxic exposure for 7 hours) and surface levels of AnxA2 (70% increase on hypoxic exposure for 7 hours) compared with that in the normoxic control. Confocal microscopy of RMVECs exposed to hypoxic conditions for the indicated periods of time (from 30 minutes to 7.5 hours) and immunostained for AnxA2 showed an increased intracellular expression of AnxA2 in cells incubated under hypoxic conditions compared with that in cells incubated under normoxic conditions (Fig. 1C). In order to test the involvement of AnxA2 in the hypoxia-induced neovascular responses of the retina, we used the mouse oxygen-induced retinopathy (OIR) model (Sone et al., 1999). Immunostaining of paraffin-embedded OIR mouse retinal for AnxA2 revealed abundant expression of AnxA2 in the neovascular tufts of OIR sections at postnatal day 17 (p17) (Fig. 1D). The nonvascular tufts were observed to be extending above the inner limiting membrane. Thus, the localization of AnxA2 in the neovascular tufts of the OIR sections suggests its involvement in the vaso-obliterative phase of oxygen-induced retinopathy.
Hypoxia-induced upregulation of secreted and membrane-bound levels of AnxA2 and tPA in RMVECs
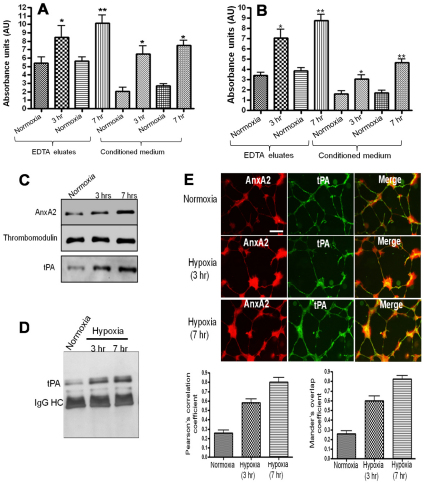
RMVECs exposed to 3 and 7 hours of hypoxia secreted elevated levels of tPA into the conditioned medium compared with that by the normoxic RMVECs; the majority of the secreted tPA was associated with the cell surface (twofold increase in cells exposed to hypoxia for 7 hours) as it was recovered by incubating the cells with EDTA (Fig. 2A). Under hypoxic conditions, AnxA2 was also predominantly associated with the cell surface, with very little present in the conditioned medium (Fig. 2B). These results were confirmed by immunoblotting of the EDTA eluates, which showed an increase in the cell-surface levels of AnxA2 and tPA under hypoxic conditions (Fig. 2C). Thrombomodulin, an EDTA-elutable endothelial cell-surface protein was used as a control for loading. Immunoprecipitation of the RMVEC EDTA eluates also revealed an increase in the levels of AnxA2–tPA complex in cells exposed to hypoxic conditions when compared with the basal levels under normoxic conditions (Fig. 2D). Furthermore, RMVECs exposed to hypoxic conditions for the indicated periods of time revealed an increased colocalization of AnxA2 and tPA in the tube-like structures formed on the Matrigel basement membrane compared with that in the RMVECs exposed to normoxic conditions (Fig. 2E). The extent of AnxA2–tPA colocalization was quantified by measuring the Pearson's correlation coefficient (PCC) and Mander's overlap coefficient (MOC). The PCC and MOC are reliable estimates for the degree of colocalization; PCC describes the correlation of the intensity distributions between channels and MOC indicates the overlap of the signals (Adler and Parmryd, 2010; Zinchuk and Grossenbacher-Zinchuk, 2009). The PCC of the red fluorophore (for AnxA2) to green fluorophore (for tPA) was calculated for control cells exposed to hypoxic conditions as ~0.258±0.096, and for cells exposed to 3 hours and 7 hours of hypoxia as ~0.582±0.0840 and ~0.802±0.110, respectively. The MOC also revealed an increase in the colocalization of AnxA2 and tPA in cells exposed to 3 hours (0.6±0.104) and 7 hours (0.826±0.074) of hypoxia compared with that in cells exposed to normoxic conditions (0.258±0.069).
Fig. 2.
Colocalization of the AnxA2–tPA complex in RMVECs exposed to hypoxic conditions. (A) RMVECs were exposed to 3 and 7 hours of hypoxia. The conditioned medium and EDTA eluates were subjected to ELISA with anti-tPA antibody. After the addition of TMB substrate, the reaction was measured spectrophotometrically at 450 nm. (B) ELISA of the EDTA eluates and conditioned medium from RMVECs exposed to hypoxic (3 and 7 hours) and normoxic conditions with the anti-AnxA2 antibody. (C) Western immunoblot analysis of the EDTA eluates from RMVECs exposed to hypoxic conditions with anti-AnxA2 and anti-tPA antibodies. Thrombomodulin was used as a loading control for the EDTA eluates. (D) RMVEC lysates exposed to hypoxic conditions (3%) were subjected to immunoprecipitation with anti-AnxA2 antibody and immunoblotted with anti-tPA antibody. (E) RMVECs grown to confluence on coverslips coated with Matrigel were exposed to hypoxic conditions (3%) for 7 hours. The cells were fixed and subjected to immunocytochemistry followed by confocal microscopy (Zeiss LSM 510; 40× water-immersion objective) with anti-AnxA2 (red) and anti-tPA (green) antibodies. Scale bar: 20 μm. The extent of colocalization was measured by calculating the Pearson's correlation coefficient and Mander's overlap coefficient from ten independent fields of cells in two different experiments. Error bars represent the standard error from 20 fields in two independent experiments.
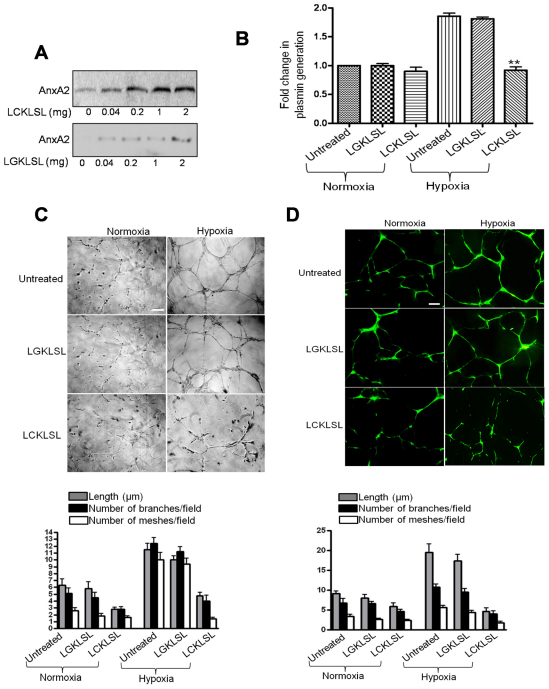
The N-terminal hexapeptide LCKLSL inhibits the efficiency of tPA-mediated plasmin generation
To demonstrate the binding specificity of the hexapeptides LCKLSL and LGKLSL to AnxA2, hypoxic RMVECs (7 hours of hypoxia) were incubated in the presence of increasing concentrations of LCKLSL or LGKLSL peptides biotinylated at the C-terminus. The surface-bound biotinylated peptides were pulled down by incubating with Sepharose-conjugated streptavidin and subjected to western immunoblot analysis for AnxA2. The presence of AnxA2 in the streptavidin pulldown extracts recovered from the cells treated with increasing concentrations of the biotinylated LCKLSL peptide suggests that the peptide interacts with cell-surface AnxA2 (Fig. 3A, upper panel). There was only a modest recovery of AnxA2 on treatment with the LGKLSL biotinylated peptide, suggesting a lack of specificity for AnxA2 (Fig. 3A, lower panel). The efficiency of the LCKLSL peptide in inhibiting the cell-surface binding of tPA was also confirmed by performing a fluorogenic plasmin generation assay in the presence of 100 nM plasminogen and a chromogenic plasmin substrate. Hypoxic exposure for 3 hours resulted in a ~2.0-fold increase in the levels of plasmin compared with that for the normoxic controls. The hexapeptide LCKLSL, at a concentration of 5 μM, resulted in a 1.1±0.2-fold (s.e.; n=5) increase in the generation of plasmin compared with the 1.7±0.1-fold (s.e.; n=4) increase in the presence of the control peptide, LGKLSL. The LCKLSL peptide seemed to have only moderate effects on the baseline levels of plasmin generation in cells exposed to normoxic conditions (Fig. 3B). The hexapeptide was also tested for its ability to inhibit the capillary tube formation (Fig. 3C). In the presence of LCKLSL, the length of the capillary-tube-like structures in hypoxic RMVECs cultured on Matrigel was reduced by ~60% compared with the ~15% reduction in the tube length in the presence of the control peptide (LGKLSL), with respect to the length of untreated cells under normoxic conditions (Fig. 3C). With regard to the topological parameters, the number of branches and the number of meshes per field were reduced by ~60% and ~75%, respectively, in cells treated with the LCKLSL peptide (Fig. 3C).
