Abstract
Escherichia and Salmonella do not synthesize quorum sensing signaling molecules of the N-acyl-L-homoserine lactone (AHL) type but they can detect AHLs produced by other species of bacteria. AHLs are present in the bovine rumen but not in the remainder of the gastrointestinal tract. Enterohemorrhagic E. coli (EHEC) responds to AHLs extracted from the bovine rumen. Salmonella fails to detect AHLs in the gastrointestinal tracts of pathogen-free mice or pigs, suggesting that AHLs are not present. However, Salmonella does detect the AHL production of Yersinia enterocolitica in mouse Peyer’s patches. In response to AHLs, EHEC represses flagellar genes and the LEE pathogenicity island while it activates the acid fitness island, whereas Salmonella activates the rck operon and a gene, srgE, encoding a putative Type III secreted effector.
Introduction
Bacteria can communicate with their own species and other species using small diffusible molecules. The presence of these molecules is thought to indicate the population density of a species and/or the diffusion characteristics of their environment. This process has been termed quorum sensing, efficiency sensing, or in a broader context, telesensing [1**,2]. Within small confined volumes, or spaces with low rates of diffusion, the accumulation of quorum sensing molecules indicates either a high Manuscript population density or alternatively, a low population density that has been producing quorum sensing molecules for a sufficient length of time [3]. The quorum sensing molecules, which are typically freely diffusible across bacterial membranes, are detected by transcription factors that control significant cell processes such as host interaction, bioluminescence, conjugation, competence, sporulation and biofilm formation [4,5]. In this review we will concentrate on recent advances in our understanding of one type of quorum sensing by Escherichia coli and Salmonella enterica, the detection of N-acyl-Lhomoserine lactones (AHLs).
LuxR and AHLs
The prototypical quorum sensing system is the production and detection of AHLs by Vibrio fischeri [4,5]. LuxI synthesizes the AHL signal molecules and LuxR is the transcription factor that detects the presence of AHL. The LuxR-AHL complex activates the transcription of the luxICDABE operon required for bioluminescence. The regulation of this operon is important to the symbiotic relationship between Vibrio fischeri and its host, the squid Euprymna scolopes [6,7].
Homologs of LuxR and LuxI have been found throughout the Gram-negative bacteria. A particular LuxI homolog predominantly synthesizes one or two AHL variants, although other variants are produced in lesser quantities. The AHL synthesized by a particular LuxR homolog can have an acyl chain length of between 4 and 18 carbon atoms (abbreviated here as C4, C6, etc.) (Figure 1). Additionally, the 3 position of the acyl chain can be either unmodified or modified with a carbonyl or hydroxyl group (abbreviated here by placing “oxo” or “OH” in front of the acyl chain length, eg., oxoC8). Although the acyl chain is most commonly present in the fully saturated form, varying degrees of saturation can also be found. The LuxI protein of Vibrio fischeri produces an AHL with a fully saturated acyl chain length of six and a carbonyl group at position 3 (N-(3-oxohexanoyl)-L-homoserine lactone, abbreviated here as oxoC6). The LuxR homolog typically has a binding preference for the AHL synthesized by its cognate LuxI enzyme. The variations in AHL structure produced and detected provide some degree of species specificity to the system.
Figure 1.
