Abstract
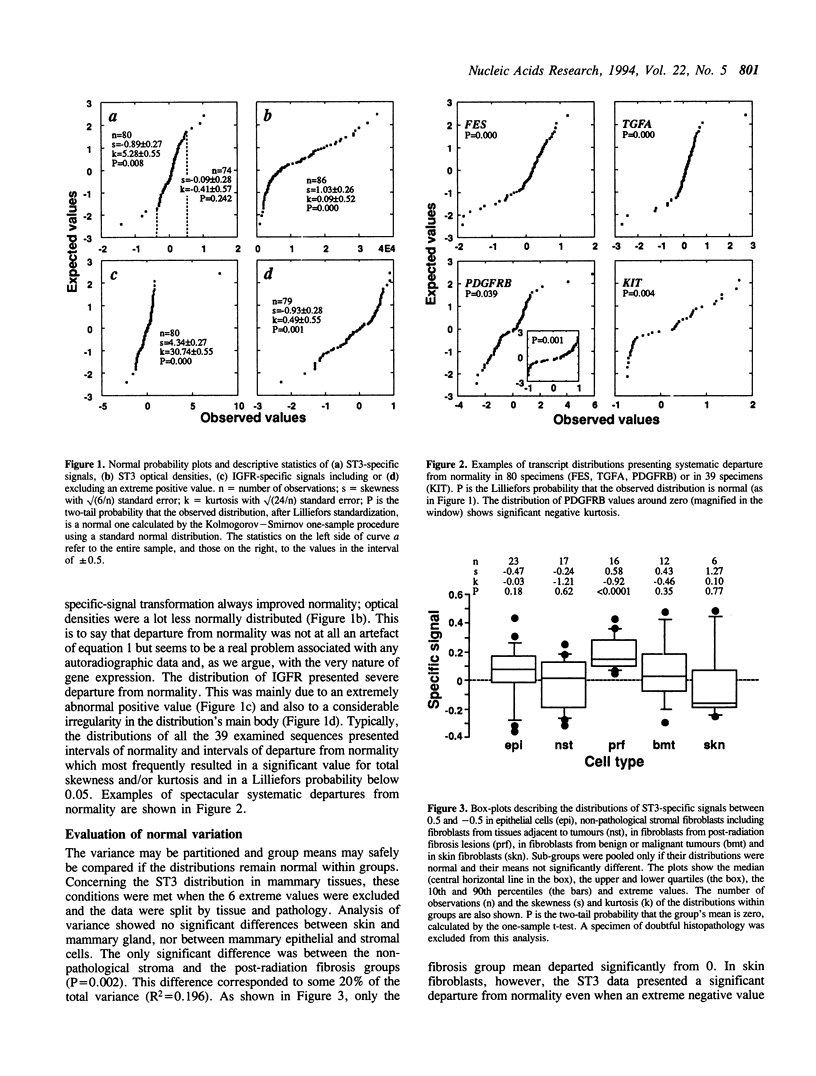
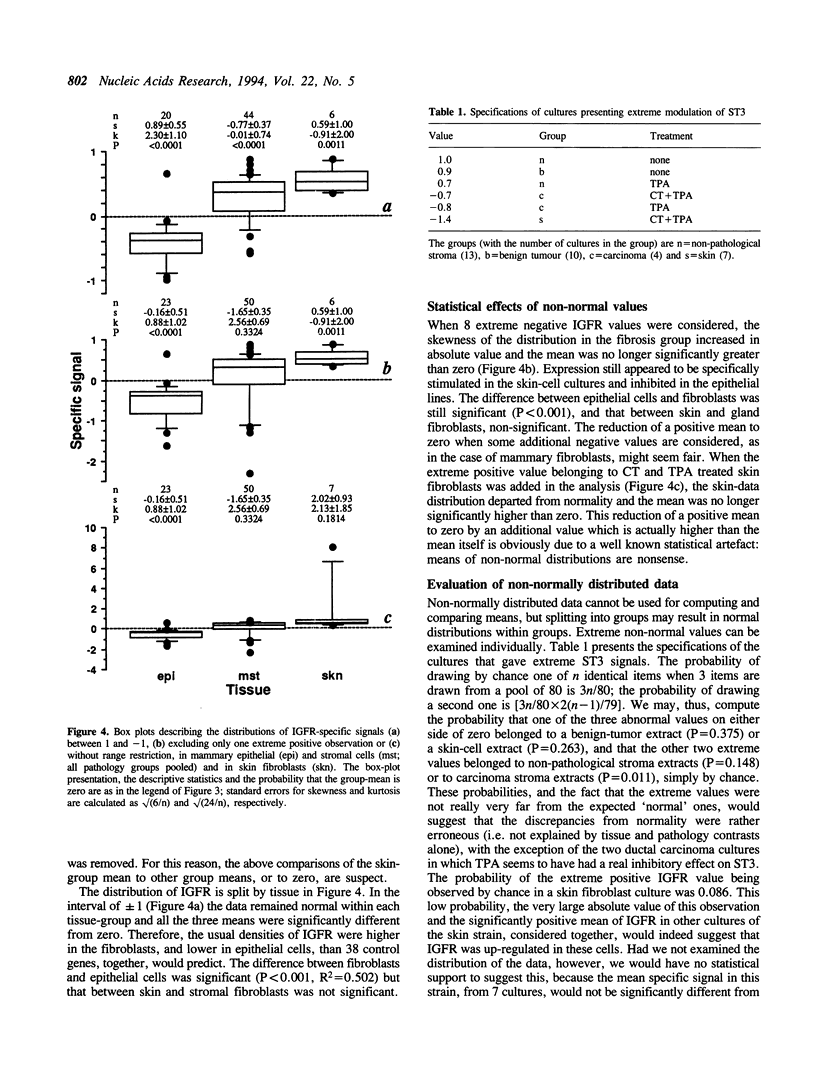
We investigate the behaviour of the gene-expression rate as a statistical variable using autoradiographic data for 39 transcripts from a heterogeneous set of 80 breast-tissue cultures. Despite standardization, the data distributions of all transcripts showed intervals of normality and intervals of systematic departure from normality which most frequently resulted in a significant skewness and/or kurtosis. Non-normal shapes are attributed to modulation of gene expression. This statistical particularity creates difficulties in the evaluation of differences among specimens. Using classical parametric and non-parametric procedures for normal and non-normal variation, respectively, we demonstrate that large differences in optical density are neither necessary nor sufficient for associating expression rates with biological factors. The transcripts coding for the metalloprotease stromelysin-3 (ST3) and for the receptor to insulin-like growth factors (IGFR) are used as examples and their variation is presented in detail. ST3 expression appeared to be specifically associated with mammary stroma fibroblasts derived from post-radiation fibrosis lesions. IGFR was expressed at higher rates in mammary gland and skin fibroblasts than in mammary epithelial cells and was subject to frequent and strong modulation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arkins S., Rebeiz N., Biragyn A., Reese D. L., Kelley K. W. Murine macrophages express abundant insulin-like growth factor-I class I Ea and Eb transcripts. Endocrinology. 1993 Nov;133(5):2334–2343. doi: 10.1210/endo.133.5.8404686. [DOI] [PubMed] [Google Scholar]
- Basset P., Bellocq J. P., Wolf C., Stoll I., Hutin P., Limacher J. M., Podhajcer O. L., Chenard M. P., Rio M. C., Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990 Dec 20;348(6303):699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- Camps J. L., Chang S. M., Hsu T. C., Freeman M. R., Hong S. J., Zhau H. E., von Eschenbach A. C., Chung L. W. Fibroblast-mediated acceleration of human epithelial tumor growth in vivo. Proc Natl Acad Sci U S A. 1990 Jan;87(1):75–79. doi: 10.1073/pnas.87.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F. H., Bourne H. R. Cholera toxin induces cAMP-independent degradation of Gs. J Biol Chem. 1989 Apr 5;264(10):5352–5357. [PubMed] [Google Scholar]
