Abstract
Background
BCL-2 (B-cell leukemia/lymphoma 2) gene has been demonstrated to be associated with breast cancer development and a single nucleotide polymorphism (SNP; -938C > A) has been identified recently. To investigate whether this polymorphism functions as a modifier of breast cancer development, we analyzed the distribution of genotype frequency, as well as the association of genotype with clinicopathological characteristics. Furthermore, we also studied the effects of this SNP on Bcl-2 expression in vitro.
Methods
We genotyped the BCL-2 (-938C > A) in 114 patients and 107 controls, and analyzed the estrogen receptor (ER), progestogen receptor (PR), C-erbB2 and Ki67 status with immunohistochemistry (IHC). Different Bcl-2 protein levels in breast cancer cell lines were determined using western blot. Logistic regression model was applied in statistical analysis.
Results
We found that homozygous AA genotype was associated with an increased risk (AA vs AC+CC) by 2.37-fold for breast cancer development and significant association was observed between nodal status and different genotypes of BCL-2 (-938C > A) (p = 0.014). AA genotype was more likely to develop into lobular breast cancer (p = 0.036). The result of western blot analysis indicated that allele A was associated with the lower level of Bcl-2 expression in breast cancer cell lines.
Conclusions
AA genotype of BCL-2 (-938C > A) is associated with susceptibility of breast cancer, and this genotype is only associated with the nodal status and pathological diagnosis of breast cancer. The polymorphism has an effect on Bcl-2 expression but needs further investigation.
Background
Breast cancer has become the most common female malignancy around the world. Each year, there're over one million women diagnosed with breast cancer, with approximately 400,000 deaths [1]. Like other carcinomas, breast cancer occurs based on an interaction between genetic heterogeneity and the environment. It has been reported that an accumulation of genetic variants is involved in the process of breast carcinogenesis[2]. Among these genetic variants, many of them play roles in apoptosis or cellular proliferation, since the balance between the two decides which direction to go: normal mammary development or carcinogenesis of the mammary gland [3].
Apoptosis is a highly programmed cell death, and it can be achieved by two major pathways: death-receptor pathway and mitochondrial pathway[4]. Bcl-2 family, as the most important regulator in the mitochondrial pathway, contains both anti-apoptotic proteins such as Bcl-2 and Bcl-xL and pro-apoptotic proteins such as Bax, Bad and Bak [5]. Although Bcl-2 is an oncogenic protein, the association between its expression and patient survival result is quite conflicting and seems tissue-specific. Increased Bcl-2 expression is associated with poor survival in B-cell chronic lymphocytic leukemia (CLL), prostate cancer and urinary tract transitional cell cancer [6-9]; while its high expression is associated to favorable outcome in colorectal cancer, breast cancer, non-small-cell lung cancer, renal cancer and head and neck cancer [10-15].
BCL-2 (B-cell leukemia/lymphoma 2) gene, located at 18q21.3 [16], consists of three exons and two promoters (P1 and P2), which have different functions. The second promoter, P2, is located 1,400 bp upstream of the translation initiation site and functions as a negative regulatory element to the P1 promoter [17,18]. Park et al. investigated the genetic variants of BCL-2 genes by sequencing the 24 Korean DNA samples and identified a novel single nucleotide polymorphism (SNP; -938C > A) in P2[19]. According to the findings from Nuckel et al., the -938C allele is associated with significantly increased P2 activity and binding of nuclear proteins compared with the A allele. Due to the negatively regulatory function of P2, Bcl-2 protein expression was significantly decreased in B cells derived from CLL patients carrying the -938CC genotype [20]. However, Majid et al. reported no association of Bcl-2 protein expression level with the promoter SNP or any clinical or laboratory parameters [21]. On the other hand, it has been suggested that the (-938C > A) polymorphism could serve as a survival prognosticator as well as high-risk indicator within the lymph node-negative breast cancer [22]. In order to investigate whether BCL-2 (-938C > A) genotype can serve as a susceptible and/or progressive factor in breast cancer, we analyzed the distribution of genotype frequency among breast cancer cases and controls, as well as the association of genotype with clinicopathological characteristics. In addition, we also chose 4 breast cancer cell lines to investigate the association between this polymorphism and Bcl-2 expression in vitro.