Fig. 3.
Effect of the LCKLSL hexapeptide on hypoxia-induced plasmin generation. (A) Confluent RMVECs exposed to hypoxia for 7 hours were incubated in the presence of biotinylated test (LCKLSL) and control (LGKLSL) hexapeptides, and the biotinylated proteins were isolated by conjugation with streptavidin. The pulled down extracts were subjected to immunoblotting with anti-AnxA2 antibody. (B) A chromogenic plasmin generation assay of RMVECs treated with the LCKLSL and LGKLSL peptides, and exposed to hypoxic conditions for 7 hours in the presence of recombinant plasminogen and a plasmin substrate. The fold increase in plasmin generation was calculated by normalizing the initial rates of plasmin generation in untreated cells, which was assigned a value of 1. The data are means+s.e.m. (n=7 for untreated controls and n=5 for peptide treatments). (C) Phase-contrast images and quantification of capillary tube formation of RMVECs seeded on Matrigel basement membrane and incubated with the LCKLSL or LGKLSL peptide in the presence of recombinant plasminogen. The cells exposed to normoxic conditions were used as controls to measure the baseline levels of tube formation. Images were acquired using a phase-contrast microscope and quantified using the NIH ImageJ software. The data are the mean tube length (+s.e.m.), and the tubes were quantified in five different microscope fields per sample. Each experiment was performed in triplicate and repeated at least twice. The topological parameters (number of branching points per field and the number of meshes per field) were evaluated and the data are represented. Scale bar: 200 μm. (D) Co-culture of RMVEC and HAoSMCs, seeded on Matrigel, with the extent of capillary-like structure formation was evaluated. Quantification of the length of the tube-like structures and the topological parameters were evaluated. Scale bar: 50 μm.
Although endothelial tube formation assays on Matrigel, a matrix derived from mouse tumors, is widely accepted as an important tool to study angiogenesis in vitro (Ponce, 2009), these assays do not replicate the in vivo environment, as they do not incorporate the complex interactions of endothelial cells with stromal cells. Hence, we performed a co-culture assay of endothelial cells with human aortic smooth muscle cells (HAoSMCs) on Matrigel, as this allows for the formation of a scaffold of capillary-like networks (Donovan et al., 2001). As shown in the Fig. 3D, the tube-like structures formed in the co-culture assay were longer than those formed in the single endothelial cell culture. The length of the tube-like structures was measured under normoxic and hypoxic conditions in the presence of the experimental and control peptide. We observed an increase in the length of the tube-like structures in the co-culture of RMVECs with HAoSMCs compared with that in the singe culture of endothelial cells alone (Fig. 3D). The length of the tube-like structures under hypoxic conditions was reduced by ~75% and ~15% in the LCKLSL- and LGKLSL-treated cells, respectively, compared with that in the untreated cells under hypoxic conditions. The number of branches and meshes per field was also reduced by ~60% and ~80%, respectively, in the LCKLSL-peptide-treated cells compared with that in the untreated cells under hypoxia (Fig. 3D). These results suggest that the interaction of endothelial cells with smooth muscle cells results in a matrix provided by the smooth muscle cells that influence the ability of endothelial cells to form capillary-like structures in response to angiogenic insults.
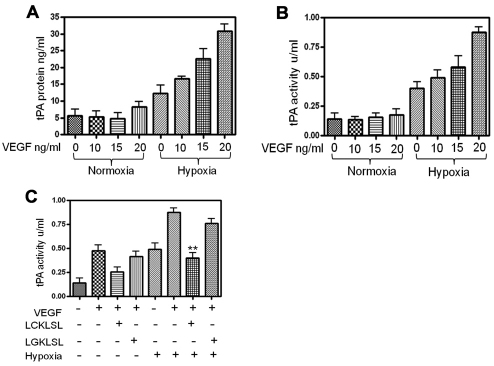
The AnxA2 N-terminal hexapeptide LCKLSL inhibits VEGF-induced tPA activity in RMVECs under hypoxic conditions
Vascular obliteration and ischemia are common features of microvascular angiopathies (Nguyen and Wong, 2009). Retinal hypoxia is suggested to be an important factor in the induction of vascular leakage and is followed by an upregulation in the secretion and activity of VEGF, a major angiogenic cytokine (Arjamaa and Nikinmaa, 2006). VEGF is known to regulate many steps in the angiogenic process and is primarily involved in inducing extracellular proteolysis by upregulating the expression and activity of plasminogen activators (Pepper, 2001). The effects of increasing concentrations of VEGF in modulating the levels of cell-surface-associated tPA was tested in RMVECs under normoxic and hypoxic conditions. On exposure to hypoxic conditions for 7 hours, RMVECs exhibited a dose-dependent increase in the cell-surface levels of tPA in response to VEGF (Fig. 4A). We also measured the activity of cell-surface tPA in the presence of VEGF. We observed a time- and dose-dependent increase in the antigen activity of cell-surface tPA in hypoxic RMVECs. The increase in the antigen activity of tPA was consistent with the observed increase in the cell-surface levels of tPA under hypoxia (Fig. 4B).
Fig. 4.
Effect of the LCKLSL hexapeptide on VEGF-induced activity of tPA in RMVECs under hypoxic conditions. (A) Effect of VEGF on the secreted and cell-surface levels of tPA was determined by ELISA of the EDTA eluates in the presence of the indicated concentrations of VEGF. Exposure to VEGF resulted in a significantly increased production of secreted and cell-surface levels of tPA. Data are the mean concentration of tPA in ng/ml. The experiments were performed in triplicate at least three times. (B) VEGF-induced increase in the activity of tPA was measured in the EDTA eluates using an ELISA-based activity assay. Data are the mean tPA activity in units per ml. The experiments were performed in triplicate at least three times. (C) RMVECs were exposed to hypoxia by co-incubating the cells with VEGF and the LCKLSL or LGKLSL peptides. The efficiency of the peptides in inhibiting VEGF-induced plasmin generation was measured by performing chromogenic plasmin generation assay. The data are represented as absorbance units (AUs). Cells exposed to normoxic conditions and treated with vehicle control were used as negative controls. The experiments were performed in triplicate at least three times. *P<0.05; **P<0.01 (compared with the control).
