Abstract
This paper reviews evidence suggesting that nicotine and tobacco smoke profoundly modulate the effects of alcohol on γ-aminobutyric acid (GABA) neuronal function, specifically at the GABAA-benzodiazepine receptor (GABAA-BZR). The focus of this paper is on recent neuroimaging evidence in preclinical models as well as clinical experiments. First, we review findings implicating the role of alcohol at the GABAA-BZR and discuss the changes in GABAA-BZR availability during acute and prolonged alcohol withdrawal. Second, we discuss preclinical evidence that suggests nicotine affects GABA neuronal function indirectly by a primary action at neuronal nicotinic acetylcholine receptors. Third, we show how this evidence converges in studies that examine GABA levels and GABAA-BZRs in alcohol-dependent smokers and nonsmokers, suggesting that tobacco smoking attenuates the chemical changes that occur during alcohol withdrawal. Based on a comprehensive review of literature, we hypothesize that tobacco smoking minimizes the changes in GABA levels that typically occur during the acute cycles of drinking in alcohol-dependent individuals. Thus, during alcohol withdrawal, the continued tobacco smoking decreases the severity of the withdrawal-related changes in GABA chemistry.
Keywords: GABA, alcohol dependence, tobacco smoking, brain imaging
Introduction
γ-aminobutyric acid (GABA) neuronal function is modulated by ethanol and nicotine (Kumar et al., 2009; Markou, 2008). Neuroadaptations within GABA neurons contribute to the development of both ethanol and nicotine dependence, as well as complications associated with the abrupt discontinuation of ethanol or nicotine in dependent individuals (Hughes, 2009). Similarly, GABA systems have been implicated in the recovery from ethanol and nicotine dependence (Mason et al., 2006; Staley et al., 2005a). Given the high rate of alcohol and nicotine dependence comorbidity (Grant et al., 2004), studying the convergent effects of acute and chronic exposure to these substances might provide clinically relevant insights about the underlying mechanistic interactions of ethanol and nicotine. In this review we examine evidence suggesting that GABA neuronal function, and specifically the GABAA-benzodiazepine receptor (GABAA-BZR), is a major neurochemical target mediating the comorbidity of alcohol and nicotine dependence. We hypothesize that nicotine profoundly alters both the acute and chronic effects of ethanol on GABAA receptors and thus increases the risk for both alcoholism (by suppressing ethanol neuroadaptations and withdrawal) and nicotine dependence (because the increased tolerability of ethanol and ethanol withdrawal serves as a negative reinforcer for continued smoking). Important contributions of neuroadaptions associated with GABAA receptors to ethanol dependence have been reviewed previously (Krystal et al., 2006) and we refer readers to other recently published reviews on the interaction of nicotine and alcohol such as genetic components (Flatscher-Bader and Wilce, 2009; Schlaepfer et al., 2008) and reward mechanisms (Schlaepfer et al., 2008) that contribute to the comorbidity.
Comorbidity of Alcohol and Nicotine Dependence
The high rate of comorbidity between alcohol and nicotine dependence is well established. While in the general population it is estimated that 25% of people are current cigarette smokers, up to 45% of alcohol-dependent individuals smoke, and as many as 14% of smokers are also alcohol-dependent (Grant et al., 2004). Smokers consume twice as much alcohol as nonsmokers (Carmody et al., 1985), alcohol problems are approximately 10 times more prevalent in smokers vs. nonsmokers (DiFranza and Guerrera, 1990), and alcohol-dependent smokers use more cigarettes per day than non-alcohol-dependent smokers (Dawson, 2000). Unfortunately, individuals with a current or past alcohol problem have a more difficult time quitting smoking than non-alcoholics (Bobo et al., 1987; Kahler et al., 2010a; Romberger and Grant, 2004) and they are more likely to lapse to smoking while they are drinking (Kahler et al., 2010b). However, quitting smoking does not appear to affect rates of heavy drinking (Kahler et al., 2010a), suggesting that quitting smoking and drinking must both be targeted in vulnerable individuals. There are several factors that may underlie the high comorbidity of smoking and drinking (reviewed in (Meyerhoff et al., 2006). First, the effects of the drugs, when used together, may be additive or synergistic with regard to the reinforcing properties. Second, pharmacological effects or interactions of the drugs, such as changes in metabolism or cross-tolerance, may lead to co-abuse. Third, genetic factors likely contribute to this comorbidity.
We suggest that there is a critical influence of nicotine on alcohol's actions at the GABAA receptor, as an additional factor that may contribute to the comorbidity of these disorders. The cycle of addiction begins with acquisition or initiation of drug taking, which escalates to a steady-state maintenance phase and then cycles of withdrawal and relapse typically occur. It is likely that during these phases, discrete brain areas and neurochemical systems are recruited. Traditionally, the initiation phase is characterized by positive reinforcement, which is critically tied to the mesolimbic dopamine system (Di Chiara and Imperato, 1988). The shift from controlled to uncontrolled use can be associated with a shift from positive to negative reinforcement, i.e., when addicted individuals switch from taking drugs for the euphoric effects to taking drugs to prevent withdrawal symptoms. At that point it becomes critical to maintain homeostasis between the inhibitory (GABAergic) and excitatory (glutamatergic) systems. During alcohol withdrawal, the drug-maintained homeostasis is severely disrupted, most clearly evidenced by seizures, and it is at this critical juncture that nicotine may be conferring protection against changes in neurochemicals and in alcohol withdrawal symptoms. Specifically, the profound effects of nicotine on alcohol-induced alterations of GABAA receptors may drive the continued use of both substances.
Effects of Alcohol and Alcohol Withdrawal on GABAA Receptors
GABAA receptors are ligand-gated ion channels that are the primary mechanism for modulating inhibitory synaptic transmission in the brain and have a central role in modulating the effects of alcohol in the central nervous system (Davies, 2003; Krystal et al., 2006; Kumar et al., 2004). GABA plays a number of important roles in the brain, including maintaining homeostatic balance between excitation and inhibition (Sivilotti and Nistri, 1991), tuning the activity of glutamate neurons (Sesack et al., 2003), and entraining the coherent oscillatory interactions within cortical networks (Mann and Paulsen, 2007; Wang and Buzsaki, 1996). The GABAA receptor is a member of a family of homologous transmitter-gated ion channels along with the nicotinic acetylcholine receptor (nAChR), glycine and 5-HT3 receptors (Sigel and Buhr, 1997). GABAA receptors are pentamers typically comprised of two α1-6, two ß1-3, and one γ1-3 subunit. Benzodiazepines bind to a distinct site at the interface between an α and γ subunit on the GABAA receptor, a site commonly referred to as the GABAA-benzodiazepine receptor (GABAA-BZR).
Although acute ethanol exposure has been reported to potentiate GABA-gated currents (Suzdak and Paul, 1987; Tatebayashi et al., 1998), direct ethanol effects on synaptic GABAA receptors only occur at ethanol levels that are lethal in humans (Koski et al., 2002; Koski et al., 2005). Potent effects of ethanol in vivo likely reflect direct actions of ethanol at extrasynaptic GABAA receptors (Krystal et al., 2006; Sundstrom-Poromaa et al., 2002; Wallner et al., 2003). Acute ethanol may also act indirectly at synaptic GABAA receptors (via increased glutamate release), thereby increasing the synaptic release of GABA (Ariwodola and Weiner, 2004; Carta et al., 2004; Moghaddam and Bolinao, 1994). Of interest is the capacity of ethanol to raise levels of neurosteroids that interact with GABAA receptors (Barbaccia et al., 1999; Morrow et al., 1999) in a manner similar to other GABAA-receptor positive modulators that may influence tolerance to alcohol (Morrow et al., 2006). Importantly, GABA-ergic neurosteroids do not exhibit cross-tolerance like benzodiazepines, and have been shown to be protective during ethanol withdrawal (Devaud et al., 1996; Devaud et al., 1995). Neurosteroids act at extrasynaptic GABAA receptors that mediate tonic inhibition, rather than at synaptic GABAA receptors that mediate phasic inhibition (Stell et al., 2003; Wohlfarth et al., 2002). This is important because as previously mentioned the extrasynaptic GABAA receptors have been proposed as a high-affinity target for ethanol (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003). Long-term ethanol exposure stimulates changes in GABAA receptor subunit composition (Charlton et al., 1997; Devaud et al., 1997; Petrie et al., 2001), and the consequence of this adaptation is that GABAA receptors show higher affinity for GABA, but disrupted chloride channel conductance (Liang et al., 2007; Morrow et al., 1988; Sanna and Harris, 1993). When ethanol is removed, the lowered functionality of the GABAA receptors contributes to the heightened excitatory tone (Kang et al., 1996), which is clearly evidenced by irritability, sympathetic activation, seizure activity, and neurotoxicity associated with the ethanol withdrawal syndrome (Hoffman, 1995; Kokka et al., 1993).
