Abstract
Purpose
Sphingosine 1-phosphate (S1P) is an important mediator of cancer cell growth and proliferation. Production of S1P is catalyzed by sphingosine kinase 1 (SphK). Safingol, (L-threo-dihydrosphingosine) is a putative inhibitor of SphK. We conducted a phase I trial of safingol (S) alone and in combination with cisplatin (C).
Experimental Design
A 3+3 dose escalation was used. For safety, S was given alone 1 week before the combination. S + C were then administered every 3 weeks. S was given over 60–120 minutes (min), depending on dose. 60 min later, C was given over 60 min. The C dose of 75 mg/m2 was reduced in cohort 4 to 60 mg/m2 due to excessive fatigue.
Results
43 patients were treated. 41 were evaluable for toxicity and 37 for response. The maximum tolerated dose (MTD) was S 840 mg/m2 over 120 min C 60 mg/m2, every 3 weeks. DLTs attributed to cisplatin included fatigue and hyponatremia. DLT from S was hepatic enzyme elevation. S pharmacokinetic parameters were linear throughout the dose range with no significant interaction with C. Patients treated at or near the MTD achieved S levels of > 20 µM and maintained levels ≥ 5 µM for 4 hours. The best response was stable disease in 6 patients for on average 3.3 months (range 1.8 – 7.2 m). One patient with adrenal cortical cancer had significant regression of liver and lung metastases and another had prolonged stable disease. S was associated with a dose-dependent reduction in S1P in plasma.
Conclusions
Safingol, the first putative SphK inhibitor to enter clinical trials, can be safely administered in combination with cisplatin. Reversible dose-dependent hepatic toxicity was seen, as expected from preclinical data. Target inhibition was achieved with downregulation of S1P. The recommended phase 2 dose is S 840 mg/m2 and C 60 mg/m2, every 3 weeks.
Keywords: Drug-mediated stimulation of cell death pathways, Pharmacokinetics and pharmacodynamics, Kinase and phosphatase inhibitors, Novel antitumor agents, Sphingosine Kinase, Sphingolipids
Introduction
The role of sphingolipid compounds is increasingly recognized in cancer. The metabolites ceramide, sphingosine and sphingosine 1-phosphate (S1P) have crucial roles in intracellular and extracellular signaling.(1) Sphingosine kinase 1 (SphK) catalyzes the phosphorylation of sphingosine to form sphingosine 1-phosphate (S1P).(2) S1P acts downstream as a second messenger and via G protein-coupled receptors regulates multiple cellular processes including apoptosis inhibition, cell proliferation and angiogenesis.(2) SphK therefore regulates a rheostat, balancing the effects of pro-apoptotic ceramide and pro-proliferative S1P. Inhibiting SphK and downregulating S1P is thus a rational therapeutic target in cancer.(3)
Safingol (L-threo-dihydrosphingosine) is a potent competitive inhibitor of SphK with a Ki of about 5 µM.(4) Safingol is the first SphK inhibitor to enter clinical trials as an anti-cancer agent. Safingol has significant in vitro anti-cancer activity. It increases the in vitro antitumor effect of various chemotherapeutic agents such as doxorubicin, cisplatin and mitomycin C by enhancing chemotherapy-induced apoptosis.(5) We have also shown that safingol alone induces cell death by autophagy.(6) Although safingol has limited single-agent activity in vivo, xenograft experiments have shown that it can increase the antitumor activity of cisplatin without increasing toxicity.(5, 7) The exact mechanism of its action is, however, uncertain. Safingol has previously been studied extensively as an inhibitor of Protein Kinase C, although the Ki is somewhat higher at 33 µM for PKC than for SphK.(8) Inhibition of SphK is a plausible mechanism of action based on safingol’s ability to increase ceramide levels, alter the S1P/ceramide rheostat, and decrease tumor cell survival.(9)
We have previously reported a phase I study of safingol in combination with doxorubicin.(10) Doses of safingol up to 120 mg/m2 were administered every three weeks with no dose-limiting toxicity observed. However, the clinical trial was terminated before the maximum tolerated dose could be determined because of insufficient drug supply. The only safingol-associated toxicity was a single episode of grade 1 hemolysis. No significant pharmacokinetic interaction with doxorubicin was detected. Further clinical development of safingol was delayed by drug supply issues and commercial circumstances. Recently, however, safingol has gained renewed attention as an putative inhibitor of SphK.(11) We now report the results of a complete phase I study of safingol in combination with cisplatin.
The primary objective of this trial was to determine the maximum tolerated dose (MTD) of safingol when administered in combination with cisplatin in patients with advanced solid tumors. Safingol was administered over 60 to 120 minutes, prior to cisplatin dosing. Secondary objectives were to investigate the clinical pharmacokinetics of safingol and cisplatin, to study the pharmacodynamics of SphK inhibition, and to obtain preliminary data on the therapeutic activity of this regimen.