As our data indicated that VEGF acts as a potent inducer of tPA in hypoxic RMVECs, we investigated whether inhibition of tPA binding to AnxA2 could dampen the VEGF-induced, tPA-mediated activation of plasminogen into plasmin. Previous studies have shown the involvement of AnxA2 in VEGF-induced neovascular responses and the role of AnxA2 as a cell-surface catalytic center for the accelerated conversion of plasminogen into plasmin is directly implicated in these processes (Zhao et al., 2010). Here, we wanted to demonstrate the efficacy of the LCKLSL hexapeptide in inhibiting the VEGF-induced activity of tPA. Our results demonstrate that VEGF at a concentration of 20 ng/ml significantly increased the plasmin-generating activity of tPA under hypoxic conditions, which was substantially inhibited by treatment with the LCKLSL competitive peptide. By contrast, treatment with the LGKLSL control peptide was not efficient in inhibiting VEGF-induced activation of tPA, suggesting the importance of the cysteine residue in the binding of tPA to AnxA2 (Fig. 4C). VEGF was also observed to moderately induce the secretion of tPA in cells exposed to normoxic culture conditions.
AnxA2 N-terminal hexapeptide abrogates the neovascular responses in an in vivo model of angiogenesis
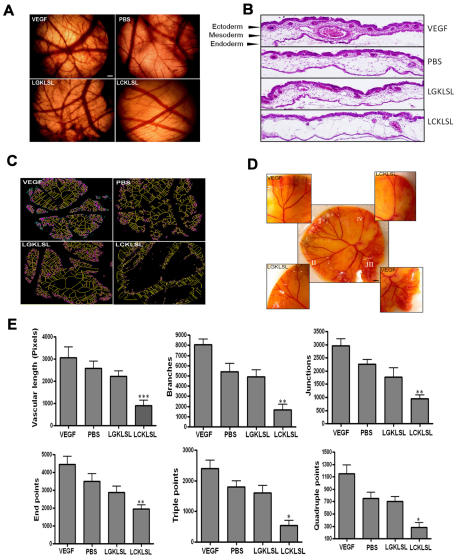
We further demonstrate the role of AnxA2 in the neovascular responses by performing a chicken chorioallantoic membrane (CAM) assay whereby collagen sponges supplemented with 5 μg of the N-terminal competitive peptide LCKLSL or the control peptide LGKLSL were implanted on the allantoic membrane of fertilized chicken eggs. The allantoic membrane was recovered and assayed for a vascular response. A significant decrease in the number of vascular branch points and regression of angiogenesis was observed in the LCKLSL-peptide-treated embryos compared with the group treated with control peptide (Fig. 5A). The embryos treated with VEGF showed abundant vessels and intense vascular proliferation, which was absent in the PBS-treated CAMs (Fig. 5A). Histological analysis of hematoxylin- and eosin-stained CAM membranes revealed the structural details of the CAM. The CAM is composed of three well-distinguishable layers, a multilayer epithelium at the air interface, called ectoderm, a lose layer of stroma and a single layer of inner epithelium at the interface with the allantoic sac, called the endoderm (Valdes et al., 2002). Application of 1 μg of VEGF resulted in the formation of a second layer of capillary network in the subectodermal region. Careful examination of the VEGF-treated CAMs revealed that the newly formed layer of capillaries in the subectodermal position was extended much further into the mesoderm (Fig. 5B). In CAMs treated with PBS, and the control peptide LGKLSL, the capillary network in the subectodermal region was much thinner and contained fewer vessels than the VEGF-treated CAMs. By contrast, in CAMs treated with the LCKLSL peptide, the subectodermal region showed a reduction in the number of blood vessels compared with that in the CAMs treated with PBS and the LGKLSL control peptide, and the subectodemal capillary network was barely visible (Fig. 5B). The CAMs were quantified to measure the number and type of vascular branching using the NIH ImageJ software. For this purpose, CAM images were initially binarized to black and white, and were then skeletonized. Fig. 5C is a multicolor representation of the skeletonized images with the primary vessels shown in yellow, the secondary vessels in pink and the tertiary vessels in green. To address the inherent variability in the angiogenic responses of the eggs, we also performed a shell-less CAM assay (Fig. 5D). At embryonic day 5, the embryo and its embryonic membranes were transferred into a glass petridish for further development. The yolk sac was divided into four sectors, and the test substances were applied on collagen sponges on day 12. As a positive control, collagen sponges dipped in VEGF were placed in two of the four sectors. Phase-contrast images of the CAM indicated increased vasoobliteration in the LCKLSL-peptide-treated sector compared with upon the LGKLSL treatment (Fig. 5D).
Fig. 5.
CAM assay to test the vascular responses to the LCKLSL and LGKLSL peptides. (A) A opening (1 cm) was made on the top of the egg, and the egg was then incubated with collagen sponges supplemented with 5 μg/ml of the LCKLSL and LGKLSL peptides, as well as VEGF and PBS. Scale bar: 200 μm. (B) Hematoxylin and eosin staining of the paraffin-embedded CAM sections showing the vascular obliteration in the presence of the LCKLSL peptide, which was absent on treatment with LGKLSL peptide. The vehicle control had no effect and vascular hyperproliferation was observed in the presence of VEGF. Scale bar: 100 μm. (C) A multicolor representation of the skeletonized images in different treatment groups. Primary, secondary and tertiary vessels are shown in yellow, pink and green, respectively. (D) A shell-less egg assay, segregated into four sectors treated with the experimental and control peptides, along with VEGF, that serves as positive control. Scale bar: 200 μm. (E) Skeletonized CAM images were scored for the total vascular length, number of branches, junctions, end-points, and triple and quadruple points. The results are means+s.e.m. *P<0.05; **P<0.01; ***P<0.005.
The length of the vessels and other vascular parameters were quantified by use of computer-assisted digital image analysis using ImageJ. In order to compare the treatment groups, the vessel width was normalized by skeletonizing the images and the total vessel length was calculated. We observed that treatment with the LCKLSL peptide significantly decreased the vascular length, whereas the LGKLSL peptide did not result in significant changes in the vascular length (Fig. 5E). In addition, we analyzed the vascular architecture by counting the number of vascular branches, junctions and vessel ends per image. At a dose of 5 μg/ml, the LCKLSL peptide significantly decreased the number of branches, junctions and end-points, indicating the efficiency of the peptide in inhibiting the microvascular sprouting from existing vessels (Fig. 5E). We also quantified the total number of triple and quadruple points, which correlate with the microvascular sprouting. The LCKLSL-treated CAMs showed a significant increase in the number of triple and quadruple points in comparison with the LGKLSL-treated CAMs (Fig. 5E).
