Abstract
Previous studies have shown that plasmin cleaves monocyte chemoattractant protein 1 (MCP1; officially known as C-C motif chemokine 2, CCL2) at K104, and this cleavage enhances its chemotactic potency significantly. Accumulating evidence reveals that MCP1 also disrupts the integrity of the blood–brain barrier (BBB). Here, we show that K104Stop-MCP1, truncated at the K104 where plasmin would normally cleave, is more efficient than the full-length protein (FL-MCP1) in compromising the integrity of the BBB in in vitro and in vivo models. K104Stop-MCP1 increases the permeability of BBB in both wild-type mice and mice deficient for tissue plasminogen activator (tPA), which converts plasminogen into active plasmin, suggesting that plasmin-mediated truncation of MCP1 plays an important role in BBB compromise. Furthermore, we show that the mechanisms underlying MCP1-induced BBB disruption involve redistribution of tight junction proteins (occludin and ZO-1) and reorganization of the actin cytoskeleton. Finally, we show that the redistribution of ZO-1 is mediated by phosphorylation of ezrin-radixin-moesin (ERM) proteins. These findings identify plasmin as a key signaling molecule in the regulation of BBB integrity and suggest that plasmin inhibitors might be used to modulate diseases accompanied by BBB compromise.
Key words: Monocyte chemoattractant protein 1 (CCL2), Blood–brain barrier, Plasmin, Tight junction, Endothelia cells
Introduction
Chemokines are a superfamily of structurally related small proteins with strong chemotactic activity. By binding to their specific G-protein-coupled receptors, chemokines induce cell-specific migration and activation of immune cells (Glabinski et al., 1996; Hulkower et al., 1993; Lahrtz et al., 1998; Miller and Meucci, 1999). Monocyte chemoattractant protein-1 (MCP1; officially known as C-C motif chemokine 2, CCL2) is one of the most highly and transiently expressed chemokines in many central nervous system (CNS) injuries, including ischemia, hemorrhage and excitotoxic injury (Capoccia et al., 2008; Dimitrijevic et al., 2006; Frangogiannis et al., 2007; Hanisch, 2002; Kim et al., 2008; Morimoto et al., 2008; Sheehan et al., 2007; Yan et al., 2007). The rodent MCP1 differs from the human protein at the C-terminus, with the rodent protein having a longer highly glycosylated C-terminus. We have previously shown that mouse MCP1 can be cleaved by plasmin at K104, and the truncated MCP1 is more potent than the full-length MCP1 in inducing microglial migration (Sheehan et al., 2007; Yao and Tsirka, 2010) through its receptor CCR2.
Besides chemotaxis, MCP1 is also involved in blood–brain barrier (BBB) compromise (Dimitrijevic et al., 2006; Stamatovic et al., 2006; Stamatovic et al., 2003; Stamatovic et al., 2005). Here, we investigated whether plasmin-mediated cleavage regulates MCP1-induced BBB breakdown. It has been reported that components of the plasmin(ogen) system can regulate BBB integrity. Specifically, tissue-type plasminogen activator (tPA), which converts inactive plasminogen into active plasmin, promotes BBB disruption and subsequent peripheral blood mononuclear cell (PBMC) infiltration (Reijerkerk et al., 2008). Additionally, BBB compromise and PBMC infiltration have been found in mice deficient for plasminogen activator inhibitor 1 (PAI-1) (Kataoka et al., 2000), suggesting that plasmin-mediated cleavage of MCP1 might play a role in BBB disruption.
The BBB, a specialized structure that prevents the infiltration of components from the peripheral blood into the CNS, mainly comprises brain microvascular endothelial cells (BMECs), pericytes and astrocytes (Guillemin and Brew, 2004). BMECs form an impermeable monolayer through tight junctions, where both transmembrane and cytoplasmic tight junction proteins (TJPs) are expressed. The transmembrane proteins, such as occludin and claudin-1, -5 and -11, function to seal gaps between adjacent cells, whereas cytoplasmic accessory proteins, such as the zonula occluden proteins (ZO-1, ZO-2 and ZO-3) (Citi and Cordenonsi, 1998; Huber et al., 2001), link transmembrane proteins to the cortical actin-based cytoskeleton (Förster, 2008; Michel and Curry, 1999; Mitic and Anderson, 1998). Accumulating evidence shows that MCP1 increases BBB permeability through redistribution of TJPs and reorganization of actin cytoskeleton in a CCR2-dependent manner (Dimitrijevic et al., 2006; Stamatovic et al., 2006; Stamatovic et al., 2003; Stamatovic et al., 2005). The molecular mechanisms underlying TJP redistribution, however, are still elusive.
Here, we show that plasmin-mediated cleavage is crucial for fast MCP1-induced compromise of the BBB. Further mechanistic studies reveal that MCP1-induced redistribution of ZO-1 is mediated by ezrin-radixin-moesin (ERM) proteins and reorganization of actin cytoskeleton.
Results
Truncation by plasmin promotes MCP1-induced BBB compromise
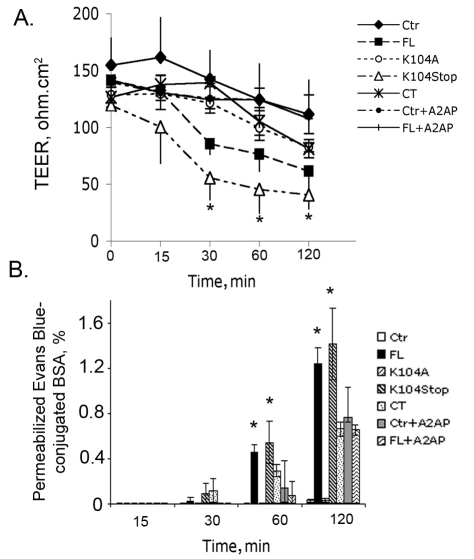
It has been shown that mouse MCP1 increases BBB permeability both in vitro and in vivo (Dimitrijevic et al., 2006; Stamatovic et al., 2005). However, it is not known whether plasmin-mediated cleavage of MCP1 at K104, which enhances its chemotactic activity (Sheehan et al., 2007), affects MCP1-induced BBB compromise. To address this question, we used four recombinant MCP1 proteins, full-length MCP1 (FL), plasmin-noncleavable MCP1 (K104A), constitutively cleaved MCP1 (K104Stop) and MCP1 C-terminal extension (CT), that we previously characterized (Yao and Tsirka, 2010). First, we evaluated the cytotoxicity of these MCP1 mutants in mouse or human BMECs (HBMECs) using lactate dehydrogenase (LDH) cytotoxicity assays. None of these MCP1 mutants was cytotoxic to BMECs (supplementary material Fig. S1). Then, we investigated the function of these MCP1 proteins on BBB permeability in a simple in vitro BBB model, which only involved HBMECs (Alter et al., 2003; Glynn and Yazdanian, 1998). Although both FL- and K104Stop-MCP1 were found to decrease transendothelial electrical resistance (TEER) values substantially, the effect of K104Stop-MCP1 was more dramatic than that of FL-MCP1 (Fig. 1A) at all timepoints. Consistent with these data, an in vitro permeability assay revealed similar results: both FL- and K104Stop-MCP1 increased the permeabilization of Evans Blue across the monolayer of HBMECs (Fig. 1B), indicating a decreased BBB integrity. Because HBMECs have endogenous plasmin expression and activity, we explored the possibility that the decrease in TEER values observed with FL-MCP1 was due to the truncation of MCP1 by endogenous plasmin. To block the endogenous plasmin generated by HBMECs, α2-antiplasmin (A2AP), a specific plasmin inhibitor, was used. In the presence of A2AP, addition of FL-MCP1 did not decrease TEER values (Fig. 1A) and was unable to promote infiltration of Evans Blue (Fig. 1B), suggesting that the effect of FL-MCP1 observed in the initial experiments was due to the endogenous HBMEC plasmin activity. Evans Blue can cross the BMEC monolayer by both transcellular and paracellular pathways (Hart et al., 1987; Kawedia et al., 2007). The addition of A2AP alone appeared to modify BBB permeability to Evans Blue but not the TEER values. This result suggested that A2AP might affect the transcellular diffusion of Evans Blue, although it is not at this point clear how A2AP would mediate this effect. When we performed the in vitro permeability experiment using the tracer FITC–Dextran (molecular mass of 4 kDa; Sigma), which only uses the paracellular pathway to go across BMEC monolayer (Hayashi et al., 2004; Neuhaus et al., 2008), only K104Stop-MCP1 was able to substantially enhance the leakage of the tracer across the co-culture system in the presence of A2AP (supplementary material Fig. S2). This result suggests that there is an important role for plasmin activity in MCP1-induced BBB disruption. Next, we investigated whether the effect of FL- and K104Stop-MCP1 on BBB permeability was dose-dependent. As shown in supplementary material Fig. S3, the activity of FL-MCP1 increased in a dose-dependent manner, reaching a plateau at 100 nM. By contrast, K104Stop-MCP1 was fully active even at lower concentrations (50 nM). These data suggest that K104Stop-MCP1 is more ‘active’ than FL-MCP1 in this permeability assay.