Several postmortem studies have been conducted to determine potential differences in numbers of GABAA-BZRs between individuals with alcohol dependence and controls; however, the results are conflicting. The studies report decreases in GABAA-BZRs of 30% in hippocampus and 25% in frontal cortex (Freund and Ballinger, 1991), no difference in GABAA-BZR density in frontal cortex or cerebellum (Korpi and Uusi-Oukari, 1992), and increases in GABAA receptor density (Tran et al., 1981) in alcohol-dependent subjects vs. controls. This variability may be due to the lack of control for smoking status, and/or to other caveats associated with the study of postmortem specimens such as postmortem interval, freezer storage time and insufficient information about the chronicity and intensity of drug use over the lifetime.
The use of receptor imaging (single photon emission computed tomography, SPECT, and positron emission tomography, PET) has significantly advanced the field of drug addiction research by allowing us to probe and quantify receptors of interest in the living human brain. SPECT in combination with the radiotracer [123I]iomazenil, a GABAA-BZR inverse agonist, and PET with [11C]flumazenil, a GABAA-BZR antagonist, provide a way to quantify numbers of GABAA-BZRs in human subjects. SPECT and PET studies consistently demonstrate decreased GABAA-BZRs in alcohol dependent subjects at approximately 1 month (Abi-Dargham et al., 1994), 3 months (Lingford-Hughes et al., 2000; Lingford-Hughes et al., 1998) and 7 months (Lingford-Hughes et al., 2005) of abstinence. The reductions in GABAA-BZR availability were consistently reported in the medial frontal cortex (Abi-Dargham et al., 1998; Lingford-Hughes et al., 1998) and cerebellum (in alcoholic women only)(Lingford-Hughes et al., 2000). The most recent study, which was the first to evaluate GABAA-BZRs longitudinally within subjects while controlling for smoking status, demonstrated increased GABAA-BZRs at about 1 week withdrawal in alcohol-dependent nonsmokers (n=8) compared to alcohol-dependent smokers (n=15) and controls (n=15), that normalized to control levels by 4 weeks of abstinence (Staley et al., 2005a). The acute increases were predominantly in the cortical regions (Staley et al., 2005a). These preliminary findings during acute abstinence highlight a temporal change in GABAA-BZRs during the recovery from alcohol dependence with increased GABAA-BZRs during early withdrawal, which then decreases during extended recovery. It is not yet clear whether GABAA-BZR availability normalizes to control levels during prolonged recovery, or whether there may be a sustained, long-lasting decrease (Lingford-Hughes et al., 2005) compared to controls.
A number of studies have also implicated the GABAA-BZR in alcohol dependence by demonstrating that alcohol-dependent subjects abstinent at least 2 months have a blunted response to benzodiazepines compared to controls (Bauer et al., 1997; Lingford-Hughes et al., 2005; Volkow et al., 1997). Further, in nonhuman primates that chronically self-administered ethanol, the benzodiazepine sensitivity of GABAA receptors in the amygdala was decreased (Anderson et al., 2007). In combination with the neuroreceptor findings, this suggests several main points. First, the upregulation of GABAA-BZRs observed in alcohol-dependent nonsmokers (Staley et al., 2005a) seemed to develop over the first week of abstinence and was related to the severity of withdrawal symptoms, suggesting that synaptic GABAA-BZRs were recruited in response to alcohol withdrawal. Second, the upregulation/recruitment of receptors was transient, suggesting that the increase represented a transitional stage between two populations of receptors. This is consistent with the preclinical studies suggesting that alcohol potently facilitates the extrasynaptic, BZ-insensitive GABAA receptors (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003), and that the synaptic, BZ-sensitive GABAA-receptors reemerge during acute withdrawal. Third, the blunted sensitivity to BZs during prolonged abstinence is consistent with the idea that protracted alcohol withdrawal is associated with the sustained emergence of synaptic GABAA-receptors and reduction of extrasynaptic GABAA-receptors that are not stimulated by BZ agonists. These findings provide a mechanistic explanation for the cross-tolerance between alcohol and BZs.
One of the caveats to studying neurochemical changes in the brain related to a mental illness, including alcohol dependence, is the high prevalence of persistent brain atrophy in psychiatric populations. Therefore, an observation of a decrease in receptor availability may be due to a reduction in brain volume and not receptor number. Since brain atrophy in alcohol dependence can be widespread and severe (Mechtcheriakov et al., 2007) and compounded by nutritional deficiencies, if not controlled it can introduce a confound to the study. Thus, because differences in GABAA receptor density were reported in the absence of changes in gray matter in alcohol-dependent subjects (Lingford-Hughes et al., 1998), it could be speculated that changes in density may not be due to global or regional brain atrophy but to changes in neurochemistry at the receptor level. Another limitation of measuring the GABAA-BZR with SPECT and PET is that changes in GABAA receptor density may be due to changes in the composition of the receptor subunits, which cannot be differentiated using existing radiotracers. For example, preclinical (Floyd et al., 2004; Kumar et al., 2003; Luddens and Korpi, 1995; Sarviharju et al., 2006) and postmortem human (Mitsuyama et al., 1998) studies suggest differential changes in subunit expression after chronic ethanol exposure. However, [123I]iomazenil SPECT and [11C]flumazenil PET measure all GABAA-BZRs containing various subunits and we cannot distinguish between them. Thus, continuing translational neuroscience research will be critical to determine specific receptor subunit compositions that may be involved in specific targeted behaviors and whether any respond preferentially to treatment medications (Atack, 2010; Wallner and Olsen, 2008).
Neuroreceptor imaging studies in human alcohol-dependent subjects have yet to control for heritable or developmental (gene by environment interactions) factors that influence ligand binding to GABAA receptors. This issue is important because several GABAA receptor subunit genes have been reported to contribute to the heritable risk for alcoholism. The GABAA α2 subunit gene, GABRA2, is involved in modulating acute alcohol sensitivity (Haughey et al., 2008) and subsequently alcohol dependence (Bierut et al., 2010; Covault et al., 2004; Edenberg et al., 2004; Soyka et al., 2007). Additionally, the GABRG1 polymorphism, which encodes the GABAA receptor γ1 subunit and is in linkage disequilibrium with the GABRA2 polymorphism, has been associated with alcohol dependence (Covault et al., 2008). Subsequent studies matching for genotype will be needed to address this issue.