Patients and Methods
Eligibility
Eligible patients were ≥ 18 years of age with a diagnosis of pathologically confirmed measurable or evaluable advanced solid tumor, with disease that was refractory to standard therapy or for which there was no standard therapy. Eligible patients had Karnofsky performance status of ≥ 70%, total white blood cell count ≥ 3,500/mm3, absolute neutrophil count ≥ 1,500/mm3, platelet count of ≥ 100,000/mm3, hemoglobin of ≥ 9.5 g/dL, haptoglobin ≥ 30 mg/dL, and adequate hepatic and renal function. Patients may have received prior chemotherapy (including cisplatin) but 4 weeks from last dose had to elapse before study entry (6 weeks for nitrosoureas and mitomycin C). Patients with central nervous system metastases or a primary central nervous system neoplasm were not eligible.
The protocol was approved by the Institutional Review Board of MSKCC and all patients provided written informed consent.
Treatment plan
This was an open-label, non-randomized, dose escalation study to determine the maximum tolerated dose (MTD) of intravenous safingol when administered 1 hour prior to a standard dose of cisplatin, once every 21 days.
Groups of three to six patients were treated sequentially according to the dose escalation in Table 1. Safingol was administered as a 60- to 120-minute IV infusion, depending on the volume of the dose. 60 minutes after the completion of the safingol infusion, cisplatin was given as a 60-minute infusion. Each treatment cycle lasted 3 weeks, except for Cycle 1, which lasted 4 weeks. In Cycle 1, in order to assess safety and pharmacokinetics, safingol was given alone on Day 1. Following this, on Day 8 of Cycle 1, both safingol and cisplatin were administered.
Table 1.
Dose escalation
| Cohort | Safingol (mg/m2) |
Cisplatin (mg/m2) |
N |
|---|---|---|---|
| 1 | 60 | 75 | 4 |
| 2 | 120 | 75 | 3 |
| 3 | 240 | 75 | 3 |
| 4 | 240 | 60 | 6 |
| 5 | 360 | 60 | 3 |
| 6 | 480 | 60 | 3 |
| 7 | 600 | 60 | 3 |
| 8 | 750 | 60 | 6 |
| 9 | 930 | 60 | 6 |
| 10 | 840 | 60 | 6 |
Because of concerns for hemolysis, urinalysis, haptoglobin, RBC morphology and CBC were assessed before and after the first infusion of safingol.
All treatments were administered in the outpatient setting and, once assigned to a dose level, intra-patient dose escalation was not permitted.
Toxicity was graded in accordance with the Common Toxicity Criteria version 3.0.(12) Dose Limiting Toxicity (DLT) was defined as the occurrence of Grade 4 hematologic toxicity, Grade 3 or 4 non-hematologic toxicity attributable to the study drug in combination with cisplatin, or any delay in treatment of more than one week. The MTD was defined as one level below the dose at which two or more of the patients experience DLT during the first cycle. Patients who experienced a DLT or toxicity attributed to study treatment could continue to receive treatment after recovery, with appropriate dose modifications as defined per protocol.
To be evaluable for response and to be assessable for determination of MTD, patients had to have received at least one full cycle of therapy. Responses were evaluated after every two cycles with computed tomography scans or other diagnostic tests, as appropriate. Response Evaluation Criteria in Solid Tumors (RECIST) were used by an independent protocol radiologist.(13)
Drug supply
Safingol (NSC 714503) was supplied through the Rapid Access to Interventions Development (RAID) program of the National Cancer Institute, Cancer Therapy Evaluation Program. Safingol is the non-proprietary name for the L-threo enantiomer of dihydrosphingosine. The chemical name is (2S,3S)-2-amino-1,3-octadecanediol. Safingol was supplied as a sterile, pyrogen free emulsion containing, per mL, 2 mg of Safingol, 20 mg of Lipoid 80 (Egg Phospholipids), 45.4 mg of dextrose, and 1.2 mg lactic acid in water. The final safingol concentration was made to 1 mg/ml with normal saline. Cisplatin was obtained commercially.
Statistical design
The main objective of this study was to determine the MTD of safingol when administered in combination with cisplatin. Standard 3+3 design was used for dose escalation. The incidence of toxicities was summarized separately by safingol cohort. Secondary analyses included pharmacokinetic analyses of safingol by non-compartmental methods, and pharmacodynamic analyses of S1P levels.
Pharmacokinetics
For each patient, blood samples for safingol pharmacokinetics were collected during cycle 1, day 1 (treatment with safingol alone), and during cycle 1, day 8 (treatment with safingol and cisplatin). Plasma samples were obtained prior to administration of safingol, and 0, 15, 30 and 60 min, and 2, 4, 6 and 24 h following. For patients treated in cohorts 8–10, PK sampling was limited to cycle 1, day 1, prior to administration of safingol, and 0, 15, 30 and 60 min, and 4 h following. Assessment of safingol(14) and sphingolipid profile(15) was measured by high-performance liquid chromatography (HPLC) with mass spectroscopy (HPLS/MS) for quantitation. Pharmacokinetic parameters were estimated using a non-compartmental model.