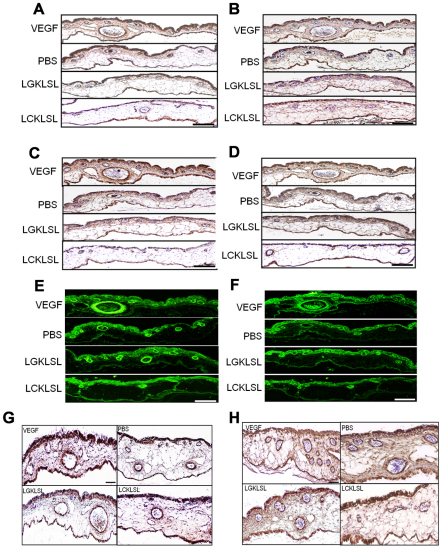
Immunohistochemical staining of the CAMs with anti-PECAM-1 antibody (Fig. 6A) revealed the presence of PECAM-1-positive vessels in the subectodermal and mesodermal regions. PECAM-1-positive cells were also found in the ectodermal region. Formation of a dense subectodermal capillary network containing PECAM-1-positive vessels was found in CAMs treated with VEGF. The number of PECAM-1-positive vessels in the subectodermal position was found to be significantly reduced in CAMs treated with the LCKLSL peptide compared with the CAMs treated with PBS and the LGKLSL control peptide (Fig. 6A). Immunostaining with anti-VEGF antibody showed the VEGF-expressing endothelial cells lining the blood vessels in the CAMs (Fig. 6B). The localization of VEGF was similar to that of PECAM-1 under different treatment conditions (Fig. 6B). Immunostaining of the CAMs with anti-AnxA2 antibody (Fig. 6C) revealed the expression of AnxA2 in the vasculature that comprises the dense subectodermal capillary network in the CAMs treated with VEGF. Expression of AnxA2 was also found in the endothelial cells lining the blood vessels in the thin layer of the subectodermal capillary network in the PBS- and LGKLSL-peptide-treated CAMs. In addition, fewer AnxA2-positive blood vessels were found in the highly compromised capillary network in the CAMs treated with the LCKLSL peptide (Fig. 6C). Serial sections of the CAM were immunostained for tPA to reveal the expression of tPA in the endothelial cells lining the blood vessels in the CAMs treated with VEGF, PBS, control and experimental peptide (Fig. 6D). The number of tPA-positive vessels in the LCKLSL-peptide-treated CAMs was significantly reduced compared with that in the PBS- and the control-peptide-treated CAMs (Fig. 6D). We also observed that CAMs treated with the LCKLSL experimental peptide showed fewer α-smooth muscle actin (α-SMA)-positive vessels compared with that in the CAMs treated only with VEGF and the CAMs treated with PBS and the control peptide (Fig. 6E). These results suggest that the peptide resulted in a reduction in the number of blood vessels associated with α-SMA-positive mural cells.
Fig. 6.
Immunohistochemical characterization of the CAM. (A–H) The CAMs were immunostained for PECAM-1 (A) and VEGF (B), AnxA2 (C), tPA (D), α-smooth muscle actin (E), fibronectin (F), laminin (G) and Ki-67 (H). Scale bar: 50 μm.
In addition, the CAMs were also immunostained for makers of ECM proteins, fibronectin and laminin (Fig. 6F and G, respectively). Our data suggest that the newly formed vasculature in the VEGF-treated CAMs is positive for these angiogenic makers. The expression of fibronectin and laminin was markedly compromised in CAMs treated with the LCKLSL peptide compared with that in the PBS- and LGKLSL-control-peptide-treated CAMs. CAMs were also immunostained for Ki-67, a marker for proliferating cells. Ki-67-positive endothelial cells, lining the blood vessels, were abundant in the CAMs treated with VEGF compared with in the PBS- and LGKLSL-control-peptide-treated CAMs. In CAMs treated with the LCKLSL peptide, a significant reduction in the number of Ki-67 positive vessels was observed (Fig. 6H). The reduction in the number of Ki-67-positive vessels indicates that the LCKLSL peptide inhibited the new vessel formation formed by sprouting angiogenesis. These data collectively suggest that several activated angiogenic vessels are present in the control CAMs and the formation of new blood vessels is comprised in the experimental-peptide-treated CAMs.
AnxA2 N-terminal competitive peptide inhibits angiogenesis in a mouse Matrigel plug assay
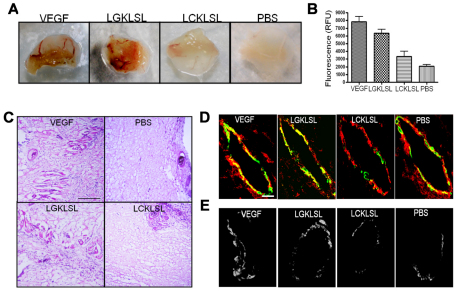
The role of the N-terminal peptide in inhibiting angiogenesis was tested in the in vivo murine Matrigel plug assay, as previously described (Auerbach et al., 2003; Merchan et al., 2010). Mice were injected with Matrigel in the presence of PBS alone (negative control) or Matrigel mixed with VEGF and basic FGF (bFGF; positive control). The LGKLSL and LCKLSL peptides were incorporated into the Matrigel plugs in the presence of VEGF and bFGF. At 5 days after the implantation of Matrigel, FITC–Dextran was injected and the extent of Matrigel infiltration was assessed by measuring the relative fluorescence of the Matrigel plugs. The angiogenic responses in the Matrigel plugs are shown in Fig. 7A. In vivo angiogenesis was inhibited by ~55% in the Matrigel plugs containing the LCKLSL experimental peptide compared with that in the positive control group. By contrast, the plugs containing the LGKLSL peptide showed an ~15% reduction in the fluorescence intensity compared with that in the positive control group (Fig. 7B). Hematoxylin and eosin staining of the plugs also revealed a marked reduction in the number of the capillaries in the plugs containing the LCKLSL peptide compared with those containing the LGKLSL peptide (Fig. 7C). Immunostaining of the frozen section of the plugs indicated a modest colocalization of AnxA2 and tPA in the microvessels from the plugs containing the LCKLSL peptide compared with that in the plugs containing the LGKLSL peptide (Fig. 7D). Elevated levels of AnxA2 and tPA were present in the microvessels from the VEGF-treated group. The plugs containing PBS alone showed only basal levels of immunostaining (Fig. 7D). For the detection of plasmin activity in the frozen sections, we used enzyme overlay membranes (EOMs) incorporated with the fluorogenic plasmin substrate D-Val-Leu-Lys-7-amino-4-trifluoro-methylcoumarin (D-Val-Leu-Lys-AFC) (Day and Neufeld, 1997). When the EOMs were placed in contact with the frozen sections, a fluorescent pattern of enzymatic activity was detected, which is a representation of localization of plasmin activity in the tissue sections. Extensive plasmin enzymatic activity was detected in the microvessels from the Matrigel plugs containing VEGF and bFGF (Fig. 7E). A comparable activity was observed in the microvessels of the plugs containing the LGKLSL peptide. By contrast, a weak plasmin enzymatic activity was detected in the microvessles from the plugs containing the LCKLSL peptide. Basal levels of plasmin enzymatic activity were detected in the PBS-containing plugs (Fig. 7E).
Fig. 7.
Angiogenic responses of the AnxA2 N-terminal peptide in a mouse Matrigel plug assay. (A) Mice were injected with Matrigel in the presence of PBS alone (negative control), or bFGF and VEGF (VEGF; positive control), or with bFGF and VEGF with the experimental and control peptides. After 5 days, the plugs were removed and the angiogenic responses were evaluated. (B) Matrigel plugs, as in A, were injected with FITC–Dextran, and plugs were removed and perfusion was determined by fluorescence. (C) Frozen sections of Matrigel plugs were stained with hematoxylin and eosin to reveal the presence of red-blood-cell-filled microvessels. (D) Frozen sections of the Matrigel plugs were subjected to immunostaining for AnxA2 (red) and tPA (green) to reveal the colocalization of AnxA2 and tPA. (E) EOM localization of plasmin activity in freshly frozen sections of the Matrigel plugs.