Effects of Nicotine on GABAA Receptors
The primary addictive chemical in tobacco smoke is nicotine, and the majority of work on nicotine's effects on neuronal receptor systems has involved the nicotinic acetylcholine receptor (nAChR) and the catecholaminergic system (Penton and Lester, 2009; Picciotto et al., 1998). However, activation of the nAChR by nicotine or nicotinic agonists has been shown to increase GABA release (Dani and De Biasi, 2001; Lena and Changeux, 1997; Maggi et al., 2001) and influence GABA-mediated inhibition (Freund et al., 1990) within the mesolimbic dopamine system (Balfour et al., 2000; Di Chiara, 2000) and the septohippocampal pathway (Wu et al., 2003). The effects of nicotine on GABA activity likely occur indirectly as a result of nicotine's actions at the nAChRs which are located directly on the soma, preterminal, and presynaptic regions of GABAergic neurons (Colquhoun and Patrick, 1997). Activation of nAChRs enhances GABA activity (Alkondon et al., 1997; Genzen and McGehee, 2005) as well as other neurotransmitter activity (Colquhoun and Patrick, 1997). Acute nicotine administration increases GABA activity (Lena et al., 1993; McMahon et al., 1994a, b), and both acute nicotine (Porcu et al., 2003) and more chronic administration in tobacco smokers (Bjornerem et al., 2004; Field et al., 1994) is associated with increased concentrations of GABA-ergic neurosteroids, which are correlated with negative affect and craving (Marx et al., 2006). The effects of chronic nicotine administration on GABA activity are less clear. While a preclinical study indicated chronic nicotine treatment increased the density of BZ receptors in the cerebral cortex (Magata et al., 2000), a recent neuroimaging study in our laboratory demonstrated no difference in GABAA-BZR availability between current healthy smokers and nonsmokers (Esterlis et al., 2009). An important caveat to this study is that smokers were imaged at 7 hours of smoking abstinence. Since tobacco smoke contains over 4000 chemicals in addition to the primary addictive chemical, nicotine, including the harmala alkaloids which are inverse agonists at the GABAA-BZR (Rommelspacher et al., 1981; Totsuka et al., 1999), these may have interfered with [123I]iomazenil binding to the receptor if they were still present in the brain. However, a subset of smokers (n=4) was imaged again after 5 weeks continued smoking abstinence, and the initial findings of no difference in receptor availability between smokers and nonsmokers were confirmed. Due to the high comorbidity of alcohol dependence and tobacco smoking, it will be important in future studies to determine the effects of nicotine and other components in tobacco smoke on the regulation of the GABAA receptor. It is possible that the harmala alkaloids act either alone or in concert with nicotine to oppose the effects of alcohol at the GABAA receptor.
Interactions of Ethanol and Nicotine on GABAA Receptors
Preclinical and clinical studies suggest a potential mechanism by which nicotine may modulate alcohol's effects at the GABAA receptor. Specifically, modulation of nAChRs by nicotine and a nicotinic antagonist reduced the sensitivity of GABAA-receptors to the benzodiazepine diazepam (Lof et al., 2007). The authors suggest that this may translate into the co-abuse of alcohol and tobacco if nicotine acts to decrease the sedative effects of alcohol. This is consistent with findings in humans that nicotine reduces alcohol's sedating effects (Perkins et al., 2000; Perkins et al., 1995) and thus underscores a potential increased risk for both alcohol (the reduced sensitivity to sedation may lead to more drinking) and nicotine dependence (the increased tolerability of alcohol drives continued smoking).
Despite the high comorbidity of tobacco smoking and alcohol dependence, few clinical studies have been conducted on the interaction of alcohol and nicotine on GABAA receptors. Our group (Staley et al., 2005a) investigated changes in GABAA-BZRs during the recovery from alcohol dependence in tobacco smokers versus nonsmokers using [123I]iomazenil and SPECT brain imaging. There was a significantly increased availability of GABAA-BZRs in alcohol-dependent nonsmokers versus smokers during acute alcohol withdrawal (1 week) suggesting that tobacco smoke suppressed the alcohol-induced GABAA-BZR increase. This acutely increased GABAA-BZR availability is likely primarily composed of synaptic GABAA receptors that are recruited during early alcohol withdrawal. This elevation was correlated with more severe alcohol withdrawal symptoms in alcohol-dependent nonsmokers, which were not apparent in alcohol-dependent smokers, suggesting that tobacco smoking may block some symptoms of alcohol withdrawal by suppressing the increased availability of GABAA-BZRs during acute withdrawal from alcohol.
Preliminary evidence from an ongoing study in our laboratory suggests that even in long-term abstinent alcohol-dependent individuals (abstinent at least 6 months) smoking continues to modify cortical GABAA-BZR availability, such that abstinent alcoholics who continued to smoke had lower GABAA-BZR availability than abstinent alcoholics who remained nonsmokers (Esterlis et al., 2010). Thus, during both acute and long-term alcohol withdrawal, tobacco smoking profoundly regulates alcohol-induced brain chemistry changes.
In addition to SPECT and PET imaging modalities, proton magnetic resonance spectroscopy (MRS) can be used to measure brain chemicals, such as GABA, in vivo. In a preliminary study, Mason and colleagues (2006) measured occipital GABA levels in alcohol-dependent smokers and nonsmokers over the course of recovery from alcohol dependence. Alcohol-dependent nonsmokers at 1 week of alcohol abstinence had higher GABA levels than alcohol-dependent smokers (alcohol-dependent smokers had levels similar to healthy controls), and at 1 month of abstinence GABA levels decreased in nonsmokers and remained unchanged in smokers (Mason et al., 2006). However, there is a limitation to MRS measures of extracellular GABA. MRS measures the total amount of GABA in the volume of tissue, but because the extracellular GABA levels exist in a low concentration, they could be significantly changed without affecting the total concentration of GABA measured in the tissue volume.
Taken together, these initial studies suggest that during acute withdrawal (1 week of abstinence) there may be increased cortical GABA levels and cortical synaptic GABAA-BZRs in nonsmokers, but not smokers, and that by 1 month of abstinence, both GABA levels and GABAA-BZRs have “normalized” in the nonsmokers, while remaining constant in the smokers (Mason et al., 2006; Staley et al., 2005b). In long-term abstinent alcoholics (greater than 1 month), there is consistent evidence for a reduction in GABAA-BZRs (Abi-Dargham et al., 1998; Gilman et al., 1996; Lingford-Hughes et al., 1998), and preliminary evidence indicates that tobacco smoking may mediate this reduction (Esterlis et al., 2010). Thus, prior to chronic alcohol dependence, available GABAA-receptors are BZ sensitive; but during alcohol dependence, the GABAA-BZ insensitive receptors, which are extrasynaptic, are recruited. These extrasynaptic GABAA receptors have a higher affinity for ethanol, but lower conductance than synaptic GABAA receptors, which accounts for alcohol tolerance. With the shift from synaptic to more extrasynaptic GABAA receptors, the overall conductance capacity for chloride is reduced leading to a smaller response to alcohol and a vulnerability to withdrawal due to a functional GABA deficiency. When alcohol is removed from the system, during acute recovery, there is a re-emergence of the GABAA-BZ sensitive receptors in addition to the insensitive receptors resulting in an overall upregulation of GABAA-BZRs as noted in Staley et al., 2005, which resolves and then decreases during extended abstinence.
Nicotine and alcohol may also interact via their actions on glutamic acid decarboxylase (GAD) 67, one of the two enzymes that synthesize GABA in the brain. GAD67 and 65 appear to be responsible for GABA synthesis in terminals and cell bodies, respectively, with GAD67 maintaining levels of GABA (Soghomonian and Martin, 1998). Nicotine has been shown to upregulate GAD67 expression by its actions at nAChRs located on GABAergic interneurons in the cortex and hippocampus (Satta et al., 2008). Chronic alcohol consumption has been linked to a reduction in GAD 67 mRNAs (Falco et al., 2009). Another potential mechanism by which GABA levels could be changed is rapid change of GAD between its inactive apoenzyme to its active holoenzyme. The conversions from the active to inactive forms can occur within minutes (Porter and Martin, 1988; Soghomonian and Martin, 1998). Thus, nicotine may reverse the alcohol-induced suppression in GAD by acute and chronic stimulation of GAD. Indeed, acute nicotine inhalation has been reported to increase the rate of GABA synthesis in the human brain (Mason et al., 2007). It may be by its action on GAD67 that nicotine compensates for alcohol-induced alterations in GABAA-BZRs.
