Abstract
Background
Previous studies examining the relationship between micronutrient intakes and survival following diagnosis of breast cancer have reported mixed results. This may be partly due to considerable variance in amounts of micronutrients consumed from diet and supplements across studies.
Methods
Early stage breast cancer survivors (n=3081) completed four 24-hour dietary and supplement recalls at the baseline assessment (1995 to 2000) and were followed for a median of 9.0 years. Mean micronutrient intakes were compared to dietary reference intakes (DRI) to assess micronutrient adequacy for both users and non-users of supplements. Cox regressions were performed to assess whether intakes of selected micronutrients were associated with all-cause mortality.
Results
412 deaths occurred between baseline and August 2009. Among these women, more supplement users had adequate micronutrient intakes than non-users for 15 out of 17 micronutrients. Less than 10% of supplement users (< 2% of non-supplement users) reported levels that exceeded the tolerable upper limit for each micronutrient except magnesium. After adjusting for age, tumor characteristics, and health status variables, micronutrient intakes were not significantly associated with all-cause mortality.
Conclusion
Dietary supplements may improve overall micronutrient intakes of breast cancer survivors. However, vitamin and mineral intakes were not associated with all-cause mortality.
Keywords: dietary intake, supplement use, breast cancer survival
INTRODUCTION
The 5-year relative survival rates for women who have been diagnosed with breast cancer is approximately 89 % (1), resulting in approximately 2.5 million women in the US with a history of breast cancer (2). Mortality within the first few years of a breast cancer diagnosis is clearly associated with the tumor characteristics and treatment (3); however, other factors become important in later years. Lifestyle variables, such as dietary habits, may have a more important role in long-term than short-term survival for women with a history of breast cancer.
Observational studies have shown mixed results regarding the relationship between micronutrient intakes and all-cause mortality in women diagnosed with breast cancer (4). Studies reported a decreased risk of death for those with higher intakes of beta-carotene and vitamin C (5–7); others have reported a similar effect but the finding did not reach significance (8–9) and some reported there was no association between individual micronutrients and all-cause mortality (10–12). The variation in study methodologies could explain the lack of consistencies across these studies. The major differences include the micronutrient source (food versus supplemental), total micronutrient intake level and approach to measurement. For example, early studies reported only the food source (5, 11) while later studies reported total intake from both food and supplements (8, 10). Irrespective of source, a comparison of micronutrient intakes is also limited since four out of the six studies do not provide sufficient information to determine the quantity that was associated with the decreased risk of death (5, 9–11). Often the conclusions of the study have compared the risk of the top quintile to the lowest quintile or a p-value for trend, but it is difficult to determine how the micronutrient intake distribution reported in one study compared to another study without the values for the micronutrient intake. Thus far, investigations of breast cancer survivors have not evaluated dietary intakes against the established dietary reference intakes (DRI) set forth by the Food and Nutrition Board (13–16).
These studies have identified and cited other limitations such as lack of statistical power and inadequate information regarding covariates such as type of treatment. In order to determine the impact of micronutrient intake on breast cancer outcomes, a study is needed that has a robust sample, appropriate data for both the food and supplemental sources and a guideline to identify the estimated intake that may be associated with risk for recurrence or mortality.
The present analysis, which is based on a robust dataset collected in the Women’s Healthy Eating and Living (WHEL) Study, had a large sample size, a relatively long follow-up period, and sufficient data for the relevant covariates such as tumor and treatment characteristics (17). The study uses the following DRIs to determine how breast cancer survivors compare to national guidelines: 1) recommended dietary allowance (RDA), 2) adequate intake (AI) and 3) tolerable upper limit (UL) (18–19). The specific aims were 1) To evaluate the micronutrient contribution of dietary supplements to the total intake of breast cancer survivors and 2) To evaluate whether the micronutrient intakes either below the RDA (or AI) or above the UL, as defined per micronutrient, were associated with all-cause mortality over a median follow-up of 9.0 years.