Methods
Patients and Samples
The study involved 114 patients diagnosed with breast cancer in Qilu Hospital (Shandong, China) between September 2008 and April 2010. All the malignant cases were classified and assessed according to the WHO classification of tumor of the breast. Among all the patients, 7 had lobular carcinoma, 87 had tubular carcinoma and 20 suffered from other malignant types. The size of the primary tumor was defined as the largest tumor diameter (cm) reported by pathologists after surgical excision. To investigate whether BCL-2 -938 SNP is a susceptible biomarker, 107 healthy women were involved as control group in this study. The average age of cases and controls are 49.1 ± 5.51 and 47.8 ± 11.4 years separately, and the Student's t test showed no significant difference between the two groups (p = 0.374). For both patient and control group, 1.5 ml whole blood sample was extracted from each participant and stored at -80°C. Written informed consent was signed by each subject and the study design was approved by the Ethical Committee of Shandong University.
Cell culture
Breast cancer cell lines (MCF-7, MDA-MB-453, MDA-MB-468 and T-47 D cell lines) were all obtained from American Type Culture Collection (ATCC, Manassas, VA, USA), and routinely cultured in appropriate medium supplemented with 10% FBS (Haoyang, Tianjin, China), 100 U/ml penicillin, and 100 μg/ml streptomycin, under the condition of 5% CO2 at 37°C.
DNA extraction
DNA from both whole blood cells and cultured breast cancer cells was extracted with TIANamp Genomic DNA Kit (Tiangen, Beijing, China), following the manufacturer's instructions. DNA concentration and purity of each sample were measured by ultraviolet spectrophotometer (GE Healthcare, Piscataway, NJ, USA). DNA samples were routinely stored at -20°C.
PCR-restriction fragment length polymorphism analysis of BCL2 -938 polymorphism
Genotyping of the SNP -938C > A polymorphism was determined by PCR-restriction fragment length polymorphism (PCR-RFLP) method. Primers were designed according to the sequence of rs2279115 as follows: forward primer 5'-TTATCCAGCAGCTTTTCGG-3' and reverse primer 5'-GGCGGCAGATGAATTACAA-3'. In each 25 μl reaction, 1 μl genomic DNA (100 ng/μl) was amplified by 1.25 U EasyTaq DNA polymerase (Transgen, Beijing, China) with 2 μl of 2.5 mM dNTPs and 0.5 μl of each primer. The PCR conditions were set as follows: 94°C for 5 min, 35 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s and a final extension step of 72°C for 10 min.
After PCR reaction, 10 μl product from each sample was digested by BccI Enzyme (NEB, Beijing, China) for 2 hours. After electrophoresis on 3% ethidium bromide added agarose gel, photographs were taken under ultraviolet light transilluminator. To confirm our results of genotyping, several PCR products were randomly picked for sequencing.
Immunohistochemistry (IHC) and evaluation of IHC staining
To evaluate the association between BCL-2 SNP-938C > A and clinicopathological parameters (estrogen receptor (ER), progestogen receptor (PR), C-erbB2 and Ki67), tissue microarrays were made and stained as described previously[23]. An already known positive case was included as the positive control, while the primary antibody was replaced with non-immune mouse serum as the negative control. Evaluation of the IHC staining results was proceeded by pathologists without knowing the Bcl-2 -938C > A genotypes. The tumor was scored positive for ER and PR when ≥10% of the tumor cells on the slide irrespective of the staining intensity. C-erbB2 was evaluated according to the DAKO score; complete membranous staining observed in ≥10% of tumor cells (DAKO score 2+ and 3+) was defined as C-erbB2 positive. More than 30% of tumor cells with the positive stained nuclear indicated a highly proliferative activity of Ki67.
Western-blot analysis of Bcl-2 expression in breast cancer cell lines
Breast cancer cell lines were routinely cultured and the total protein of each cell lines was collected with RIPA lysis buffer (Sigma, St Louis, MO, USA) in the presence of protease inhibitors. Briefly, 100 μg of protein were loaded onto 12% SDS-PAGE gels and transferred to a PVDF membrane with a semi-dry blotting apparatus (Bio-Rad, Hercules, CA, USA). After blocking with 5% non-fat milk, the membrane was incubated overnight at 4°C with primary anti-Bcl-2 (1:250; Carpinteria, CA, USA), followed by binding with the secondary antibody. β-actin was used as endogenous control. The expression of each protein was quantified as the densitometry value analyzed by ImageJ. The figure shown is representative of three independent experiments.