Fig. 1.
Truncated MCP1 compromises the integrity of the HBMEC monolayer. HBMECs seeded in Transwell inserts were treated with the indicated form of MCP1 (100 nM; in the lower chamber, abluminal side) in the presence or absence of A2AP. Ctr, control (saline). (A) Changes in the transendothelial electrical resistance (TEER) over time. Results are means±s.d. (n=3). *P<0.05 (analyzed using one-way ANOVA followed by Newman–Keuls multiple comparison test) compared with results with FL-MCP1 without A2AP. (B) Chances in the permeability of Evan Blue over time. Results are means±s.d. (n=6). *P<0.05 (comparison with Ctr).
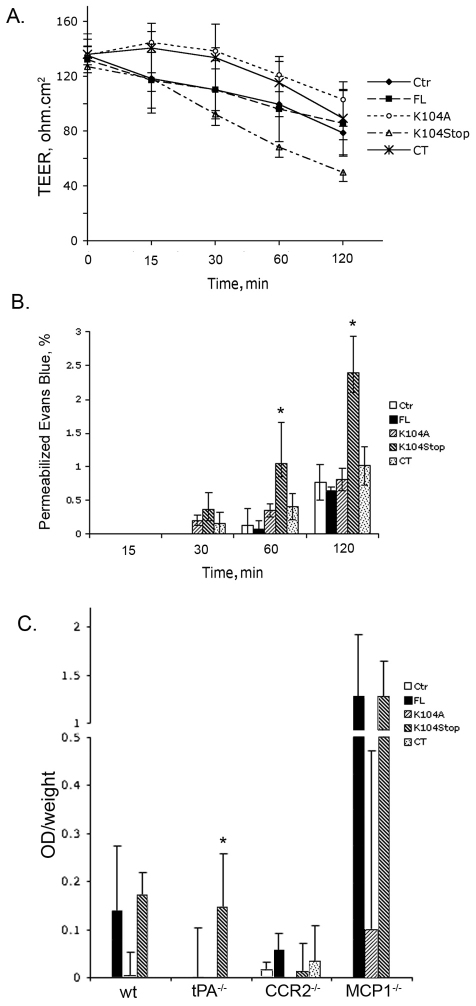
To better replicate the in vivo BBB conditions, we used a mouse BMEC-astrocyte co-culture system, as astrocytes contribute to the impermeability of BBB by covering more than 99% of the microvessels (Kacem et al., 1998; Simard et al., 2003). Consistent with previous data, in the absence of A2AP, FL- and K104Stop-MCP1 reduced TEER values and increased Evans Blue infiltration substantially (data not shown), whereas, in the presence of A2AP, only K104Stop-MCP1 was effective in compromising the integrity of this in vitro BBB model (Fig. 2A,B). These data further support the hypothesis that plasmin-mediated truncation of MCP1 plays a vital role in MCP1-induced BBB compromise.
Fig. 2.
Truncated MCP1 compromises BBB integrity. (A) The indicated form of MCP1 (100 nM) was added to the lower chamber of the BMEC-astrocyte co-culture system. TEER values were determined over time in the presence of A2AP. Results are means±s.d. (n=3). Ctr, control (saline). (B) Cells were treated as in A. The leakage of Evans Blue across the co-culture system was determined spectrophotometrally after treatment with A2AP. Results are means±s.d. (n=4). *P<0.05 (analyzed using one-way ANOVA followed by Newman–Keuls multiple comparison test) compared with results with FL-MCP1. (C) Infiltration of Evans Blue across the compromised BBB into brain parenchyma was assayed 12 hours after a single injection of MCP1 (1 μg). Mice injected with saline were used as control. Results [OD (optical density; absorbance) per g of body weight] are means+s.d. (n=4). *P<0.05 (analyzed using one-way ANOVA followed by Newman–Keuls multiple comparison test) compared with results with FL-MCP1 without A2AP.
We investigated the integrity of the BBB in wild-type, tPA−/−, MCP1−/− and Ccr2−/− mice, as in vivo the shearing stress effects on BBB are also present. In wild-type or MCP1−/− mice, both FL- and K104Stop-MCP1 enhanced the diffusion of Evans Blue into the brain parenchyma. In tPA-deficient mice, the FL-MCP1 was not as efficient as the K104Stop-MCP1 in enhancing BBB permeability. As the absence of tPA results in the virtual absence of plasmin activity, the decreased efficiency of FL-MCP1 to compromise the BBB suggests that MCP1-induced disruption of BBB depends upon the activity of plasmin. Although MCP1−/− mice showed virtually no compromise of the BBB, adding back recombinant MCP1 to them (Fig. 2C) resulted in significant BBB compromise by both FL- and K104Stop-MCP1. By contrast, in Ccr2−/− mice, none of these MCP1 proteins was able to increase the diffusion of Evans Blue from the blood to the brain parenchyma (Fig. 2C), indicating an indispensable role for CCR2 in MCP1-induced BBB leakage.
Plasmin-mediated truncation of MCP1 promotes redistribution of TJPs
Redistribution of TJPs has been reported in MCP1-treated BMECs (Stamatovic et al., 2006; Stamatovic et al., 2003; Stamatovic et al., 2005). We further studied whether plasmin-mediated cleavage of MCP1 is involved in this process. Using HBMECs, we found that FL- and K104Stop-MCP1 disrupted the integrity of occludin distribution on the cell membrane. K104Stop-MCP1 was more potent, in that disruption of the BBB was evident from the 30-minute timepoint onwards (Fig. 3A,B showing the 120-minute timepoint). By contrast, K104A- and CT-MCP1 did not change the staining pattern of occludin. Similarly, FL- and K104Stop-MCP1 treatment led to punctate ZO-1 staining in the cell border, whereas K104A- and CT-MCP1 treatment minimally affected ZO-1 staining (Fig. 3A). We also investigated the redistribution of ZO-1 and occludin in primary BMECs, and the results were the same as those from HBMECs (Fig. 3C at the 120-minute timepoint).
Fig. 3.
Truncated MCP1 induces the redistribution of tight junction proteins. (A) HBMECs were treated with the indicated form of MCP1 (100 nM). Ctr, control (saline); WT, wild-type. At 15, 30, 60 and 120 minutes after treatment, the cells were fixed and immunostained for occludin and ZO-1. (B) Higher magnification of cells incubated with the MCP-1 variants and stained for occludin at the 120-minute timepoint. Scale bar: 15 μm. (C) Primary mouse BMECs were exposed to the MCP1 variants (100 nM) for 2 hours. Then, the cells were fixed and immunostained for ZO-1 and occludin. Blue, DAPI staining.
