Abstract
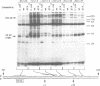
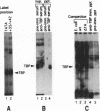
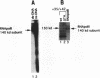
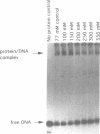
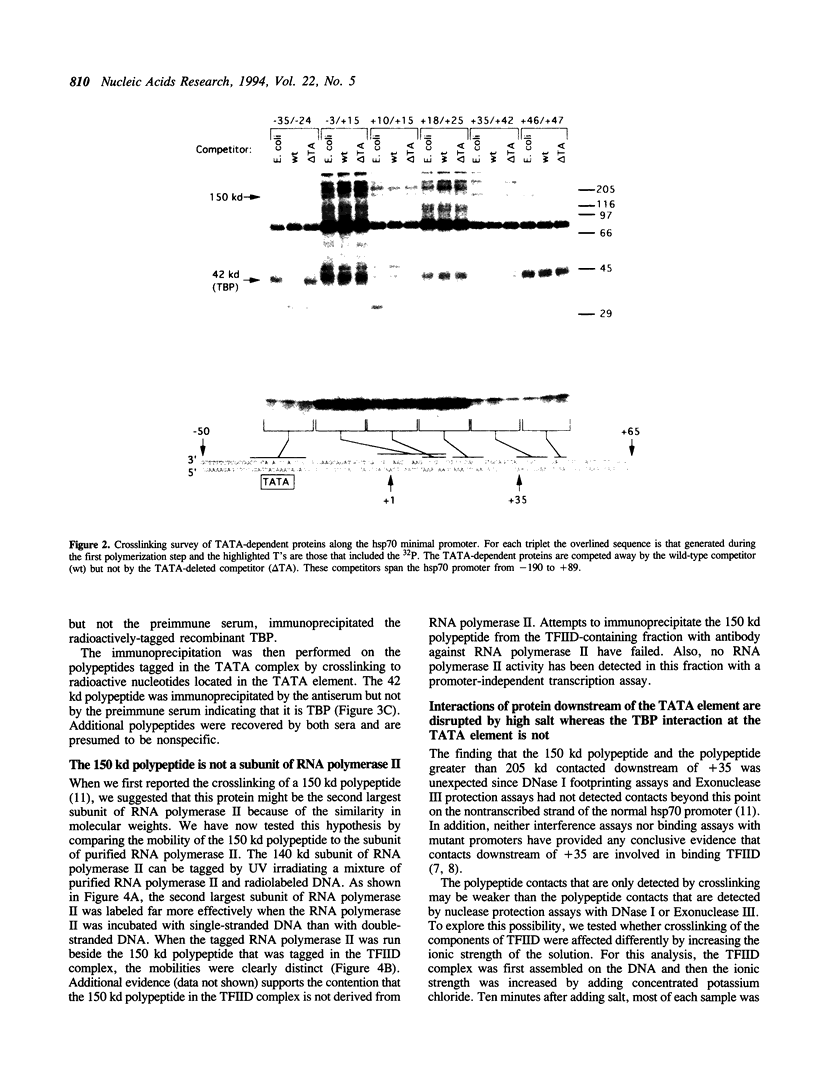
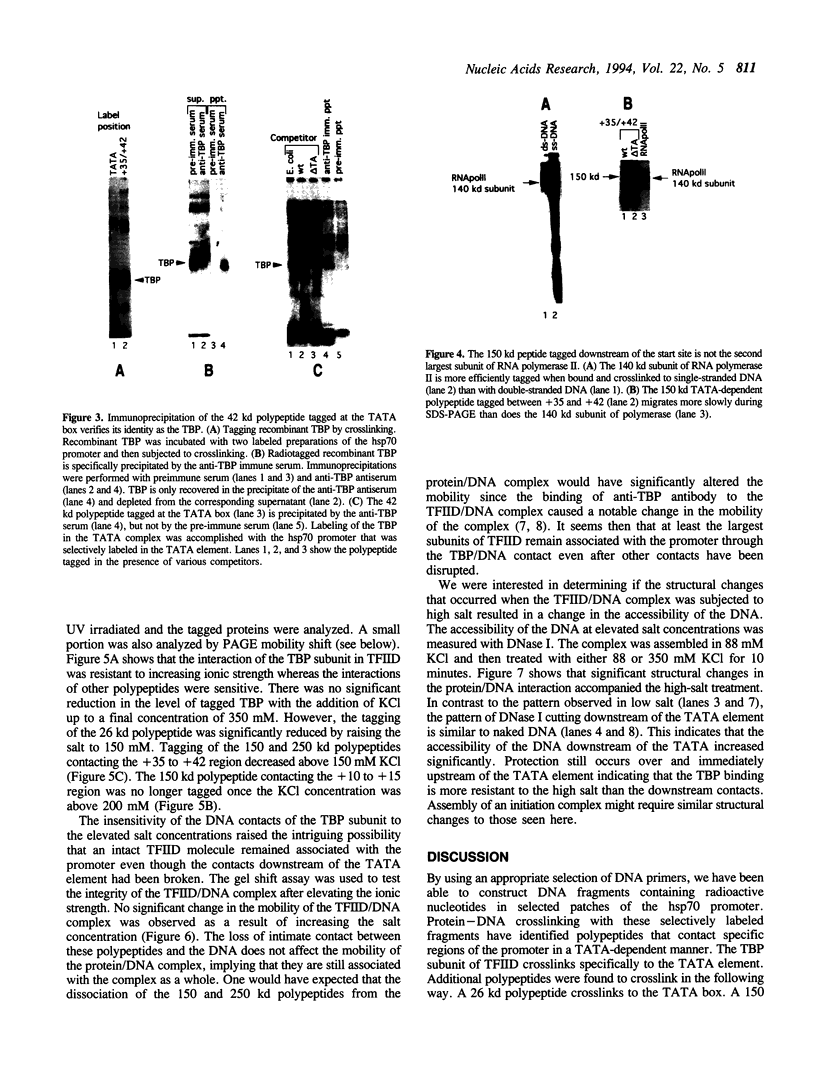
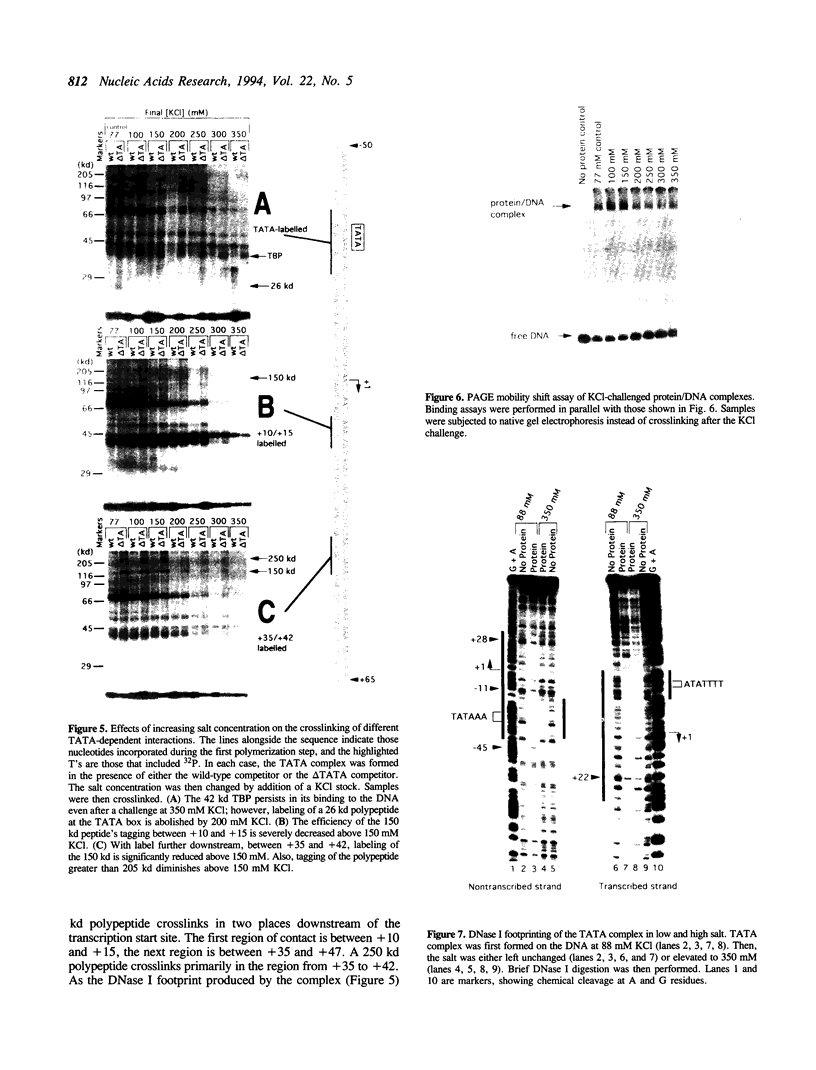
A protein--DNA complex containing TFIID has been analyzed by crosslinking. The TBP subunit of TFIID crosslinked to the TATA element but not to any of the regions further downstream which were tested. A 150 kd polypeptide, which corresponds in size to one of the TBP-associated factors (TAFs), crosslinked to a region between +10 and +15 and a second region between +35 and +47. Another polypeptide of greater than 205 kd (also a potential TAF) crosslinked preferentially to the region between +35 and +42. The +10 to +15 region has been recently implicated in hsp70 promoter recognition by TFIID, and the most downstream contacts overlap with the region where RNA polymerase II pauses on the hsp70 promoter in noninduced cells. Crosslinking revealed that as the salt concentration was increased, the TBP interaction was largely unaffected whereas the protein/DNA interactions downstream of the TATA element were disrupted. We propose that during the formation of a transcription complex, TATA-dependent interactions could be disrupted in the vicinity of the start site and the region immediately downstream. A protein contact downstream of +35 might function in pausing polymerase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dynlacht B. D., Hoey T., Tjian R. Isolation of coactivators associated with the TATA-binding protein that mediate transcriptional activation. Cell. 1991 Aug 9;66(3):563–576. doi: 10.1016/0092-8674(81)90019-2. [DOI] [PubMed] [Google Scholar]
- Emanuel P. A., Gilmour D. S. Transcription factor TFIID recognizes DNA sequences downstream of the TATA element in the Hsp70 heat shock gene. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8449–8453. doi: 10.1073/pnas.90.18.8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina C., Pérez-Riba M., Lis J. T. Promoter melting and TFIID complexes on Drosophila genes in vivo. Genes Dev. 1992 Nov;6(11):2190–2200. doi: 10.1101/gad.6.11.2190. [DOI] [PubMed] [Google Scholar]