Statistical Analysis
The genotype and allele frequency of BCL-2 SNP938 were tested using the public statistical web-tool http://www.oege.org/software/hwe-mr-calc.shtml for Hardy-Weinberg equilibrium (HWE). P-value > 0.05 was considered not deviate from the equilibrium. Logistic regression model was carried out to analyze the distribution of BCL-2 SNP938 polymorphism between case and control group and the clinicopathological characteristics of breast cancer. All statistical tests were considered significant with a level of p ≤ 0.05. Odds ratios (OR) adjusted for age were calculated. All statistical analyses in our study were carried out with SPSS Statistics 17.0 (SPSS Inc. Chicago, Illinois, USA).
Results
The BCL-2 (-938C > A) genotype distribution
The breast cancer patients and healthy controls were all Chinese mainland women and we didn't find statistically significant difference between the two groups in the matching characteristic. Chi-square test was used to determine whether the subjects met the Hardy-Weinberg equilibrium. We confirmed that both case and control groups were compatible with the HWE, for in case and control groups the χ2 value was 0.11 and 1.91 respectively, both p > 0.05.
As shown in Table 1, in the logistic regression model, BCL-2 SNP938 variant genotype CC was associated with a decreased risk for breast cancer by 0.40-fold compared with the homozygote AA. Additionally, genotype AA was associated with an increased risk for breast cancer by 2.37-fold compared with the combined genotype AC+CC. Several samples from cases were randomly chosen to show the result of genotyping (Figure 1), and the genotype distribution of BCL-2 SNP938 polymorphism between case and control group was shown in Table 1. Our data indicated that the homozygous AA genotype may be a susceptible genotype for breast cancer development and may increase the risk of breast cancer among Chinese women.
Table 1.
The BCL-2 (-938C > A) genotype distribution in patients and controls
| Genotype | No. of subjects (%) | Non-adjusted b | Adjusted c | |||
|---|---|---|---|---|---|---|
| Case a | Control a | p value | OR | p value | OR | |
| AA | 19(16.7) | 8(7.5) | 1.00 | 1.00 | ||
| AC | 53(46.5) | 53(49.5) | 0.062 | 0.42 | 0.081 | 0.44 |
| CC | 42(36.8) | 46(43.0) | 0.043 | 0.38 | 0.054 | 0.40 |
| AC+CC | 95(83.3) | 99(92.5) | 1.00 | 1.00 | ||
| AA | 19(16.7) | 8(7.5) | 0.042 | 2.47 | 0.055 | 2.37 |
| AC+AA | 72(63.2) | 61(57.0) | 1.00 | 1.00 | ||
| CC | 42(36.8) | 46(43.0) | 0.351 | 0.77 | 0.358 | 0.77 |
| AA+CC | 61(53.5) | 54(50.5) | 1.00 | 1.00 | ||
| AC | 53(46.5) | 53(49.5) | 0.651 | 0.89 | 0.711 | 0.90 |
| All | 114 | 107 | ||||
a The χ2 for HWE of case and control group is 0.11 and 1.91 respectively (both p > 0.05).
b Logistic regression model, non-adjusted.
c Logistic regression model, adjusted for diagnostic age.
All statistical tests were two-sided with a significance level of p ≤ 0.05.
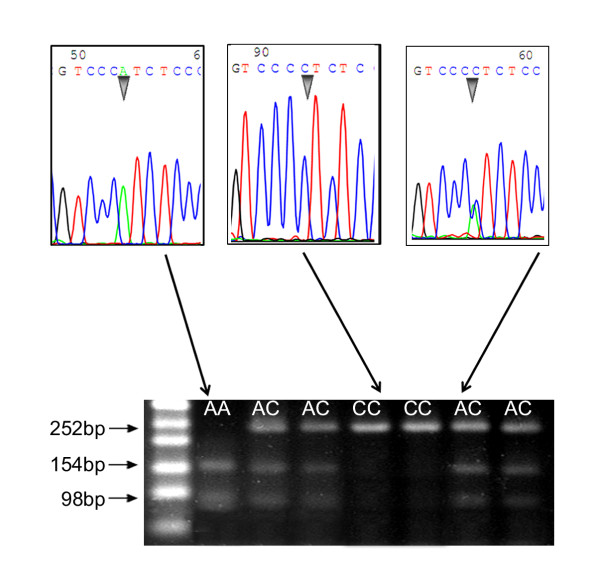
Figure 1.
Bcl-2 (-938C > A) genotyping results. BCL-2 (-938C > A) polymorphism genotyping with PCR-RFLP and DNA sequencing. Several samples from cancer group were chosen randomly to show the PCR-RFLP results, and DNA sequencing was used to confirm our result of genotyping. Undigested 252 bp products represent CC genotype, and totally digested 154 and 98 bp products represent AA genotype; AC heterozygous genotype showed both the undigested 252 bp band and digested 154 and 98 bp bands.