METHODS
Study Design and Participants
This project was part of a large multi-site clinical trial investigating the efficacy of a dietary intervention to reduce risk for breast cancer recurrence (the WHEL Study). The WHEL Study enrolled participants at seven clinical sites between 1995 and 2000: each of the institutional review boards at these sites approved the protocol, and all participants provided written, informed consent. Details of the study protocol are described elsewhere (20). Major eligibility criteria included diagnosis within the past 4 years of primary operable invasive stage I (≥ 1 cm), II, or IIIA breast carcinoma; age 18–70 years at the time of diagnosis; no current or planned chemotherapy; no evidence of recurrent disease or new breast cancer since completion of initial treatment; and no other cancer in the past 10 years. As previously reported, the intervention aimed to promote the adoption of a diet very high in vegetables, fruit, and fiber and low in fat; however, the intervention did not address the use of dietary supplements (neither encouraged nor restricted) (17).
The present analyses included WHEL Study participants who completed one set of 24-hour dietary and supplement recalls at the baseline assessment, which included 3–4 prescheduled recalls during a 3-week period, stratified to include both weekdays (Monday-Thursday) and weekend days (Friday-Sunday). Dietary recalls were collected via telephone interviews by trained dietary assessors. Vitamin and mineral intakes from food and from supplement sources was averaged over the four days. The Minnesota Nutrition Data System (NDS) was used for the collection and nutrient analysis of the dietary data.
Measures
Demographic and medical information
Demographic and medical information was self-reported by participants at study enrollment. Medical information was confirmed by reviewing the participant’s medical record.
Dietary supplements
Detailed data on dietary supplement use, including dosage verification from the label or distributor, was collected at baseline and at each follow-up throughout the study (21). During each recall, participants were asked to name all dietary supplement formulations that they had ingested during the previous 24 hours and to report the number of tablets or capsules ingested from each formulation. Throughout the study, each new supplement reported was entered into the database using the DataEase software program and verified by the WHEL Coordinating Center. For each supplement, the database includes the name, manufacturer and/or brand name, and the nutrient content in standardized units per tablet or capsule. Detailed protocols regarding dietary and supplement data collection, which include verification by label review and manufacturer information, have been described elsewhere (21–22).
Health behaviors: Smoking status and physical activity
At baseline, participants completed a “Personal Habits” questionnaire (23), adapted from the Women’s Health Initiative Study. This questionnaire assessed lifetime smoking history (current, past, never) as well as physical activity (9-item measure). Responses were converted to metabolic equivalent tasks (METs) in minutes per week (24) and 540 MET/min/week was determined as the threshold for adequate energy expenditure.
Outcome
All-cause mortality (survival time) is the time from enrollment to reported/confirmed death from all causes. Death was confirmed through acquisition of death certificates and review of social security death records. Follow-up time was censored at the last documented staff contact date or follow-up completion (August 24, 2009).
Analytic methods
Descriptive statistics were computed on all the micronutrient variables from both sources (food and supplements) and each variable was logarithmically transformed to improve the normality of the distribution. The mean micronutrient intakes from food of non-supplement users were compared to that of supplement users. We examined the additional micronutrient intake from supplements among the users. Supplemental micronutrients were calculated from the participant reports of both single and multiple-ingredient products. We stratified the sample by nonusers and users of supplements; then using the raw scores combined from both sources, each stratum was categorized into the following groups: 1) ‘Below the RDA’ 2) ‘Adequate Consumption’, an amount that was between the RDA level and the upper limit (UL) and 3) ‘Exceeding the UL’. The women were categorized according to the recommended dietary allowance for their age category (13–16). If the recommended dietary allowance had not been set for any particular micronutrient, we used the Adequate Intake (AI) level. AI was substituted for vitamin D, vitamin, K and calcium. In addition, we only examined preformed vitamin A, retinol; however, the RDA and UL applied in this analysis was for total vitamin A. For each of the selected micronutrients, we compared the percent with adequate intake between nonusers and users of supplements.
Cox proportional hazard regression was used to determine the unadjusted association between adequate micronutrient intakes and all-cause mortality for the entire sample. The Hazard ratios (and associated 95% confidence intervals) were the measure of association. Univariate associations were also examined for variables known to affect all-cause mortality such as: patient characteristics, lifestyle factors, and tumor characteristics. A sensitivity analysis was conducted to determine whether the results varied as a function of micronutrient intake. We redefined the categories of dietary adequacy at the level of two-thirds of the RDA. For those micronutrients that had an adequate sample size, we conducted Cox proportional hazard regressions that compared those who had reported inadequate intakes to those with adequate intakes.
