Abstract
Background
Hyperoxaluria and increased calcium oxalate stone formation occur after Rouxen- Y gastric bypass (RYGB) surgery for morbid obesity. The etiology of this hyperoxaluria is unknown. We hypothesized that after bariatric surgery, intestinal hyperabsorption of oxalate contributes to increases in plasma oxalate and urinary calcium oxalate supersaturation.
Methods
We prospectively examined oxalate metabolism in 11 morbidly obese subjects prior to and 6 and 12 months after RYGB (n = 9) and biliopancreatic diversion-duodenal switch (n =2). We measured 24 hour urinary supersaturations for calcium oxalate, apatite, brushite, uric acid, and sodium urate, fasting plasma oxalate, 72 hour fecal fat, and increases in urine oxalate following an oral oxalate load.
Results
Six and 12 months after RYGB surgery, plasma oxalate and urine calcium oxalate supersaturation increased significantly compared to similar measurements obtained prior to surgery (P values all ≤0.02). Fecal fat excretion at 6 and 12 months was increased (P-value, 0.026 and 0.055, 0 vs 6 and 12 months). An increase in urine oxalate excretion after an oral dose of oxalate was observed at 6 and 12 months (P-values ≤0.02 each). Therefore, after bariatric surgery, increases in fecal fat excretion, urinary oxalate excretion after an oral oxalate load, plasma oxalate, and urinary calcium oxalate supersaturation values were observed.
Conclusions
Enteric hyperoxaluria is often present in patients after the operations of RYGB and BPD-DS that utilize an element of intestinal malabsorption as a mechanism for weight loss.
Keywords: Oxalate, urinary supersaturation, nephrolithiasis, bariatric surgery, gastric bypass, fat malabsorption, intestinal oxalate absorption
Introduction
As a result of the dramatic increases in the prevalence of obesity in the last two decades (1) there has been a marked increase in the numbers of individuals undergoing bariatric surgery for weight loss (2). Estimates from the National Center for Health Statistics show that the prevalence of obesity in the United States exceeds 30% in most age groups and amongst both sexes (1). The prevalence of grade 2 and 3 obesity combined (BMI>/=35) ranges between 10.5% and 14.4% in men, and from 16.6% and 27.9% in women depending on racial grouping. The overall prevalence of grade 3 obesity (BMI >/=40) is an alarming 5.7% (1). Consensus guidelines recommend that subjects with morbid obesity (BMI>40), and some with a BMI>35 who have clinically important weight-related comorbid conditions attributable to obesity, be considered for bariatric surgery (3, 4). The number of bariatric procedures performed in 2004 in the United States was estimated at 121,055; in 2010, the number of bariatric operations will likely exceed 220,000 (2). A variety of surgical approaches have been used to decrease weight in the morbidly obese (5, 6). Roux-en-Y gastric bypass is the most frequently used procedure, and multiple trials have demonstrated it successfully decreases the prevalence of carbohydrate, lipid, and cardiovascular morbidities associated with extreme obesity (6–9), and can improve overall mortality (9).
Although there is a decrease in overall mortality and an improvement in lipid and carbohydrate metabolism in patients after RYGB, the incidence of nephrolithiasis and hyperoxaluria increases after RYGB (10–20). We previously described a series of 60 patients at Mayo Clinic-Rochester who developed nephrolithiasis after RYGB (11). Calcium oxalate stones were found in 19 and mixed calcium oxalate/uric acid stones in 2, while hyperoxaluria and increased calcium oxalate supersaturation were prevalent in these patients. In a recent study of 4,639 RYGB patients who had undergone bariatric surgery and 4,639 obese control patients, Matlaga et al noted that 7.65% (355 of 4,639) of RYGB patients developed urolithiasis compared to 4.63% (215 of 4,639) of the obese control patients in the control group (19).
To develop effective methods to prevent hyperoxaluria and nephrolithiasis, knowledge of the mechanisms underlying the pathogenesis of hyperoxaluria is necessary. The mechanism by which hyperoxaluria occurs in patients post-RYGB has not been clearly delineated. We now demonstrate that patients develop fat malabsorption, intestinal hyperabsorption of oxalate, increases in plasma oxalate and higher urinary calcium oxalate supersaturation after RYGB.
Methods and Materials
Subjects were recruited from amongst patients undergoing bariatric surgery at the Mayo Clinic. All subjects had BMI in excess of 40 kg/m2 and met criteria for bariatric surgery as outlined by the NIH consensus conference in bariatric surgery. In addition, subjects successfully completed a multidisciplinary evaluation with nutrition and medical specialists, dietitians, psychologists and bariatric surgeon. Goals of preoperative evaluation included: optimal management of existing medical morbidities, promotion of lifestyle changes in preparation for surgery, patient education of bariatric operation recommended. Nine patients underwent standard RYGB and two patients underwent BPD-DS (21). For a standard RYGB procedure, the volume of the gastric pouch is 15–20 mL, the length of the Roux segment is 100–150 cm, and the length of jejunum from the ligament of Treitz and the site of jejunojejunal anastomosis is 30–60 cm. this anatomy leaves a common channel of anywhere between 250–600 cm based on the usual length of the jejunoileum of 350–800 cm. For the BPD-DS procedure, the common channel is 100 cm in length, the segment from the gastric anastomosis to the jejunal anastomosis is 150 cm, and the "bypassed" duodenoileum is 100–550 cm. All patients received empiric vitamin supplementation: multivitamin/mineral preparation 2 tablets orally daily, calcium supplementation 1500 mg/daily, and Vitamin B12 1,000 µg subcutaneously monthly. Modifications to supplementation regimen were guided by laboratory results completed at 6 and 12 months after surgery. Specific goals for calcium and vitamin D supplementation included a serum 25-hydroxyvitamin D of 30 ng/mL and 24 urine calcium greater than 100 mg. Subjects reported an average intake of approximately 5000 IU of vitamin D3/per day and 1600 mg of elemental calcium/day after surgery.