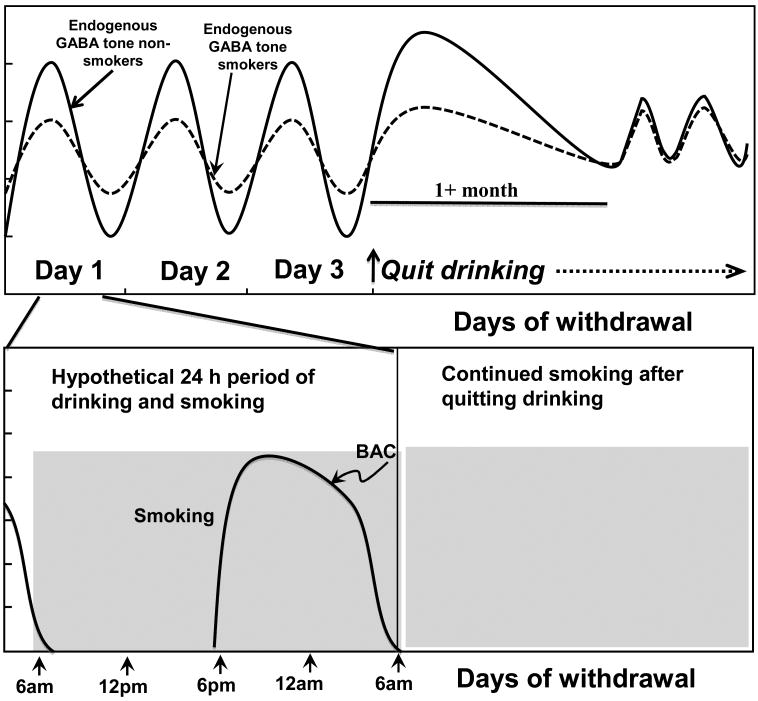
It remains unclear at which point in the addiction process tobacco smoking modulates the alcohol-induced changes on GABA neuronal function. We hypothesize that tobacco smoking is functionally changing or dampening GABA tone in the alcohol maintenance phase during the repeated cycles of drinking that occur every few days during chronic episodes of drinking. There are a variety of drinking patterns in alcohol-dependent individuals. While some individuals engage in daily drinking (Danel et al., 2003; Hatton et al., 2009), others engage in several days of drinking followed by acute withdrawal periods (Mello and Mendelson, 1971). Among younger individuals a common pattern is to engage in binge-drinking on weekends (Harrison et al., 2008). Chronic tobacco smokers do engage in daily smoking, so an alcohol-dependent smoker may have either alcohol or nicotine continuously in their system. In alcohol-dependent smokers, during acute alcohol withdrawal, the neuronal excitability may remain attenuated by continued tobacco smoking. In general, studies suggest that people do not change their smoking status during alcohol and substance abuse treatment (Friend and Pagano, 2005; Toneatto et al., 1995), but there is a lack of research on smoking consumption changes during early alcohol withdrawal. It will be important to determine in future studies whether alcohol-dependent smokers transiently increase their smoking consumption during acute alcohol withdrawal, which may relieve withdrawal symptoms. In summary, during acute withdrawal, there is increased GABA-ergic tone in both alcohol-dependent smokers and nonsmokers, but it is significantly greater in nonsmokers. Over the course of protracted abstinence (greater than 1 month) we hypothesize the GABA oscillations return to baseline conditions in both smokers and nonsmokers (Figure 1).
Figure 1.
The top panel illustrates a hypothetical series of several days of drinking in a chronic alcohol-dependent individual followed by acute and prolonged withdrawal. Repeated dosing of alcohol and withdrawal with regular episodes of smoking shown over the course of one day (bottom panel) may cycle endogenous GABAergic tone up protectively during periods of alcohol withdrawal and down during periods of drinking (top panel), when alcohol supplements the endogenous tone. Smoking may compensate for a loss of alcohol during periods of acute withdrawal (top panel), allowing alcohol-dependent smokers to maintain greater stability of GABAergic tone. Specifically, during acute alcohol withdrawal, there is an initial increase in GABAergic tone in alcohol-dependent smokers and nonsmokers, which is more severe in nonsmokers. During prolonged withdrawal there is likely a return to baseline, or normal cycling of GABA.
Implications
The interaction of ethanol and nicotine at the GABA receptor may contribute to the high rates of the persistence of co-abuse of the drugs resulting in increased intake of alcohol in tobacco smokers and of tobacco in alcohol-dependent individuals. These findings have implications for the treatment of alcohol-dependent smokers and nonsmokers during alcohol withdrawal. Specifically, smokers may be benefiting from a protective effect of nicotine or tobacco smoke on GABA-ergic alcohol-withdrawal symptoms. If nicotine does suppress the alcohol-induced increase in GABAA-BZRs, this suggests that nicotine replacement therapies and nicotinic agonist medications may be useful treatment strategies in both smokers and nonsmokers (nicotine replacement is not typically addictive) during alcohol withdrawal. There is recent preclinical (Steensland et al., 2007) and clinical (McKee et al., 2009) evidence to support the use of varenicline, a partial ß2-nAChR agonist, for reducing alcohol consumption. Additionally, the nicotine patch reduced alcohol self-administration in a human laboratory paradigm in heavy drinking daily smokers (McKee et al., 2008). Although studies examining the direct effects of ethanol on ß2-nAChRs are mixed (Gorbounova et al., 1998; Ribeiro-Carvalho et al., 2009; Robles and Sabria, 2008), prolonged abstinence from chronic alcohol consumption was recently associated with decreased ß2-nAChR availability compared to baseline in nonhuman primates (Cosgrove et al., 2010). If this decrease is found to be associated with withdrawal symptoms and increased relapse rates in humans, this suggests that nicotinic agonist drugs may be useful to help keep the ß2-nAChRs at baseline levels. Thus, nicotinic agonist therapy (varenicline, nicotine replacement) may be indicated for both alcohol and nicotine withdrawal symptoms in alcohol-dependent smokers encouraged to quit drinking and smoking at the same time. Because preclinical evidence suggests varenicline is useful in reducing alcohol-seeking behaviors in animals not exposed to nicotine (Hendrickson et al., 2010; Steensland et al., 2007), future research should also evaluate the potential benefit of nicotinic agonist therapy during initial abstinence from alcohol in alcohol-dependent nonsmokers.
Further, both ethanol and nicotine have been shown to increase levels of GABA-ergic neurosteroids that act at the GABAA receptor in a manner similar to positive modulators. Thus, an alcohol-dependent smoker who quits drinking may benefit from the protective effects of neurosteroids that are promoted as they continue to smoke. A recent study suggests that neuroactive steroids may be a novel treatment for smoking cessation (Marx et al., 2006); and, they may also prove useful during alcohol withdrawal and for dual alcohol and smoking cessation, especially because neurosteroids do not exhibit cross-tolerance as do benzodiazepines.
In addition to viewing nicotine and nicotinic agonists as potential therapeutics for alcohol dependence, it is possible that GABA-ergic agents may be helpful in tobacco smoking cessation. Preclinical (Corrigall et al., 2001; Paterson et al., 2004) and clinical (Franklin et al., 2009) studies suggest that baclofen attenuates nicotine and cigarette consumption. A clinical limitation of baclofen is that it must be taken every 4-6 hours. Thus, effective GABA-ergic drugs for smoking cessation will be dependent on new medications development.
Recommendations for Future Research
1. Continued development of neuroimaging ligands
It is important to continue development of compounds for in vivo imaging. Specifically, ligands that preferentially measure different subunit compositions and differentiate between synaptic and extrasynaptic GABAA receptors, based on the evidence that subunit composition of the GABAA receptor changes with the development of alcohol dependence (Krystal et al., 2006). The importance of a polymorphism in GABRA2 (Edenberg et al., 2004; Soyka et al., 2007) and GABRG1 (Covault et al., 2008) for alcohol dependence highlights the need for an α2 and γ1 selective ligands. The GABAB-receptor is also a site of interest and a radiotracer for this receptor is currently under development.
2. Continued development of imaging methodology
Currently, MRS has been used to measure a specific voxel of interest in the occipital cortex in alcohol-dependent smokers and nonsmokers. With the advancing technology, GABA and other chemicals measurements in other brain regions of interest have been examined in other populations and it will be critical to examine alternative brain regions such as the frontal cortex that are critically involved in alcohol dependence.
3. Better characterization of alcohol and tobacco smoking use in imaging studies
Recommendations for assessing patterns of alcohol consumption have been given (Leeman et al., 2007). We would additionally recommend obtaining measures in tobacco smokers that assess tobacco smoking dependence, craving and withdrawal, including the Fagerström Test for Nicotine Dependence (FTND) (Heatherton et al., 1991), the Tiffany Questionnaire for Smoking Urges (QSU) (Tiffany et al., 1993), and the Nicotine Withdrawal Scale (Hughes and Hatsukami, 1986). Biochemical measures of recent smoking, including carbon monoxide and cotinine levels, should be used for confirmation of smoking and nonsmoking status in addition to self-reports of cigarettes smoked. Time since last cigarette should be reported and standardized in imaging protocols. Obtaining these measures in all subjects will increase cohesion between imaging groups and the interpretation of findings across studies.