Discussion
One of the most well-studied tissue responses to hypoxia is the induction of angiogenesis by several angiogenic growth factors that are generated in response to hypoxia (Shweiki et al., 1992). The initial generation of hypoxia is followed by the increase in angiogenic growth factors, primarily VEGF, which upregulates the expression of several extracellular proteases by microvascular endothelial cells (Pepper et al., 1991). Remodeling of the extracellular environment by proteolytic components is an indispensable prerequisite for angiogenesis (Pepper et al., 1996; Roy et al., 2006). Among the different proteolytic components secreted by the cells, the plasmin–plasminogen system plays an important role by directly or indirectly activating the matrix metalloproteinases or liberating the sequestered growth factors or cytokines trapped in the ECM (van Hinsbergh et al., 2006). Upregulation of proteolysis is associated with an increase in the migratory ability of the endothelial cells, hence immunoneutralization or chemical inhibition of the proteolytic system can have potential implications in inhibiting angiogenesis.
Several intracellular and extracellular proteases are known to be involved in angiogenesis. Although the intracellular proteases play a role in the processing and maturation of angiogenic proteins, extracellular proteases are involved in the degradation of ECM proteins (van Hinsbergh et al., 2006). Furthermore, a well-coordinated pericellular degradation of matrix proteins is also known to be involved in the degradation of matrix proteins, facilitating in the migration and invasion of endothelial cells during angiogenesis (Werb et al., 1999). The pericellular degradation of ECM proteins is facilitated by the activation of a proteolytic cascade that controls the availability of active and sequestered angiogenic growth factors and cytokines (Werb et al., 1999). A pericellular proteolytic cascade involving the plasmin–plasminogen system is known to be one of the fundamental processes influencing cell migration and invasion (Parfyonova et al., 2002; Werb et al., 1999). Upon angiogenic stimuli, quiescent endothelial cells secrete plasminogen activators, such as tPA and uPA, to degrade the basement membrane and facilitate the formation of a new blood vessel (Parfyonova et al., 2002). Because activation of plasminogen activators triggers an active proteolytic cascade that is further amplified by the activation of matrix metalloproteinases, this process is tightly controlled by several regulatory mechanisms (Pepper et al., 1996). One of the mechanisms by which unrestricted degradation of the basement membrane can be controlled is by the presence of cell-surface receptors that result in localized generation of proteolytically active plasmin, the end-product of the plasmin–plasminogen system (Pepper et al., 1996).
By virtue of acting as the only known receptor for both tPA and plasminogen, AnxA2 plays an important role in the localized generation of plasmin on the cell surface. Inhibiting the binding of tPA and plasminogen to the cell surface can have potential implications in reducing the localized generation of plasmin and, consequently, in inhibiting the degradation of the basement membrane, which is an important event during angiogenesis.
AnxA2 is known to be abundantly expressed in the endothelial cells and is regarded as a potential receptor for the generation of cell-surface plasmin (Hajjar and Acharya, 2000). Additional support for the role of AnxA2 as a profibrinolytic receptor comes from the observation that AnxA2-knockout mice show a complete reduction in the generation of extracellular plasmin on the surface of endothelial cells (Ling et al., 2004). Here, we report the potential anti-angiogenic roles of the previously identified competitive LCKLSL hexapeptide inhibitor of tPA binding to AnxA2 in neovascularization (Roda et al., 2003). We used hypoxia-mediated vascular induction as a model to study the influence of the peptide on neovascularization. Hypoxia triggers angiogenesis by the induction of angiogenic growth factors, which results in the formation and growth of new blood vessels (Chen et al., 2009). Development of new blood vessels occurs by two distinct processes, vasculogenesis, in which new blood vessels are formed from endothelial progenitor cells, and angiogenesis, in which new blood vessels are formed from pre-existing vessels (Chen et al., 2009). Angiogenesis is a sequential multistep process that occurs in a series of three steps: initiation, proliferation-invasion and maturation (Kumar et al., 1998). In the first step, angiogenic inducers stimulate vascular cell proliferation and invasion. The activated endothelium secretes ECM-degrading proteases that permit the dissolution of the endothelial cell basement membrane, thereby promoting endothelial cell invasion and migration. In the last step, the new vessel is stabilized by the recruitment of accessory cells, such as pericytes, and they form a lumen for the blood flow (Folkman, 2003). Extracellular proteolysis is an indispensable process in angiogenesis that is required for degradation of the ECM and the regulation of cytokine activity (Cheresh and Stupack, 2008).
We studied the expression of AnxA2 in the neovascular regions in a mouse model of OIR. During retinopathy, neovascular nuclei protrude from the inner limiting membrane into the vitreous (Connor et al., 2009). We observed that AnxA2 was abundantly expressed in the vascular tufts penetrating from the inner nuclear layer of OIR mice, suggesting its involvement in vasoproliferative retinopathy. In isolated RMVECs exposed to hypoxic conditions, an upregulation in the cell-surface and intracellular levels of AnxA2 was observed followed by a concomitant increase in the cell-surface levels of the AnxA2–tPA complex. These results are supported by previous observations that suggest that AnxA2 is upregulated in VEGF-mediated neovascular responses in response to hypoxia (Genetos et al., 2010; Zhao et al., 2009). Under hypoxia, both VEGF and AnxA2 are upregulated by hypoxia-inducible factor 1 alpha (HIF-1α) thereby potentiating ischemia-induced neovascularization (Genetos et al., 2010).
Our observation that VEGF increased both the levels and activity of tPA in hypoxic RMVECs suggests that the AnxA2–tPA complex is important in hypoxia-induced angiogenesis. In addition, we also demonstrate that the hypoxia-induced increase in the activity of tPA under hypoxic conditions is inhibited by LCKLSL peptide, which blocks the binding of tPA to AnxA2. In addition, we demonstrate that an increase in the levels of AnxA2 and tPA in hypoxic RMVECs is accompanied by a concomitant upregulation in the generation of plasmin. Furthermore, the competitive hexapeptide inhibitor that binds to AnxA2 functions as a potent angiogenic inhibitor by inhibiting several aspects of angiogenesis, including endothelial cell tube formation on Matrigel, generation of plasmin and inhibition of neovascular responses. Application of the LCKLSL hexapeptide inhibited both the vascular density and vascular sprouting in the in vivo models of angiogenesis. Taken together, the data presented here identify the anti-angiogenic role of a competitive hexapeptide inhibitor in preventing AnxA2-mediated neovascular responses.
Design and development of peptide therapeutics to inhibit angiogenesis has gained considerable importance in anti-angiogenesis research. Anti-angiogenic peptides have several advantages over proteins because of their small size, stability, solubility and lack of toxicity to the host system. In addition, they can be synthetically made and modified to enhance their therapeutic efficiency (Griffioen and Molema, 2000; Sulochana and Ge, 2007). The data presented here identifies the anti-angiogenic role of the competitive hexapeptide inhibitor LCKLSL in preventing AnxA2-mediated neovascular responses under hypoxic conditions.
Materials and Methods
Isolation and culture of human retinal microvascular endothelial cells
Human eyes were procured from the Lions Eye Institute for Transplant & Research LEITR, Tampa, FL, The Eye-Bank for Sight Restoration, New York, NY, The Cullen Eye Institute, Houston, TX and Florida Lions Eye Bank, Miami, FL. The eyes were obtained in compliance with good clinical practice, with informed consent under institutional review board regulations, and in accordance with the tenets of the Declaration of Helsinki. The eyes were enucleated, hemisected and the lenses and vitreous were removed. The retinas were removed and placed in HBSS buffer containing 1% penicillin and streptomycin (Gibco). The retinas were digested with 0.5% collagenase (Worthington Biochemical) in Ca2+- and Mg2+-free PBS at 37°C for 35–40 minutes. Following digestion, the retinal digests were incubated in endothelial growth medium (EGM-2; Lonza) with 10% fetal bovine serum (FBS; Gibco), and the digests were dissociated by trituration and centrifugation. The cells were passed through a double-layer of sterile 40-μm sterile nylon mesh (Falcon) and were pelleted by centrifugation at 400 g for 10 minutes. After washing the cells twice with EGM-2, the cell suspension was incubated with 200 μl of anti-PECAM antibody coated magnetic dyanabeads (Invitrogen). The RMVECs bound to the magnetic beads were recovered using a magnetic particle collector and resuspended in EGM-2. The cells were seeded on fibronectin (Sigma) coated flasks and grown in EGM-2 supplemented with growth factors. The cells were passaged for 6–8 passages.