Patients were counseled intensely regarding increasing her daily oral intake of fluids based on our previous work. In addition, multiple sessions pre-operatively, perioperatively, and post operatively with specific bariatric dietitians as well as bariatricians emphasized the importance of calorie/sugar restriction and emphasized protein intake (10, 11). Most patients go through a 13 week program supervised by a psychologist (22, 23). This "course" addresses healthy living, healthy eating, healthy choices and other multiple aspects of changes in lifestyle after bariatric surgery.
Metabolic studies were conducted on three occasions - immediately prior to, and 6 and 12 months after bariatric surgery. Patients collected a 24-hour urine sample the day prior to admission to the Clinical Research Unit (CRU) at the Mayo Clinic on their choice diet. The 24 hour urine sample was used to measure the determinants of urinary supersaturation (a measure of the propensity of a given type of crystal to form at a given concentrations of analytes and pH) including pH, volume, and urine chemistries (sodium, potassium, chloride, calcium, magnesium, citrate, phosphorus, uric acid, creatinine, and oxalate) in the Mayo Clinic Renal Function Laboratory (24). Results are expressed as Delta G (DG), the Gibbs free energy of transfer from a supersaturated to a saturated solution. DG is negative for under-saturated solutions and positive for supersaturated solutions. On average, the DG value for a given variety of kidney stone is more positive among individuals who form that type of stone. Dietary intake was estimated by the Women's Health Initiative FFQ as adapted by Viocare (25–27).
To assess response to an oral oxalate load, a fasting blood sample was collected at 0800 hours on admission to the CRU for the measurement of calcium, ionized calcium, phosphorus, uric acid in the Mayo Clinic Central Chemistry Laboratory and plasma oxalate by oxalate oxidase in the Mayo Renal Function Laboratory (28). At 0900 hours, an oral dose of 120 mg of disodium oxalate was administered orally and all urine was collected for the next 24 hours. Urine oxalate was measured in Mayo Renal Function Laboratory by oxalate oxidase. Patients were allowed to eat their usual diet for lunch and dinner.
Computerized tomography was used to determine the presence of stones prior to the commencement of the study and at 12 months after bariatric surgery. Stone number, volume, and calcification scores were assessed by radiologists unaware of the nature of the study.
Statistical analysis was first performed using mixed effects models to test for an overall (fixed) time effect (baseline, 6 months, 12 months) with random repeated subject effects. Paired t-tests were then done to assess specific differences from baseline to 6 months and 12 months after surgery. The two methods were very consistent in terms of significance. As such only the paired t-test results are presented. In view of the large number of tests, the possibility of some false positive findings increased. While the working alpha level was 0.05, it is suggested that the most credence be given to effects with p-values of 0.01 or less. Analyses were done by using SAS, version 9.1 (SAS institute, Cary, NC).
Results
Eleven women, age 49.5 ± 11.5 years (mean ± SD, range 28–68 years) participated in a prospective study. Nine subjects underwent standard RYGB surgery. Two subjects underwent a BPD-DS procedure. Prior to the operation, the subjects had a weight of 121.7 ± 18.39 kg with a BMI = 45.7 ± 5.03 kg/m2. Six months after bariatric surgery, their weight had decreased to 87.1 ± 18.19 kg and BMI to 32.5 ± 5.18 kg/m2 (P-value, paired t test, baseline to 6 months, P<0.001 each), and 12 months following bariatric surgery, weight and BMI were 75.7 ± 9.19 kg and 28.4 ± 2.03 kg/m2 (P-value, paired t test, baseline to 6 months, P<0.001 each).
Estimated nutrient intake is given in Table 1. Caloric, carbohydrate, and fat intake were significantly decreased 6 and 12 months after bariatric surgery but changes in dietary calcium intake were not significant (p>0.05). With the physician-recommended supplementation of calcium citrate instituted after bariatric surgery, calcium intake at 6 and 12 months was an additional 1.6 g/day above dietary calcium intake. Estimated dietary oxalate did not change significantly at 6 and 12 months after surgery compared with the intake prior to surgery. Dietary sodium intake diminished significantly, as did the intake of water.
Table 1.