RESULTS
Participant Characteristics
Participants (n=3081) had a mean age at enrollment of 53 years. The majority of the sample was white non-Hispanic (85.3%), college graduates (84.9%) and married (70.6%). Slightly over half of the sample had Stage II breast cancer at diagnosis (56.4%) and 70% reported having had chemotherapy. Of the sample, 85% reported using dietary supplements; and among supplement users, the median number of supplement formulations used per day was four. The most frequently used supplements were multivitamins/minerals and calcium.
Table 1 shows that the demographic characteristics are significantly different in supplement users compared to non-supplement users. The supplement users tended to be older, non-Hispanic white, and more educated. Supplement users were also less likely to be obese and more likely to be physically active. Only 24% of the supplement users were obese (body mass index [BMI] greater than 30 kg/m2) compared to 36% of the non-supplement users. More supplement users reported greater than 540 MET/min/week than non-supplement users, 57% and 40%, respectively. Although smoking rates are low in this sample, non-supplement users were more likely to be a current smoker. Supplement users were more likely to be currently using anti-estrogen therapy and have less time since their diagnosis, but there were no other group differences in the types of cancer treatments the women had received prior to the study.
Table 1.
Sample characteristics of a cohort of breast cancer survivors enrolled in the WHEL study.
| Nonusers of Supplements | Users of Supplements | F-test or Chi-square | ||
|---|---|---|---|---|
| Sample Size (n) | n=556 | n=2525 | ||
| Age (mean (SD)) | 50.6 (9.31) | 53.8 (8.79) | 59.18*** | |
| Ethnicity (% non-Hispanic White) | 79.9 | 86.5 | 16.19*** | |
| Education (% College & Beyond) | 50.4 | 55.0 | 4.08* | |
| % Obese (BMI > 30 kg/m2) | 36 | 23.6 | 43.98*** | |
| % Adequate Physical Activity | 39.4 | 56.9 | 53.9*** | |
| % Current Smokers | 7.1 | 3.9 | 10.33*** | |
| Years since Diagnosis (mean (SD)) | 2.1 (1.09) | 1.9 (1.02) | 5.53* | |
| Stage at Diagnosis | I | 39.5 | 38.4 | 1.05 |
| IIA | 31.5 | 33.6 | ||
| IIB | 13.2 | 12.3 | ||
| IIIA | 12.1 | 12.1 | ||
| IIIC | 3.7 | 3.7 | ||
| % Chemotherapy | 73.1 | 69.3 | 3.2 | |
| % Radiation | 61.4 | 61.6 | 0.01 | |
| % Lumpectomy | 52.7 | 52.1 | 0.05 | |
| Anti-estrogen Use (% current) | 53.6 | 62.8 | 16.41*** |
Significance values are as follows:
p < .05,
p < .01,
p < .001
Patterns of micronutrient intake
Mean micronutrient intakes from both food and supplement sources are reported for nonusers and users of supplements in Table 2. These data show no evidence that this cohort of breast cancer survivors exceeded the upper intake levels (i.e., safe levels) through either solely food intake or food and supplements in combination. Among 11 vitamins examined, the average food intakes of retinol, folate, and vitamin E were below the RDA. Supplement users obtained nearly adequate intakes of folate and vitamin E from supplements and foods combined. It is notable that for vitamin E only, the amount obtained from supplements greatly exceeded that obtained from foods. Food intakes of vitamin D, on average, were below the adequate intake level but supplementation increased the mean level above the adequate intake. Among 7 minerals examined, only calcium and magnesium intakes from foods were below the RDA/AI. However, supplement users obtained nearly adequate amounts of these minerals from food and supplements combined. In addition, among the supplement users who specifically took these minerals, the majority reached an adequate intake for their age and gender.
Table 2.