- Gilmour D. S., Dietz T. J., Elgin S. C. TATA box-dependent protein-DNA interactions are detected on heat shock and histone gene promoters in nuclear extracts derived from Drosophila melanogaster embryos. Mol Cell Biol. 1988 Aug;8(8):3204–3214. doi: 10.1128/mcb.8.8.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour D. S., Dietz T. J., Elgin S. C. UV cross-linking identifies four polypeptides that require the TATA box to bind to the Drosophila hsp70 promoter. Mol Cell Biol. 1990 Aug;10(8):4233–4238. doi: 10.1128/mcb.10.8.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour D. S., Lis J. T. RNA polymerase II interacts with the promoter region of the noninduced hsp70 gene in Drosophila melanogaster cells. Mol Cell Biol. 1986 Nov;6(11):3984–3989. doi: 10.1128/mcb.6.11.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour D. S., Thomas G. H., Elgin S. C. Drosophila nuclear proteins bind to regions of alternating C and T residues in gene promoters. Science. 1989 Sep 29;245(4925):1487–1490. doi: 10.1126/science.2781290. [DOI] [PubMed] [Google Scholar]
- Hahn S., Buratowski S., Sharp P. A., Guarente L. Yeast TATA-binding protein TFIID binds to TATA elements with both consensus and nonconsensus DNA sequences. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5718–5722. doi: 10.1073/pnas.86.15.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumm A., Meulia T., Brunvand M., Groudine M. The block to transcriptional elongation within the human c-myc gene is determined in the promoter-proximal region. Genes Dev. 1992 Nov;6(11):2201–2213. doi: 10.1101/gad.6.11.2201. [DOI] [PubMed] [Google Scholar]
- Lee H., Kraus K. W., Wolfner M. F., Lis J. T. DNA sequence requirements for generating paused polymerase at the start of hsp70. Genes Dev. 1992 Feb;6(2):284–295. doi: 10.1101/gad.6.2.284. [DOI] [PubMed] [Google Scholar]