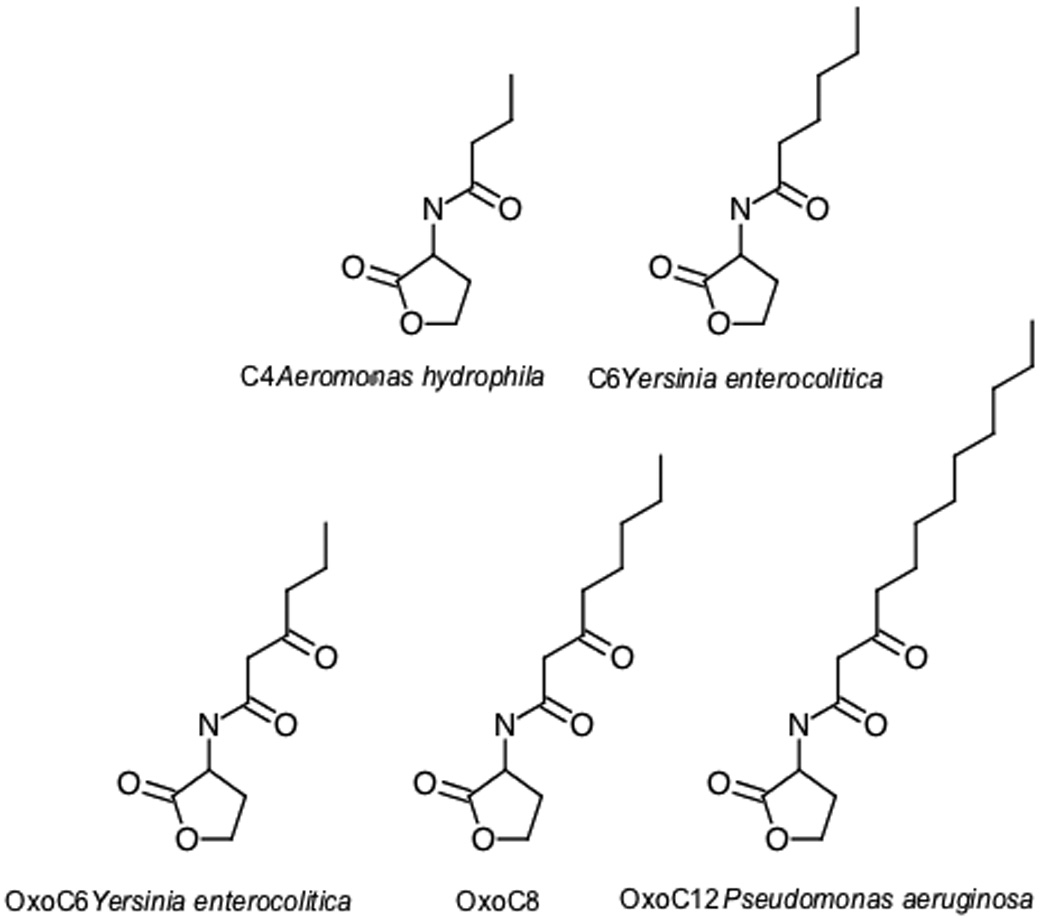
Structures of some AHLs that can be detected by SdiA. The organisms listed next to the structures have been shown to produce those particular AHLs. Those organisms can also be detected by Salmonella sdiA-dependent biosensor strains in a cross-streak assay.
The LuxR homolog of Escherichia and Salmonella, SdiA
Escherichia, Salmonella and related bacteria such as Klebsiella, Enterobacter, and Citrobacter encode a single LuxR homolog named SdiA, but lack a corresponding LuxI homolog and do not produce AHLs [8]. Thus, SdiA is considered an orphan or solo LuxR homolog [9]. The SdiA proteins of Escherichia and Salmonella detect AHLs produced by other species of bacteria [10,11,12**,13*,14,15*]. SdiA of Salmonella can detect an unusually wide range of AHL structures, but preferentially binds oxoC8 (Figure 1) [16,17]. SdiA can also detect AHLs that are not modified at position 3 and can detect chain lengths of 4 to 12 (C4, C6, C8, etc.). The detection sensitivity of SdiA for the various AHLs is in the range of 1 nM to 1 µM. Even the detection of non-ideal AHLs with micromolar sensitivities may be physiologically relevant since Salmonella can detect Pseudomonas aeruginosa, an organism that produces primarily C4 and oxoC12, when studied using a cross-streak assay on LB agar plates [17]. The cross-streak assay was also used to demonstrate that Salmonella can detect Aeromonas hydrophila, an organism that produces C4 [18] and Yersinia enterocolitica, which produces a mixture of C6 and oxoC6 [19].