Association between BCL-2 (-938C > A) polymorphism and known clinicopathological variables
Table 2 listed the association of AA and AC+CC genotypes with clinicopathological characteristics, including age at diagnosis, pathological diagnosis, tumor size and grade, nodal metastasis and status of several biological markers. The AA genotype and the occurrence of lobular breast cancer showed a strong association (p = 0.036). Furthermore, the association of different genotypes with the lymph nodal status was significant (p = 0.014). However, we found no association of the polymorphism with age at diagnosis, tumor size and grade. Regarding to the biological markers, which can predict disease prognosis and treatment outcomes, the genotypes were similarly distributed irrespective of the status of ER, PR, erbB2 and Ki67, neither do the receptor status or triple-negative breast cancer. Thus, the BCL-2 (-938C > A) polymorphism may be an indicator of lymph nodal metastasis in female breast cancer.
Table 2.
Relationship between BCL-2 (-938C > A) polymorphism and known clinicopathological variables
| Clinicopathological Variables | All | Genotype (%) | Adjusted a | ||
|---|---|---|---|---|---|
| AA | AC+CC | p value | OR | ||
| Age | |||||
| ≤ 40 | 21(18.9) | 3(14.3) | 18(85.7) | 1.00 | |
| > 40 | 90(81.1) | 15(16.7) | 75(83.3) | 0.790 | 1.20 |
| Pathological diagnosis | |||||
| Ductal | 87(76.3) | 14(16.1) | 73(83.9) | 1.00 | |
| Lobular | 7(6.1) | 3(42.9) | 4(57.1) | 0.036 | 6.42 |
| Others | 20(17.6) | 2(10.0) | 18(90.0) | 0.605 | 0.66 |
| Tumor size(cm) | |||||
| ≤ 2 | 50(53.2) | 9(18.0) | 41(82.0) | 1.00 | |
| > 2 | 44(46.8) | 9(20.4) | 35(79.6) | 0.704 | 1.23 |
| Grade | |||||
| I, II | 59(79.7) | 10(16.9) | 49(83.1) | 1.00 | |
| III | 15(20.3) | 2(13.3) | 13(86.7) | 0.725 | 0.74 |
| Positive lymph node | |||||
| 0 | 34(44.7) | 3(8.8) | 31(91.2) | 1.00 | |
| 1-3 | 25(32.9) | 3(12.0) | 22(88.0) | 0.672 | 1.44 |
| ≥ 4 | 17(22.4) | 7(41.2) | 10(58.8) | 0.014 | 6.95 |
| ER | |||||
| Negative | 28(26.9) | 6(21.4) | 22(78.6) | 1.00 | |
| Positive | 76(73.1) | 12(15.8) | 64(84.2) | 0.493 | 0.68 |
| PR | |||||
| Negative | 37(35.9) | 8(21.6) | 29(78.4) | 1.00 | |
| Positive | 66(64.1) | 10(15.2) | 56(84.8) | 0.688 | 0.80 |
| Receptor status | |||||
| Negative | 26(25.2) | 6(23.1) | 20(76.9) | 1.00 | |
| Positive | 77(74.8) | 12(15.6) | 65(84.4) | 0.115 | 3.06 |
| c-erbB2 | |||||
| Negative | 73(70.2) | 13(17.8) | 60(82.2) | 1.00 | |
| Positive | 31(29.8) | 5(16.1) | 26(83.9) | 0.936 | 1.05 |
| Triple-negative | |||||
| No | 87(84.5) | 14(16.1) | 73(83.9) | 1.00 | |
| Yes | 16(15.5) | 4(25.0) | 12(75.0) | 0.444 | 1.67 |
| Ki67 | |||||
| Negative | 59(58.4) | 10(16.9) | 49(83.1) | 0.875 | 1.00 |
| Positive | 42(41.6) | 7(16.7) | 35(83.3) | 1.09 | |
aLogistic regression model adjusted for diagnostic age.
All statistical tests were two-sided with a significance level of p ≤ 0.05.