- O'Brien T., Lis J. T. Rapid changes in Drosophila transcription after an instantaneous heat shock. Mol Cell Biol. 1993 Jun;13(6):3456–3463. doi: 10.1128/mcb.13.6.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. H., Sluder A. E., Greenleaf A. L. Fractionation of transcription factors for RNA polymerase II from Drosophila Kc cell nuclear extracts. J Biol Chem. 1987 Mar 5;262(7):3244–3255. [PubMed] [Google Scholar]
- Purnell B. A., Gilmour D. S. Contribution of sequences downstream of the TATA element to a protein-DNA complex containing the TATA-binding protein. Mol Cell Biol. 1993 Apr;13(4):2593–2603. doi: 10.1128/mcb.13.4.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen E. B., Lis J. T. In vivo transcriptional pausing and cap formation on three Drosophila heat shock genes. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7923–7927. doi: 10.1073/pnas.90.17.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougvie A. E., Lis J. T. Postinitiation transcriptional control in Drosophila melanogaster. Mol Cell Biol. 1990 Nov;10(11):6041–6045. doi: 10.1128/mcb.10.11.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer V. L., Wobbe C. R., Struhl K. A wide variety of DNA sequences can functionally replace a yeast TATA element for transcriptional activation. Genes Dev. 1990 Apr;4(4):636–645. doi: 10.1101/gad.4.4.636. [DOI] [PubMed] [Google Scholar]
- Yokomori K., Admon A., Goodrich J. A., Chen J. L., Tjian R. Drosophila TFIIA-L is processed into two subunits that are associated with the TBP/TAF complex. Genes Dev. 1993 Nov;7(11):2235–2245. doi: 10.1101/gad.7.11.2235. [DOI] [PubMed] [Google Scholar]
- Zehring W. A., Lee J. M., Weeks J. R., Jokerst R. S., Greenleaf A. L. The C-terminal repeat domain of RNA polymerase II largest subunit is essential in vivo but is not required for accurate transcription initiation in vitro. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3698–3702. doi: 10.1073/pnas.85.11.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]