The SdiA regulon of Salmonella
There are over 2600 serovars of Salmonella enterica and it is likely that the SdiA regulon is different among the serovars. The sdiA gene of Salmonella enterica serovar Typhimurium (S. Typhimurium) regulates two loci, the rck operon and the srgE gene (Figure 2). The rck operon is encoded on the 90 kb virulence plasmid of S. Typhimurium, pSLT, and contains six genes pefI, srgD, srgA, srgB, rck, and srgC. PefI, SrgD, and SrgC are homologous to transcription factors and PefI exhibits regulatory effects on the neighboring pef operon (plasmid encoded fimbriae) [20]. SrgA is a DsbA homolog that is involved with folding of the PefA fimbrial subunit and the SsaC (SpiA) protein of the SPI2-encoded Type III secretion system (TTSS2) [21,22]. Rck is an outer membrane β-barrel protein that confers resistance to complement killing, adhesion to fibronectin and laminin, and a zippering-type of host cell invasion [23–26,27*,28*]. The srgE gene appears to be a single gene horizontal acquisition in the chromosome [29]. A computer algorithm suggests that this gene is likely to encode a Type III secreted effector [30]. Interestingly, the rck operon is not expressed at temperatures below 37°C whereas srgE can be expressed at lower temperatures [29]. The temperature requirement of the rck operon and the apparent host interaction functions of the entire SdiA regulon suggest that SdiA is important in the host, rather than the external environment.
Figure 2.
The SdiA system of Salmonella enterica serovar Typhimurium. AHLs produced by other bacterial species diffuse across the membrane and are bound by SdiA. SdiA then increases the expression of the genes colored blue. These genes include pefI, srgD, srgA, srgB, rck and srgC found on the virulence plasmid, pSLT. STM1554 (srgE) is a chromosomal gene regulated by sdiA that appears to be a single gene horizontal acquisition that may encode a Type III secreted effector.
Salmonella SdiA activity in nature
Salmonella can detect the AHL production of numerous bacterial species during growth on agar plates, but the organisms detected in nature and the environmental settings and consequences of these detection events are largely unknown. The most obvious hypothesis, that Salmonella detects the gut environment by sensing the AHLs produced by the resident gut flora, turns out to be incorrect. A Salmonella strain that reports SdiA activity failed to respond in any region of the gastrointestinal tracts of a guinea pig, a rabbit, a cow, 5 mice, 6 pigs, or 12 chickens [12**,18]. However, this reporter strain did detect AHLs in mice colonized with the AHL-producing pathogen Yersinia enterocolitica [12**]. The signaling between Salmonella and Yersinia occurred primarily in the Peyer’s patches. This interspecies signaling required both the sdiA gene of Salmonella and the yenI gene (a luxI homolog) of Yersinia enterocolitica. These data indicate that the normal flora of animals does not produce AHLs, and that Salmonella is detecting the AHL production of specific pathogens instead. The microbial communities of the gastrointestinal tract are some of the most diverse and highly concentrated communities known. The lack of AHL in this environment is surprising. However, maybe it is the lack of AHL signaling by the normal flora that allows AHL-mediated quorum sensing to be of use to pathogens like Yersinia enterocolitica. If the normal flora produced high concentrations of AHLs, then Yersinia would not be able to use AHLs to measure its population density.
Yersinia enterocolitica has LuxR and LuxI homologs named YenR and YenI. In vitro, yenI does not affect the quantity of Yop effectors secreted into the culture supernatant [19]. Instead, yenI is required for expression of the flagellin protein, FleB, and for swimming and swarming motility [19]. In BALB/c mice, which are susceptible to lethal Yersinia infection, a yenI mutant has no discernible fitness or virulence defect [12**]. In CBA/J mice, which are resistant to lethal Yersinia infection, the yenI mutant has a mild fitness defect. In competition with the wild-type, the yenI mutant is recovered from feces in 10-fold lower numbers than the wild-type for the first seven days of infection, but the two strains are recovered in equal numbers for the next 21 days [12**]. It should be noted that in both the BALB/c and CBA/J mouse experiments, the animals were also infected with Salmonella, which may have affected the yenI mutant phenotypes [12**]. No other virulence phenotypes have been published for the yenI/yenR quorum sensing system of Yersinia enterocolitica. It is possible that more significant virulence phenotypes for these genes will be observed with other animal models or when virulence is examined in more detail.