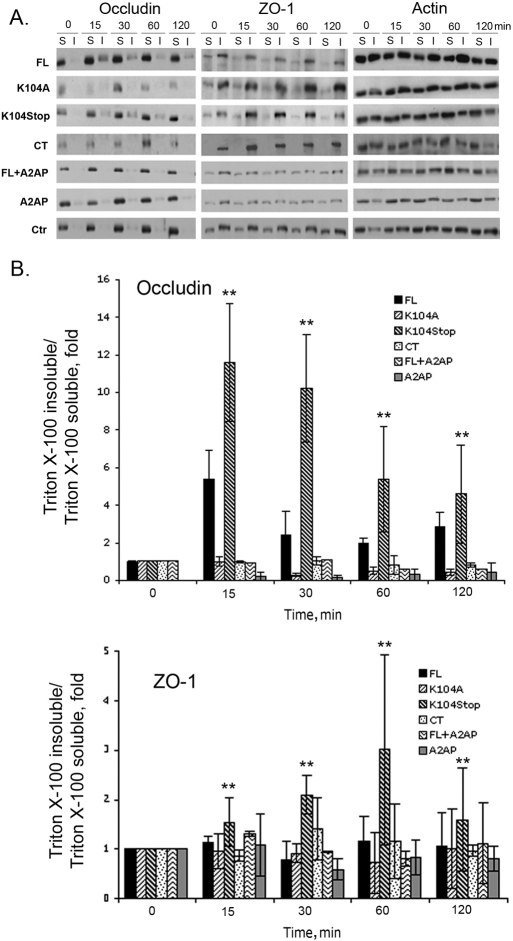
Next, we quantified the redistribution of TJPs with semi-quantitative western blotting using Triton-X-100 fractions. Consistent with previous reports (Stamatovic et al., 2006; Stamatovic et al., 2003; Stamatovic et al., 2005; Tsukamoto and Nigam, 1997; Tsukamoto and Nigam, 1999), FL-MCP1 shifted occludin and ZO-1 from the Triton-X-100-soluble fraction to the Triton-X-100-insoluble fraction. K104Stop-MCP1, however, had a dramatically amplified change compared with that induced by FL-MCP1. By contrast, K104A- and CT-MCP1 failed to induce the shift of the TJPs (Fig. 4). As the effect of K104Stop-MCP1 was more dramatic than that of FL-MCP1, we tested again the possibility that endogenous plasmin generated by BMECs might truncate MCP1, as described for Fig. 1. In the presence of A2AP the shift of the TJPs induced by FL-MCP1 was abrogated, whereas A2AP alone did not affect their distribution (Fig. 4).
Fig. 4.
Truncated MCP1 shifts TJPs from the Triton-X-100-soluble fraction to the Triton-X-100-insoluble fraction. HBMECs were exposed to the indicated form of MCP1 (100 nM) for different periods of time in the presence or absence of A2AP. Ctr, control (saline). The shift of occludin and ZO-1 from the Triton-X-100-soluble fraction (S) to the Triton-X-100-insoluble fraction (I) was analyzed by western blotting. Actin was used a loading control. (A) Representative images of western blots. (B) Quantitative data from western blots. The intensity of target bands was determined using Scion Image. The intensity of occludin and ZO-1 was normalized to that of actin and the ratios were further normalized to the zero timepoint. Results are means±s.d. (n=3). **P<0.01 (analyzed using one-way ANOVA followed by Newman–Keuls multiple comparison test) for K104Stop-MCP1 compared with FL-MCP1 at each timepoint.
Plasmin-mediated truncation of MCP1 changes the actin cytoskeleton
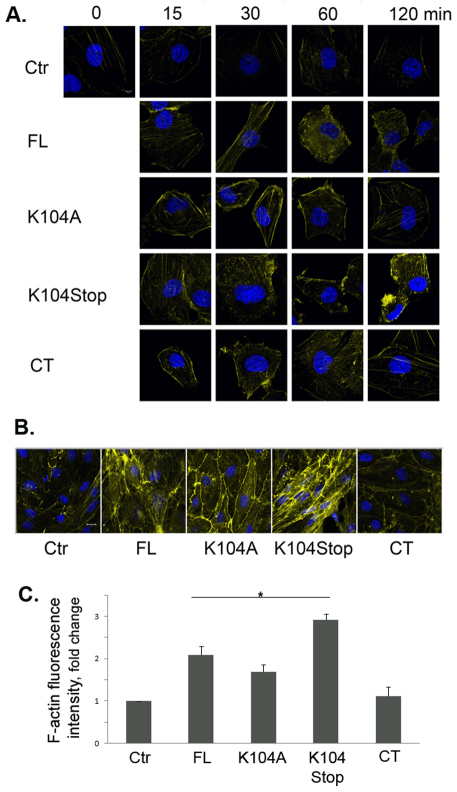
As MCP1 treatment leads to the redistribution of TJPs, which are connected to the actin cytoskeleton directly or indirectly, we examined the reorganization of F-actin in HBMECs over time. FL-MCP1 increased F-actin over time (Fig. 5A). It peaked at 1 hour after treatment. K104Stop-MCP1 treatment led to a significant enhancement in the amount of F-actin as early as 15 minutes after treatment. This effect was even more dramatic at 2 hours after treatment. Although K104A- and CT-MCP1 elevated F-actin slightly at early timepoints, F-actin mainly remained distributed at the cell border. F-actin levels returned to baseline levels at 2 hours after treatment (Fig. 5A). We also treated primary BMECs with various MCP1 proteins for 2 hours. Consistently, FL-MCP1 increased F-actin expression compared with that in untreated cells. K104Stop-MCP1 treatment resulted in a dramatic enhancement of the intensity of F-actin (Fig. 5B). K104A-MCP1 only elevated F-actin staining slightly in the cell border (indicative of the presence of cortical actin), whereas CT-MCP1 had no effect on F-actin levels or distribution (Fig. 5B). Quantification of the intensity of F-actin fluorescence is shown in Fig. 5C. Although K104A-MCP1 also significantly increased F-actin intensity, the effect was also limited to the cell border (Fig. 5B). Compared with FL-MCP1, K104Stop-MCP1 more efficiently enhanced F-actin (Fig. 5C). These results further support the hypothesis that plasmin-mediated truncation is necessary for MCP1-induced BBB compromise.
Fig. 5.
Truncated MCP1 promotes reorganization of the actin cytoskeleton. (A) HBMECs were treated with saline (Ctr) or the indicated form of MCP1 (100 nM) for different times, and the actin cytoskeleton was visualized using Alexa-Fluor-647–phalloidin. (B) Primary mouse BMECs treated with the indicated form of MCP1 (100 nM) for 2 hours were immunostained to visualize the actin cytoskeleton. (C) Quantification of F-actin fluorescence intensity. Values are mean+s.d. (n=9). *P<0.05 (analyzed using Student's t-tests).
Plasmin-truncated MCP1 induces phosphorylation of ERM proteins
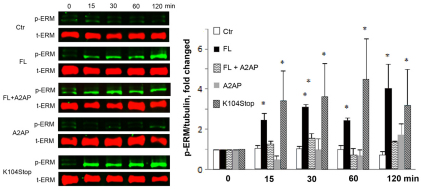
In an effort to understand how TJPs are redistributed during BBB compromise, we focused on the ERM proteins, a family of highly conserved proteins that serve as a linker between the plasma membrane and the actin cytoskeleton (Louvet-Vallee, 2000). These proteins have two states: a dormant state (during which they are located in the cytoplasm) and an active state (during which they are located just beneath the plasma membrane) (Louvet-Vallee, 2000). The activation process involves post-translational and conformational changes. It has been shown that phosphorylation on a conserved threonine residue (Thr567 for ezrin, Thr564 for radixin and Thr558 for moesin) suppresses the intramolecular interaction between the N-terminus and C-terminus of ERM proteins and promotes a subsequent conformational change (Bretscher et al., 1995; Louvet-Vallee, 2000; Pearson et al., 2000). This then results in the N-terminus interacting with membrane proteins [e.g. CD44 (Tsukita et al., 1994), CD43 (Yonemura et al., 1993) and ICAM-2 (Helander et al., 1996), ICAM-3 (Serrador et al., 1997)] and the C-terminus interacting with the actin cytoskeleton (Matsui et al., 1998; Nakamura et al., 1995; Pestonjamasp et al., 1995; Turunen et al., 1994). Here, we investigated whether activation of ERM proteins is involved in MCP1-induced BBB disruption. Using a phosphorylation-specific antibody, we found that FL- and K104Stop-MCP1 significantly enhanced the phosphorylation of ERM proteins over time (Fig. 6), suggesting that phosphorylation of ERM proteins is involved in the MCP1-induced BBB compromise. In the presence of A2AP, however, FL-MCP1 failed to induce phosphorylation of ERM proteins, suggesting that plasmin-mediated truncation of MCP1 plays a crucial role.