4. Integration of receptor imaging with other biochemical tools, i.e., multimodal imaging
Because many imaging studies are limited in their findings, e.g., to a difference in receptor availability between two groups, or a change over time, it is important to increase the amount of biochemical data obtained in subjects. Examples include obtaining MRS in the same subjects, measuring functional brain changes, obtaining additional receptor imaging scans to examine changes in more than one receptor system in the same subjects, and correlating promising biomarkers with the brain measures.
Summary
Despite the high prevalence of comorbid tobacco smoking and alcohol dependence, studies on the neurochemical mechanisms underlying these disorders and their interactions are scarce. Moreover, because of the high rate of comorbidity and the devastating medical consequences of using both drugs, additional studies are needed to address changes that occur during acute withdrawal from alcohol alone, and from dual alcohol and nicotine withdrawal, to delineate the best strategy for abstinence in alcohol-dependent individuals who abuse both drugs, i.e., stop alcohol first vs. nicotine first, or both simultaneously. In this review we have described evidence that nicotine profoundly alters both the acute and chronic effects of ethanol on GABAA receptors, which may underlie the high comorbidity of alcohol and nicotine dependence. This relationship has significant implications for our understanding of the recovery from alcohol dependence in both smokers and nonsmokers. Future studies should systematically examine the effects of comorbid tobacco smoking during the recovery from alcohol dependence in order to successfully inform the clinical treatment of alcoholism.
Acknowledgments
National Institute of Health (K01DA20651, K05AA014715), VA CDA-1, VA Alcohol Research Center
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abi-Dargham A, Krystal J, Anjivel S, Scanley B, Zoghbi S, Baldwin R, Rajeevan N, Ellis S, Petrakis I, Seibyl J, Charney D, Laruelle M, Innis R. Alterations of benzodiazepine receptors in type II alcoholic subjects measured with SPECT and [123I]iomazenil. Am J Psychiatry. 1998;155:1550–1555. doi: 10.1176/ajp.155.11.1550. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Laruelle M, Seibyl J, Rattner Z, Baldwin R, Zoghbi S, Zea-Ponce Y, Bremner J, Hyde T, Charney D, Hoffer P, Innis R. SPECT measurement of benzodiazepine receptors in human brain with [123I]Iomazenil: kinetic and equilibrium paradigms. J Nucl Med. 1994;35:228–238. [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther. 1997;283:1396–1411. [PubMed] [Google Scholar]
- Anderson NJ, Daunais JB, Friedman DP, Grant KA, McCool BA. Long-Term Ethanol Self-Administration by the Nonhuman Primate, Macaca fascicularis, Decreases the Benzodiazepine Sensitivity of Amygdala GABA(A) Receptors. Alcohol Clin Exp Res. 2007 doi: 10.1111/j.1530-0277.2007.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atack JR. Preclinical and clinical pharmacology of the GABAA receptor alpha5 subtype-selective inverse agonist alpha5IA. Pharmacol Ther. 2010;125:11–26. doi: 10.1016/j.pharmthera.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Balfour DJ, Wright AE, Benwell ME, Birrell CE. The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behav Brain Res. 2000;113:73–83. doi: 10.1016/s0166-4328(00)00202-3. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Affricano D, Trabucchi M, Purdy RH, Colombo G, Agabio R, Gessa GL. Ethanol markedly increases “GABAergic” neurosteroids in alcohol-preferring rats. Eur J Pharmacol. 1999;384:R1–2. doi: 10.1016/s0014-2999(99)00678-0. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Gross JB, Meyer RE, Greenblatt DJ. Chronic alcohol abuse and the acute sedative and neurophysiologic effects of midazolam. Psychopharmacology (Berl) 1997;133:293–299. doi: 10.1007/s002130050404. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Agrawal A, Bucholz KK, Doheny KF, Laurie C, Pugh E, Fisher S, Fox L, Howells W, Bertelsen S, Hinrichs AL, Almasy L, Breslau N, Culverhouse RC, Dick DM, Edenberg HJ, Foroud T, Grucza RA, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Krueger RF, Kuperman S, Lynskey M, Mann K, Neuman RJ, Nothen MM, Nurnberger JI, Jr, Porjesz B, Ridinger M, Saccone NL, Saccone SF, Schuckit MA, Tischfield JA, Wang JC, Rietschel M, Goate AM, Rice JP. A genome-wide association study of alcohol dependence. Proc Natl Acad Sci U S A. 107:5082–5087. doi: 10.1073/pnas.0911109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Agrawal A, Bucholz KK, Doheny KF, Laurie C, Pugh E, Fisher S, Fox L, Howells W, Bertelsen S, Hinrichs AL, Almasy L, Breslau N, Culverhouse RC, Dick DM, Edenberg HJ, Foroud T, Grucza RA, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Krueger RF, Kuperman S, Lynskey M, Mann K, Neuman RJ, Nothen MM, Nurnberger JI, Jr, Porjesz B, Ridinger M, Saccone NL, Saccone SF, Schuckit MA, Tischfield JA, Wang JC, Rietschel M, Goate AM, Rice JP. A genome-wide association study of alcohol dependence. Proc Natl Acad Sci U S A. 2010;107:5082–5087. doi: 10.1073/pnas.0911109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornerem A, Straume B, Midtby M, Fonnebo V, Sundsfjord J, Svartberg J, Acharya G, Oian P, Berntsen GK. Endogenous sex hormones in relation to age, sex, lifestyle factors, and chronic diseases in a general population: the Tromso Study. J Clin Endocrinol Metab. 2004;89:6039–6047. doi: 10.1210/jc.2004-0735. [DOI] [PubMed] [Google Scholar]
- Bobo J, Gilchrist L, Schilling R, Noach B, Schinke S. Cigarette smoking cessation attempts by recovering alcoholics. Addict Behav. 1987;12:209–215. doi: 10.1016/0306-4603(87)90030-x. [DOI] [PubMed] [Google Scholar]
- Carmody T, Brischetto C, Matarazzo J, O'Donnell R, Connor W. Co-occurrent use of cigarettes, alcohol, and coffee in healthy, community-living men and women. Health Psychol. 1985;4:323–335. doi: 10.1037//0278-6133.4.4.323. [DOI] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton ME, Sweetnam PM, Fitzgerald LW, Terwilliger RZ, Nestler EJ, Duman RS. Chronic ethanol administration regulates the expression of GABAA receptor alpha 1 and alpha 5 subunits in the ventral tegmental area and hippocampus. J Neurochem. 1997;68:121–127. doi: 10.1046/j.1471-4159.1997.68010121.x. [DOI] [PubMed] [Google Scholar]
- Colquhoun LM, Patrick JW. Pharmacology of neuronal nicotinic acetylcholine receptor subtypes. Adv Pharmacol. 1997;39:191–220. doi: 10.1016/s1054-3589(08)60072-1. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Zhang J, Adamson KL. GABA mechanisms in the pedunculopontine tegmental nucleus influence particular aspects of nicotine self-administration selectively in the rat. Psychopharmacology (Berl) 2001;158:190–197. doi: 10.1007/s002130100869. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Kloczynski T, Bois F, Pittman B, Tamagnan G, Seibyl JP, Krystal JH, Staley JK. Decreased Beta(2)*-nicotinic acetylcholine receptor availability after chronic ethanol exposure in nonhuman primates. Synapse. 2010;64:729–732. doi: 10.1002/syn.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004;129B:104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Jensen K, Anton R, Kranzler HR. Markers in the 5′-region of GABRG1 associate to alcohol dependence and are in linkage disequilibrium with markers in the adjacent GABRA2 gene. Neuropsychopharmacology. 2008;33:837–848. doi: 10.1038/sj.npp.1301456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danel T, Jeanson R, Touitou Y. Temporal pattern in consumption of the first drink of the day in alcohol-dependent persons. Chronobiol Int. 2003;20:1093–1102. doi: 10.1081/cbi-120025533. [DOI] [PubMed] [Google Scholar]
- Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacol Biochem Behav. 2001;70:439–446. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- Davies M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J Psychiatry Neurosci. 2003;28:263–274. [PMC free article] [PubMed] [Google Scholar]
- Dawson D. Drinking as a risk factor for sustained smoking. Drug and Alcohol Dependence. 2000;59:235–249. doi: 10.1016/s0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Bidirectional alterations of GABA(A) receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. Sensitization of gamma-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J Pharmacol Exp Ther. 1996;278:510–517. [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Morrow AL. The neurosteroid, 3 alpha-hydroxy-5 alpha-pregnan-20-one, protects against bicuculline-induced seizures during ethanol withdrawal in rats. Alcohol Clin Exp Res. 1995;19:350–355. doi: 10.1111/j.1530-0277.1995.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza J, Guerrera M. Alcoholism and smoking. J Stud Alcohol. 1990;51:130–135. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr, O'Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterlis I, Cosgrove K, Kirk K, Petrakis I, Guidone E, Tamagnan G, Bois F, Baldwin R, Seibyl J, Staley J, Krystal J. Research Society on Alcoholism. San Antonio, TX: 2010. Preliminary evidence that smoking influences cortical GABA-A-BZR availability in long term sober alcohol dependent subjects; p. 23A. [Google Scholar]
- Esterlis I, Cosgrove KP, Batis JC, Bois F, Kloczynski TA, Stiklus SM, Perry E, Tamagnan GD, Seibyl JP, Makuch R, Krishnan-Sarin S, O'Malley S, Staley JK. GABA(A)-benzodiazepine receptor availability in smokers and nonsmokers: Relationship to subsyndromal anxiety and depression. Synapse. 2009;63:1089–1099. doi: 10.1002/syn.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco AM, Bergstrom HC, Bachus SE, Smith RF. Persisting changes in basolateral amygdala mRNAs after chronic ethanol consumption. Physiol Behav. 2009;96:169–173. doi: 10.1016/j.physbeh.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Field AE, Colditz GA, Willett WC, Longcope C, McKinlay JB. The relation of smoking, age, relative weight, and dietary intake to serum adrenal steroids, sex hormones, and sex hormone-binding globulin in middle-aged men. J Clin Endocrinol Metab. 1994;79:1310–1316. doi: 10.1210/jcem.79.5.7962322. [DOI] [PubMed] [Google Scholar]
- Flatscher-Bader T, Wilce PA. The effect of alcohol and nicotine abuse on gene expression in the brain. Nutr Res Rev. 2009;22:148–162. doi: 10.1017/S0954422409990114. [DOI] [PubMed] [Google Scholar]
- Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, McCool BA. Long-term ethanol self-administration by cynomolgus macaques alters the pharmacology and expression of GABAA receptors in basolateral amygdala. J Pharmacol Exp Ther. 2004;311:1071–1079. doi: 10.1124/jpet.104.072025. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Harper D, Kampman K, Kildea-McCrea S, Jens W, Lynch KG, O'Brien CP, Childress AR. The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study. Drug Alcohol Depend. 2009;103:30–36. doi: 10.1016/j.drugalcdep.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund G, Ballinger WE. Loss of synaptic receptors can precede morphologic changes induced by alcoholism. Alcohol Alcohol Suppl. 1991;1:385–391. [PubMed] [Google Scholar]
- Freund RK, Luntz-Leybman V, Collins AC. Nicotine interferes with GABA-mediated inhibitory processes in mouse hippocampus. Brain Res. 1990;527:286–291. doi: 10.1016/0006-8993(90)91148-a. [DOI] [PubMed] [Google Scholar]
- Friend KB, Pagano ME. Changes in cigarette consumption and drinking outcomes: findings from Project MATCH. J Subst Abuse Treat. 2005;29:221–229. doi: 10.1016/j.jsat.2005.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genzen JR, McGehee DS. Nicotinic modulation of GABAergic synaptic transmission in the spinal cord dorsal horn. Brain Res. 2005;1031:229–237. doi: 10.1016/j.brainres.2004.10.042. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koeppe R, Adams K, Johnson-Greene D, Junck L, Kluin K, Brunberg J, Martorello S, Lohman M. Positron emission tomographic studies of cerebral benzodiazepine -receptor binding in chronic alcoholics. Ann Neurol. 1996;40:163–171. doi: 10.1002/ana.410400207. [DOI] [PubMed] [Google Scholar]
- Gorbounova O, Svensson A, Jonsson P, Mousavi M, Miao H, Hellstrom-Lindahl E, Nordberg A. Chronic ethanol treatment decreases [3H]epibatidine and [3H]nicotine binding and differentially regulates mRNA levels of nicotinic acetylcholine receptor subunits expressed in M10 and SH-SY5Y neuroblastoma cells. J Neurochem. 1998;70:1134–1142. doi: 10.1046/j.1471-4159.1998.70031134.x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Harrison EL, Desai RA, McKee SA. Nondaily smoking and alcohol use, hazardous drinking, and alcohol diagnoses among young adults: findings from the NESARC. Alcohol Clin Exp Res. 2008;32:2081–2087. doi: 10.1111/j.1530-0277.2008.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton J, Burton A, Nash H, Munn E, Burgoyne L, Sheron N. Drinking patterns, dependency and life-time drinking history in alcohol-related liver disease. Addiction. 2009;104:587–592. doi: 10.1111/j.1360-0443.2008.02493.x. [DOI] [PubMed] [Google Scholar]
- Haughey HM, Ray LA, Finan P, Villanueva R, Niculescu M, Hutchison KE. Human gamma-aminobutyric acid A receptor alpha2 gene moderates the acute effects of alcohol and brain mRNA expression. Genes Brain Behav. 2008;7:447–454. doi: 10.1111/j.1601-183X.2007.00369.x. [DOI] [PubMed] [Google Scholar]
- Heatherton T, Kozlowski L, Frecker R, Fagerstrom K. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionairre. Brit J Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Pang X, Gardner PD, Tapper AR. Activation of alpha4* nAChRs is necessary and sufficient for varenicline-induced reduction of alcohol consumption. J Neurosci. 2010;30:10169–10176. doi: 10.1523/JNEUROSCI.2601-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman PL. Glutamate receptors in alcohol withdrawal-induced neurotoxicity. Metab Brain Dis. 1995;10:73–79. doi: 10.1007/BF01991784. [DOI] [PubMed] [Google Scholar]
- Hughes J, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Alcohol withdrawal seizures. Epilepsy Behav. 2009;15:92–97. doi: 10.1016/j.yebeh.2009.02.037. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Borland R, Hyland A, McKee SA, O'Connor RJ, Fong GT, Cummings KM. Quitting smoking and change in alcohol consumption in the International Tobacco Control (ITC) Four Country Survey. Drug Alcohol Depend. 2010a;110:101–107. doi: 10.1016/j.drugalcdep.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Metrik J. Alcohol use and initial smoking lapses among heavy drinkers in smoking cessation treatment. Nicotine Tob Res. 2010b doi: 10.1093/ntr/ntq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Spigelman I, Sapp DW, Olsen RW. Persistent reduction of GABA(A) receptor-mediated inhibition in rat hippocampus after chronic intermittent ethanol treatment. Brain Res. 1996;709:221–228. doi: 10.1016/0006-8993(95)01274-5. [DOI] [PubMed] [Google Scholar]
- Kokka N, Sapp DW, Taylor AM, Olsen RW. The kindling model of alcohol dependence: similar persistent reduction in seizure threshold to pentylenetetrazol in animals receiving chronic ethanol or chronic pentylenetetrazol. Alcohol Clin Exp Res. 1993;17:525–531. doi: 10.1111/j.1530-0277.1993.tb00793.x. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Uusi-Oukari M. Cerebellar GABAA receptors and alcohol-related behaviors: focus on diazepam-insensitive [3H]Ro 15-4513 binding. Adv Biochem Psychopharmacol. 1992;47:289–299. [PubMed] [Google Scholar]