But why does Salmonella detect AHL signals from Yersinia? More importantly, is Yersinia an organism that Salmonella detects in nature? If so, in which animal(s) does this detection normally occur and for what purpose? The identification of the “natural” scenario for AHL detection by a solo LuxR homolog is not straightforward, especially when discussing an organism with a very broad host range like S. Typhimurium. For example, one hypothesis was that Salmonella uses sdiA to detect the AHL production of plant pathogens in produce [15*]. This hypothesis was based on the observation that Salmonella SdiA is able to detect the AHL production of Pectobacterium carotovorum in vitro. However, during coinfection of produce, Salmonella was unable to detect the AHL production of Pectobacterium. It was determined that the environmental conditions present in produce are not correct for sdiA expression [15*]. A second hypothesis is that the function of Salmonella SdiA is to detect the AHL production of Aeromonas hydrophila in turtles [18]. SdiA becomes activated when Salmonella transits through the gastrointestinal tract of turtles and the only AHL producing organism that could be cultured from these animals was Aeromonas hydrophila [18]. However, in competition assays between the wild-type and sdiA mutant strains, the sdiA mutant had no fitness phenotype in turtles suggesting that this may not be the environmental setting where sdiA is important. One caveat is that the Salmonella serovar used for the experiments, Typhimurium, is not commonly associated with reptiles, leaving open the possibility that sdiA-dependent detection of Aeromonas may be relevant to other serovars.
This leads back to the question of whether the detection of AHL production by Yersinia is the “natural” function of sdiA in Salmonella. In competition experiments between wild-type and sdiA mutant Salmonella, both strains are recovered in equal numbers from the feces of CBA/J mice infected with Yersinia [12**]. As with the turtle experiments, this suggests that Salmonella does not benefit from the detection of Yersinia in mice. However, it was possible that only a few Salmonella cells were detecting Yersinia AHLs and the benefit conferred upon those cells could not be detected among the much larger population of Salmonella that had not detected AHLs. Therefore, the competition was repeated with Salmonella strains in which the Yersinia yenI gene was incorporated into the Salmonella chromosome (so that all members of the Salmonella population would detect AHLs). In this yenI+ background, the sdiA+ Salmonella quickly and dramatically outcompete an isogenic sdiA mutant in CBA/J mice [12**]. Both the srgE gene and the rck operon are required for this phenotype. This indicates that the SdiA regulon is indeed functional and advantageous when expressed in the mouse. This experiment has not been performed with turtles.
Although possible, it seems unlikely that the “natural” function of S. Typhimurium SdiA is the detection of Aeromonas in turtles or Yersinia in mice. However, a natural reservoir for both S. Typhimurium and Yersinia enterocolitica is swine. Roughly 15% to 25% of animals are infected with either organism on U.S. swine farms. Therefore, Salmonella and Yersinia should encounter each other on a regular basis in these animals. Preliminary results indicate that Salmonella can detect Yersinia in pigs (Ahmer, unpublished). Further work is needed to determine the prevalence and consequence of this interaction in nature.
The role of SdiA in E. coli and other bacteria
The role of sdiA in other Salmonella serovars is unknown, although it is known to be present in all 101 serovars that have been examined to date [31]. The role of sdiA in other genera like Citrobacter, Enterobacter, and Klebsiella is also unknown, although we have identified AHL-responsive fusions in Enterobacter cloacae and Klebsiella pneumoniae (Ahmer, unpublished). In E. coli, the SdiA regulon is entirely different than the regulon in S. Typhimurium. SdiA activates the gad genes of the acid fitness island of E. coli K-12 and EHEC [10,13*,14]. SdiA also represses flagellar genes and the genes of the LEE pathogenicity island (LEE) of EHEC [14,32]. A model has been proposed in which EHEC SdiA detects AHLs in the bovine rumen where acid resistance is increased and LEE expression is repressed [14]. AHL is no longer encountered beyond the rumen allowing the LEE to be expressed so that the recto-anal junction can be colonized [14]. The organisms that produce AHLs in the rumen have not been identified. It was also determined that AHL can induce lambda prophage induction in an sdiA-dependent manner and that environmental prophages respond to AHL as well [33**].