Results
Patient characteristics
From April 2004 to October 2008, 43 patients with advanced solid tumors were registered and treated. Two of these were not evaluable for determining DLT because they did not complete one cycle (4 weeks) of treatment. The reasons were clinical deterioration (1 patient) and adverse effects of cisplatin (1 patient). A further four patients were not evaluable for response because they did not continue treatment until the first restaging scan. The reasons were withdrawal of consent due to adverse events (3 patients) and clinical deterioration (1 patient). In total, 41 patients were evaluable for toxicity and 37 for response.
The median age was 54 years (range, 33–82 years) and the median Karnofsky performance status was 80% (range, 70–100%). There were 17 men and 26 women. The cancers treated and patient numbers were colorectal (11), adrenal cortical (7), sarcoma (6), pancreas (4), melanoma (3), hepatocellular (3), gastric (3), and others (6). 26 patients (60%) had received prior chemotherapy. 6 patients (14%) had received prior cisplatin.
Cycle 1 toxicities
Table 3 lists the significant grade 2 to 4 toxicities for the first cycle of therapy. The starting dose was safingol 60 mg/m2 with cisplatin 75 mg/m2. Dose escalation of safingol continued until cohort 3 (safingol 240 mg/m2 with cisplatin 75 mg/m2) where 2 of 3 patients experienced DLT in the form of grade 3 fatigue. Since this was considered to be caused by cisplatin, the protocol was amended and the cisplatin dose for subsequent cohorts was reduced to 60 mg/m2. Safingol escalation continued without evidence of further fatigue. In cohort 4 (safingol 240 mg/m2 with cisplatin 60 mg/m2), one of three patients had a DLT with thrombocytopenia (grade 3 for > 7 days). The cohort was expanded to include six evaluable patients with no further DLT observed. In cohort 8 (safingol 750 mg/m2 with cisplatin 60 mg/m2), one DLT occurred (grade 3 AST/ALT). Again, the cohort was expanded to include six evaluable patients with no further DLT observed. In cohort 9 (safingol 930 mg/m2 with cisplatin 60 mg/m2), two DLTs occurred (grade 3 and 4 AST/ALT) among the six patients. This was the maximum administered dose and by definition exceeded the MTD. To further define the MTD, a dose intermediate between cohorts 8 and 9 was tested. In this cohort 10 (safingol 840 mg/m2 with cisplatin 60 mg/m2), one DLT occurred (grade 3 AST/ALT) among six patients. Thus, the MTD had been reached. The DLT rate for the MTD cohort was 17% (1/6). This was considered acceptable using pre-specified guidelines since the upper limit of the 95% posterior interval was 40%.(16)
Table 3.
Cycle 1 toxicity
| AST/ALT | Fatigue | Hyponatremia | Lymphopenia | Anemia | Platelets | Nausea/vomiting | Creatinine | Neuropathy | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | N | G2 | G3 | G4 | G2 | G3 | G4 | G2 | G3 | G4 | G2 | G3 | G4 | G2 | G3 | G4 | G2 | G3 | G4 | G2 | G3 | G4 | G2 | G3 | G4 | G2 | G3 | G4 |