- Koski A, Ojanpera I, Vuori E. Alcohol and benzodiazepines in fatal poisonings. Alcohol Clin Exp Res. 2002;26:956–959. doi: 10.1097/01.ALC.0000021337.78063.67. [DOI] [PubMed] [Google Scholar]
- Koski A, Vuori E, Ojanpera I. Relation of postmortem blood alcohol and drug concentrations in fatal poisonings involving amitriptyline, propoxyphene and promazine. Hum Exp Toxicol. 2005;24:389–396. doi: 10.1191/0960327105ht542oa. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Staley J, Mason G, Petrakis IL, Kaufman J, Harris RA, Gelernter J, Lappalainen J. Gamma-aminobutyric acid type A receptors and alcoholism: intoxication, dependence, vulnerability, and treatment. Arch Gen Psychiatry. 2006;63:957–968. doi: 10.1001/archpsyc.63.9.957. [DOI] [PubMed] [Google Scholar]
- Kumar S, Fleming RL, Morrow AL. Ethanol regulation of gamma-aminobutyric acid A receptors: genomic and nongenomic mechanisms. Pharmacol Ther. 2004;101:211–226. doi: 10.1016/j.pharmthera.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kralic JE, O'Buckley TK, Grobin AC, Morrow AL. Chronic ethanol consumption enhances internalization of alpha1 subunit-containing GABAA receptors in cerebral cortex. J Neurochem. 2003;86:700–708. doi: 10.1046/j.1471-4159.2003.01894.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Huffman CJ, O'Malley SS. Alcohol history and smoking cessation in nicotine replacement therapy, bupropion sustained release and varenicline trials: a review. Alcohol Alcohol. 2007;42:196–206. doi: 10.1093/alcalc/agm022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lena C, Changeux JP. Role of Ca2+ ions in nicotinic facilitation of GABA release in mouse thalamus. J Neurosci. 1997;17:576–585. doi: 10.1523/JNEUROSCI.17-02-00576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lena C, Changeux JP, Mulle C. Evidence for “preterminal” nicotinic receptors on GABAergic axons in the rat interpeduncular nucleus. J Neurosci. 1993;13:2680–2688. doi: 10.1523/JNEUROSCI.13-06-02680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27:12367–12377. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes A, Acton P, Gacinovic S, Boddington S, Costa D, Pilowsky L, Ell P, Marshall E, Kerwin R. Levels of g-aminobutyric acid-benzodiazepine receptors in abstinent, alcohol dependent women: Preliminary findings from an 123I-iomazenil single photon emission tomography study. Alcoholism, Clin and Exp Res. 2000;24:1449–1455. [PubMed] [Google Scholar]
- Lingford-Hughes A, Acton P, Gacinovic S, Suckling J, Busatto G, Boddington S, Bullmore E, Woodruff P, Costa D, Pilowsky L, Ell P, Marshall E, Kerwin R. Reduced levels of GABA-benzodiazepine receptor in alcohol dependency in the absence of grey matter atrophy. Br J Psychiatry. 1998;173:116–122. doi: 10.1192/bjp.173.2.116. [DOI] [PubMed] [Google Scholar]
- Lingford-Hughes AR, Wilson SJ, Cunningham VJ, Feeney A, Stevenson B, Brooks DJ, Nutt DJ. GABA-benzodiazepine receptor function in alcohol dependence: a combined 11C-flumazenil PET and pharmacodynamic study. Psychopharmacology (Berl) 2005 doi: 10.1007/s00213-005-2271-x. [DOI] [PubMed] [Google Scholar]
- Lof E, Chau PP, Stomberg R, Soderpalm B. Ethanol-induced dopamine elevation in the rat--modulatory effects by subchronic treatment with nicotinic drugs. Eur J Pharmacol. 2007;555:139–147. doi: 10.1016/j.ejphar.2006.10.056. [DOI] [PubMed] [Google Scholar]
- Luddens H, Korpi E. Biological function of GABAA/benzodiazepine receptor heterogeneity. J Psychiatry Res. 1995;29:77–94. doi: 10.1016/0022-3956(94)00040-x. [DOI] [PubMed] [Google Scholar]
- Magata Y, Kitano H, Shiozaki T, Iida Y, Nishizawa S, Saji H, Konishi J. Effect of chronic (-) nicotine treatment on rat cerebral benzodiazepine receptors. Nuclear Med & Biol. 2000;27:57–60. doi: 10.1016/s0969-8051(99)00078-5. [DOI] [PubMed] [Google Scholar]
- Maggi L, Sher E, Cherubini E. Regulation of GABA release by nicotinic acetylcholine receptors in the neonatal rat hippocampus. J Physiol. 2001;536:89–100. doi: 10.1111/j.1469-7793.2001.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci. 2007;30:343–349. doi: 10.1016/j.tins.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Markou A. Review. Neurobiology of nicotine dependence. Philos Trans R Soc Lond B Biol Sci. 2008;363:3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx CE, Trost WT, Shampine L, Behm FM, Giordano LA, Massing MW, Rose JE. Neuroactive steroids, negative affect, and nicotine dependence severity in male smokers. Psychopharmacology (Berl) 2006;186:462–472. doi: 10.1007/s00213-005-0226-x. [DOI] [PubMed] [Google Scholar]
- Mason GF, Boumezbeur F, Sanacora G, Guidone E, Watzl J, Novotny E, Weinzimer S, Shulman I, Krystal JH, Rothman DL, O'Malley S. Acute nicotine stimulates GABA synthesis in human brain. Proc Intern Soc Magn Reson Med. 2007:770. [Google Scholar]
- Mason GF, Petrakis IL, de Graaf RA, Gueorguieva R, Guidone E, Coric V, Epperson CN, Rothman DL, Krystal JH. Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biol Psychiatry. 2006;59:85–93. doi: 10.1016/j.biopsych.2005.06.009. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O'Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline Reduces Alcohol Self-Administration in Heavy-Drinking Smokers. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, O'Malley SS, Shi J, Mase T, Krishnan-Sarin S. Effect of transdermal nicotine replacement on alcohol responses and alcohol self-administration. Psychopharmacology (Berl) 2008;196:189–200. doi: 10.1007/s00213-007-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LL, Yoon KW, Chiappinelli VA. Electrophysiological evidence for presynaptic nicotinic receptors in the avian ventral lateral geniculate nucleus. J Neurophysiol. 1994a;71:826–829. doi: 10.1152/jn.1994.71.2.826. [DOI] [PubMed] [Google Scholar]
- McMahon LL, Yoon KW, Chiappinelli VA. Nicotinic receptor activation facilitates GABAergic neurotransmission in the avian lateral spiriform nucleus. Neuroscience. 1994b;59:689–698. doi: 10.1016/0306-4522(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Mechtcheriakov S, Brenneis C, Egger K, Koppelstaetter F, Schocke M, Marksteiner J. A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78:610–614. doi: 10.1136/jnnp.2006.095869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. A quantitative analysis of drinking patterns in alcoholics. Arch Gen Psychiatry. 1971;25:527–539. doi: 10.1001/archpsyc.1971.01750180047009. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Tizabi Y, Staley JK, Durazzo TC, Glass JM, Nixon SJ. Smoking comorbidity in alcoholism: neurobiological and neurocognitive consequences. Alcohol Clin Exp Res. 2006;30:253–264. doi: 10.1111/j.1530-0277.2006.00034.x. [DOI] [PubMed] [Google Scholar]
- Mitsuyama H, Little KY, Sieghart W, Devaud LL, Morrow AL. GABA(A) receptor alpha1, alpha4, and beta3 subunit mRNA and protein expression in the frontal cortex of human alcoholics. Alcohol Clin Exp Res. 1998;22:815–822. [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML. Biphasic effect of ethanol on extracellular accumulation of glutamate in the hippocampus and the nucleus accumbens. Neurosci Lett. 1994;178:99–102. doi: 10.1016/0304-3940(94)90299-2. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, Grant KA. Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action? Alcohol Clin Exp Res. 1999;23:1933–1940. doi: 10.1111/j.1530-0277.1999.tb04094.x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Porcu P, Boyd KN, Grant KA. Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues Clin Neurosci. 2006;8:463–477. doi: 10.31887/DCNS.2006.8.4/amorrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Karanian JW, Paul SM. Chronic ethanol administration alters gamma-aminobutyric acid, pentobarbital and ethanol-mediated 36Cl- uptake in cerebral cortical synaptoneurosomes. J Pharmacol Exp Ther. 1988;246:158–164. [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. The GABAB receptor agonists baclofen and CGP44532 decreased nicotine self-administration in the rat. Psychopharmacology (Berl) 2004;172:179–186. doi: 10.1007/s00213-003-1637-1. [DOI] [PubMed] [Google Scholar]