Mean daily intakes (95% CIs) from food and supplement sources stratified by supplement use among a cohort of breast cancer survivors enrolled in the WHEL study.
| Nonusers of Supplements (n=556) | Users of Supplements (n=2525) | Recommended Dietary Allowance (RDA)* | Upper Intake Level (UL) | ||
|---|---|---|---|---|---|
| Food Only | Food | Supplements | |||
| Vitamins | |||||
| Retinol (RE) | 343 (333, 352) | 349 (344,354) | 102 (95, 109) | 700 | 3000 |
| Thiamin (mg) | 2.5 (2.5, 2.5) | 2.6 (2.6, 2.6) | 4.8 (4.6, 4.9) | 1.1 | nd |
| Riboflavin (mg) | 2.6 (2.6, 2.6) | 2.7 (2.7, 2.7) | 4.7 (4.6, 4.8) | 1.1 | nd |
| Niacin (mg) | 20 (20, 20) | 21 (21, 21) | 14 (14, 14) | 14 | 35 |
| Vitamin B6 (mg) | 2.7 (2.6, 2.7) | 2.9 (2.9, 2.9) | 5.8 (5.6, 6.0) | 1.3 – 1.5 | 100 |
| Folate (mcg) | 273 (267, 277) | 315 (312, 317) | 87 (83, 92) | 400 | 1000 |
| Vitamin B12 (mcg) | 4.3 (4.2, 4.4) | 4.6 (4.5, 4.6) | 11.0 (10.6, 11.4) | 2.4 | nd |
| Vitamin C (mg) | 105 (102, 107) | 132 (130, 137) | 171 (163, 179) | 75 | 2000 |
| Vitamin E (alpha-Toc eq) | 9 (9, 9) | 10 (10, 10) | 92 (88, 95) | 15 | 1000 |
| Vitamin D (mcg) | 4.7 (4.6, 4.8) | 5.0 (5.0, 5.1) | 6.0 (5.9, 6.1) | 5 – 10 | 50 |
| Vitamin K (mcg) | 94 (92, 97) | 114 (112, 114) | 4 (4, 4) | 90 | nd |
| Minerals | |||||
| Calcium (mg) | 683 (671, 695) | 752 (746, 758) | 202 (193, 211) | 1000 – 1200 | 2500 |
| Copper (mg) | 2 (2, 2) | 2 (2, 2) | 2 (2, 2) | 2 | 10 |
| Iron (mg) | 14 (14, 14) | 16 (15, 16) | 5 (5, 5) | 8 – 18 | 45 |
| Magnesium (mg) | 270 (266, 273) | 306 (304, 308) | 32 (31, 34) | 320 | 350 |
| Phosphorus (mg) | 1041 (1027, 1053) | 1105 (1099, 1111) | 9 (8, 9) | 700 | 4000 |
| Selenium (mcg) | 95 (94, 96) | 100 (99, 100) | 13 (12, 13) | 55 | 400 |
| Zinc (mg) | 10 (10, 10) | 10 (10, 10) | 8 (8, 8) | 8 | 40 |
Adequate Intake (AI) levels were substituted for RDA for vitamin D, vitamin K, and calcium
Adequate intakes
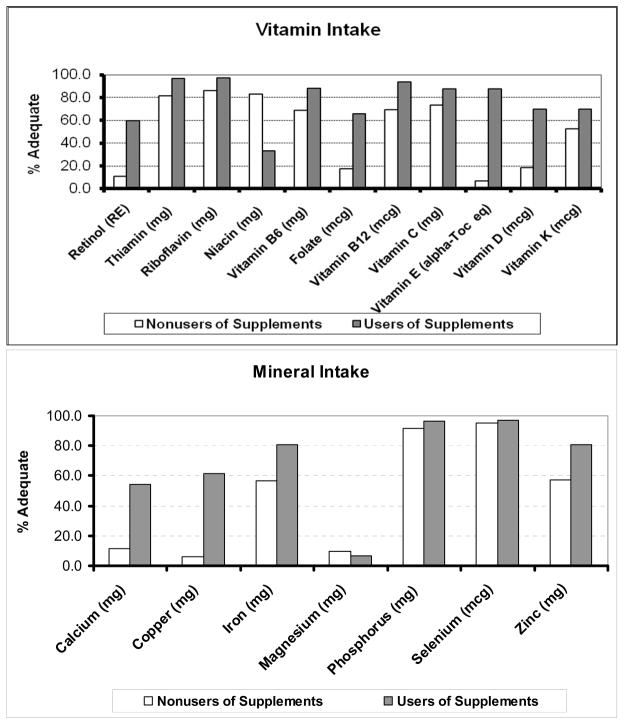
As shown in Figure 1, we stratified by supplement use and reported the percentages of women who had reached the reported adequate intakes through food sources or in combination with supplements per micronutrient. For vitamin intakes, the percentage of supplement users who reached adequate micronutrient levels was greater than that of the non-supplement users, with the exception of niacin. In the case of niacin, 64% of supplement users exceeded the upper intake level. The vitamins with the most prominent differences in intakes between the groups were retinol, folate, vitamin E and vitamin D. As depicted in Figure 1, mineral intake differences were primarily seen in calcium, copper and zinc. For these minerals, the use of supplements made it more likely that the intake reached an adequate level. It should be noted that less than 10% of the women were consuming an adequate amount of magnesium, which could be due to the narrow range for adequate consumption (320 mg/d to 350 mg/d). The majority of the supplement users (65 %) exceeded the upper limit for magnesium and the majority of the non-supplement users were below the RDA level (73 %). Overall, a greater percentage of supplement users consumed an adequate amount of each micronutrient compared to nonusers of supplements.