| 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| 2 | 3 | 0 | 1* | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| 3 | 3 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 6 | 1 | 2† | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 6 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 6 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
DLTs are noted in bold.
One patient had G3 AST/ALT attributed to liver metastases and not considered a DLT.
In one patient this was attributed to progressive liver metastases; in the second this was attributed to drug and considered a DLT.
Pharmacokinetics
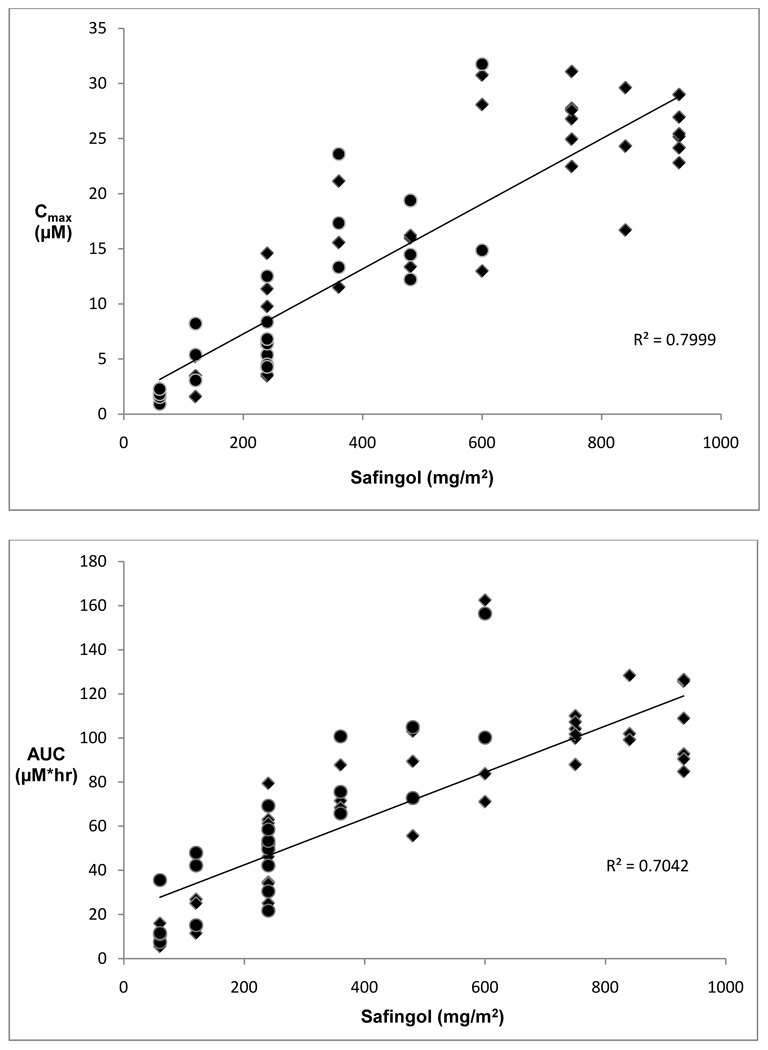
Blood samples for pharmacokinetic analyses were obtained for 39 patients. Table 4 summarizes safingol pharmacokinetic parameters for patients by cohort. Overall, safingol Cmax and AUC increased in a linear and dose-proportional manner, although there was significant inter-patient variability (Figure 1). Mean day 1 safingol Cmax ranged from 1.7 µM (at safingol 60 mg/m2) up to 23.9 – 26.8 µM (at safingol 600 – 930 mg/m2). Mean day 1 safingol AUC ranged from 11.2 µM*hr (at safingol 60 mg/m2) up to 101.9 – 109.9 µM*hr (at safingol 600 – 930 mg/m2). On average, each 100 mg/m2 increase in safingol dose resulted in 3 µM increase in Cmax and 10 µM*hr increase in AUC.
Table 4.
Safingol pharmacokinetic parameters by cohort
| Day 1 | Day 1 | Day 8 | Day 8 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Safingol (mg/m2) |
Cisplatin (mg/m2) |
N | mean Cmax (µM) |
SD | mean AUC (µM*hr) |
SD | N | mean Cmax (µM) |
SD | mean AUC (µM*hr) |
SD |
| 1 | 60 | 75 | 4 | 1.7 | 0.7 | 11.2 | 4.3 | 4 | 1.6 | 0.6 | 16.5 | 12.9 |
| 2 | 120 | 75 | 3 | 3.4 | 1.8 | 21.2 | 8.4 | 3 | 5.6 | 2.6 | 35.1 | 17.5 |
| 3 | 240 | 75 | 3 | 7.5 | 6.2 | 40.9 | 19.6 | 3 | 8.8 | 3.6 | 54.5 | 13.7 |
| 4 | 240 | 60 | 5 | 7.4 | 3.1 | 56.8 | 17.3 | 5 | 5.8 | 1.3 | 42.8 | 15.8 |
| 5 | 360 | 60 | 3 | 16.1 | 4.8 | 76.0 | 10.4 | 3 | 18.1 | 5.2 | 80.7 | 18.0 |
| 6 | 480 | 60 | 3 | 15.2 | 1.6 | 82.7 | 24.4 | 3 | 15.4 | 3.7 | 94.2 | 18.6 |
| 7 | 600 | 60 | 3 | 23.9 | 9.6 | 105.8 | 49.5 | 2 | 23.3 | 11.9 | 128.3 | 39.8 |
| 8 | 750 | 60 | 6 | 26.8 | 2.9 | 101.9 | 7.7 | 0 | ND | ND | ND | ND |
| 9 | 930 | 60 | 6 | 25.6 | 2.2 | 104.9 | 18.3 | 0 | ND | ND | ND | ND |
| 10 | 840 | 60 | 3 | 23.5 | 6.5 | 109.9 | 16.1 | 0 | ND | ND | ND | ND |
For patients in Cohorts 8–10, samples were only obtained on Day 1, when safingol was given alone.
Figure 1.
Pharmacokinetic dose-ranging for Cmax (top) and AUC (bottom) of safingol. Cmax and AUC for each patient studied are plotted against safingol dose. Diamonds represent doses of safingol given alone. Circles represent doses of safingol given with cisplatin.
Results for day 8, when safingol was given with cisplatin, were available for 23 patients. These were similar to day 1 PK assessments. Mean day 8 safingol Cmax ranged from 1.6 µM (at safingol 60 mg/m2) up to 23.3 µM (at safingol 600 mg/m2). Mean day 8 safingol AUC ranged from 16.5 µM*hr (at safingol 60 mg/m2) up to 128.3 µM*hr (at safingol 600 mg/m2). There was no statistically significant change in PK parameters from day 1 to day 8 and thus no evidence for any significant drug-drug interaction with cisplatin.