Peptide synthesis
The LCKLSL and control LGKLSL peptides were synthesized using solid-phase methods (Genscript), and their sequence was analyzed by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry. Biotinylated peptides were prepared by adding biotin groups to the C-terminal lysine residue of the peptides with an amide bond. The positive charge on the lysine residue was later removed.
Generation of hypoxic culture conditions
Cells from P2 to P4 were incubated under a 95% N2 and 5% CO2 gas mixture in a hypoxic chamber (Proox C21; BioSperix), under the control of the Proox controller (model 110; BioSpherix) set to 3% O2 at 37°C and 100% relative humidity. Cells incubated under standard normoxic conditions (95% air and 5% CO2) from the same batch and passage were used as normoxic controls.
Antibodies and reagents
Antibodies and reagents were purchased from the following sources: anti-AnxA2 antibody (BD Pharmingen), anti-tPA antibody (American Diagnostica), Glu-plasminogen (American Diagnostica), recombinant human tPA (American Diagnostica), anti-vWF antibody (Sigma). Matrigel (Invitrogen), sulfo-NHS-LC-biotin (Pierce), Sepharose-conjugated streptavidin (Sigma), D-Val-Leu-Lys-7-amino-4-trifluoromethylcoumarin (Sigma), recombinant human tPA, tPA concentration and activity ELISA kits (Innovative Research).
EDTA elution and cell-surface biotinylation
RMVECs were washed with 0.5 mM EDTA and PBS buffer (Gibco) for 20 minutes at 37°C. The EDTA eluates were collected as described previously (Mai et al., 2000) and subjected to western immunoblot analysis. In this report, the EDTA washes are referred to as EDTA eluates. Cell-surface proteins were subjected to biotinylation with 0.5 mg/ml of EZ-link-sulfo-NHS biotin (Pierce) and recovered with avidin-conjugated Sepharose (Sigma), as previously described (Peterson et al., 2003).
Confocal microscopy and TIRF microscopy
Double immunostaining for AnxA2 and tPA followed by confocal microscopy was performed as described previously (Liu et al., 2003).
Plasmin generation assay
The assay was performed according to the previously established protocols (Jacovina et al., 2001). RMVECs were treated with 5 μM LCKLSL peptide or 5 μM LGKLSL control peptide, and exposed to hypoxic conditions. After several washes, the cells were incubated with 100 nM Glu-plasminogen for 1 hour and subsequently treated with the fluorogenic plasmin substrate D-VLK-AMC (D-Val-Leu-Lys-7-amido-4-methylcoumarin; Sigma). Substrate hydrolysis was measured at 4-minute intervals and results are given as relative fluorescence units (RFUs) as determined using a fluorescence spectrophotometer (380 nm excitation and 460 nm emission wavelengths). The readings were collected from four replicates and the rate of plasmin generation was determined using linear regression analysis of the plots of RFUs against time2, as described previously (Jacovina et al., 2001).
ELISA detection for total tPA and active tPA
The levels of total tPA and active tPA in the EDTA eluates and conditioned medium were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Innovative Research) following the manufacturer's instructions. Conditioned medium and EDTA eluates from normoxic and hypoxic RMVECs were added to the wells of a 96-well microtiter plate along with the tPA standards. The primary anti-human tPA antibody was later added followed by the addition of a horseradish peroxidase (HRP)-conjugated secondary antibody and the reaction was read by the addition of the TMB substrate at 450 nm. Data are expressed as means±s.d. and the samples were plated in triplicate wells for each experiment; every experiment was repeated at least three times.
Chicken chorioallantoic membrane assay
Fertilized white leghorn chicken embryos (9-day-old; Charles River SPAFAS) were maintained at 37°C and in 60% relative humidity. The extent of vascularization in the eggs was examined with the aid of an egg lamp, and a small hole was made in the air sack at the area where prominent blood vessels are located. A window was cut with the aid of a scapel blade and the air sack was gently displaced. Collagen filter discs saturated with 1 μg/ml of VEGF were placed on the CAMs of 9-day-old chicken embryos followed by the daily topical application of 5 μg/ml of LCKLSL or LGKLSL peptides. Filter discs saturated with VEGF and PBS served as positive and negative controls, respectively. Each group, consisting of 6–9 eggs, was treated daily for 3 days, and on the fourth day, the CAMs underlying the filter discs were isolated and analyzed. Angiogenesis was assessed in the CAMs by counting the number of visible blood vessel branch points in each field; the images were taken using a Nikon Diaphot camera. Paraffin-embedded CAM sections were stained with hematoxylin and eosin.
Shell-less chicken embryo culture
Fertilized white leghorn chicken eggs (5-day-old) were rinsed with 70% ethanol and opened into 100-mm glass petri dishes. The embryos were incubated under aseptic conditions at 37°C for 7–9 additional days. The LCKLSL or LGKLSL peptides (20 μg) and VEGF (100 ng/μl) were added to collagen filter discs (4 mm×4 mm squares). The filter discs were later placed onto the CAM of a 12-day-old embryo in the area devoid of major blood vessels. The embryos were returned to a humidified incubator at 37°C with 3% CO2 for 48 hours. After the incubation period, the CAMs were visualized under an inverted light-microscope, and the images were taken using a Nikon Diaphot camera.
Quantification of angiogenesis
Quantitative assessment of angiogenesis in the CAM images was performed using the computer-assisted CAM image analysis (Vu et al., 1985). Changes in the vascular length, branch, junction, end, triple and quadruple points were quantified by creating the binary images after application of a common threshold value. The binary images were converted into skeletonized images using NIH ImageJ software (http://rsbweb.nih.gov/ij/) and later quantified using the ‘analyze skeleton’ plugin of ImageJ.
Mouse model of oxygen-induced retinopathy
Hypoxia–normoxia-induced proliferative retinopathy was induced in a mouse model, as previously described (Scott and Fruttiger, 2010). Briefly, postnatal day 7 (P7), littermates along with their nursing mothers were exposed to 75% O2 for 5 days, and returned to room air (21% oxygen) for 5 days. The control littermates were placed in room air for the entire period. At the end of the experimental period, the animals were euthanized, and the eye globes were enucleated, fixed in 4% paraformaldehyde and embedded in paraffin. Serial sections (5 μm) of the retina were subjected to immunostaining with the anti-AnxA2 antibody, as described previously. The animal experiments were performed according to the ARVO statement for the Use of Animals in Ophthalmic and Vision Research.
In vivo angiogenesis (Matrigel plug) assay
The Matrigel plug assay was performed as described previously (Merchan et al., 2010). Briefly, 500 μl of unpolymerized Matrigel (BD Biosciences) (~20 mg/ml), either alone (negative control), mixed with bFGF and VEGF (100 ng/ml each), or in the presence of 5 μM of the LGKLSL or LCKLSL peptides in combination with bFGF and VEGF (treatment group), was injected subcutaneously at the left lower abdominal wall of four groups of athymic nude mice (4- to 6-weeks-old) (Harlan, Madison, WI). Mice were killed 5 days after the Matrigel injections, and the plugs were recovered, photographed, fixed in formaldehyde and embedded in OCT. Quantification of FITC–Dextran levels in the plugs was performed as described previously (Merchan et al., 2010; Klement et al., 2000).