Figure 1.
Percentages of women who reported adequate intake (AI) levels for each micronutrient through food sources or in combination with supplements.
All-Cause Mortality
As shown in Table 3, micronutrient intakes that were below the RDA were not significantly associated with all-cause mortality. In the unadjusted regression model, those women who had vitamin B-12 intakes below the RDA had a reduced risk of mortality. Low vitamin B-12 intake may be an indicator of consuming less animal products, but this could have been a chance finding given that the effect was no longer significant after adjusting the model for the covariates. Results of the sensitivity analysis suggested that lowering the value to two-thirds of the RDA value did not change the findings or conclusions of this study.
Table 3.
Adjusted a hazard ratios (HR) of all-cause mortality in relation to daily micronutrient intakes from food and supplements in a cohort of US breast cancer survivors followed for a median 9.0 years.
| All-Cause Mortality b n=2939; Events =388 HR (95 % CI) | ||
|---|---|---|
| Micronutrients | Below RDA | Above UL |
| Retinol (RE) | 1.05 (.86, 1.30) | 0.9 (.61, 1.48) |
| Thiamin (mg) | 0.9 (.59, 1.49) | na |
| Riboflavin (mg) | 1.1 (.70, 1.81) | na |
| Niacin (mg) | 0.8 (.52, 1.51) | 1.02 (.83, 1.26) |
| Vitamin B6 (mg) | 1.02 (.74, 1.42) | 0.9 (.63, 1.53) |
| Folate (mcg) | 1.1 (.88, 1.36) | 1.3 (.89, 1.78) |
| Vitamin B12 (mcg) | 0.7 (.50, 1.05) | na |
| Vitamin C (mg) | 1.3 (.93, 1.92) | 1.1 (.79, 1.60) |
| Vitamin E (alpha-Toc eq) | 0.9 (.77, 1.25) | 1.6 (.73, 3.71) |
| Vitamin D (mcg) | 0.9 (.79, 1.18) | 0.9 (.13, 7.11) |
| Vitamin K (mcg) | 1.06 (.86, 1.31) | na |
| Minerals | ||
| Calcium (mg) | 0.9 (.74, 1.12) | 1.1 (.68, 1.71) |
| Copper (mg) | 1.06 (.86, 1.30) | na |
| Iron (mg) | 1.6 (.91, 2.90) | 1.3 (.93, 1.97) |
| Magnesium (mg) | 1.02 (.68, 1.53) | 1.03 (.69, 1.53) |
| Phosphorus (mg) | 1.04 (.64, 1.67) | na |
| Selenium (mcg) | 1.2 (.55, 2.50) | 1.5 (.78, 2.96) |
| Zinc (mg) | 1.1 (.83, 1.45) | 1.00 (.68, 1.48) |
Cox regression models were adjusted for age at randomization, tumor stage, tumor grade, time since diagnosis, body mass index, smoking, randomization group, hot flashes, group by hot flash interaction and physical health.
Reference group for each survival analysis includes those with ‘adequate’ micronutrient intake, which is set between the recommended dietary intake and the upper limit for each micronutrient.
Micronutrient intakes above the UL were not significantly associated with a higher risk of mortality. Intakes of folate, iron and selenium that were above the UL had an elevated hazard ratio but these estimates were not statistically different from the reference group (adequate intake).