Fig. 6.
Truncated MCP1 induces phosphorylation of ERM proteins. Mouse BMECs were treated with saline (Ctr, control) or the indicated form of recombinant MCP1 proteins (100 nM) with or without A2AP over time. The phosphorylated ERM proteins (p-ERM) were determined using a phosphorylation-specific antibody for ERM proteins. The level of total ERM (t-ERM) proteins is also shown. Quantitative data of western blots are shown as mean+s.d. (n=3). *P<0.05 (compared with the zero timepoint control).
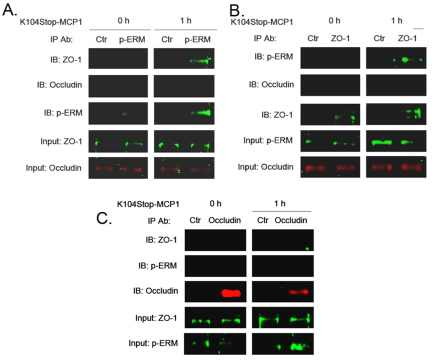
Plasmin-truncated MCP1 promotes interaction between phosphorylated ERM proteins and ZO-1
As K104Stop-MCP1 induced phosphorylation of ERM proteins, we next asked whether the N-termini of ERM proteins interact with TJPs. By performing co-immunoprecipitation experiments we found that, in the absence of MCP1, phosphorylated ERM proteins did not interact with ZO-1 (Fig. 7). Treatment of endothelial cells with K104Stop-MCP1 for 1 hour, however, promoted interaction between phosphorylated ERM proteins and ZO-1 (Fig. 7). By contrast, occludin failed to interact with phosphorylated ERM proteins, suggesting that the redistribution of occludin is not regulated through ERM proteins.
Fig. 7.
Truncated MCP1 promotes interaction between phosphorylated ERM proteins and ZO-1. Mouse BMECs were treated with K104Stop-MCP1 or saline (0 h) for 1 hour. Cell lysates were collected and used for co-immunoprecipitation experiments. (A) Immunoprecipitation (IP) with an anti-(phosphorylated ERM) antibody (p-ERM) and immunoblotting (IB) with anti-ZO-1 and anti-occludin antibodies. (B) Immunoprecipitation with an anti-ZO-1 antibody and immunoblotting with anti-(phosphorylated ERM) and anti-occludin antibodies. (C) Immunoprecipitation with an anti-occludin antibody and immunoblotting with anti-(phosphorylated ERM) and anti-ZO-1 antibodies. Anti-(mouse IgG) antibody was used as control (Ctr).
Discussion
The BBB is a unique structure that separates the CNS from the peripheral nervous system. It mainly comprises BMECs, pericytes and astrocytes (Guillemin and Brew, 2004); the BMECs, which connect to each other through TJPs, forming the primary barrier, whereas astrocytes, which cover more than 99% of the microvessels with their endfeet, confer a secondary barrier (Kacem et al., 1998; Simard et al., 2003). Here, we used two different in vitro BBB models to assess the effect of plasmin on MCP1-induced BBB compromise. In both models, we found that the constitutively active K104Stop-MCP1 was functional in increasing BBB permeability, suggesting that the activation of MCP1 by plasmin is indispensable for MCP1-induced BBB compromise.
Although the in vitro systems replicate some of the anatomical structures of BBB, they lack the shear stress, which plays an important role in maintaining the dynamic properties and integrity of BBB. Hence, we also performed the Evans Blue permeability assay in vivo (in wild-type and MCP1−/− mice) and obtained similar results to those described above for the in vitro model. However, the effect was more potent in MCP1−/− mice than in wild-type mice. It has been shown that MCP1 binds to and reduces membrane CCR2 levels (probably through endocytosis) (Jung et al., 2009; Yao and Tsirka, 2010). The dramatic change observed in MCP1−/− mice could be due to higher membrane CCR2 density, as in the absence of MCP1 there would be no recycling of CCR2 off the plasma membrane. To examine whether plasmin activity is indispensable, we also conducted experiments in mice deficient for tPA, which converts plasminogen into active plasmin. As expected, only K104Stop-MCP1 was functional in tPA−/− mice. Ccr2−/− mice were used as negative controls. Consistent with previous reports that Ccr2−/− mice were resistant to MCP1-induced BBB compromise, none of the recombinant MCP1 proteins disrupted BBB integrity in these mice, suggesting that MCP1 exerts its BBB-compromising effect solely through CCR2.
Stamatovic et al. showed that loss of CCR2 on astrocytes did not affect MCP1-induced BBB disruption in the BMEC-astrocyte co-culture system, whereas loss of CCR2 on BMECs significantly decreased BBB permeability (Stamatovic et al., 2005), suggesting that MCP1 exerts its function by binding to CCR2 on BMECs. To maintain the stability of the microenvironment in the brain, BMECs connect to each other through tight junctions, where a lot of adhesion molecules, such as the TJPs, bind to each other, sealing the intercellular gaps. This structure makes the permeability of the barrier highly dependent on the expression and localization of TJPs. Our immunofluorescence data showed that plasmin-truncated MCP1 disrupted occludin and ZO-1 staining in the borders of HBMECs, suggesting a disruption of tight junction structure. As tight junction disassembly involves formation of large protein complexes and increased association of TJPs with the actin cytoskeleton, which is Triton-X-100 insoluble, we utilized detergent extractability to analyze the integrity of BBB biochemically. Semi-quantitative western blotting revealed a shift of occludin and ZO-1 from the Triton-X-100-soluble fraction to the Triton-X-100-insoluble fraction. Although TJPs are connected to the actin cytoskeleton directly or indirectly, they are not normally affixed to it, which allows them to be extracted mostly in the Triton-X-100-soluble fraction. The translocation from the Triton-X-100-soluble fraction to the Triton-X-100-insoluble fraction supports the compromise of tight junction structures, suggesting that truncated MCP1 induces BBB disruption through redistribution of TJPs. Consistently, we found that K104Stop-MCP1 significantly enhanced stress fiber formation in endothelial cells, suggesting reorganization of actin skeleton. These data suggest that upon MCP-1 binding to endothelial CCR2, a property that all MCP-1 mutants that retain the N-terminal portion of the protein exhibit, an increase in cortical actin is observed. However, the next step of the process, the internalization of CCR2 and re-arrangements of actin cytoskeleton to allow the cells to retract/reorganize the TJPs, is taking place only when the active FL- and K104Stop-MCP-1 proteins are present (Yao and Tsirka, 2010).
