Abstract
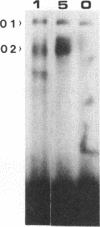
Herpes simplex virus (HSV) is capable of lytic replication in most cells, such replication in epithelial cells resulting in the mucocutaneous lesions observed following in vivo infection. In addition however, the virus also establishes asymptomatic latent infections in sensory neurons which serve as a reservoir for further cycles of peripheral lytic infections. These latent infections are dependent upon the inhibition of viral immediate-early (IE) gene expression via the octamer-related TAATGARAT motif in the IE promoters resulting in the failure of the viral lytic cycle. Here we show that the ectopic expression of neuronal isoforms of the octamer/TAATGARAT-binding transcription factor Oct-2 in permissive BHK cells represses IE gene expression following HSV infection and inhibits the viral lytic cycle whereas the B lymphocyte isoform of Oct-2 does not have this effect. These results suggest that the neuronal isoforms of Oct-2 play a critical role in rendering neuronal cells non-permissive for the viral lytic cycle thereby allowing the establishment of latent infection. Moreover, this is the first time that the ectopic expression of a cellular transcription factor has been shown to inhibit infection with any virus, raising the possibility of therapeutically inhibiting lytic viral infections by inducing such ectopic expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campbell M. E., Palfreyman J. W., Preston C. M. Identification of herpes simplex virus DNA sequences which encode a trans-acting polypeptide responsible for stimulation of immediate early transcription. J Mol Biol. 1984 Nov 25;180(1):1–19. doi: 10.1016/0022-2836(84)90427-3. [DOI] [PubMed] [Google Scholar]
- Croen K. D., Ostrove J. M., Dragovic L. J., Smialek J. E., Straus S. E. Latent herpes simplex virus in human trigeminal ganglia. Detection of an immediate early gene "anti-sense" transcript by in situ hybridization. N Engl J Med. 1987 Dec 3;317(23):1427–1432. doi: 10.1056/NEJM198712033172302. [DOI] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Gerster T., Roeder R. G. A herpesvirus trans-activating protein interacts with transcription factor OTF-1 and other cellular proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6347–6351. doi: 10.1073/pnas.85.17.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp L. M., Brickell P. M., La Thangue N. B., Latchman D. S. Transcriptional induction of cellular gene expression during lytic infection with herpes simplex virus. Biosci Rep. 1986 Nov;6(11):945–951. doi: 10.1007/BF01114970. [DOI] [PubMed] [Google Scholar]
- Kemp L. M., Dent C. L., Latchman D. S. Octamer motif mediates transcriptional repression of HSV immediate-early genes and octamer-containing cellular promoters in neuronal cells. Neuron. 1990 Feb;4(2):215–222. doi: 10.1016/0896-6273(90)90096-x. [DOI] [PubMed] [Google Scholar]
- Latchman D. S. Current status review: molecular biology of herpes simplex virus latency. J Exp Pathol (Oxford) 1990 Feb;71(1):133–141. [PMC free article] [PubMed] [Google Scholar]
- Lebeau M. C., Alvarez-Bolado G., Braissant O., Wahli W., Catsicas S. Ribosomal protein L27 is identical in chick and rat. Nucleic Acids Res. 1991 Mar 25;19(6):1337–1337. doi: 10.1093/nar/19.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop K. A., Dent C. L., Wheatley S. C., Beech M. N., Ninkina N. N., Wood J. N., Latchman D. S. The octamer-binding protein Oct-2 represses HSV immediate-early genes in cell lines derived from latently infectable sensory neurons. Neuron. 1991 Sep;7(3):381–390. doi: 10.1016/0896-6273(91)90290-g. [DOI] [PubMed] [Google Scholar]