The bovine paradox
There is an apparent contradiction in the observations that AHL can be chemically extracted from the bovine rumen but a Salmonella reporter of SdiA activity failed to respond in this environment [14,18,34,35*]. The Salmonella reporter was recovered from only a single calf so it is possible that this particular animal was of the wrong age or on the wrong diet to have AHLs in its rumen. It also appears that the time of year plays a major role in whether or not AHLs are found in the rumen [35*]. Another possibility is that the sdiA gene of Salmonella is not expressed in this particular environment. This was found to be the case when the same Salmonella reporter system failed to detect the AHL production of Pectobacterium carotovorum in a tomato soft rot [15*]. The sdiA gene was not expressed so SdiA was not available to detect AHL. Further research is required on the parameters affecting AHL concentration in the bovine rumen, the role of AHL in the rumen community, and on the regulation of the E. coli and Salmonella sdiA genes.
The gene downstream of sdiA may also respond to other bacteria
Interestingly, in all of the bacterial genera that contain sdiA, a gene named sirA (Salmonella invasion regulator) is located downstream [36,37]. In fact, sirA is distributed more broadly than sdiA, being present throughout the gamma-proteobacteria. In other genera, the sirA ortholog is known as gacA, uvrY, varA, letA, etc. SirA is the response regulator of a two component regulatory system that responds to short chain fatty acids (SCFA) [38,39**,40**]. Since Salmonella produces SCFA, SirA is active in pure culture. However, in the host, Salmonella and other sirA-encoding organisms may use SirA to detect the SCFA production of the normal flora [38]. Thus, both SdiA and SirA may respond to signals from other bacterial species.
Conclusions
SdiA of Escherichia and Salmonella functions to detect the AHLs produced by other bacteria. The scenarios in which this occurs in nature, however, are still largely unknown and many questions remain. It is clear that the gastrointestinal tracts of most animals, excluding the bovine rumen, do not contain AHL. It appears that EHEC uses sdiA in the bovine rumen to repress the LEE pathogenicity island and to increase acid resistance. What organism(s) are producing the AHLs and what role do the AHLs play in the rumen community? Is detection of bacteria in the rumen the only scenario in which sdiA provides a benefit to EHEC? What are the scenarios in which sdiA provides a benefit to the other serogroups of E. coli? What are the scenarios for Citrobacter, Klebsiella, and Enterobacter? Is the role of sdiA in Salmonella truly to detect the AHLs of other gastrointestinal pathogens like Yersinia enterocolitica? If this is true, in which eukaryotic hosts do these bacterial interactions occur? How is pathogenesis altered when Salmonella detects Yersinia or other pathogens? What are the scenarios in which sdiA provides a benefit to the other 2600 serovars of Salmonella, many of which have different host ranges and disease manifestations? Are all of these scenarios similar or has each bacterial species or serovar adapted SdiA to a very specific situation? So far, the EHEC and S. enterica serovar Typhimurium scenarios look quite different. How is the sdiA gene regulated so that SdiA protein is not available for AHL detection in inappropriate environments like the tomato soft rot? Clearly, the study of AHL detection by Escherichia and Salmonella has only just begun.
Figure 3.
The SdiA system of EHEC. AHLs produced by other bacterial species diffuse across the membrane and are bound by SdiA. SdiA then increases the expression of the acid fitness island and induces prophage induction. SdiA also represses flagellar genes and the LEE pathogenicity island.
Acknowledgements
This work was supported by grant number AI073971 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. Roux A, Payne SM, Gilmore MS. Microbial telesensing: Probing the environment for friends, foes, and food. Cell Host Microbe. 2009;6(2):115–124. doi: 10.1016/j.chom.2009.07.004. **A review of broad mechanisms by which bacteria use small molecules to sense their environment.