- Penton RE, Lester RA. Cellular events in nicotine addiction. Semin Cell Dev Biol. 2009;20:418–431. doi: 10.1016/j.semcdb.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Grobe JE. Sex differences in the acute effects of cigarette smoking on the reinforcing value of alcohol. Behav Pharmacol. 2000;11:63–70. doi: 10.1097/00008877-200002000-00007. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Sexton JE, DiMarco A, Grobe JE, Scierka A, Stiller RL. Subjective and cardiovascular responses to nicotine combined with alcohol in male and female smokers. Psychopharmacology (Berl) 1995;119:205–212. doi: 10.1007/BF02246162. [DOI] [PubMed] [Google Scholar]
- Petrie J, Sapp DW, Tyndale RF, Park MK, Fanselow M, Olsen RW. Altered gabaa receptor subunit and splice variant expression in rats treated with chronic intermittent ethanol. Alcohol Clin Exp Res. 2001;25:819–828. [PubMed] [Google Scholar]
- Picciotto M, Zoli M, Rimondin R, Lena C, Marubio L, Pich E, Fuxe K, Changeux J. Acetycholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Porcu P, Sogliano C, Cinus M, Purdy RH, Biggio G, Concas A. Nicotine-induced changes in cerebrocortical neuroactive steroids and plasma corticosterone concentrations in the rat. Pharmacol Biochem Behav. 2003;74:683–690. doi: 10.1016/s0091-3057(02)01065-1. [DOI] [PubMed] [Google Scholar]
- Porter TG, Martin DL. Stability and activation of glutamate apodecarboxylase from pig brain. Journal of Neurochemistry. 1988;51:1886–1891. doi: 10.1111/j.1471-4159.1988.tb01173.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Carvalho A, Lima CS, Medeiros AH, Siqueira NR, Filgueiras CC, Manhaes AC, Abreu-Villaca Y. Combined exposure to nicotine and ethanol in adolescent mice: effects on the central cholinergic systems during short and long term withdrawal. Neuroscience. 2009;162:1174–1186. doi: 10.1016/j.neuroscience.2009.05.032. [DOI] [PubMed] [Google Scholar]
- Robles N, Sabria J. Effects of moderate chronic ethanol consumption on hippocampal nicotinic receptors and associative learning. Neurobiol Learn Mem. 2008;89:497–503. doi: 10.1016/j.nlm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Romberger DJ, Grant K. Alcohol consumption and smoking status: the role of smoking cessation. Biomed Pharmacother. 2004;58:77–83. doi: 10.1016/j.biopha.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Rommelspacher H, Nanz C, Borbe HO, Fehske KJ, Muller WE, Wollert U. Benzodiazepine antagonism by harmane and other beta-carbolines in vitro and in vivo. Eur J Pharmacol. 1981;70:409–416. doi: 10.1016/0014-2999(81)90173-4. [DOI] [PubMed] [Google Scholar]
- Sanna E, Harris RA. Recent developments in alcoholism:neuronal ion channels. Recent Dev Alcohol. 1993;11:169–186. [PubMed] [Google Scholar]
- Sarviharju M, Hyytia P, Hervonen A, Jaatinen P, Kiianmaa K, Korpi ER. Lifelong ethanol consumption and brain regional GABAA receptor subunit mRNA expression in alcohol-preferring rats. Alcohol. 2006;40:159–166. doi: 10.1016/j.alcohol.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Satta R, Maloku E, Zhubi A, Pibiri F, Hajos M, Costa E, Guidotti A. Nicotine decreases DNA methyltransferase 1 expression and glutamic acid decarboxylase 67 promoter methylation in GABAergic interneurons. Proc Natl Acad Sci U S A. 2008;105:16356–16361. doi: 10.1073/pnas.0808699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer IR, Hoft NR, Ehringer MA. The genetic components of alcohol and nicotine co-addiction: from genes to behavior. Curr Drug Abuse Rev. 2008;1:124–134. doi: 10.2174/1874473710801020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Sigel E, Buhr A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci. 1997;18:425–429. doi: 10.1016/s0165-6147(97)01118-8. [DOI] [PubMed] [Google Scholar]
- Sivilotti L, Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol. 1991;36:35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends in Pharmacological Sciences. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- Soyka M, Preuss UW, Hesselbrock V, Zill P, Koller G, Bondy B. GABA-A2 receptor subunit gene (GABRA2) polymorphisms and risk for alcohol dependence. J Psychiatr Res. 2007 doi: 10.1016/j.jpsychires.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Staley J, Gottschalk C, Petrakis I, Gueorguieva R, O'Malley S, Baldwin R, Jatlow P, Verhoeff N, Perry E, Weinzimmer D, Frohlich E, Ruff E, van Dyck C, Seibyl J, Innis R, Krystal J. Cortical GABAA/benzodiazepine receptors in recovery from alcohol dependence: relationship to features of alcohol dependence and cigarette smoking. Arch Gen Psychiatry. 2005a;62:877–888. doi: 10.1001/archpsyc.62.8.877. [DOI] [PubMed] [Google Scholar]
- Staley JK, Gottschalk C, Petrakis IL, Gueorguieva R, O'Malley S, Baldwin R, Jatlow P, Verhoeff NP, Perry E, Weinzimmer D, Frohlich E, Ruff E, van Dyck CH, Seibyl JP, Innis RB, Krystal JH. Cortical gamma-aminobutyric acid type A-benzodiazepine receptors in recovery from alcohol dependence: relationship to features of alcohol dependence and cigarette smoking. Arch Gen Psychiatry. 2005b;62:877–888. doi: 10.1001/archpsyc.62.8.877. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzdak PD, Paul SM. Ethanol stimulates GABA receptor-mediated Cl- ion flux in vitro: possible relationship to the anxiolytic and intoxicating actions of alcohol. Psychopharmacol Bull. 1987;23:445–451. [PubMed] [Google Scholar]
- Tatebayashi H, Motomura H, Narahashi T. Alcohol modulation of single GABA(A) receptor-channel kinetics. Neuroreport. 1998;9:1769–1775. doi: 10.1097/00001756-199806010-00018. [DOI] [PubMed] [Google Scholar]
- Tiffany S, Singleton E, Haertzer CA, et al. The development of a craving questionnaire. Drug Alcohol Depend. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- Toneatto A, Sobell LC, Sobell MB, Kozlowski LT. Effect of cigarette smoking on alcohol treatment outcome. J Subst Abuse. 1995;7:245–252. doi: 10.1016/0899-3289(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Totsuka Y, Ushiyama H, Ishihara J, Sinha R, Goto S, Sugimura T, Wakabayashi K. Quantification of the co-mutagenic beta-carbolines, norharman and harman, in cigarette smoke condensates and cooked foods. Cancer Lett. 1999;143:139–143. doi: 10.1016/s0304-3835(99)00143-3. [DOI] [PubMed] [Google Scholar]
- Tran V, Synder S, Major L, Hawley R. GABA receptors are increased in the brains of alcoholics. Ann Neurol. 1981;9:289–292. doi: 10.1002/ana.410090312. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Overall JE, Hitzemann R, Fowler JS, Pappas N, Frecska E, Piscani K. Regional brain metabolic response to lorazepam in alcoholics during early and late alcohol detoxification. Alcohol Clin Exp Res. 1997;21:1278–1284. [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Olsen RW. Physiology and pharmacology of alcohol: the imidazobenzodiazepine alcohol antagonist site on subtypes of GABAA receptors as an opportunity for drug development? Br J Pharmacol. 2008;154:288–298. doi: 10.1038/bjp.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Buzsaki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci. 1996;16:6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Hajszan T, Leranth C, Alreja M. Nicotine recruits a local glutamatergic circuit to excite septohippocampal GABAergic neurons. Eur J Neurosci. 2003;18:1155–1168. doi: 10.1046/j.1460-9568.2003.02847.x. [DOI] [PubMed] [Google Scholar]