- Chomette G., Auriol M., Tranbaloc P., Blondon J. Stromal changes in early invasive breast carcinoma. An immunohistochemical, histoenzymological and ultrastructural study. Pathol Res Pract. 1990 Feb;186(1):70–79. doi: 10.1016/s0344-0338(11)81012-5. [DOI] [PubMed] [Google Scholar]
- Cullen K. J., Smith H. S., Hill S., Rosen N., Lippman M. E. Growth factor messenger RNA expression by human breast fibroblasts from benign and malignant lesions. Cancer Res. 1991 Sep 15;51(18):4978–4985. [PubMed] [Google Scholar]
- Dupriez V. J., Darville M. I., Antoine I. V., Gegonne A., Ghysdael J., Rousseau G. G. Characterization of a hepatoma mRNA transcribed from a third promoter of a 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-encoding gene and controlled by ets oncogene-related products. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8224–8228. doi: 10.1073/pnas.90.17.8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J. E., Schor A. M., Howell A., Ferguson M. W. Tenascin distribution in the normal human breast is altered during the menstrual cycle and in carcinoma. Differentiation. 1990 Feb;42(3):199–207. doi: 10.1111/j.1432-0436.1990.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Glatt C. E., Snyder S. H. Cloning and expression of an adenylyl cyclase localized to the corpus striatum. Nature. 1993 Feb 11;361(6412):536–538. doi: 10.1038/361536a0. [DOI] [PubMed] [Google Scholar]
- Luo Y., Raible D., Raper J. A. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993 Oct 22;75(2):217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Pieroni J. P., Miller D., Premont R. T., Iyengar R. Type 5 adenylyl cyclase distribution. Nature. 1993 Jun 24;363(6431):679–680. doi: 10.1038/363679a0. [DOI] [PubMed] [Google Scholar]
- Spanakis E. Problems related to the interpretation of autoradiographic data on gene expression using common constitutive transcripts as controls. Nucleic Acids Res. 1993 Aug 11;21(16):3809–3819. doi: 10.1093/nar/21.16.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hooff A. Stromal involvement in malignant growth. Adv Cancer Res. 1988;50:159–196. doi: 10.1016/s0065-230x(08)60437-6. [DOI] [PubMed] [Google Scholar]