Association between BCL-2 (-938C > A) polymorphism and Bcl-2 expression in vitro
We further investigated the possible linkage of BCL-2 (-938C > A) polymorphism with Bcl-2 expression level in vitro; we tested the genotypes of the polymorphism and Bcl-2 expression in 4 breast cancer cell lines. Figure 2 showed the result of genotyping and western blot of the 4 breast cancer cell lines (MCF-7, MDA-MB-453, MDA-MB-468 and T-47D). From the result, we can identify an association that CC genotype correlated with high Bcl-2 expression and AA genotype displayed an intermediate level, while AC genotype correlated with the lowest expression. The ratio of Bcl-2/actin was almost 5-fold higher comparing CC genotype (1.00 ± 0.11) with AC genotype (0.18 ± 0.10; p = 0.0006; Student's t test), whereas no significant effect was found when comparing AA genotype (0.24 ± 0.05) to AC genotype (0.18 ± 0.10; p = 0.372; Student's t test). Overall, BCL-2 (-938C > A) polymorphism showed a contribution to Bcl-2 expression, namely allele A was associated with lower level of Bcl-2 expression and vice versa.
Figure 2.
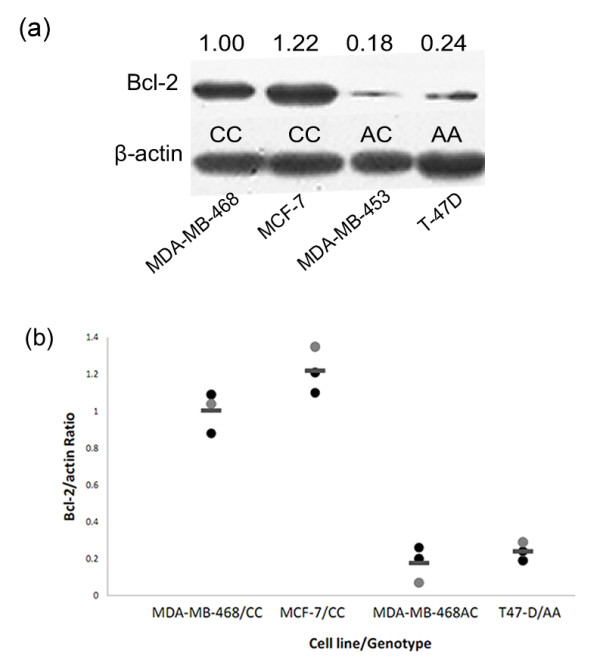
Genotype-dependent expression of Bcl-2 protein in breast cancer cell lines. (a) Effect of BCL-2 (-938C > A) polymorphism on Bcl-2 expression level in breast cancer cell lines. β-actin was used as an internal control and the expression of Bcl-2 protein was quantified as densitometry value analyzed by ImageJ. The figure shown is representative of three independent experiments. (b) Quantitative analysis of protein expression shown in the figure. Densitometry was performed using ImageJ statistical analysis. Statistical analysis was performed using the Student's t test. Horizontal bars represent the mean value of the three values of each cell line/genotype.
Discussion
Anti-apoptotic BCL-2 gene plays an important role in regulation of apoptosis and triggering cell cycle arrest. Due to its role in CLL development, many studies which focused on its function and variants were carried out in leukemia previously. The BCL-2 (-938C > A) polymorphism, which was first identified by Park and colleagues[19], has attracted a lot of attention recently; the result of these studies[20,21,24-26] in CLL patients appeared in great controversial and no agreement has been reached. However, studies carried out in solid tumors showed a more consistent result [14,15,22,27,28], indicating BCL-2 (-938C > A) polymorphism may function in a tissue specific way. Since Bcl-2 protein has been reported to contribute much to breast malignancy development (or progression), we conducted this study to investigate whether this polymorphism functioned as a modifier of breast cancer development.
In our study, we found that homozygous AA genotype may be a susceptible genotype for breast cancer development and this genotype is associated with the pathological diagnosis and nodal status of breast cancer. In addition, we investigated the effect of this polymorphism on Bcl-2 expression in vitro and found that allele A was associated with the lower level of Bcl-2 expression.
As indicated in previous literatures, this polymorphism was located in the second promoter of the BCL-2 gene, which worked as a negative modulator on the first promoter and subsequently, BCL-2 gene expression [18]. According to the study of Nuckel et al., the presence of AA genotype was associated with decreased activity of P2 and increased Bcl-2 protein expression and was demonstrated as an unfavorable genetic marker in patients with B-CLL [20]. In contrast, we showed that allele A was associated with low Bcl-2 expression in breast cancer cell lines and AA genotype is in positive relation to breast cancer susceptibility. This result can partly explain the reason why high Bcl-2 expression is associated with better prognosis of breast cancer [12,29,30]. The different results in CLL and breast cancer may be determined by the balance between the dual function of Bcl-2 protein[31]. On one hand it plays an important role as an oncogene to inhibit apoptosis; on the other hand, it can initiate cell cycle arrest at G0 stage [32] and cause growth inhibitory effects similar to those of p53 [33]. Nevertheless, a recent study by Bachmann et al. reported no significant association was observed between Bcl-2 expression status and BCL-2 (-938C > A) polymorphism (p = 0.485) [22]. Therefore, the association of BCL-2 (-938C > A) polymorphism with Bcl-2 expression in primary tumor should be studied in a larger sample size and the involved molecular mechanisms need further investigation. Additionally, it was reported that Bcl-2 high expression contributed to a series of cytotoxic drugs resistance [34]. Thus, we can also investigate whether the BCL-2 (-938C > A) polymorphism has an effect on chemotherapy resistance in the future work.