EOM technique for the detection of plasmin enzymatic activity
The assay was preformed as described previously (Berndt et al., 2000; Day and Neufeld, 1997). Frozen sections (8–10 μm) of the Matrigel plugs were placed on coverslips, air dried for 1 hour, fixed in methanol for 10 minutes at −20°C and rinsed with PBS. A 10 mm×10 mm EOM (Enzyme system products) was soaked in PBS and placed over the tissue sections. A sandwich was prepared by covering with a second microscope slide. Enzyme activity was visualized as blue–green fluorescence under UV excitation, and the intensity was monitored at 5–10-min intervals for 30 minutes to 1 hour. For the detection of plasmin-specific enzymatic activity, membranes containing the plasmin substrate D-Val-Leu-Lys-AFC were used.
Statistical analysis
Statistical analysis was performed using GraphPad 5.0 software. A Mann–Whitney U test was used when comparing two groups, and the Kruskal–Wallis test with Dunn's post-test for three or more multiple comparisons. Significance was defined as P<0.05. Results are presented as means±s.e.m.
Supplementary Material
Acknowledgments
This research was supported in part by a grant from the National Center on Minority Health and Health Disparities (NIH) (MD001633). The authors thank Jwalitha Shankardas and Dan Dimitrijevich for technical assistance. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/124/9/1453/DC1
References
- Adler J., Parmryd I. (2010). Quantifying colocalization by correlation: the Pearson correlation coefficient is superior to the Mander's overlap coefficient. Cytometry A 77, 733-742 [DOI] [PubMed] [Google Scholar]
- Almér L. O., Pandolfi M., Osterlin S. (1975). The fibrinolytic system in patients with diabetes mellitus with special reference to diabetic retinopathy. Ophthalmologica 170, 353-361 [DOI] [PubMed] [Google Scholar]
- Arjamaa O., Nikinmaa M. (2006). Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp. Eye Res. 83, 473-483 [DOI] [PubMed] [Google Scholar]
- Auerbach R., Lewis R., Shinners B., Kubai L., Akhtar N. (2003). Angiogenesis assays: a critical overview. Clin. Chem. 49, 32-40 [DOI] [PubMed] [Google Scholar]
- Berndt A., Johannesson J., Haas M., Arkona C., Katenkamp D., Wiederanders B., Hyckel P., Kosmehl H. (2000). Simultaneous enzyme overlay membrane (EOM)-based in situ zymography and immunofluorescence technique reveals cathepsin B-like activity in a subset of tumour vessels. Histochem. Cell Biol. 114, 63-68 [DOI] [PubMed] [Google Scholar]
- Castellino F. J., Ploplis V. A. (2005). Structure and function of the plasminogen/plasmin system. Thromb. Haemost. 93, 647-654 [DOI] [PubMed] [Google Scholar]
- Cesarman G. M., Guevara C. A., Hajjar K. A. (1994). An endothelial cell receptor for plasminogen/tissue plasminogen activator (t-PA). II. Annexin II-mediated enhancement of t-PA-dependent plasminogen activation. J. Biol. Chem. 269, 21198-21203 [PubMed] [Google Scholar]
- Chen L., Endler A., Shibasaki F. (2009). Hypoxia and angiogenesis: regulation of hypoxia-inducible factors via novel binding factors. Exp. Mol. Med. 41, 849-857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Stupack D. G. (2008). Regulation of angiogenesis: apoptotic cues from the ECM. Oncogene 27, 6285-6298 [DOI] [PubMed] [Google Scholar]
- Connor K. M., Krah N. M., Dennison R. J., Aderman C. M., Chen J., Guerin K. I., Sapieha P., Stahl A., Willett K. L., Smith L. E. (2009). Quantification of oxygen-induced retinopathy in the mouse: A model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 4, 1565-1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day F. A., Neufeld D. A. (1997). Use of enzyme overlay membranes to survey proteinase activity in frozen sections: cathepsin-like and plasmin-like activity in regenerating newt limbs. J. Histochem. Cytochem. 45, 779-783 [DOI] [PubMed] [Google Scholar]
- Donovan D., Brown N. J., Bishop E. T., Lewis C. E. (2001). Comparison of three in vitro human ‘angiogenesis’ assays with capillaries formed in vivo. Angiogenesis 4, 113-121 [DOI] [PubMed] [Google Scholar]
- Folkman J. (2003). Fundamental concepts of the angiogenic process. Curr. Mol. Med. 3, 643-651 [DOI] [PubMed] [Google Scholar]
- Fukao H., Ueshima S., Okada K., Matsuo O. (1997). The role of the pericellular fibrinolytic system in angiogenesis. Jpn. J. Physiol. 47, 161-171 [DOI] [PubMed] [Google Scholar]
- Genetos D. C., Wong A., Watari S., Yellowley C. E. (2010). Hypoxia increases annexin A2 expression in osteoblastic cells via VEGF and ERK. Bone 47, 1013-1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M. B., Guay C. (1991). Plasminogen activator production by human retinal endothelial cells of nondiabetic and diabetic origin. Invest. Ophthalmol. Vis. Sci. 32, 53-64 [PubMed] [Google Scholar]
- Griffioen A. W., Molema G. (2000). Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol. Rev. 52, 237-268 [PubMed] [Google Scholar]
- Hajjar K. A., Acharya S. S. (2000). Annexin II and regulation of cell surface fibrinolysis. Ann. N. Y. Acad. Sci. 902, 265-271 [DOI] [PubMed] [Google Scholar]
- Hajjar K. A., Deora A. (2000). New concepts in fibrinolysis and angiogenesis. Curr. Atheroscler. Rep. 2, 417-421 [DOI] [PubMed] [Google Scholar]
- Hajjar K. A., Menell J. S. (1997). Annexin II: A novel mediator of cell surface plasmin generation. Ann. N. Y. Acad. Sci. 15, 337-349 [DOI] [PubMed] [Google Scholar]
- Hajjar K. A., Jacovina A. T., Chacko J. (1994). An endothelial cell receptor for plasminogen/tissue plasminogen activator. I. identity with annexin II. J. Biol. Chem. 269, 21191-21197 [PubMed] [Google Scholar]
- Hajjar K. A., Mauri L., Jacovina A. T., Zhong F., Mirza U. A., Padovan J. C., Chait B. T. (1998). Tissue plasminogen activator binding to the annexin II tail domain. direct modulation by homocysteine. J. Biol. Chem. 273, 9987-9993 [DOI] [PubMed] [Google Scholar]
- Hattenbach L. O., Allers A., Gümbel H. O., Scharrer I., Koch F. H. (1999). Vitreous concentrations of TPA and plasminogen activator inhibitor are associated with VEGF in proliferative diabetic vitreoretinopathy. Retina 19, 383-389 [DOI] [PubMed] [Google Scholar]
- Houck K. A., Leung D. W., Rowland A. M., Winer J., Ferrara N. (1992). Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J. Biol. Chem. 267, 26031-26037 [PubMed] [Google Scholar]
- Jacovina A. T., Zhong F., Khazanova E., Lev E., Deora A. B., Hajjar K. A. (2001). Neuritogenesis and the nerve growth factor-induced differentiation of PC-12 cells requires annexin II-mediated plasmin generation. J. Biol. Chem. 276, 49350-49358 [DOI] [PubMed] [Google Scholar]