Dietary Intake of Nutrients Estimated by FFQ (mean ± SD)
| Nutrient | Baseline, 0 m |
6 m | 12 m | P-value, paired t-test, 0–6 m |
P-value, paired t-test, 0–12m |
|---|---|---|---|---|---|
| Calories, Kcal/day | 2247.8 ± 816.2 | 1209.0 ± 795.1 | 1508.7 ± 783.9 | 0.018 | 0.033 |
| Carbohydrates, grams/day | 284.9 ± 116.2 | 156.6 ± 114.3 | 199.8 ± 130.4 | 0.010 | 0.033 |
| Fat, grams/day | 79.5 ± 35.2 | 39.3 ± 25.1 | 50.9 ± 25.3 | 0.018 | 0.038 |
| Protein, grams/day | 108.2 ± 51.5 | 64.6 ± 41.6 | 71.0 ± 38.3 | 0.077 | 0.070 |
| Sugar, grams/day | 129.4 ± 75.2 | 89.7 ± 79.2 | 105.3 ± 87.1 | 0.031 | 0.140 |
| Cholesterol, mg/day | 289.4 ± 151.8 | 145.3 ± 60.9 | 187.5 ± 94.2 | 0.027 | 0.110 |
| Water, mL/day | 3682.2 ± 816.7 | 2356.2 ± 600.13 | 2342.1 ± 724.6 | 0.004 | 0.003 |
| Sodium, mg/day | 3850.1 ± 1212.8 | 2075.1 ± 1100.3 | 2550.3 ± 1425.2 | 0.017 | 0.033 |
| Calcium, mg/day | 1478.2 ± 718.2 | 1136.9 ± 899.8 | 1144.0 ± 821.3 | 0.182 | 0.085 |
| Phosphorus, mg/day | 1902.5 ± 837.2 | 1250.6 ± 917.1 | 1374.8 ± 873.7 | 0.060 | 0.061 |
| Oxalate, mg/day | 205.7 ± 77.4 | 139.7 ± 126.8 | 193.9 ± 84.6 | 0.157 | 0.656 |
Values for serum creatinine, blood urea nitrogen, total calcium, ionized calcium, phosphorus, uric acid and magnesium concentrations did not change significantly before, and 6 and 12 months after bariatric surgery (Table 2). Serum uric acid decreased significantly at 12 months (Table 2). Notably, plasma oxalate increased from 1.2 ± 0.36 µmol/L prior to bariatric surgery to 2.2 ± 1.26 µmol/L and 1.9 ± 0.84 µmol/L 6 and 12 months after bariatric surgery, respectively (P = 0.018 and 0.016 vs. baseline at 6 and 12 months, respectively, Table 2). Cholesterol decreased significantly at 6 and 12 months (P = 0.008 and P = 0.0107 vs. baseline at 6 and 12 months). Triglycerides decreased at 12 months (P = 0.245 and P = 0.004 vs. baseline at 6 and 12 months). LDL cholesterol decreased at 12 months (P = 0.051 and P = 0.02 vs. baseline at 6 and 12 months).
Table 2.
Concentrations of Blood Analytes (mean ± SD)
| Serum Analyte | Baseline, 0 m |
6 m | 12 m | P-value, paired t-test, 0–6 m |
P-value, paired t-test, 0–12 m |
|---|---|---|---|---|---|
| Creatinine, mg/dL | 0.8 ± 0.19 | 0.8 ± 0.14 | 0.7 ± 0.10 | 0.583 | 0.212 |
| BUN, mg/dL | 16.8 ± 4.12 | 15.1 ± 7.25 | 16.1 ± 4.93 | 0.496 | 0.684 |
| Total calcium, mg/dL | 9.4 ± 0.35 | 9.5 ± 0.33 | 9.3 ± 0.27 | 1.0 | 0.386 |
| Ionized serum calcium, mg/dl | 4.9 ± 0.13 | 5.0 ± 0.17 | 5.0 ± 0.15 | 0.179 | 0.098 |
| Phosphorus, mg/dL | 3.8 ± 0.65 | 3.8 ± 0.43 | 4.2 ± 0.39 | 0.766 | 0.051 |
| Uric acid, mg/dL | 5.6 ± 1.05 | 4.6 ± 1.11 | 4.2 ± 0.71 | 0.100 | 0.004 |
| Magnesium, mg/dL | 2.2 ± 0.16 | 2.2 ± 0.31 | 2.1 ± 0.09 | 0.751 | 0.085 |
| Total cholesterol, mg/dL | 188.4 ± 30.5 | 163.3 ± 29.7 | 154.8 ± 37.4 | 0.008 | 0.0107 |