Conclusions
In summary, we can conclude that AA genotype of BCL-2 (-938C > A) may be one susceptible genotype for breast cancer, and this genotype is associated with the nodal status of breast cancer. Since Bcl-2 protein has been demonstrated to be correlated with treatment outcomes and prognosis of breast cancer, it is of great significance to find the function pattern of this polymorphism and the possible association with Bcl-2 expression. Further research is necessary to elucidate whether BCL-2 (-938C > A) polymorphism can serve as a prognostic biomarker for breast cancer patients.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
NZ, XL, KT did the genotype analysis and IHC staining. LJ, TM, SY, CY collected all the clinical samples. FL did the statistical analyses. MM, BH, QY designed the study. All authors read and approved the final manuscript
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Ning Zhang, Email: zhangning0816@gmail.com.
Xiaoyan Li, Email: xiaoyanli1219@gmail.com.
Kai Tao, Email: daiyaokai@msn.com.
Liyu Jiang, Email: breastonco@hotmail.com.
Tingting Ma, Email: ttm81@163.com.
Shi Yan, Email: gggg2182@163.com.
Cunzhong Yuan, Email: yuancunzhong@126.com.
Meena S Moran, Email: meena.moran@yale.edu.
Faming Liang, Email: fliang@stat.tamu.edu.
Bruce G Haffty, Email: hafftybg@umdnj.edu.
Qifeng Yang, Email: qifengy@gmail.com.
Acknowledgements
This project is partly supported by National Natural Science Foundation of China (No. 81072150), Program for New Century Excellent Talents in University 2007, Key Project of Chinese Ministry of Education (No. 108080), and Independent Innovation Foundation of Shandong University (IIFSDU, No.2009JQ007) to Prof. Yang Q.. We appreciate Ning Yang for collecting data for this study and thank Jiang Zhu, Jiezhi Zhu, Jie Li, Haifeng Qiu, and Xiaoli Kong, Ying Zhao for technical support and critical discussions.
References
- Igene H. Global health inequalities and breast cancer: an impending public health problem for developing countries. Breast J. 2008;14(5):428–434. doi: 10.1111/j.1524-4741.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- Mavaddat N, Antoniou AC, Easton DF, Garcia-Closas M. Genetic susceptibility to breast cancer. Mol Oncol. pp. 174–191. [DOI] [PMC free article] [PubMed]
- Kumar R, Vadlamudi RK, Adam L. Apoptosis in mammary gland and cancer. Endocr Relat Cancer. 2000;7(4):257–269. doi: 10.1677/erc.0.0070257. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Brown R. The bcl-2 family of proteins. Br Med Bull. 1997;53(3):466–477. doi: 10.1093/oxfordjournals.bmb.a011624. [DOI] [PubMed] [Google Scholar]
- Faderl S, Keating MJ, Do KA, Liang SY, Kantarjian HM, O'Brien S, Garcia-Manero G, Manshouri T, Albitar M. Expression profile of 11 proteins and their prognostic significance in patients with chronic lymphocytic leukemia (CLL) Leukemia. 2002;16(6):1045–1052. doi: 10.1038/sj.leu.2402540. [DOI] [PubMed] [Google Scholar]
- Keshgegian AA, Johnston E, Cnaan A. Bcl-2 oncoprotein positivity and high MIB-1 (Ki-67) proliferative rate are independent predictive markers for recurrence in prostate carcinoma. Am J Clin Pathol. 1998;110(4):443–449. doi: 10.1093/ajcp/110.4.443. [DOI] [PubMed] [Google Scholar]
- Stackhouse GB, Sesterhenn IA, Bauer JJ, Mostofi FK, Connelly RR, Srivastava SK, Moul JW. p53 and bcl-2 immunohistochemistry in pretreatment prostate needle biopsies to predict recurrence of prostate cancer after radical prostatectomy. J Urol. 1999;162(6):2040–2045. doi: 10.1016/S0022-5347(05)68095-0. [DOI] [PubMed] [Google Scholar]