- Lillycrop K. A., Latchman D. S. Alternative splicing of the Oct-2 transcription factor RNA is differentially regulated in neuronal cells and B cells and results in protein isoforms with opposite effects on the activity of octamer/TAATGARAT-containing promoters. J Biol Chem. 1992 Dec 15;267(35):24960–24965. [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Marashi F., Prokopp K., Stein J., Stein G. Evidence for a human histone gene cluster containing H2B and H2A pseudogenes. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1936–1940. doi: 10.1073/pnas.81.7.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis T. P., Sedarati F., Dobson A. T., Feldman L. T., Stevens J. G. Pathways of viral gene expression during acute neuronal infection with HSV-1. Virology. 1992 Jul;189(1):150–160. doi: 10.1016/0042-6822(92)90690-q. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Brauer D. H. Complete DNA sequence of the short repeat region in the genome of herpes simplex virus type 1. Nucleic Acids Res. 1986 Feb 25;14(4):1727–1745. doi: 10.1093/nar/14.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M. M., Ruppert S., Schaffner W., Matthias P. A cloned octamer transcription factor stimulates transcription from lymphoid-specific promoters in non-B cells. Nature. 1988 Dec 8;336(6199):544–551. doi: 10.1038/336544a0. [DOI] [PubMed] [Google Scholar]
- O'Hare P., Goding C. R. Herpes simplex virus regulatory elements and the immunoglobulin octamer domain bind a common factor and are both targets for virion transactivation. Cell. 1988 Feb 12;52(3):435–445. doi: 10.1016/s0092-8674(88)80036-9. [DOI] [PubMed] [Google Scholar]
- Patel R., Chan W. L., Kemp L. M., La Thangue N. B., Latchman D. S. Isolation of cDNA clones derived from a cellular gene transcriptionally induced by herpes simplex virus. Nucleic Acids Res. 1986 Jul 25;14(14):5629–5640. doi: 10.1093/nar/14.14.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry L. J., Rixon F. J., Everett R. D., Frame M. C., McGeoch D. J. Characterization of the IE110 gene of herpes simplex virus type 1. J Gen Virol. 1986 Nov;67(Pt 11):2365–2380. doi: 10.1099/0022-1317-67-11-2365. [DOI] [PubMed] [Google Scholar]
- Preston C. M. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979 Jan;29(1):275–284. doi: 10.1128/jvi.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M., Frame M. C., Campbell M. E. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell. 1988 Feb 12;52(3):425–434. doi: 10.1016/s0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- Roizman B., Sears A. E. An inquiry into the mechanisms of herpes simplex virus latency. Annu Rev Microbiol. 1987;41:543–571. doi: 10.1146/annurev.mi.41.100187.002551. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990 Feb 9;60(3):375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Clements J. B. A herpes simplex virus type 1 function continuously required for early and late virus RNA synthesis. Nature. 1980 May 29;285(5763):329–330. doi: 10.1038/285329a0. [DOI] [PubMed] [Google Scholar]
- Wheatley S. C., Kemp L. M., Wood J. N., Latchman D. S. Cell lines derived from dorsal root ganglion neurons are nonpermissive for HSV and express only the latency-associated transcript following infection. Exp Cell Res. 1990 Oct;190(2):243–246. doi: 10.1016/0014-4827(90)90192-d. [DOI] [PubMed] [Google Scholar]
- Wirth T., Priess A., Annweiler A., Zwilling S., Oeler B. Multiple Oct2 isoforms are generated by alternative splicing. Nucleic Acids Res. 1991 Jan 11;19(1):43–51. doi: 10.1093/nar/19.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. N., Bevan S. J., Coote P. R., Dunn P. M., Harmar A., Hogan P., Latchman D. S., Morrison C., Rougon G., Theveniau M. Novel cell lines display properties of nociceptive sensory neurons. Proc Biol Sci. 1990 Sep 22;241(1302):187–194. doi: 10.1098/rspb.1990.0084. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Lillycrop K. A., Dent C. L., Ninkina N. N., Beech M. M., Willoughby J. J., Winter J., Latchman D. S. Regulation of expression of the neuronal POU protein Oct-2 by nerve growth factor. J Biol Chem. 1992 Sep 5;267(25):17787–17791. [PubMed] [Google Scholar]