Pharmacodynamics
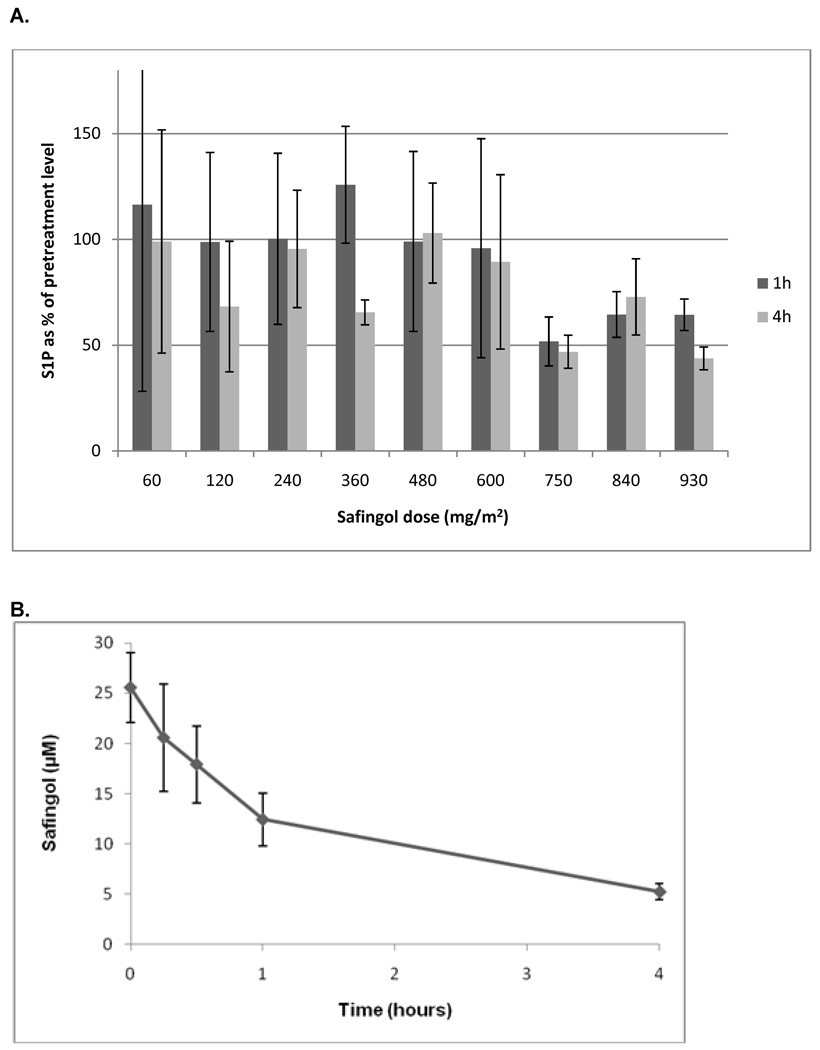
Blood samples were also assayed for levels of sphingosine-1-phophate (S1P). As an inhibitor of SphK, safingol would be expected to decrease S1P levels, which thus serve as a pharmacodynamic readout. The results are shown in figure 2A. The average S1P levels 1 hour and 4 hours after safingol treatment are shown, with patients grouped by safingol dose cohort. S1P levels are normalized to the pre-treatment levels for each patient cohort and standard error bars are shown. The results show a dose-dependent decrease in S1P levels. Specifically, at safingol doses of over 750 mg/m2, S1P levels are decreased by about half (p < 0.001 by Student’s t-test). At 930 mg/m2, there may also be a time-dependent effect, since S1P levels were lower 4 hours post-treatment than at 1 hour post-treatment (p = 0.001). The decreases in S1P levels were associated with safingol levels of ≥ 5 µM out to 4 hours following drug administration (figure 2B).
Figure 2.
(A) Pharmacodynamics of sphingosine-1-phophate (S1P) in peripheral blood. The mean S1P levels 1 hour and 4 hours after safingol treatment are shown, with patients grouped by safingol dose cohort. S1P levels are normalized to the pre-treatment S1P levels for each cohort with standard error bars shown. (B) Safingol plasma concentration over time for the patients treated at or near the MTD. Safingol doses of 750–930 mg/m2 were administered. Plasma levels were measured beginning at the end of safingol infusion (time = 0).