| Triglycerides, mg/dL | 162.1 ± 82.7 | 130.0 ± 55.3 | 83.3 ± 27.7 | 0.245 | 0.004 |
| LDL cholesterol, mg/dL | 108.5 ± 34.5 | 90.8 ± 28.6 | 84.8 ± 26.1 | 0.051 | 0.02 |
| Plasma oxalate, µmoles/L | 1.2 ± 0.36 | 2.2 ± 1.26 | 1.9 ± 0.84 | 0.018 | 0.016 |
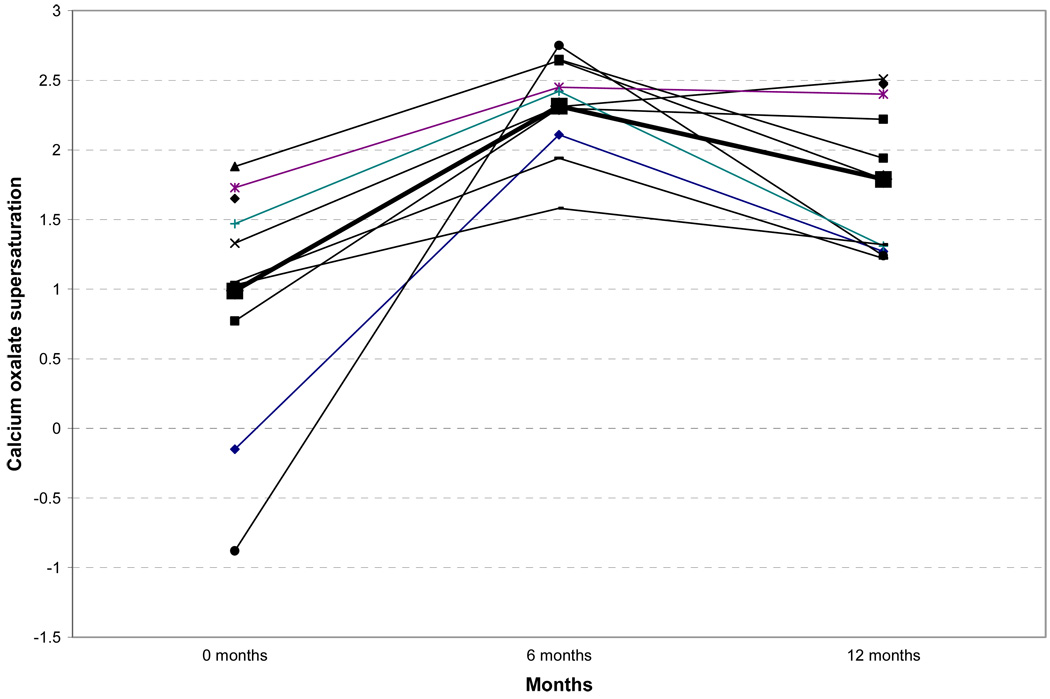
Twenty four-hour urine chemistries are shown in Table 3. Significant decreases in urinary excretion of phosphate, sulfate, and uric acid were noted 6 and 12 months after bariatric surgery. Urinary sodium and magnesium excretion were significantly decreased 6 months after surgery. Urinary calcium, citrate, chloride, and oxalate excretions did not change significantly. Urinary volume was significantly (p=0.018) diminished at 6 months. Urine supersaturation studies are shown in Table 4. Although urine oxalate did not increase significantly, urinary calcium oxalate supersaturation was significantly higher 6 and 12 months after bariatric surgery (Figure 1). Other urinary supersaturations did not change.
Table 3.
Twenty Four-hour urine Analytes Prior to, and 6 and 12 Months after Bariatric Surgery (mean ± SD)
| Urine test | Baseline, 0 m |
6 m | 12 m | P-value, paired t-test, 0–6 m |
P-value, paired t-test, 0–12 m |
|---|---|---|---|---|---|
| Sodium mmol/24 h | 170.3 ± 103.8 | 93.5 ± 39.6 | 135.6 ± 59.9 | 0.04 | 0.216 |
| Potassium mmol/24 h | 59.9 ± 22.7 | 46.8 ± 28.8 | 52.5 ± 28.2 | 0.083 | 0.178 |
| Calcium mg/24 h | 120.1 ± 76.6 | 141.1 ± 60.9 | 111.4 ± 52.1 | 0.826 | 0.576 |
| Magnesium mg/24 h | 92.2 ± 22.1 | 76.6 ± 34.8 | 101.6 ± 32.1 | 0.0169 | 0.348 |
| Chloride mmol/24 h | 164.0 ± 97.1 | 98.5 ± 46.5 | 130.8 ± 63.6 | 0.085 | 0.201 |
| Phosphorus mg/24 h | 896.9 ± 416.9 | 446.4 ± 171.6 | 552.9 ± 167.8 | 0.005 | 0.007 |
| Uric acid mg/24 h | 670.0 ± 359.7 | 359.9 ± 129.5 | 403.6 ± 135.3 | 0.019 | 0.008 |
| Citrate mmol/24 h | 526.8 ± to 283.5 | 523.0 ± 344.8 | 535.7 ± 415.1 | 0.979 | 0.749 |
| Sulfate mmol/24 h | 23.0 ± 12.3 | 11.3 ± 5.3 | 13.7 ± 5.8 | 0.018 | 0.006 |
| Oxalate mg/24 h | 26.4 ± 13.3 | 27.2 ± 8.2 | 32.6 ± 11.4 | 0.899 | 0.185 |
| Volume mL/24 h | 2091.2 ± 768.4 | 1316.8 ± 540.1 | 1595.8 ± 568.9 | 0.018 | 0.106 |
Table 4.
Twenty Four-hour Urine Supersaturations Prior to, and 6 and 12 Months after Bariatric Surgery (mean ± SD)
| Urine test | Baseline, 0 m |
6 m | 12 m | P-value, paired t-test, 0–6 m |
P-value, paired t-test, 0–12m |
|---|---|---|---|---|---|
| CaOx DG | 1.0 ± 0.88 | 2.3 ± 0.36 | 1.8 ± 0.54 | 0.003 | 0.009 |
| Brushite DG | −1.5 ± 0.02 | −1.4 ± 1.41 | −1.1 ± 1.01 | 0.918 | 0.483 |
| Apatite DG | 1.8 ± 1.27 | 1.9 ± 0.87 | 3.0 ± 1.5 | 0.907 | 0.147 |
| Uric acid DG | 1.7 ± 2.09 | 1.8 ± 2.01 | −0.4 ± 2.3 | 0.773 | 0.082 |
| Sodium urate DG | 0.9 ± 1.20 | 0.5 ± 0.77 | 0.8 ± 0.7 | 0.603 | 0.809 |
Figure 1.
Twenty four-hour urine calcium oxalate supersaturation prior to and 6 and 12 months following bariatric surgery.