- Kim J., Hajjar K. A. (2002). Annexin II: A plasminogen-plasminogen activator co-receptor. Front. Biosci. 1, 341-348 [DOI] [PubMed] [Google Scholar]
- Klement G., Baruchel S., Rak J., Man S., Clark K., Hicklin D. J., Bohlen P., Kerbel R. S. (2000). Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J. Clin. Invest. 105, R15-R24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Yoneda J., Bucana C. D., Fidler I. J. (1998). Regulation of distinct steps of angiogenesis by different angiogenic molecules. Int. J. Oncol. 12, 749-757 [DOI] [PubMed] [Google Scholar]
- Lijnen H. R. (2001). Elements of the fibrinolytic system. Ann. N. Y. Acad. Sci. 936, 226-236 [DOI] [PubMed] [Google Scholar]
- Ling Q., Jacovina A. T., Deora A., Febbraio M., Simantov R., Silverstein R. L., Hempstead B., Mark W. H., Hajjar K. A. (2004). Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. J. Clin. Invest. 113, 38-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Rothermund C. A., Ayala-Sanmartin J., Vishwanatha J. K. (2003). Nuclear annexin II negatively regulates growth of LNCaP cells and substitution of ser 11 and 25 to glu prevents nucleo-cytoplasmic shuttling of annexin II. BMC Biochem. 4, 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai J., Finley R.L., Jr, Waisman D.M., Sloane B.F. (2000). Human procathepsin B interacts with the annexin II tetramer on the surface of tumor cells. J. Biol. Chem. 275, 12806-12812 [DOI] [PubMed] [Google Scholar]
- Merchan J. R., Kovacs K., Railsback J. W., Kurtoglu M., Jing Y., Pina Y., Gao N., Murray T. G., Lehrman M. A., Lampidis T. J. (2010). Antiangiogenic activity of 2-deoxy-D-glucose. PLoS ONE 5, e13699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milkiewicz M., Panovic E., Doyle J. L., Haas T. L. (2006). Regulators of angiogenesis and strategies for their therapeutic manipulation. Int. J. Biochem. Cell Biol. 38, 333-357 [DOI] [PubMed] [Google Scholar]
- Nguyen T. T., Wong T. Y. (2009). Retinal vascular changes and diabetic retinopathy. Curr. Diab. Rep. 9, 277-283 [DOI] [PubMed] [Google Scholar]
- Parfyonova Y. V., Plekhanova O. S., Tkachuk V. A. (2002). Plasminogen activators in vascular remodeling and angiogenesis. Biochemistry (Mosc.) 67, 119-134 [DOI] [PubMed] [Google Scholar]
- Pepper M. S. (2001). Extracellular proteolysis and angiogenesis. Thromb. Haemost. 86, 346-355 [PubMed] [Google Scholar]
- Pepper M. S., Ferrara N., Orci L., Montesano R. (1991). Vascular endothelial growth factor (VEGF) induces plasminogen activators and plasminogen activator inhibitor-1 in microvascular endothelial cells. Biochem. Biophys. Res. Commun. 181, 902-906 [DOI] [PubMed] [Google Scholar]
- Pepper M. S., Montesano R., Mandriota S. J., Orci L., Vassalli J. D. (1996). Angiogenesis: A paradigm for balanced extracellular proteolysis during cell migration and morphogenesis. Enzyme Protein 49, 138-162 [DOI] [PubMed] [Google Scholar]
- Peterson E. A., Sutherland M. R., Nesheim M. E., Pryzdial E. L. (2003). Thrombin induces endothelial cell-surface exposure of the plasminogen receptor annexin 2. J. Cell. Sci. 116, 2399-2408 [DOI] [PubMed] [Google Scholar]
- Ponce M. L. (2009). Tube formation: an in vitro Matrigel angiogenesis assay. Methods Mol. Biol. 467, 183-188 [DOI] [PubMed] [Google Scholar]
- Roda O., Valero M. L., Peiró S., Andreu D., Real F. X., Navarro P. (2003). New insights into the tPA-annexin A2 interaction. is annexin A2 CYS8 the sole requirement for this association? J. Biol. Chem. 278, 5702-5709 [DOI] [PubMed] [Google Scholar]
- Roy R., Zhang B., Moses M. A. (2006). Making the cut: protease-mediated regulation of angiogenesis. Exp. Cell Res. 312, 608-622 [DOI] [PubMed] [Google Scholar]
- Scott A., Fruttiger M. (2010). Oxygen-induced retinopathy: a model for vascular pathology in the retina. Eye (Lond.) 24, 416-421 [DOI] [PubMed] [Google Scholar]
- Shweiki D., Itin A., Soffer D., Keshet E. (1992). Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359, 843-845 [DOI] [PubMed] [Google Scholar]
- Simpson A. J., Booth N. A., Moore N. R., Lewis S. J., Gray R. S. (1999). Circulating tissue-type plasminogen activator and plasminogen activator inhibitor type 1 in proliferative diabetic retinopathy: a pilot study. Acta Diabetol. 36, 155-158 [DOI] [PubMed] [Google Scholar]
- Sone H., Kawakami Y., Segawa T., Okuda Y., Sekine Y., Honmura S., Segawa T., Suzuki H., Yamashita K., Yamada N. (1999). Effects of intraocular or systemic administration of neutralizing antibody against vascular endothelial growth factor on the murine experimental model of retinopathy. Life Sci. 65, 2573-2580 [DOI] [PubMed] [Google Scholar]
- Sulochana K. N., Ge R. (2007). Developing antiangiogenic peptide drugs for angiogenesis-related diseases. Curr. Pharm. Des. 13, 2074-2086 [DOI] [PubMed] [Google Scholar]
- Valdes T. I., Kreutzer D., Moussy F. (2002). The chick chorioallantoic membrane as a novel in vivo model for the testing of biomaterials. J. Biomed. Mater. Res. 62, 273-282 [DOI] [PubMed] [Google Scholar]
- van Hinsbergh V. W., Engelse M. A., Quax P. H. (2006). Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler. Thromb. Vasc. Biol. 26, 716-728 [DOI] [PubMed] [Google Scholar]
- Vu M. T., Smith C. F., Burger P. C., Klintworth G. K. (1985). An evaluation of methods to quantitate the chick chorioallantoic membrane assay in angiogenesis. Lab. Invest. 53, 499-508 [PubMed] [Google Scholar]
- Werb Z., Vu T. H., Rinkenberger J. L., Coussens L. M. (1999). Matrix-degrading proteases and angiogenesis during development and tumor formation. APMIS 107, 11-18 [DOI] [PubMed] [Google Scholar]
- Zhao S., Huang L., Wu J., Zhang Y., Pan D., Liu X. (2009). Vascular endothelial growth factor upregulates expression of annexin A2 in vitro and in a mouse model of ischemic retinopathy. Mol. Vis. 15, 1231-1242 [PMC free article] [PubMed] [Google Scholar]
- Zhao S. H., Pan D. Y., Zhang Y., Wu J. H., Liu X., Xu Y. (2010). Annexin A2 promotes choroidal neovascularization by increasing vascular endothelial growth factor expression in a rat model of argon laser coagulation-induced choroidal neovascularization. Chin. Med. J. (Engl.) 123, 713-721 [PubMed] [Google Scholar]
- Zinchuk V., Grossenbacher-Zinchuk O. (2009). Recent advances in quantitative colocalization analysis: focus on neuroscience. Prog. Histochem. Cytochem. 44, 125-172 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.