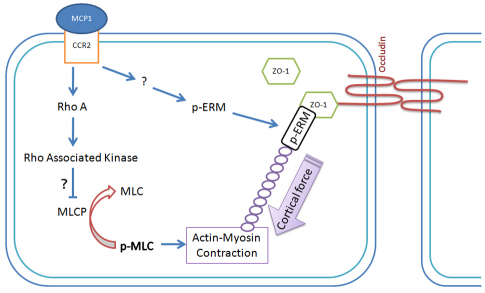
Although the redistribution of TJPs, which leads to the formation of gaps between endothelial cells, and the reorganization of actin cytoskeleton, which promotes the formation of contractile forces, have been shown to cause the compromise of BBB integrity, the mechanisms underlying the shift of TJPs are not clear. It is thought that a phosphorylation event contributes to the translocation of TJPs (Clarke et al., 2000; Farshori and Kachar, 1999; Hirase et al., 2001; Stamatovic et al., 2006; Stamatovic et al., 2003; Ward et al., 2002). The kinases responsible for the phosphorylation involve members of PKC family and Rho-associated kinase (ROCK) (Stamatovic et al., 2006; Stamatovic et al., 2003), although it is not clear how phosphorylation of TJPs leads to their subcellular redistribution. MCP1 has been shown to activate ROCK through RhoA in endothelial cells (Stamatovic et al., 2006). One of the substrates for ROCK is myosin light chain phosphatase (MLCP), which functions in opposition to myosin light chain kinase (MLCK) (Hicks et al., 2010) [MLCK phosphorylates myosin light chain (MLC) and promotes actin–myosin contraction, resulting in contractile forces (Hicks et al., 2010; Stamatovic et al., 2003; Stephan and Brock, 1996; van Nieuw Amerongen et al., 2000)]. Our data clearly show that the presence of MCP1 leads to increased phosphorylation of ERM proteins, which subsequently bind to ZO-1 and the actin cytoskeleton. Our model (Fig. 8) is that by binding to CCR2, MCP1 activates intracellular kinases, which induce phosphorylation of ERM proteins, leading to their conformation change. The phosphorylated ERM proteins then bind to ZO-1 and the actin cytoskeleton. In addition, MCP1 also activates RhoA, which further activates ROCK (Stamatovic et al., 2006). ROCK is then expected to phosphorylate the regulatory subunits of MLCP, leading to inhibition of phosphatase activity. The effect of MLCK would outweigh that of MLCP, resulting in increased phosphorylation of MLC, which would cause enhanced and prolonged actin–myosin contraction. The contraction generates contractile forces and pulls ZO-1 away from TJ, leading to disruption of BBB integrity. It should be noted, however, that the phosphorylated ERM proteins do not interact with occludin upon MCP1 treatment, suggesting the translocation of occludin is not mediated by ERM proteins. Whether there is another ERM-like protein that mediates shift of occludin or if the redistribution of occludin is mediated by other mechanisms is unclear.
Fig. 8.
Proposed model for MCP1-induced BBB compromise. Upon MCP1 treatment, ERM proteins are phosphorylated by unknown kinases. The phosphorylated ERM proteins then bind to ZO-1 and the actin cytoskeleton. By binding to CCR2, MCP1 also activates RhoA, and Rho-associated kinases, which phosphorylate and inactivate MLCP, leading to enhanced phosphorylation of MLC and thus prolonged actin–myosin contraction. The contractile forces then pull ZO-1 away from the cell-cell border, resulting in a disrupted BBB.
Besides MCP1, many other cytokines, including interleukin (IL)-1, IL-4, IL-8, IL-10, IL-13, tumor necrosis factor (TNF)-α and interferon (IFN)-γ (Ahdieh et al., 2001; Blamire et al., 2000; Coyne et al., 2002; Paul et al., 2003; Ross and Joyner, 1997; Wojciak-Stothard et al., 1998; Yang et al., 1999; Youakim and Ahdieh, 1999), have been shown to change the structure of tight junctions and promote the formation of stress fibers in epithelial and endothelial cells. Whether these molecules induce BBB compromise using the same signaling pathways is not clear; however, on the basis of the conserved function and high expression level of ERM proteins in epithelial and endothelial cells, we would assume the same signaling pathways are activated upon the cytokine treatment.
Our work suggests that plasmin-mediated truncation of MCP1 is indispensable for MCP1-induced BBB compromise. Moreover, we have shown that BBB compromise involves reorganization of the actin cytoskeleton and redistribution of TJPs, with the redistribution of ZO-1 mediated by ERM proteins. However, which kinase(s) phosphorylates ERM proteins and what mediates the redistribution of occludin need further investigation.
Materials and Methods
Animal experimentation
All the mice used were bred in the Division of Laboratory Animal Resources (DLAR) at Stony Brook University. They had free access to food and water, and were maintained in a 12-hour light–12-hour dark cycle. All the procedures were approved by the Institutional Animal Care and Use Committee (IACUC).
Generation of His6-tagged MCP1 proteins
FL-, K104A-, and K104Stop-MCP1 were subcloned without the signal peptide into pET vectors with an N-terminal His6 tag (Sheehan et al., 2007). CT-MCP1 was subcloned into a pTYB1 vector with an N-terminal His6 tag (Yao and Tsirka, 2010). BL21 cells were transformed with these constructs and expression of the target proteins was induced for 5 hours using isopropyl-β-D-thiogalactopyranoside. Recombinant proteins were purified using a cobalt affinity resin (Clontech) according to the manufacturer's instructions. The column fractions were analyzed by Tris-Tricine SDS-PAGE (16% gels) by Coomassie Blue staining. The purified proteins were confirmed by immunoblotting using 1:1000 anti-MCP1 antibodies (Serotec and Cell Sciences) or 1:1000 anti-His6 antibody (Santa Cruz Biotechnology).
Cell culture
Human BMECs (HBMECs) were kindly provided by Monique Stins (Johns Hopkins University, Baltimore, MD). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum (FBS), 10% NuSerum, 1× sodium pyruvate, 1× nonessential amino acid, 1× vitamin mixtures, 15 units/ml heparin, 30 μg/ml endothelial cell growth supplement (ECGS), 100 units/ml penicillin and 100 μg/ml streptomycin, at 37°C under a 5% CO2 atmosphere. Mouse BMEC (CRL2299) and mouse astrocyte (CRL2541) cell lines were purchased from the ATCC and cultured in DMEM supplemented with 10% FBS, 100 units/ml penicillin and 100 μg/ml streptomycin at 37°C under a 5% CO2 atmosphere.
Primary mouse BMECs were isolated and cultured as previously described with minor modifications (Calabria et al., 2006; Deli et al., 2003). Briefly, brains were removed and cut into two hemispheres. The meninges were removed by rolling the hemispheres on sterile Whatman chromatography paper. The cortices were minced and triturated, followed by collagenase (0.7 mg/ml) and DNase I (39 units/ml) digestion at 37°C for 1 hour. Then the solution was diluted with DMEM and centrifuged at 1000 g for 8 minutes at 4°C. The pellet was resuspended in 20% BSA and centrifuged at 1000 g for 20 minutes at 4°C. The pellet was further digested with collagenase and dispase (1 mg/ml), and DNase I (39 units/ml) at 37°C for 1 hour. Then the solution was diluted with DMEM and centrifuged at 700 g at 4°C for 7 minutes. The pellet was re-suspended and layered over a 33% continuous Percoll gradient and centrifuged in a JA-20 rotor at 3000 rev. per minute for 10 minutes at 4°C. The microvessel layer was collected using an 18-gauge needle and diluted into DMEM. After centrifuging at 700 g for 10 minutes at 4°C, the pellet was resuspended in complete culture medium [DMEM supplemented with 20% bovine platelet-poor plasma-derived serum (Biomedical Technologies), 100 μg/ml heparin, 1 ng/ml basic fibroblast growth factor, 4 μg/ml puromycin, 100 units/ml penicillin and 100 μg/ml streptomycin] and plated on type IV collagen (400 μg/ml) and fibronectin (100 μg/ml) pre-coated plates. The culture medium was changed every 24 hours after initial plating. From day three after the initial plating, complete culture medium without puromycin was used.
Cytotoxicity assay
Cytotoxicity was performed using the LDH Detection Kit (Roche) according to the manufacturer's instructions. Briefly, mouse BMECs or HBMECs were seeded into 96-well plates at a density of 1×104 cells per well and maintained in DMEM with 1% FBS. The next day, the medium was replaced with fresh DMEM plus 1% FBS and the cells were treated with 100 nM mouse FL-, K104A-, K104Stop-, CT-, or human MCP1 overnight. The medium was collected and used to determine LDH levels. Cells treated with 2% Triton X-100 were used as control to determine the maximal LDH release. The data were converted into a percentage of the maximal LDH release.