Antitumor activity
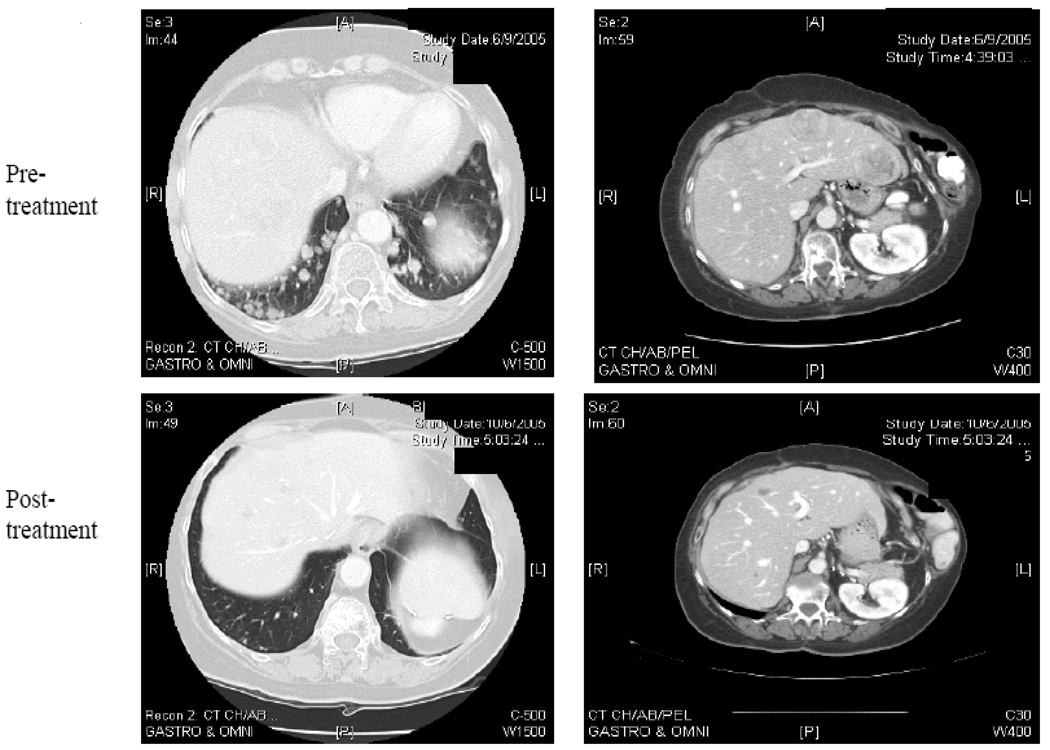
37 patients were evaluable for response assessment. The best response was stable disease in 6 patients for on average 3.3 m (range 1.8 – 7.2 m). One patient with adrenal cortical cancer had significant regression of liver and lung metastases (Figure 3). This patient, treated in cohort 3, developed grade 3 fatigue and hyponatremia. This was considered related to the cisplatin treatment, not to the safingol, and led to the modification of the cisplatin dose for subsequent cohorts. Because of these side effects, the patient withdrew consent and did not receive a second cycle of therapy. She was therefore not evaluable for the response endpoint as defined in the protocol. Nonetheless, her follow-up CT scans over several months with no further therapy demonstrated a major decrease in the size and number of metastases. This would have qualified as a confirmed partial response by RECIST and the response lasted 5.8 months. We also note that the other patient with significant clinical benefit, a long period of stable disease (7.2 m), also had adrenal cortical cancer.
Figure 3.
Regression of pulmonary and hepatic metastases from a patient with adrenal cortical carcinoma, 16 weeks after a treatment with safingol and cisplatin.
Discussion
This clinical trial describes the safety and pharmacokinetics of safingol in combination with cisplatin in patients with solid tumors. The MTD of safingol was determined to be 840 mg/m2 over 120 minutes with cisplatin 60 mg/m2 once every 3 weeks.
Overall, the drug combination was well tolerated with no major increase in toxicities over those of cisplatin alone. Remarkably, with 60 mg/m2 of cisplatin there was no significant renal insufficiency, a common complication of cisplatin treatment. The principal DLT was hepatic enzyme elevation. Although mild LFT changes were seen following the initial dose of safingol alone, significant DLT (Grade 3/4 LFT) occurred only after the combination treatment. In view of this, it is not possible to determine whether the observed toxicity is a consequence of the combination of safingol and cisplatin, or a result of repeat dosing of safingol. However, hepatic toxicity was predicted from preclinical studies that showed that administration of safingol alone to mice causes increases in ALT and histological changes consistent with hepatocyte autophagy.(14) The doses of safingol administered here were significantly higher than those in the other clinical trial of safingol with doxorubicin in which no hepatic toxicity was observed. In that study, safingol doses did not exceed 120 mg/m2 due to drug supply constraints. Here we identify for the first time a true MTD associated with safingol treatment. In the previous study, one case of hemolysis was considered a possible toxicity of safingol. In the current study, despite careful monitoring, no hemolysis was observed in any patient.
Extensive pharmacokinetic sampling was performed. There was no significant interaction of safingol with cisplatin. For patients treated in cohorts 1–7, safingol Cmax and AUC were not significantly changed from Day 1, when safingol was administered alone, to Day 8, when safingol was administered with cisplatin (Table 4). Based on this observation, pharmacokinetic sampling was therefore limited to only Day 1 for those patients treated in cohorts 8–10.