- Bilim V, Tomita Y, Kawasaki T, Katagiri A, Imai T, Takeda M, Takahashi K. Prognostic value of Bcl-2 and p53 expression in urinary tract transitional cell cancer. J Natl Cancer Inst. 1996;88(10):686–688. doi: 10.1093/jnci/88.10.686-a. [DOI] [PubMed] [Google Scholar]
- Ofner D, Riehemann K, Maier H, Riedmann B, Nehoda H, Totsch M, Bocker W, Jasani B, Schmid KW. Immunohistochemically detectable bcl-2 expression in colorectal carcinoma: correlation with tumour stage and patient survival. Br J Cancer. 1995;72(4):981–985. doi: 10.1038/bjc.1995.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestrini R, Benini E, Veneroni S, Daidone MG, Tomasic G, Squicciarini P, Salvadori B. p53 and bcl-2 expression correlates with clinical outcome in a series of node-positive breast cancer patients. J Clin Oncol. 1996;14(5):1604–1610. doi: 10.1200/JCO.1996.14.5.1604. [DOI] [PubMed] [Google Scholar]
- Zhang GJ, Kimijima I, Tsuchiya A, Abe R. The role of bcl-2 expression in breast carcinomas (Review) Oncol Rep. 1998;5(5):1211–1216. doi: 10.3892/or.5.5.1211. [DOI] [PubMed] [Google Scholar]
- Fontanini G, Vignati S, Bigini D, Mussi A, Lucchi M, Angeletti CA, Basolo F, Bevilacqua G. Bcl-2 protein: a prognostic factor inversely correlated to p53 in non-small-cell lung cancer. Br J Cancer. 1995;71(5):1003–1007. doi: 10.1038/bjc.1995.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Hinoda Y, Nakajima K, Kikuno N, Suehiro Y, Tabatabai ZL, Ishii N, Dahiya R. The bcl2-938CC Genotype Has Poor Prognosis and Lower Survival in Renal Cancer. Journal of Urology. 2009;182(2):721–727. doi: 10.1016/j.juro.2009.03.081. [DOI] [PubMed] [Google Scholar]
- Lehnerdt GF, Franz P, Bankfalvi A, Grehl S, Kelava A, Nuckel H, Lang S, Schmid KW, Siffert W, Bachmann HS. The regulatory BCL2 promoter polymorphism (-938C > A) is associated with relapse and survival of patients with oropharyngeal squamous cell carcinoma. Ann Oncol. 2009;20(6):1094–1099. doi: 10.1093/annonc/mdn763. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Gorham J, Cossman J, Jaffe E, Croce CM. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985;229(4720):1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- Seto M, Jaeger U, Hockett RD, Graninger W, Bennett S, Goldman P, Korsmeyer SJ. Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-Ig fusion gene in lymphoma. EMBO J. 1988;7(1):123–131. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RL, Korsmeyer SJ. A negative regulatory element in the bcl-2 5'-untranslated region inhibits expression from an upstream promoter. Mol Cell Biol. 1993;13(6):3686–3697. doi: 10.1128/mcb.13.6.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BL, Kim LH, Cheong HS, Cho HY, Kim EM, Shin HD, Kim YS, Lee C. Identification of variants in cyclin D1 ( CCND1) and B-Cell CLL/lymphoma 2 ( BCL2) J Hum Genet. 2004;49(8):449–454. doi: 10.1007/s10038-004-0173-0. [DOI] [PubMed] [Google Scholar]
- Nuckel H, Frey UH, Bau M, Sellmann L, Stanelle J, Durig J, Jockel KH, Duhrsen U, Siffert W. Association of a novel regulatory polymorphism (-938C > A) in the BCL2 gene promoter with disease progression and survival in chronic lymphocytic leukemia. Blood. 2007;109(1):290–297. doi: 10.1182/blood-2006-03-007567. [DOI] [PubMed] [Google Scholar]
- Majid A, Tsoulakis O, Walewska R, Gesk S, Siebert R, Kennedy DB, Dyer MJ. BCL2 expression in chronic lymphocytic leukemia: lack of association with the BCL2 938A > C promoter single nucleotide polymorphism. Blood. 2008;111(2):874–877. doi: 10.1182/blood-2007-07-098681. [DOI] [PubMed] [Google Scholar]