Transendothelial electrical resistance assay
For the simple BBB model, HBMECs were plated in the upper chamber of 0.4 μm Transwell inserts. For the co-culture system, mouse astrocytes were plated onto the lower side of the 0.4 μm Transwell inserts. After 4 hours, the inserts were inverted and mouse BMECs were seeded in the upper chamber, as described previously (Dimitrijevic et al., 2006; Stamatovic et al., 2005). When the cells reached confluence, 100 nM FL-, K104A-, K104Stop- or CT-MCP1 was added to the lower chambers. After different periods of time, TEER values were measured using the EVOM Epithelial Volt/Ohm meter (World Precision Instruments). The resistance of empty inserts were also measured and subtracted for calculation of final TEER values (Ω/cm2).
In vitro permeability assay
The permeability of BBB was assessed in vitro, as described previously (Eugenin, 2006). Briefly, HBMECs or the co-culture system was prepared as described above. Sterile Evans Blue or FITC–Dextran was added into the upper chamber and 100 nM FL-, K104A-, K104Stop- and CT-MCP1 were added to the lower chambers. At different timepoints, 100-μl samples from the lower chamber were collected and 100 μl of fresh medium with 100 nM MCP1 was added back to the lower chamber. The collected samples were read at 620 nm (for Evans Blue dye) or 488 nm (for FITC-Dextran) to quantify the concentrations of infiltrated tracers. Empty inserts were used to measure the maximal absorbance reading, which signifies the complete disruption of the BBB. The absorbance values were converted to percentage of permeability (%) with respect to the maximal absorbance reading.
In vivo permeability assay
In vivo permeability assays were performed as described previously with minor modifications (Ohno et al., 1980; Parathath et al., 2006; Wu and Tsirka, 2009; Yepes et al., 2003). Briefly, wild-type (C57BL6, wt), MCP1−/−, Ccr2−/−, and tPA−/− mice were anesthetized by injecting atropine (0.6 μg per g of body weight) and avertin (0.02 ml per g of body weight) intraperitoneally. A burr hole was then drilled for hippocampal injection at stereotaxic coordinates of −2.5 mm posterior to bregma, 1.7 mm lateral from midline and 1.6 mm in depth. Then, 1 μg of FL-, K104A-, K104Stop- or CT-MCP1 was delivered in 2.5 μl of saline over 2 minutes. The needle was kept in place for 2 minutes to prevent reflux. A total of 2.5 μl of saline was injected as a control. Evans Blue (2%) was injected into the systematic circulation via the eye. At 12 hours after the MCP1 injection, the mice were anesthetized again, followed by transcardiac perfusion with PBS to remove the blood from the vessels. Brains were removed, divided into ipsilateral (ipsi) and contralateral (cont) hemispheres and weighed (W). Each hemisphere was homogenized in PBS supplemented with 0.5% Triton X-100, and centrifuged at 21,000 g. Evans Blue in the supernatant was calculated as follows: Evans Blue=A620 ipsi/Wipsi–A620 cont/Wcont.
Immunofluorescent staining
HBMECs or primary BMECs were plated onto coverslips and treated with MCP1 as above. After fixation, in −20°C methanol for 10 minutes or in 4% paraformaldehyde for 20 minute, the cells were incubated overnight with mouse anti-occludin antibody (1:300; Zymed) (Sugimoto et al., 2008) and rabbit anti-ZO-1 antibody (1:300; Zymed) followed by fluorescent anti-(mouse Ig) or anti-(rabbit Ig) antibodies (1:1000; Invitrogen). Next, the cells were washed and mounted on slides using Fluoromount with DAPI. For phalloidin staining, the cells were fixed with 4% paraformaldehyde and stained with 1:30 Alexa-Fluor-647–phalloidin overnight at 4°C. The stained samples were examined using confocal microscopy (Zeiss LSM510).
Triton X-100 fractionation
Triton X-100 fractionation was performed as described previously with minor modifications (Fey et al., 1984; Stamatovic et al., 2006; Stamatovic et al., 2003; Stamatovic et al., 2005). Briefly, extracting buffer (10 mM Tris-HCl pH 7.4, 100 mM NaCl, 300 mM sucrose, 0.5% Triton X-100 and protease inhibitor cocktail) was added on top of a confluent monolayer of HBMECs. Extraction was performed by gently rocking at 4°C for 20 minutes. Collected supernatants were defined as the Triton-X-100-soluble fraction. The residue of cells, which still adheres to the culture dishes, was washed twice with PBS supplemented with protease inhibitor cocktail and lysed with radioimmunoprecipitation assay buffer (10 mM Tris-HCl pH 7.4, 140 mM NaCl, 1% sodium deoxycholate, 0.1% SDS, 1% Triton X-100 and protease inhibitor cocktail). Collected supernatants were defined as the Triton-X-100-insoluble fraction. Both Triton-X-100-soluble and -insoluble fractions were prepared by adding 4× SDS sample loading buffer and heating at 95°C for 10 minutes.
Western blotting
The samples were resolved using SDS-PAGE and transferred onto PVDF membranes. Target proteins were visualized using mouse anti-occludin, rabbit anti-ZO-1 and rabbit anti-actin antibodies (all at 1:1000), and 1:500 rabbit anti-(phosphorylated ERM) antibody (Santa Cruz Biotechnology), and the densities of the bands were measured using the NIH Image 1.63 software or the LI-COR Odyssey program.
Co-immunoprecipitation
Cells treated with or without K104Stop-MCP1 were lysed with RIPA buffer. The lysates were incubated with anti-(phosphorylated ERM) antibody, anti-ZO-1 antibody or anti-occludin antibody for 2 hours at 4°C. Then, 25 μl of protein A- and G-conjugated agarose beads (Santa Cruz Biotechnology) was added to pull down the immunocomplexes. After incubating at 4°C for 2 hours, the beads were collected by centrifugation and washed four times with PBS. After last wash, 20 μl of 2× sample loading buffer was added and the resulting protein complexes were immunoblotted with the antibodies and visualized using the LI-COR Odyssey scanner. Total Rac was determined in a similar manner, using cell lysates.
Statistics
Results are shown as means±s.d. One-way ANOVA followed by the Newman–Keuls multiple comparison test was used to analyze differences between two groups.