- 2.Hense BA, Kuttler C, Muller J, Rothballer M, Hartmann A, Kreft JU. Does efficiency sensing unify diffusion and quorum sensing? Nat Rev Microbiol. 2007;5(3):230–239. doi: 10.1038/nrmicro1600. [DOI] [PubMed] [Google Scholar]
- 3.Boedicker JQ, Vincent ME, Ismagilov RF. Microfluidic confinement of single cells of bacteria in small volumes initiates high-density behavior of quorum sensing and growth and reveals its variability. Angew Chem Int Ed Engl. 2009;48(32):5908–5911. doi: 10.1002/anie.200901550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng W-L, Bassler BL. Bacterial quorum-sensing network architectures. Annual Review of Genetics. 2009;43(1):197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson S, Williams P. Quorum sensing and social networking in the microbial world. Journal of The Royal Society Interface. 2009;6(40):959–978. doi: 10.1098/rsif.2009.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visick KL, Foster J, Doino J, McFall-Ngai M, Ruby EG. Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J Bacteriol. 2000;182(16):4578–4586. doi: 10.1128/jb.182.16.4578-4586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lupp C, Urbanowski M, Greenberg EP, Ruby EG. The vibrio fischeri quorum-sensing systems ain and lux sequentially induce luminescence gene expression and are important for persistence in the squid host. Mol Microbiol. 2003;50(1):319–331. doi: 10.1046/j.1365-2958.2003.t01-1-03585.x. [DOI] [PubMed] [Google Scholar]
- 8.Ahmer BMM, Reeuwijk Jv, Timmers CD, Valentine PJ, Heffron F. Salmonella typhimurium encodes an sdia homolog, a putative quorum sensor of the luxr family, that regulates genes on the virulence plasmid. J Bacteriol. 1998;180(5):1185–1193. doi: 10.1128/jb.180.5.1185-1193.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patankar AV, Gonzalez JE. Orphan luxr regulators of quorum sensing. FEMS Microbiol Rev. 2009;33(4):739–756. doi: 10.1111/j.1574-6976.2009.00163.x. [DOI] [PubMed] [Google Scholar]
- 10.Van Houdt R, Aertsen A, Moons P, Vanoirbeek K, Michiels CW. N-acyl-l-homoserine lactone signal interception by escherichia coli. FEMS Microbiol Lett. 2006;256(1):83–89. doi: 10.1111/j.1574-6968.2006.00103.x. [DOI] [PubMed] [Google Scholar]
- 11.Yao Y, Martinez-Yamout MA, Dickerson TJ, Brogan AP, Wright PE, Dyson HJ. Structure of the escherichia coli quorum sensing protein sdia: Activation of the folding switch by acyl homoserine lactones. J Mol Biol. 2006;355(2):262–273. doi: 10.1016/j.jmb.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 12. Dyszel JL, Smith JN, Lucas DE, Soares JA, Swearingen MC, Vross MA, Young GM, Ahmer BM. Salmonella enterica serovar typhimurium can detect acyl homoserine lactone production by yersinia enterocolitica in mice. J Bacteriol. 2010;192(1):29–37. doi: 10.1128/JB.01139-09. **This study provides a demonstration of SdiA activity in vivo and conclusive evidence of the organism being detected.
- 13. Dyszel JL, Soares JA, Swearingen MC, Lindsay A, Smith JN, Ahmer BM. E. Coli k-12 and ehec genes regulated by sdia. PLoS One. 2010;5(1):e8946. doi: 10.1371/journal.pone.0008946. *This study performs genetic screens to identify the sdiA regulons of E. coli K-12 and EHEC and revisits the hypothesis that SdiA regulates cell division and drug resistance.
- 14.Hughes DT, Terekhova DA, Liou L, Hovde CJ, Sahl JW, Patankar AV, Gonzalez JE, Edrington TS, Rasko DA, Sperandio V. Chemical sensing in mammalian host-bacterial commensal associations. Proc Natl Acad Sci U S A. 2010;107(21):9831–9836. doi: 10.1073/pnas.1002551107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Noel JT, Joy J, Smith JN, Fatica M, Schneider KR, Ahmer BM, Teplitski M. Salmonella sdia recognizes n-acyl homoserine lactone signals from pectobacterium carotovorum in vitro, but not in a bacterial soft rot. Mol Plant Microbe Interact. 2010;23(3):273–282. doi: 10.1094/MPMI-23-3-0273. *This study determined that Salmonella avoids detecting AHLs in certain environments by not expressing its AHL receptor.