DISCUSSION
We examined the micronutrient intakes of breast cancer survivors enrolled in the WHEL Study and found that dietary supplements contributed a substantial portion of the total intake for most micronutrients that were examined among those women who reported using them. The mean intake was higher in supplement users as compared to non-users for nearly every micronutrient. For such micronutrients as retinol, folate, vitamin D, and vitamin E; dietary supplement use contributed a significant proportion of the total intake. Our findings corroborate earlier findings that dietary supplements can improve dietary quality for certain micronutrients. One study reported that over half of the participants reported micronutrient intakes below the RDA level from food alone, but after accounting for supplements, less than 17% were classified as having micronutrient inadequacies (25). In addition, Burnett-Hartman (26) found that supplement use, either in the form of multivitamins or single-micronutrient supplements, was observed to be associated with adequate intakes of minerals. Additionally, vitamin E and folate are among the micronutrients for which intakes were commonly increased through supplement use in our study and in early studies (25). Overall, the previous studies in addition to the present analysis have indicated that supplement users are more likely than non-users to have adequate intakes of micronutrients.
We examined whether micronutrient intake was associated with all-cause mortality and found that for most micronutrients examined in this study, there was not a statistically significant association. Although our analytic approach differed, our findings were in accordance with the previous studies (10–12). Others have typically used the group with the lowest intake as the reference group and examined the risk according to increases in micronutrient intakes, while we have defined our reference group as those who have intakes between the levels of the RDA and UL and compared the risk for mortality to those with levels below the RDA. We found that those with micronutrient intakes below the RDA had an equivalent risk for mortality as those above the RDA. In the unadjusted regression model, those women who had vitamin B-12 intakes below the RDA had a reduced risk of all-cause mortality; however, the effect was no longer statistically significant after controlling for the covariates. Low vitamin B-12 intakes may be a reflection of a diet that consists of more plant-based foods and fewer animal products. Dietary recommendations for the prevention of chronic diseases include avoiding red meat and whole-fat dairy product, both of which are sources of vitamin B-12. Prudent dietary patterns, such as this, have been shown previously to reduce the risk of all-cause mortality among breast cancer survivors (27).
Although the term ‘mega-dose’ does not have a standardized or accepted definition, some investigators have hypothesized that a very high intake (mega-dose) of certain micronutrients could improve overall survival of cancer patients (28–29). The proposed mechanisms are uncertain but micronutrients which are known to have antioxidant properties (such as vitamins C and E, beta carotene, selenium, and zinc) may reduce the free radicals in the body and lower chronic disease risk (30–31). For the purpose of comparison, we evaluated the group who has exceeded the upper limit for these micronutrients and did not find any evidence to support this hypothesis. Those who exceeded the upper limit did not have any lower risk of death than those with adequate micronutrient intakes. Further studies are needed to examine the question of whether high intakes of micronutrients could benefit or harm breast cancer patients. Previous studies have shown mixed results (32–34) and this was study was not specifically designed for answering the question regarding the effect of excessive or mega-dose intakes.
Certain limitations of this study need to be acknowledged. The participants of the present study are not a representative sample of women with a breast cancer diagnosis since they have previously agreed to participate in a dietary intervention. These women reported healthier dietary patterns than the general population, such as a higher fruit and vegetable intake and lower fat intake. Changes in dietary patterns following diagnosis, which included dietary supplements, may limit the representation of those with micronutrient inadequacies and therefore limit our ability to show their impact on survival. Further, the micronutrient intakes are based on self-reported data, using methodologies with well-known limitations for accuracy.
Given that the WHEL study had a large sample and a long follow-up period; the study had adequate power to address the research question. The study had extensive dietary data as well as data for the relevant covariates such as tumor characteristics and treatment modalities, which were collected using quantifiable and validated measures. This study addressed the impact of dietary adequacy, using the dietary reference intakes, instead of categorizing the micronutrient intake according to quintiles. Because the large variance in micronutrient exposure across studies, reporting supplement use in a binary fashion or by quintile makes comparisons of results across studies more challenging. Should future studies use the dietary reference intakes as a standard method of describing exposures, then researchers could synthesize study results more easily and clinicians could provide more precise nutritional guidance to cancer patients. In conclusion, more supplement users had adequate micronutrient intakes than non-users of supplements; however, micronutrient intakes, from food and supplements, were not significantly associated with all-cause mortality in this cohort of breast cancer survivors.