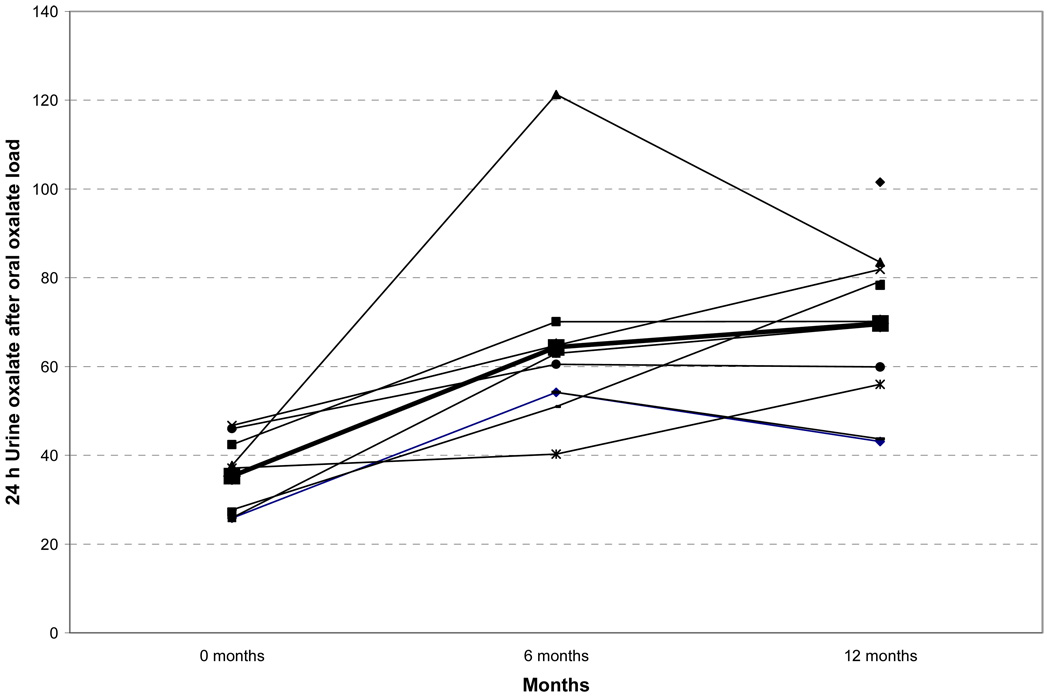
To determine the mechanism for the increase in plasma oxalate concentrations and the increase in urinary calcium oxalate supersaturation, we administered 120 mg of disodium oxalate orally to each subject and measured urinary oxalate excretion over the next 24 hours. Significant increases were observed in 24-hour urine oxalate excretion after the administration of an oral oxalate load 6 and 12 months after surgery when compared to baseline (Figure 2). These data demonstrate increased intestinal oxalate absorption after bariatric surgery.
Figure 2.
Twenty four-hour urine oxalate after an oral oxalate load prior to, and 6 and 12 months following bariatric surgery.
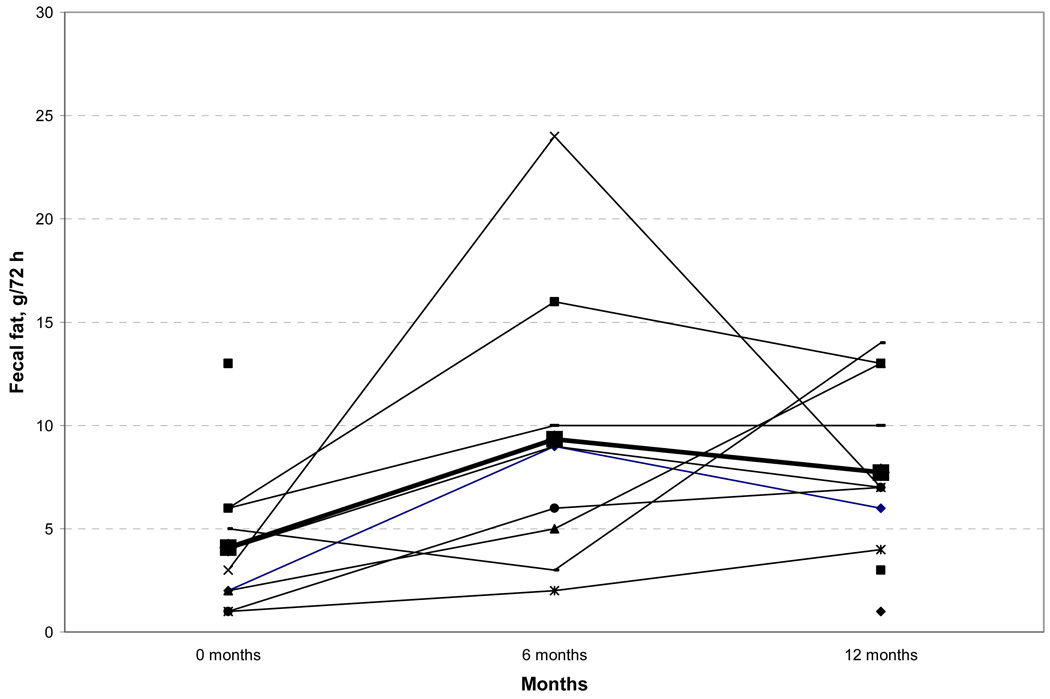
Fat malabsorption has been associated with enteric hyperoxaluria in other populations, but has yet to be systematically studied after RYGB. In the current cohort despite substantial decreases in fat intake, 72 hour fecal fat was increased in patients 6 and 12 months after bariatric surgery (prior to surgery 4.1 ± 3.5 g/72 h; 6 months 9.3 ± 6.9 g/72 h and 12 months 7.7 ± 4.3 g/72h, paired t-test P = 0.026 and P = 0.055 at 6 and 12 months respectively, Figure 3). Importantly, 7 of 11 patients had pathologic steatorrhea (< 6 g) at 6 and 12 months. An increase in fecal fat was apparent at six months following RYGB when the two with BPD-DS were eliminated from the analysis (prior to bariatric surgery 4.0 ± 3.8 g/72 h; 6 months after bariatric surgery 6.3 ± 3.1 g/72h; and 12 months after bariatric surgery 7.2 ± 4.4 g/72h, paired t-test P = 0.027 and P = 0.15 at 6 and 12 months).