- 16.Janssens JC, Metzger K, Daniels R, Ptacek D, Verhoeven T, Habel LW, Vanderleyden J, De Vos DE, De Keersmaecker SC. Synthesis of n-acyl homoserine lactone analogues reveals strong activators of sdia, the salmonella enterica serovar typhimurium luxr homologue. Appl Environ Microbiol. 2007;73(2):535–544. doi: 10.1128/AEM.01451-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michael B, Smith JN, Swift S, Heffron F, Ahmer BM. Sdia of salmonella enterica is a luxr homolog that detects mixed microbial communities. J Bacteriol. 2001;183(19):5733–5742. doi: 10.1128/JB.183.19.5733-5742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith JN, Dyszel JL, Soares JA, Ellermeier CD, Altier C, Lawhon SD, Adams LG, Konjufca V, Curtiss R, 3rd, Slauch JM, Ahmer BM. Sdia, an n-acylhomoserine lactone receptor, becomes active during the transit of salmonella enterica through the gastrointestinal tract of turtles. PLoS ONE. 2008;3(7):e2826. doi: 10.1371/journal.pone.0002826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atkinson S, Chang CY, Sockett RE, Camara M, Williams P. Quorum sensing in yersinia enterocolitica controls swimming and swarming motility. J Bacteriol. 2006;188(4):1451–1461. doi: 10.1128/JB.188.4.1451-1461.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholson B, Low D. DNA methylation-dependent regulation of pef expression in salmonella typhimurium. Mol Microbiol. 2000;35(4):728–742. doi: 10.1046/j.1365-2958.2000.01743.x. [DOI] [PubMed] [Google Scholar]
- 21.Bouwman CW, Kohli M, Killoran A, Touchie GA, Kadner RJ, Martin NL. Characterization of srga, a salmonella enterica serovar typhimurium virulence plasmid-encoded paralogue of the disulfide oxidoreductase dsba, essential for biogenesis of plasmid-encoded fimbriae. J Bacteriol. 2003;185(3):991–1000. doi: 10.1128/JB.185.3.991-1000.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miki T. Two periplasmic disulfide oxidoreductases, dsba and srga, target outer membrane protein spia, a component of the salmonella pathogenicity island 2 type iii secretion system. J Biol Chem. 2004;279(33):34631–34642. doi: 10.1074/jbc.M402760200. [DOI] [PubMed] [Google Scholar]
- 23.Hackett J, Wyk P, Reeves P, Mathan V. Mediation of serum resistance in salmonella typhimurium by an 11- kilodalton polypeptide encoded by the cryptic plasmid. J Infect Dis. 1987;155(3):540–549. doi: 10.1093/infdis/155.3.540. [DOI] [PubMed] [Google Scholar]
- 24.Heffernan EJ, Reed S, Hackett J, Fierer J, Roudier C, Guiney D. Mechanism of resistance to complement-mediated killing of bacteria encoded by the salmonella typhimurium virulence plasmid gene rck. J Clin Invest. 1992;90(3):953–964. doi: 10.1172/JCI115972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cirillo DM, Heffernan EJ, Wu L, Harwood J, Fierer J, Guiney DG. Identification of a domain in rck, a product of the salmonella typhimurium virulence plasmid, required for both serum resistance and cell invasion. Infect Immun. 1996;64(6):2019–2023. doi: 10.1128/iai.64.6.2019-2023.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crago AM, Koronakis V. Binding of extracellular matrix laminin to escherichia coli expressing the salmonella outer membrane proteins rck and pagc. FEMS Microbiol Lett. 1999;176(2):495–501. doi: 10.1111/j.1574-6968.1999.tb13703.x. [DOI] [PubMed] [Google Scholar]
- 27. Ho DK, Jarva H, Meri S. Human complement factor h binds to outer membrane protein rck of salmonella. J Immunol. 2010;185(3):1763–1769. doi: 10.4049/jimmunol.1001244. *This study provides new information regarding the mechanism of complement resistance mediated by the Rck protein, the expression of which is increased by SdiA.