Overall, safingol PK parameters increased linearly with dose (Figure 1). Results were similar to those in the prior phase I trial of safingol and doxorubicin. For example, the mean Cmax at the 60 mg/m2 and 120 mg/m2 doses were 1.3 (± 0.1) µM and 3.5 (± 0.7) µM in the study of safingol and doxorubicin, and 1.7 (± 0.7) µM and 3.4 (± 1.8) µM in the current study. Dose escalation continued beyond 120 mg/m2 to a maximum administered dose of 930 mg/m2. In patients treated at or near the MTD of this trial (cohorts 8–10; safingol dose 750–930 mg/m2), Cmax of over 20 µM of safingol was achieved. Although the duration of safingol concentration over 20 µM was relatively short (< 1 hour), safingol levels of at least 5 µM (the Ki for SphK) persist for at least 4 hours after treatment.(11)
Clinical activity was seen in two patients with adrenal cortical cancer. One had prolonged stable disease after progression on prior therapy and another patient, although not strictly evaluable per protocol, had a partial response. Although a promising result, we cannot conclude this is different from what would have been achieved with cisplatin alone. The overall low response rate may be explained by the relatively refractory cancers treated. The patient population was heavily pre-treated (median of 4 prior regimens) and included a substantial majority of patients with diseases that are resistant to cisplatin (such as colorectal cancer and sarcoma).
Treatment with safingol and cisplatin was associated with significant decreases in plasma levels of S1P. One possible explanation is that safingol effectively inhibited SphK leading to decreased S1P levels. However, S1P biology is complex and other mechanisms must be considered. SphK, when activated, translocates to the cell membrane where its substrate, sphingosine, is located.(17) S1P is generated and transported extracellularly for autocrine and paracrine signaling and can also act intracellularly through histone deacetylases to regulate gene expression (18)(19) S1P levels are closely regulated with tissue S1P generally low, and plasma S1P generally high.(20, 21) The high levels of S1P normally measured in plasma are derived from multiple sources, including platelets, erythrocytes and the vascular endothelium.(22–25)
Thus, alternative mechanisms may explain the association between safingol administration and S1P levels. The observed changes in plasma S1P could be the result of decreased S1P production or decreased S1P release from either tumor or normal tissues such as hematopoietic or endothelial cells. An increase in S1P utilization or degradation by tumor or normal tissue could also explain the result. Our pharmacokinetic data only allow us to comment on plasma levels of S1P. Whether these reflect intra-tumoral levels of S1P is unknown. Other potential causes of S1P reduction include an effect of safingol on a target other than SphK, or even a nonspecific effect of the drug formulation.
Nevertheless, the results show that high plasma levels of safingol can be achieved and sustained for up to 4 hours after dosing. These levels are in the range predicted to effectively inhibit SphK. Sustained levels of safingol in plasma were associated with decreased S1P, consistent with the putative mechanism of action. Whether S1P suppression correlates with intracellular or intratumoral SphK activity is unknown and would require tumor biopsies to elucidate.
Statement of Translational Relevance
Sphingolipid metabolites play important roles in cancer. A balance exists between the pro-apoptotic sphingolipid ceramide and its pro-proliferation counterpart sphingosine 1-phosphate (S1P). Sphingosine kinase (SphK) catalyzes the production of S1P and thus regulates this balance. Inhibition of SphK is therefore a rational therapeutic strategy. We report the first complete phase I study of a safingol, a putative inhibitor of SphK. Safingol was administered alone and, based on preclinical data, in combination with cisplatin. Safingol can be safely administered at doses that achieve plasma levels consistent with target inhibition. Manageable hepatotoxicity, as predicted from animal models, occurred. Administration of safingol and cisplatin was associated with dose-dependent decreases in plasma levels of S1P, consistent with SphK inhibition. Considering the importance of sphingolipids in inflammation, immunity and cancer, the results could have broad implication for human disease and treatment.
Table 2.
Patient Characteristics
| Characteristic | N |
|---|---|
| Total | 43 |
| Evaluable | 41 |
| Male | 17 (40%) |
| Female | 26 (60%) |
| Age, y | |
| Median | 54 |
| Range | 33–82 |
| KPS, % | |
| Median | 80 |
| Range | 70–100 |
| Prior chemotherapy | 26 (60%) |
| Median number of prior regimens | 4 |
| Primary Sites of Disease | |
| Colorectal | 11 |
| Adrenal Cortical Carcinoma | 7 |
| Sarcoma | 6 |
| Pancreas | 4 |
| Melanoma | 3 |
| Hepatocellular Carcinoma | 3 |
| Gastric | 3 |
| Head & Neck | 2 |
| Cholangiocarcinoma | 2 |
| Ovarian | 1 |
| Bladder | 1 |
Acknowledgments
This work was supported by the National Cancer Institute at the U.S. National Institutes of Health [R21CA112910 to GKS] and, in part, by LIPID Metabolites And Pathways Strategy (LIPID MAPS) [GM069338 to AHM].
For expert technical assistance in lipid biochemistry, we thank Jeremy Allegood, Sibali Bandyopadhyay, Kristin Jones, Samuel Kelly, Rebecca Shaner, and Elaine Wang.