Figure 3.
Seventy two-hour fecal fat prior to, and 6 and 12 months after bariatric surgery.
One of our subjects had nephrolithiasis prior to the commencement of the study. The patient’s stone did not increase in size during the study. None of the other patients in our study developed renal calculi as assessed by repeat CT 12 months postoperatively.
Discussion
Hyperoxaluria and nephrolithiasis are being increasingly recognized after RYGB (10–20). In a large group of patients 4,639 RYGB patients the incidence of nephrolithiasis was 7.7% compared with an incidence of 4.6% in an equally large control group of obese patients who had not undergone surgery (19). Therefore, with the increasing numbers of bariatric surgical procedures being performed (2), the numbers of patients with nephrolithiasis after such procedures will inevitably increase.
Methods to understand the pathogenesis of this disorder and to devise methods to decrease the prevalence of hyperoxaluria and the increased calcium oxalate supersaturation are clearly needed. In 2005, Nelson et al, first described the presence of hyperoxaluria in patients after a standard RYGB (10). Several reports from other groups have also subsequently noted the occurrence of hyperoxaluria after RYGB (11–20). In addition to hyperoxaluria, we showed that urinary calcium oxalate supersaturation is commonly observed in these patients after RYGB (11). Calcium oxalate stones are the predominant type observed, although mixed calcium oxalate/uric acid stones have also been documented (11). The true incidence of nephrolithiasis amongst patients undergoing RYGB procedures, however, is not known.
In the current study we demonstrate that patients post RYGB have increased plasma oxalate concentrations and an increase in calcium oxalate crystal supersaturation. These findings are consistent with previous observations made by our group (10, 11). Importantly, these are the first studies to prospectively demonstrate a serial increase in fecal fat after RYGB. Previous studies have documented that in patients with known fat malabsorption the degree of steatorrhea correlates with urinary oxalate excretion, and oral calcium supplements can blunt the hyperoxaluria in these circumstances. In the current study we documented a dramatic increase in urinary oxalate excretion after an oral oxalate in this cohort of post RYGB patients compared to their values before bariatric surgery (Figure 2). Therefore, our data fit the established paradigm that enhanced oxalate absorption could be a result of the formation of calcium fatty acid salts and a decreased amount of free calcium available to complex oxalate in the intestinal lumen. Although, as expected, fecal fat trended higher in the 2 patients undergoing BPD-DS, similar and significant changes in fecal fat, oxalate absorption and calcium oxalate supersaturation were observed when the 9 RYGB patients were analyzed alone (data not shown).
However, we did not detect a significant increase in urine oxalate excretion in subjects post RYGB, unlike our previous cross section study (11). Indeed, a recent prospective study of patients undergoing RYGB for obesity demonstrated an increase in urinary oxalate in 11 of 21 subjects from a mean of 33 mg/day at baseline to 64 mg/day at 1 year (29). One reason for this discrepancy might be an increased attention to calcium supplementation in our program over the last few years. Following the current clinical algorithms of our bariatric surgery program, subjects received an average of 1600 mg/day of elemental calcium at 12 months. Interestingly, even though urinary oxalate did not increase in the current cohort, calcium oxalate supersaturation still did, in part due to a decrease in urine volume (Table 3). However, unlike in our previous study (11) urine calcium did not decrease after RYGB but in fact increased slightly (Table 3), presumably due to the increased use of calcium supplements initiated by our group based on our previous studies. The net effect of these urinary changes on stone risk will need to be assessed.
In previous work, patients with fat malabsorption secondary to inflammatory bowel disease have been shown to develop nephrolithiasis at a rate 10–100 times that of the general population (30). Such patients have hyper-absorption of oxalate from the gut. Enteric hyperoxaluria has been documented in other diverse malabsorptive states such as after jejunoileal bypass for obesity (31–33), after several types of gastric ulcer surgery (32), and in the setting of chronic mesenteric ischemia (32). Patients often have multiple kidney stones, and those with ileocolonic disease (9–17%) are more commonly affected compared to those with ileal (6–8%) or colonic disease (3–5%) alone. The kidney stones that form are primarily composed of calcium oxalate if the ileum is involved (e.g., ileocolonic Crohn’s disease), and uric acid when patients have copious diarrhea or small bowel ostomies (30). Factors contributing to stone formation include a decrease in urinary pH, decreased urinary citrate concentration, and decreased urine volumes, all due to the diarrhea and consequent loss of bicarbonate and fluid in the stool. In addition, increased intestinal oxalate absorption from the gut produces hyperoxaluria (34). Two mechanisms of increased colonic oxalate uptake have been postulated to occur: 1) bile salt malabsorption due to the ileal disease resulting in fat malabsorption; increased colonic fats then bind to free calcium, increasing unbound oxalate that is able to cross the colonic mucosa; and 2) increased colonic permeability mediated by malabsorbed fatty acids and bile acids, perhaps causing changes in epithelial tight junctions allowing oxalate to pass from the intestine into the blood stream. In malabsorptive states, the percentage of oxalate absorbed from the gut and excreted in urine can be markedly increased, and hyperoxaluria often correlates with steatorrhea (35). Unabsorbed bile acids may also exert damaging effects on intestinal oxalate-metabolizing bacteria, thereby increasing the luminal oxalate available for absorption (36). We did not evaluate these other proposed mechanisms of enteric hyperoxaluria in the current study.