Supplementary Material
Acknowledgments
We thank the members of the Tsirka laboratory for helpful discussions and members of the Frohman laboratory for reagents. This work was partially supported by a SigmaXi grant-in-aid (to Y.Y.) and NIH R0142168 (to S.E.T.). Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/124/9/1486/DC1
References
- Ahdieh M., Vandenbos T., Youakim A. (2001). Lung epithelial barrier function and wound healing are decreased by IL-4 and IL-13 and enhanced by IFN-gamma. Am. J. Physiol. Cell Physiol. 281, C2029-C2038 [DOI] [PubMed] [Google Scholar]
- Alter A., Duddy M., Hebert S., Biernacki K., Prat A., Antel J. P., Yong V. W., Nuttall R. K., Pennington C. J., Edwards D. R., et al. (2003). Determinants of human B cell migration across brain endothelial cells. J. Immunol. 170, 4497-4505 [DOI] [PubMed] [Google Scholar]
- Blamire A., Anthony D., Rajagopalan B., Sibson N., Perry V., Styles P. (2000). Interleukin-1beta-induced changes in blood-brain barrier permeability, apparent diffusion coefficient, and cerebral blood volume in the rat brain: a magnetic resonance study. J. Neurosci. 20, 8153-8159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A., Gary R., Berryman M. (1995). Soluble ezrin purified from placenta exists as stable monomers and elongated dimers with masked C-terminal ezrin-radixin-moesin association domains. Biochemistry 34, 16830-16837 [DOI] [PubMed] [Google Scholar]
- Calabria A. R., Weidenfeller C., Jones A. R., de Vries H. E., Shusta E. V. (2006). Puromycin-purified rat brain microvascular endothelial cell cultures exhibit improved barrier properties in response to glucocorticoid induction. J. Neurochem. 97, 922-933 [DOI] [PubMed] [Google Scholar]
- Capoccia B. J., Gregory A. D., Link D. C. (2008). Recruitment of the inflammatory subset of monocytes to sites of ischemia induces angiogenesis in a monocyte chemoattractant protein-1-dependent fashion. J. Leukoc. Biol. 84, 760-768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S., Cordenonsi M. (1998). Tight junction proteins. Biochim. Biophys. Acta 1448, 1-11 [DOI] [PubMed] [Google Scholar]
- Clarke H., Marano C. W., Peralta Soler A., Mullin J. M. (2000). Modification of tight junction function by protein kinase C isoforms. Adv. Drug Deliv. Rev. 41, 283-301 [DOI] [PubMed] [Google Scholar]
- Coyne C., Vanhook M., Gambling T., Carson J., Boucher R., Johnson L. (2002). Regulation of airway tight junctions by proinflammatory cytokines. Mol. Biol. Cell 13, 3218-3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deli M. A., Abraham C. S., Niwa M., Falus A. (2003). N,N-diethyl-2-[4-(phenylmethyl)phenoxy]ethanamine increases the permeability of primary mouse cerebral endothelial cell monolayers. Inflamm. Res. 52 Suppl. 1, S39-S40 [DOI] [PubMed] [Google Scholar]
- Dimitrijevic O. B., Stamatovic S. M., Keep R. F., Andjelkovic A. V. (2006). Effects of the chemokine CCL2 on blood-brain barrier permeability during ischemia-reperfusion injury. J. Cereb. Blood Flow Metab. 26, 797-810 [DOI] [PubMed] [Google Scholar]
- Eugenin E. A., Osiecki K., Lopez L., Goldstein H., Calderon T. M., Berman J. W. (2006). CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J. Neurosci. 26, 1098-1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farshori P., Kachar B. (1999). Redistribution and phosphorylation of occludin during opening and resealing of tight junctions in cultured epithelial cells. J. Membr. Biol. 170, 147-156 [DOI] [PubMed] [Google Scholar]
- Fey E. G., Wan K. M., Penman S. (1984). Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: three-dimensional organization and protein composition. J. Cell Biol. 98, 1973-1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster C. (2008). Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 130, 55-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis N. G., Dewald O., Xia Y., Ren G., Haudek S., Leucker T., Kraemer D., Taffet G., Rollins B. J., Entman M. L. (2007). Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation 115, 584-592 [DOI] [PubMed] [Google Scholar]
- Glabinski A. R., Balasingam V., Tani M., Kunkel S. L., Strieter R. M., Yong V. W., Ransohoff R. M. (1996). Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J. Immunol. 156, 4363-4368 [PubMed] [Google Scholar]
- Glynn S. L., Yazdanian M. (1998). In vitro blood-brain barrier permeability of nevirapine compared to other HIV antiretroviral agents. J. Pharm. Sci. 87, 306-310 [DOI] [PubMed] [Google Scholar]
- Guillemin G. J., Brew B. J. (2004). Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J. Leukoc. Biol. 75, 388-397 [DOI] [PubMed] [Google Scholar]
- Hanisch U. K. (2002). Microglia as a source and target of cytokines. Glia 40, 140-155 [DOI] [PubMed] [Google Scholar]
- Hart M. N., VanDyk L. F., Moore S. A., Shasby D. M., Cancilla P. A. (1987). Differential opening of the brain endothelial barrier following neutralization of the endothelial luminal anionic charge in vitro. J. Neuropathol. Exp. Neurol. 46, 141-153 [DOI] [PubMed] [Google Scholar]
- Hayashi K., Nakao S., Nakaoke R., Nakagawa S., Kitagawa N., Niwa M. (2004). Effects of hypoxia on endothelial/pericytic co-culture model of the blood-brain barrier. Regul. Pept. 123, 77-83 [DOI] [PubMed] [Google Scholar]
- Helander T. S., Carpen O., Turunen O., Kovanen P. E., Vaheri A., Timonen T. (1996). ICAM-2 redistributed by ezrin as a target for killer cells. Nature 382, 265-268 [DOI] [PubMed] [Google Scholar]
- Hicks K., O'Neil R. G., Dubinsky W. S., Brown R. C. (2010). TRPC-mediated actin-myosin contraction is critical for BBB disruption following hypoxic stress. Am. J. Physiol. Cell Physiol. 298, C1583-C1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase T., Kawashima S., Wong E. Y., Ueyama T., Rikitake Y., Tsukita S., Yokoyama M., Staddon J. M. (2001). Regulation of tight junction permeability and occludin phosphorylation by Rhoa-p160ROCK-dependent and -independent mechanisms. J. Biol. Chem. 276, 10423-10431 [DOI] [PubMed] [Google Scholar]
- Huber J. D., Egleton R. D., Davis T. P. (2001). Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 24, 719-725 [DOI] [PubMed] [Google Scholar]
- Hulkower K., Brosnan C. F., Aquino D. A., Cammer W., Kulshrestha S., Guida M. P., Rapoport D. A., Berman J. W. (1993). Expression of CSF-1, c-fms, and MCP-1 in the central nervous system of rats with experimental allergic encephalomyelitis. J. Immunol. 150, 2525-2533 [PubMed] [Google Scholar]
- Jung H., Bhangoo S., Banisadr G., Freitag C., Ren D., White F. A., Miller R. J. (2009). Visualization of chemokine receptor activation in transgenic mice reveals peripheral activation of CCR2 receptors in states of neuropathic pain. J. Neurosci. 29, 8051-8062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacem K., Lacombe P., Seylaz J., Bonvento G. (1998). Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia 23, 1-10 [PubMed] [Google Scholar]
- Kataoka K., Asai T., Taneda M., Ueshima S., Matsuo O., Kuroda R., Kawabata A., Carmeliet P. (2000). Roles of urokinase type plasminogen activator in a brain stab wound. Brain Res. 887, 187-190 [DOI] [PubMed] [Google Scholar]
- Kawedia J. D., Nieman M. L., Boivin G. P., Melvin J. E., Kikuchi K., Hand A. R., Lorenz J. N., Menon A. G. (2007). Interaction between transcellular and paracellular water transport pathways through Aquaporin 5 and the tight junction complex. Proc. Natl. Acad. Sci. USA 104, 3621-3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G. H., Kellner C. P., Hahn D. K., Desantis B. M., Musabbir M., Starke R. M., Rynkowski M., Komotar R. J., Otten M. L., Sciacca R., et al. (2008). Monocyte chemoattractant protein-1 predicts outcome and vasospasm following aneurysmal subarachnoid hemorrhage. J. Neurosurg. 109, 38-43 [DOI] [PubMed] [Google Scholar]
- Lahrtz F., Piali L., Spanaus K. S., Seebach J., Fontana A. (1998). Chemokines and chemotaxis of leukocytes in infectious meningitis. J. Neuroimmunol. 85, 33-43 [DOI] [PubMed] [Google Scholar]
- Louvet-Vallee S. (2000). ERM proteins: from cellular architecture to cell signaling. Biol. Cell 92, 305-316 [DOI] [PubMed] [Google Scholar]