References
- 1.Pitman MR, Pitson SM. Inhibitors of the sphingosine kinase pathway as potential therapeutics. Curr Cancer Drug Targets. 2010;10:354–367. doi: 10.2174/156800910791208599. [DOI] [PubMed] [Google Scholar]
- 2.Cuvillier O, Ader I, Bouquerel P, Brizuela L, Malavaud B, Mazerolles C, et al. Activation of sphingosine kinase-1 in cancer: implications for therapeutic targeting. Curr Mol Pharmacol. 2010;3:53–65. doi: 10.2174/1874467211003020053. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds CP, Maurer BJ, Kolesnick RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004;206:169–180. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Olivera A, Kohama T, Tu Z, Milstien S, Spiegel S. Purification and characterization of rat kidney sphingosine kinase. J Biol Chem. 1998;273:12576–12583. doi: 10.1074/jbc.273.20.12576. [DOI] [PubMed] [Google Scholar]
- 5.Kedderis LB, Bozigian HP, Kleeman JM, Hall RL, Palmer TE, Harrison SD, Jr, et al. Toxicity of the protein kinase C inhibitor safingol administered alone and in combination with chemotherapeutic agents. Fundam Appl Toxicol. 1995;25:201–217. doi: 10.1006/faat.1995.1056. [DOI] [PubMed] [Google Scholar]
- 6.Coward J, Ambrosini G, Musi E, Truman JP, Haimovitz-Friedman A, Allegood JC, et al. Safingol (L-threo-sphinganine) induces autophagy in solid tumor cells through inhibition of PKC and the PI3-kinase pathway. Autophagy. 2009;5:184–193. doi: 10.4161/auto.5.2.7361. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann TK, Leenen K, Hafner D, Balz V, Gerharz CD, Grund A, et al. Antitumor activity of protein kinase C inhibitors and cisplatin in human head and neck squamous cell carcinoma lines. Anticancer Drugs. 2002;13:93–100. doi: 10.1097/00001813-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GK, Jiang J, Kelsen D, Albino AP. Protein kinase C: a novel target for inhibiting gastric cancer cell invasion. J Natl Cancer Inst. 1993;85:402–407. doi: 10.1093/jnci/85.5.402. [DOI] [PubMed] [Google Scholar]
- 9.Maurer BJ, Melton L, Billups C, Cabot MC, Reynolds CP. Synergistic cytotoxicity in solid tumor cell lines between N-(4-hydroxyphenyl)retinamide and modulators of ceramide metabolism. J Natl Cancer Inst. 2000;92:1897–1909. doi: 10.1093/jnci/92.23.1897. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz GK, Ward D, Saltz L, Casper ES, Spiess T, Mullen E, et al. A pilot clinical/pharmacological study of the protein kinase C-specific inhibitor safingol alone and in combination with doxorubicin. Clin Cancer Res. 1997;3:537–543. [PubMed] [Google Scholar]
- 11.Vessey DA, Kelley M, Zhang J, Li L, Tao R, Karliner JS. Dimethylsphingosine and FTY720 inhibit the SK1 form but activate the SK2 form of sphingosine kinase from rat heart. J Biochem Mol Toxicol. 2007;21:273–279. doi: 10.1002/jbt.20193. [DOI] [PubMed] [Google Scholar]
- 12.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Morales PR, Dillehay DL, Moody SJ, Pallas DC, Pruett S, Allgood JC, et al. Safingol toxicology after oral administration to TRAMP mice: demonstration of safingol uptake and metabolism by N-acylation and N-methylation. Drug Chem Toxicol. 2007;30:197–216. doi: 10.1080/01480540701375018. [DOI] [PubMed] [Google Scholar]
- 15.Sullards MC, Allegood JC, Kelly S, Wang E, Haynes CA, Park H, et al. Structure-specific, quantitative methods for analysis of sphingolipids by liquid chromatography-tandem mass spectrometry: "inside-out" sphingolipidomics. Methods Enzymol. 2007;432:83–115. doi: 10.1016/S0076-6879(07)32004-1. [DOI] [PubMed] [Google Scholar]
- 16.Gonen M. A Bayesian evaluation of enrolling additional patients at the maximum tolerated dose in Phase I trials. Contemp Clin Trials. 2005;26:131–140. doi: 10.1016/j.cct.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Pitson SM, Xia P, Leclercq TM, Moretti PA, Zebol JR, Lynn HE, et al. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvarez SE, Milstien S, Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab. 2007;18:300–307. doi: 10.1016/j.tem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edsall LC, Spiegel S. Enzymatic measurement of sphingosine 1-phosphate. Anal Biochem. 1999;272:80–86. doi: 10.1006/abio.1999.4157. [DOI] [PubMed] [Google Scholar]
- 21.Caligan TB, Peters K, Ou J, Wang E, Saba J, Merrill AH., Jr A high-performance liquid chromatographic method to measure sphingosine 1-phosphate and related compounds from sphingosine kinase assays and other biological samples. Anal Biochem. 2000;281:36–44. doi: 10.1006/abio.2000.4555. [DOI] [PubMed] [Google Scholar]
- 22.Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, et al. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem. 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- 23.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 24.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 25.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]