Our study suggests methods by which these patients could be effectively treated. These would include:
Additional calcium supplementation: Of note, our patients received approximately 1600 mg of added elemental calcium in the form of calcium citrate to prevent secondary hyperparathyroidism and mineral abnormalities. This amount of additional calcium appeared to prevent a decrease in urinary calcium after bariatric surgery, and may explain why urinary oxalate was increased only after an oxalate load. Additional supplemental calcium (2.5 to 3 g elemental calcium) is likely to be required to decrease urinary calcium oxalate supersaturation. However, the net effect of aggressive calcium supplementation on kidney stone risk needs to be assessed in controlled outcome studies.
Additional water intake: Our patients also drank less water and had a decreased output of urine which could have contributed to an increase in urinary calcium oxalate supersaturation. Patients after RYGB may face significant restrictions to their fluid intake despite our constant encouragement to increase then oral intake of fluid, Hence, monitoring and encouraging enhanced water intake after RYGB surgery would, therefore, be a rational method decrease urinary supersaturation.
Restriction of oxalate intake: Restriction of dietary intake of oxalate (to limit its delivery to the colon) would also be a logical intervention based on the increase in absorption of oxalate seen after an oral oxalate load.
A low fat diet: A low fat diet would limit fat malabsorption and the effects of fatty acids and bile acids in the colon (37). We should emphasize that the patients had restricted fat intake after RYGB when they were studied. Whether they could practically reduce their fat intakes further will require further study.
It is not known whether bile acid sequestrants such as cholestyramine (to limit colonic irritation by bile acids) would be effective in our patients (35, 37). Further analysis of the usefulness of various interventions noted above to prevent increases in calcium oxalate supersaturation and stone formation is needed.
Conclusion
Enteric hyperoxaluria is present in patients after the bariatric procedures of RYGB and BPD-DS with an element of intestinal malabsorption as a mechanism for weight loss. Enteric hyperoxaluria is associated with increased risk for nephrolithiasis, particularly calcium oxalate stone formation. Clinical interventions that can theoretically lower the risk for hyperoxaluria and renal stone formation include calcium supplementation, encouraging water intake, limiting dietary oxalate ingestion and adherence to a lower fat diet. However, clinical outcome studies are needed to verify the correct approach.
Acknowledgments
Supported by NIH grant DK-77669 to RK
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. Jama. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. Jama. 2005;294(15):1909–1917. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 3.NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115(12):956–961. [PubMed] [Google Scholar]
- 4.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6 Suppl 2:51S–209S. [PubMed] [Google Scholar]
- 5.Kendrick ML, Dakin GF. Surgical approaches to obesity. Mayo Clin Proc. 2006;81(10 Suppl):S18–S24. doi: 10.1016/s0025-6196(11)61177-4. [DOI] [PubMed] [Google Scholar]
- 6.Lieske JC, Kumar R, Collazo-Clavell ML. Nephrolithiasis after bariatric surgery for obesity. Semin Nephrol. 2008;28(2):163–173. doi: 10.1016/j.semnephrol.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 8.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 9.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 10.Nelson W, Houghton S, Milliner D, Lieske J, Sarr M. Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: Potentially serious and unapprecaited complications of Roux-en-Y gastric bypass. Surgery For Obesity and Related Disorders. 2005;1:481–485. doi: 10.1016/j.soard.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Sinha MK, Collazo-Clavell ML, Rule A, et al. Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int. 2007;72(1):100–107. doi: 10.1038/sj.ki.5002194. [DOI] [PubMed] [Google Scholar]
- 12.Lieske JC, Kumar R, Collazo-Clavell ML. Nephrolithiasis After Bariatric Surgery for Obesity. Semin Nephrol. 2008;28(2):163–173. doi: 10.1016/j.semnephrol.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed MH, Byrne CD. Bariatric surgery and renal function: a precarious balance between benefit and harm. Nephrol Dial Transplant. 2010 doi: 10.1093/ndt/gfq347. [DOI] [PubMed] [Google Scholar]