- Matsui T., Maeda M., Doi Y., Yonemura S., Amano M., Kaibuchi K., Tsukita S. (1998). Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J. Cell Biol. 140, 647-657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C., Curry F. (1999). Microvascular permeability. Physiol. Rev. 79 703-761 [DOI] [PubMed] [Google Scholar]
- Miller R. J., Meucci O. (1999). AIDS and the brain: is there a chemokine connection? Trends Neurosci. 22, 471-479 [DOI] [PubMed] [Google Scholar]
- Mitic L. L., Anderson J. M. (1998). Molecular architecture of tight junctions. Annu. Rev. Physiol. 60, 121-142 [DOI] [PubMed] [Google Scholar]
- Morimoto H., Hirose M., Takahashi M., Kawaguchi M., Ise H., Kolattukudy P. E., Yamada M., Ikeda U. (2008). MCP-1 induces cardioprotection against ischaemia/reperfusion injury: role of reactive oxygen species. Cardiovasc. Res. 78, 554-562 [DOI] [PubMed] [Google Scholar]
- Nakamura F., Amieva M. R., Furthmayr H. (1995). Phosphorylation of threonine 558 in the carboxyl-terminal actin-binding domain of moesin by thrombin activation of human platelets. J. Biol. Chem. 270, 31377-31385 [DOI] [PubMed] [Google Scholar]
- Neuhaus W., Plattner V. E., Wirth M., Germann B., Lachmann B., Gabor F., Noe C. R. (2008). Validation of in vitro cell culture models of the blood-brain barrier: tightness characterization of two promising cell lines. J. Pharm. Sci. 97, 5158-5175 [DOI] [PubMed] [Google Scholar]
- Ohno K., Chiueh C. C., Burns E. M., Pettigrew K. D., Rapoport S. I. (1980). Cerebrovascular integrity in protein-deprived rats. Brain Res. Bull. 5, 251-255 [DOI] [PubMed] [Google Scholar]
- Parathath S., Parathath S., Tsirka S. (2006). Nitric oxide mediates neurodegeneration and breakdown of the blood-brain barrier in tPA-dependent excitotoxic injury in mice. J. Cell Sci. 119, 339-349 [DOI] [PubMed] [Google Scholar]
- Paul R., Koedel U., Winkler F., Kieseier B., Fontana A., Kopf M., Hartung H., Pfister H. (2003). Lack of IL-6 augments inflammatory response but decreases vascular permeability in bacterial meningitis. Brain 126, 1873-1882 [DOI] [PubMed] [Google Scholar]
- Pearson M. A., Reczek D., Bretscher A., Karplus P. A. (2000). Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 101, 259-270 [DOI] [PubMed] [Google Scholar]
- Pestonjamasp K., Amieva M. R., Strassel C. P., Nauseef W. M., Furthmayr H., Luna E. J. (1995). Moesin, ezrin, and p205 are actin-binding proteins associated with neutrophil plasma membranes. Mol. Biol. Cell 6, 247-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijerkerk A., Kooij G., van der Pol S. M., Leyen T., van Het Hof B., Couraud P. O., Vivien D., Dijkstra C. D., de Vries H. E. (2008). Tissue-type plasminogen activator is a regulator of monocyte diapedesis through the brain endothelial barrier. J. Immunol. 181, 3567-3574 [DOI] [PubMed] [Google Scholar]
- Ross D., Joyner W. (1997). Resting distribution and stimulated translocation of protein kinase C isoforms alpha, epsilon and zeta in response to bradykinin and TNF in human endothelial cells. Endothelium 5, 321-332 [DOI] [PubMed] [Google Scholar]
- Serrador J. M., Alonso-Lebrero J. L., del Pozo M. A., Furthmayr H., Schwartz-Albiez R., Calvo J., Lozano F., Sanchez-Madrid F. (1997). Moesin interacts with the cytoplasmic region of intercellular adhesion molecule-3 and is redistributed to the uropod of T lymphocytes during cell polarization. J. Cell Biol. 138, 1409-1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan J. J., Zhou C., Gravanis I., Rogove A. D., Wu Y. P., Bogenhagen D. F., Tsirka S. E. (2007). Proteolytic activation of monocyte chemoattractant protein-1 by plasmin underlies excitotoxic neurodegeneration in mice. J. Neurosci. 27, 1738-1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M., Arcuino G., Takano T., Liu Q. S., Nedergaard M. (2003). Signaling at the gliovascular interface. J. Neurosci. 23, 9254-9262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic S. M., Keep R. F., Kunkel S. L., Andjelkovic A. V. (2003). Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J. Cell Sci. 116, 4615-4628 [DOI] [PubMed] [Google Scholar]
- Stamatovic S. M., Shakui P., Keep R. F., Moore B. B., Kunkel S. L., Van Rooijen N., Andjelkovic A. V. (2005). Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J. Cereb. Blood Flow Metab. 25, 593-606 [DOI] [PubMed] [Google Scholar]
- Stamatovic S. M., Dimitrijevic O. B., Keep R. F., Andjelkovic A. V. (2006). Protein kinase Calpha-RhoA cross-talk in CCL2-induced alterations in brain endothelial permeability. J. Biol. Chem. 281, 8379-8388 [DOI] [PubMed] [Google Scholar]
- Stephan C. C., Brock T. A. (1996). Vascular endothelial growth factor, a multifunctional polypeptide. P. R. Health Sci. J. 15, 169-178 [PubMed] [Google Scholar]
- Sugimoto M., Inoko A., Shiromizu T., Nakayama M., Zou P., Yonemura S., Hayashi Y., Izawa I., Sasoh M., Uji Y., et al. (2008). The keratin-binding protein Albatross regulates polarization of epithelial cells. J. Cell Biol. 183, 19-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T., Nigam S. (1997). Tight junction proteins form large complexes and associate with the cytoskeleton in an ATP depletion model for reversible junction assembly. J. Biol. Chem. 272, 16133-16139 [DOI] [PubMed] [Google Scholar]
- Tsukamoto T., Nigam S. (1999). Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am. J. Physiol. 276, F737-F750 [DOI] [PubMed] [Google Scholar]
- Tsukita S., Oishi K., Sato N., Sagara J., Kawai A. (1994). ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J. Cell Biol. 126, 391-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen O., Wahlstrom T., Vaheri A. (1994). Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. J. Cell Biol. 126, 1445-1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuw Amerongen G. P., van Delft S., Vermeer M. A., Collard J. G., van Hinsbergh V. W. (2000). Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ. Res. 87, 335-340 [DOI] [PubMed] [Google Scholar]
- Ward P. D., Klein R. R., Troutman M. D., Desai S., Thakker D. R. (2002). Phospholipase C-gamma modulates epithelial tight junction permeability through hyperphosphorylation of tight junction proteins. J. Biol. Chem. 277, 35760-35765 [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B., Entwistle A., Garg R., Ridley A. (1998). Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junction by Rho, Rac and Cdc42 in human endothelial cells. J. Cell Physiol. 176, 150-165 [DOI] [PubMed] [Google Scholar]
- Wu M., Tsirka S. (2009). Endothelial NOS-deficient mice reveal dual roles for nitric oxide during experimental autoimmune encephalomyelitis. Glia 57, 1204-1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y. P., Sailor K. A., Lang B. T., Park S. W., Vemuganti R., Dempsey R. J. (2007). Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 27, 1213-1224 [DOI] [PubMed] [Google Scholar]
- Yang G., Gong C., Qin Z., Liu X., Betz L. (1999). Tumor necrosis factor alpha expression produces increased blood–brain barrier permeability following temporary focal cerebral ischemia in mice. Brain Res. Mol. Brain Res. 69, 135-143 [DOI] [PubMed] [Google Scholar]
- Yao Y., Tsirka S. E. (2010). The C terminus of mouse monocyte chemoattractant protein 1 (MCP1) mediates MCP1 dimerization while blocking its chemotactic potency. J. Biol. Chem. 285, 31509-31516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M., Sandkvist M., Moore E., Bugge T., Strickland D., Lawrence D. (2003). Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J. Clin. Invest. 112, 1533-1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S., Nagafuchi A., Sato N., Tsukita S. (1993). Concentration of an integral membrane protein, CD43 (leukosialin, sialophorin), in the cleavage furrow through the interaction of its cytoplasmic domain with actin-based cytoskeletons. J. Cell Biol. 120, 437-449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youakim A., Ahdieh M. (1999). Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am. J. Physiol. 276, G1279-G1288 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.