Acknowledgments
The Women’s Healthy Eating and Living (WHEL) Study was initiated with the support of the Walton Family Foundation and continued with funding from NCI grant CA 69375. Some of the data were collected from General Clinical Research Centers, NIH grants M01-RR00070, M01-RR00079, and M01-RR00827. Research related to the development of this article was funded with support from NRSA National Center for Complementary and Alternative Medicine Fellowship Award 5F31AT004652-02.
References
- 1.American Cancer Society. Cancer Facts & Figures 2010. Atlanta: American Cancer Society; 2010. [Google Scholar]
- 2.Horner M, Ries L, Krapcho M, Neyman N, Aminou R, et al. SEER Cancer Statistics Review, 1975–2006. Bethesda, MD: National Cancer Institute; 2009. [Google Scholar]
- 3.Natarajan L, Pu M, Parker BA, Thomson CA, Caan BJ, et al. Time-varying effects of prognostic factors associated with disease-free survival in breast cancer. Am J Epidemiol. 2009;169(12):1463–70. doi: 10.1093/aje/kwp077. kwp077 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rock CL, Demark-Wahnefried W. Nutrition and survival after the diagnosis of breast cancer: a review of the evidence. J Clin Oncol. 2002;20(15):3302–16. doi: 10.1200/JCO.2002.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingram D. Diet and subsequent survival in women with breast cancer. Br J Cancer. 1994;69(3):592–5. doi: 10.1038/bjc.1994.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain M, Miller AB, To T. Premorbid diet and the prognosis of women with breast cancer. J Natl Cancer Inst. 1994;86(18):1390–7. doi: 10.1093/jnci/86.18.1390. [DOI] [PubMed] [Google Scholar]
- 7.Rohan TE, Hiller JE, McMichael AJ. Dietary factors and survival from breast cancer. Nutr Cancer. 1993;20(2):167–77. doi: 10.1080/01635589309514283. [DOI] [PubMed] [Google Scholar]
- 8.Fink BN, Gaudet MM, Britton JA, Abrahamson PE, Teitelbaum SL, et al. Fruits, vegetables, and micronutrient intake in relation to breast cancer survival. Breast Cancer Res Treat. 2006;98(2):199–208. doi: 10.1007/s10549-005-9150-3. [DOI] [PubMed] [Google Scholar]
- 9.Fleischauer AT, Simonsen N, Arab L. Antioxidant supplements and risk of breast cancer recurrence and breast cancer-related mortality among postmenopausal women. Nutr Cancer. 2003;46(1):15–22. doi: 10.1207/S15327914NC4601_02. [DOI] [PubMed] [Google Scholar]
- 10.Holmes MD, Stampfer MJ, Colditz GA, Rosner B, Hunter DJ, et al. Dietary factors and the survival of women with breast carcinoma. Cancer. 1999;86(5):826–35. doi: 10.1002/(sici)1097-0142(19990901)86:5<826::aid-cncr19>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Saxe GA, Rock CL, Wicha MS, Schottenfeld D. Diet and risk for breast cancer recurrence and survival. Breast Cancer Res Treat. 1999;53(3):241–53. doi: 10.1023/a:1006190820231. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Folsom AR, Sellers TA, Kushi LH, Potter JD. Better breast cancer survival for postmenopausal women who are less overweight and eat less fat. The Iowa Women's Health Study. Cancer. 1995;76(2):275–83. doi: 10.1002/1097-0142(19950715)76:2<275::aid-cncr2820760218>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Food and Nutrition Board. Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorous, Magnesium, Vitamin D, and Fluoride. Washington D.C: 1999. [Google Scholar]
- 14.Food and Nutrition Board. Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin and Choline. Washington D.C: 2000. [PubMed] [Google Scholar]
- 15.Food and Nutrition Board. Institute of Medicine. Dietary Reference Intakes: Applications in Dietary Assessment. Washington D.C: 2000. [Google Scholar]
- 16.Food and Nutrition Board. Institute of Medicine. Dietary Reference Intake for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc. Washington, D.C: 2001. [Google Scholar]
- 17.Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women's Healthy Eating and Living (WHEL) randomized trial. Jama. 2007;298(3):289–98. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrd-Bredbenner C, Moe G, Beshegetoor D, Berning J. Wardlaw's Perspectives in Nutrition. New York, NY: McGraw Hill; 2009. Tools of a Healthy Diet; pp. 35–43. Edtion ed. [Google Scholar]