- 14.Asplin JR, Coe FL. Hyperoxaluria in kidney stone formers treated with modern bariatric surgery. J Urol. 2007;177(2):565–569. doi: 10.1016/j.juro.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 15.Duffey BG, Pedro RN, Kriedberg C, et al. Lithogenic risk factors in the morbidly obese population. J Urol. 2008;179(4):1401–1406. doi: 10.1016/j.juro.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 16.Duffey BG, Pedro RN, Makhlouf A, et al. Roux-en-Y gastric bypass is associated with early increased risk factors for development of calcium oxalate nephrolithiasis. J Am Coll Surg. 2008;206(3):1145–1153. doi: 10.1016/j.jamcollsurg.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Kleinman JG. Bariatric surgery, hyperoxaluria, and nephrolithiasis: a plea for close postoperative management of risk factors. Kidney Int. 2007;72(1):8–10. doi: 10.1038/sj.ki.5002284. [DOI] [PubMed] [Google Scholar]
- 18.Lieske JC. Gastric bypass procedures and renal calculi--how should we counsel patients and bariatric surgeons? J Urol. 2009;182(5):2105–2106. doi: 10.1016/j.juro.2009.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matlaga BR, Shore AD, Magnuson T, Clark JM, Johns R, Makary MA. Effect of gastric bypass surgery on kidney stone disease. J Urol. 2009;181(6):2573–2577. doi: 10.1016/j.juro.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 20.Nasr SH, D'Agati VD, Said SM, et al. Oxalate nephropathy complicating Roux-en-Y Gastric Bypass: an underrecognized cause of irreversible renal failure. Clin J Am Soc Nephrol. 2008;3(6):1676–1683. doi: 10.2215/CJN.02940608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarr MG, Balsiger BM. Bariatric surgery in the 1990's. Swiss Surg. 2001;7(1):11–15. doi: 10.1024/1023-9332.7.1.11. [DOI] [PubMed] [Google Scholar]
- 22.Breznikar B, Dinevski D. Bariatric surgery for morbid obesity: pre-operative assessment, surgical techniques and post-operative monitoring. J Int Med Res. 2009;37(5):1632–1645. doi: 10.1177/147323000903700543. [DOI] [PubMed] [Google Scholar]
- 23.Marcus JD, Elkins GR. Development of a model for a structured support group for patients following bariatric surgery. Obes Surg. 2004;14(1):103–106. doi: 10.1381/096089204772787383. [DOI] [PubMed] [Google Scholar]
- 24.Werness PG, Brown CM, Smith LH, Finlayson B. EQUIL2: a BASiC computer program for the calculation of urinary saturation. J Urol. 1985;134(6):1242–1244. doi: 10.1016/s0022-5347(17)47703-2. [DOI] [PubMed] [Google Scholar]
- 25.Neuhouser ML, Tinker L, Shaw PA, et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women's Health Initiative. Am J Epidemiol. 2008;167(10):1247–1259. doi: 10.1093/aje/kwn026. [DOI] [PubMed] [Google Scholar]
- 26.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9(3):178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 27.Wang CY, Anderson GL, Prentice RL. Estimation of the correlation between nutrient intake measures under restricted sampling. Biometrics. 1999;55(3):711–717. doi: 10.1111/j.0006-341x.1999.00711.x. [DOI] [PubMed] [Google Scholar]
- 28.Ladwig PM, Liedtke RR, Larson TS, Lieske JC. Sensitive spectrophotometric assay for plasma oxalate. Clin Chem. 2005;51(12):2377–2380. doi: 10.1373/clinchem.2005.054353. [DOI] [PubMed] [Google Scholar]
- 29.Duffey BG, Alanee S, Pedro RN, et al. Hyperoxaluria is a long-term consequence of Roux-en-Y Gastric bypass: a 2-year prospective longitudinal study. J Am Coll Surg. 2010;211(1):8–15. doi: 10.1016/j.jamcollsurg.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Pardi DS, Tremaine WJ, Sandborn WJ, McCarthy JT. Renal and urologic complications of inflammatory bowel disease. Am J Gastroenterol. 1998;93(4):504–514. doi: 10.1111/j.1572-0241.1998.156_b.x. [DOI] [PubMed] [Google Scholar]
- 31.Hylander E, Ladefoged K, Jarnum S. The importance of the colon in calcium absorption following small-intestinal resection. Scand J Gastroenterol. 1980;15(1):55–60. doi: 10.3109/00365528009181432. [DOI] [PubMed] [Google Scholar]
- 32.Canos HJ, Hogg GA, Jeffery JR. Oxalate nephropathy due to gastrointestinal disorders. Can Med Assoc J. 1981;124(6):729–733. [PMC free article] [PubMed] [Google Scholar]
- 33.Drenick EJ, Stanley TM, Border WA, et al. Renal damage with intestinal bypass. Ann Intern Med. 1978;89(5 Pt 1):594–599. doi: 10.7326/0003-4819-89-5-594. [DOI] [PubMed] [Google Scholar]
- 34.Modigliani R, Labayle D, Aymes C, Denvil R. Evidence for excessive absorption of oxalate by the colon in enteric hyperoxaluria. Scand J Gastroenterol. 1978;13(2):187–192. doi: 10.3109/00365527809181746. [DOI] [PubMed] [Google Scholar]
- 35.McLeod RS, Churchill DN. Urolithiasis complicating inflammatory bowel disease. J Urol. 1992;148(3 Pt 2):974–978. doi: 10.1016/s0022-5347(17)36794-0. [DOI] [PubMed] [Google Scholar]
- 36.Argenzio RA, Henrikson CK, Liacos JA. Restitution of barrier and transport function of porcine colon after acute mucosal injury. Am J Physiol. 1988;255(1 Pt 1):G62–G71. doi: 10.1152/ajpgi.1988.255.1.G62. [DOI] [PubMed] [Google Scholar]
- 37.Stauffer JQ. Hyperoxaluria and calcium oxalate nephrolithiasis after jejunoileal bypass. Am J Clin Nutr. 1977;30(1):64–71. doi: 10.1093/ajcn/30.1.64. [DOI] [PubMed] [Google Scholar]