- Bachmann HS, Otterbach F, Callies R, Nuckel H, Bau M, Schmid KW, Siffert W, Kimmig R. The AA genotype of the regulatory BCL2 promoter polymorphism ( 938C > A) is associated with a favorable outcome in lymph node negative invasive breast cancer patients. Clin Cancer Res. 2007;13(19):5790–5797. doi: 10.1158/1078-0432.CCR-06-2673. [DOI] [PubMed] [Google Scholar]
- Yang Q, Moran MS, Haffty BG. Bcl-2 expression predicts local relapse for early-stage breast cancer receiving conserving surgery and radiotherapy. Breast Cancer Res Treat. 2009;115(2):343–348. doi: 10.1007/s10549-008-0068-4. [DOI] [PubMed] [Google Scholar]
- Kaderi MA, Norberg M, Murray F, Merup M, Sundstrom C, Roos G, Aleskog A, Karlsson K, Axelsson T, Tobin G. et al. The BCL-2 promoter (-938C > A) polymorphism does not predict clinical outcome in chronic lymphocytic leukemia. Leukemia. 2008;22(2):339–343. doi: 10.1038/sj.leu.2405042. [DOI] [PubMed] [Google Scholar]
- Rossi D, Rasi S, Capello D, Gaidano G. Prognostic assessment of BCL2-938C > A polymorphism in chronic lymphocytic leukemia. Blood. 2008;111(1):466–468. doi: 10.1182/blood-2007-08-106823. [DOI] [PubMed] [Google Scholar]
- Zenz T, Benner A, Duhrsen U, Durig J, Dohner H, Siffert W, Stilgenbauer S, Nuckel H. BCL2-938C > A polymorphism and disease progression in chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50(11):1837–1842. doi: 10.3109/10428190903207530. [DOI] [PubMed] [Google Scholar]
- Heubner M, Wimberger P, Otterbach F, Kasimir-Bauer S, Siffert W, Kimmig R, Nuckel H. Association of the AA genotype of the BCL2 (-938C > A) promoter polymorphism with better survival in ovarian cancer. Int J Biol Markers. 2009;24(4):223–229. doi: 10.1177/172460080902400402. [DOI] [PubMed] [Google Scholar]
- Hirata H, Hinoda Y, Kikuno N, Suehiro Y, Shahryari V, Ahmad AE, Tabatabai ZL, Igawa M, Dahiya R. Bcl2 -938C/A polymorphism carries increased risk of biochemical recurrence after radical prostatectomy. J Urol. 2009;181(4):1907–1912. doi: 10.1016/j.juro.2008.11.093. [DOI] [PubMed] [Google Scholar]
- Martinez-Arribas F, Alvarez T, Del Val G, Martin-Garabato E, Nunoz-Villar MJ, Lucas R, Sanchez J, Tejerina A, Schneider J. Bcl-2 expression in breast cancer: A comparative study at the mRNA and protein level. Anticancer Research. 2007;27(1A):219–222. [PubMed] [Google Scholar]
- Nadler Y, Camp RL, Giltnane JM, Moeder C, Rimm DL, Kluger HM, Kluger Y. Expression patterns and prognostic value of Bag-1 and Bcl-2 in breast cancer. Breast Cancer Res. 2008;10(2):R35. doi: 10.1186/bcr1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callagy GM, Webber MJ, Pharoah PD, Caldas C. Meta-analysis confirms BCL2 is an independent prognostic marker in breast cancer. Bmc Cancer. 2008;8:153. doi: 10.1186/1471-2407-8-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkel S, Gross A, Yang E. BCL2 family in DNA damage and cell cycle control. Cell Death Differ. 2006;13(8):1351–1359. doi: 10.1038/sj.cdd.4401987. [DOI] [PubMed] [Google Scholar]
- Pietenpol JA, Papadopoulos N, Markowitz S, Willson JK, Kinzler KW, Vogelstein B. Paradoxical inhibition of solid tumor cell growth by bcl2. Cancer Res. 1994;54(14):3714–3717. [PubMed] [Google Scholar]
- Yang QF, Sakurai T, Yoshimura G, Shan L, Suzuma T, Tamaki T, Umemura T, Kokawa Y, Nakamura Y, Nakamura M. et al. Expression of Bcl-2 but not Bax or p53 correlates with in vitro resistance to a series of anticancer drugs in breast carcinoma. Breast Cancer Res Treat. 2000;61(3):211–216. doi: 10.1023/A:1006474307180. [DOI] [PubMed] [Google Scholar]