- 19.Sims LS. Uses of the recommended dietary allowances: a commentary. J Am Diet Assoc. 1996;96(7):659–62. doi: 10.1016/S0002-8223(96)00184-8. S0002-8223(96)00184-8 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Pierce JP, Faerber S, Wright FA, Rock CL, Newman V, et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women's Healthy Eating and Living (WHEL) Study. Control Clin Trials. 2002;23(6):728–56. doi: 10.1016/s0197-2456(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 21.Newman V, Rock CL, Faerber S, Flatt SW, Wright FA, et al. Dietary supplement use by women at risk for breast cancer recurrence. The Women's Healthy Eating and Living Study Group. J Am Diet Assoc. 1998;98(3):285–92. doi: 10.1016/s0002-8223(98)00068-6. [DOI] [PubMed] [Google Scholar]
- 22.Rock CL, Newman V, Flatt SW, Faerber S, Wright FA, et al. Nutrient intakes from foods and dietary supplements in women at risk for breast cancer recurrence. The Women's Healthy Eating and Living Study Group. Nutr Cancer. 1997;29(2):133–9. doi: 10.1080/01635589709514614. [DOI] [PubMed] [Google Scholar]
- 23.WHI. Women's Health Initiative. [Accessed September 2, 2009];WHI Personal Habits Questionnaire. http://www.whiscience.org/data/forms/F34v2.pdf. accessed Date Accessed)|.
- 24.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 25.Sebastian RS, Cleveland LE, Goldman JD, Moshfegh AJ. Older adults who use vitamin/mineral supplements differ from nonusers in nutrient intake adequacy and dietary attitudes. J Am Diet Assoc. 2007;107(8):1322–32. doi: 10.1016/j.jada.2007.05.010. S0002-8223(07)00733-X [pii] [DOI] [PubMed] [Google Scholar]
- 26.Burnett-Hartman AN, Fitzpatrick AL, Gao K, Jackson SA, Schreiner PJ. Supplement use contributes to meeting recommended dietary intakes for calcium, magnesium, and vitamin C in four ethnicities of middle-aged and older Americans: the Multi-Ethnic Study of Atherosclerosis. J Am Diet Assoc. 2009;109(3):422–9. doi: 10.1016/j.jada.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwan ML, Weltzien E, Kushi LH, Castillo A, Slattery ML, et al. Dietary patterns and breast cancer recurrence and survival among women with early-stage breast cancer. J Clin Oncol. 2009;27(6):919–26. doi: 10.1200/JCO.2008.19.4035. JCO.2008.19.4035 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lesperance ML, Olivotto IA, Forde N, Zhao Y, Speers C, et al. Mega-dose vitamins and minerals in the treatment of non-metastatic breast cancer: an historical cohort study. Breast Cancer Res Treat. 2002;76(2):137–43. doi: 10.1023/a:1020552501345. [DOI] [PubMed] [Google Scholar]
- 29.Seifried HE, McDonald SS, Anderson DE, Greenwald P, Milner JA. The antioxidant conundrum in cancer. Cancer Res. 2003;63(15):4295–8. [PubMed] [Google Scholar]
- 30.Sigounas G, Anagnostou A, Steiner M. dl-alpha-tocopherol induces apoptosis in erythroleukemia, prostate, and breast cancer cells. Nutr Cancer. 1997;28(1):30–5. doi: 10.1080/01635589709514549. [DOI] [PubMed] [Google Scholar]
- 31.Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol Ther. 2001;92(1):57–70. doi: 10.1016/s0163-7258(01)00159-0. S0163-7258(01)00159-0 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Yang Q, Bostick RM, Friedman JM, Flanders WD. Serum folate and cancer mortality among U.S. adults: findings from the Third National Health and Nutritional Examination Survey linked mortality file. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1439–47. doi: 10.1158/1055-9965.EPI-08-0908. 18/5/1439 [pii] [DOI] [PubMed] [Google Scholar]
- 33.Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med. 2008;168(4):404–10. doi: 10.1001/archinternmed.2007.74. 168/4/404 [pii] [DOI] [PubMed] [Google Scholar]
- 34.Kabat GC, Rohan TE. Does excess iron play a role in breast carcinogenesis? An unresolved hypothesis. Cancer Causes Control. 2007;18(10):1047–53. doi: 10.1007/s10552-007-9058-9. [DOI] [PubMed] [Google Scholar]