- 28. Rosselin M, Virlogeux-Payant I, Roy C, Bottreau E, Sizaret PY, Mijouin L, Caron E, Velge P, Wiedemann A. Rck of salmonella enterica, subspecies enterica serovar enteritidis, mediates zipper-like internalization. Cell Res. 2010;20(6):647–664. doi: 10.1038/cr.2010.45. *This study characterized an alternative method by which Salmonella can invade host cells that is mediated by the Rck protein, the expression of which is increased by SdiA.
- 29.Smith JN, Ahmer BM. Detection of other microbial species by salmonella: Expression of the sdia regulon. J Bacteriol. 2003;185(4):1357–1366. doi: 10.1128/JB.185.4.1357-1366.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samudrala R, Heffron F, McDermott JE. Accurate prediction of secreted substrates and identification of a conserved putative secretion signal for type iii secretion systems. PLoS Pathog. 2009;5(4) doi: 10.1371/journal.ppat.1000375. e1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halatsi K, Oikonomou I, Lambiri M, Mandilara G, Vatopoulos A, Kyriacou A. Pcr detection of salmonella spp. Using primers targeting the quorum sensing gene sdia. FEMS Microbiol Lett. 2006;259(2):201–207. doi: 10.1111/j.1574-6968.2006.00266.x. [DOI] [PubMed] [Google Scholar]
- 32.Kanamaru K, Kanamaru K, Tatsuno I, Tobe T, Sasakawa C. Sdia, an escherichia coli homologue of quorum-sensing regulators, controls the expression of virulence factors in enterohaemorrhagic escherichia coli o157:H7. Mol Microbiol. 2000;38(4):805–816. doi: 10.1046/j.1365-2958.2000.02171.x. [DOI] [PubMed] [Google Scholar]
- 33. Ghosh D, Roy K, Williamson KE, Srinivasiah S, Wommack KE, Radosevich M. Acyl-homoserine lactones can induce virus production in lysogenic bacteria: An alternative paradigm for prophage induction. Appl Environ Microbiol. 2009;75(22):7142–7152. doi: 10.1128/AEM.00950-09. **This study determined that prophages can be induced by AHL in E. coli and in environmental samples.
- 34.Erickson DL, Nsereko VL, Morgavi DP, Selinger LB, Rode LM, Beauchemin KA. Evidence of quorum sensing in the rumen ecosystem: Detection of n-acyl homoserine lactone autoinducers in ruminal contents. Can J Microbiol. 2002;48(4):374–378. doi: 10.1139/w02-022. [DOI] [PubMed] [Google Scholar]
- 35. Edrington TS, Farrow RL, Sperandio V, Hughes DT, Lawrence TE, Callaway TR, Anderson RC, Nisbet DJ. Acyl-homoserine-lactone autoinducer in the gastrointestinal tract of feedlot cattle and correlation to season, e. Coli o157:H7 prevalence, and diet. Curr Microbiol. 2009;58(3):227–232. doi: 10.1007/s00284-008-9312-8. *This study finds that AHLs are present in the bovine rumen during spring, summer, and fall, but not in winter. This study also confirms that AHLs are not present in the remainder of the gastrointestinal tract.
- 36.Johnston C, Pegues DA, Hueck CJ, Lee A, Miller SI. Transcriptional activation of salmonella typhimurium invasion genes by a member of the phosphorylated response-regulator superfamily. Mol Microbiol. 1996;22(4):715–727. doi: 10.1046/j.1365-2958.1996.d01-1719.x. [DOI] [PubMed] [Google Scholar]
- 37.Ahmer BM. Cell-to-cell signalling in escherichia coli and salmonella enterica. Mol Microbiol. 2004;52(4):933–945. doi: 10.1111/j.1365-2958.2004.04054.x. [DOI] [PubMed] [Google Scholar]
- 38.Lawhon SD, Maurer R, Suyemoto M, Altier C. Intestinal short-chain fatty acids alter salmonella typhimurium invasion gene expression and virulence through bara/sira. Mol Microbiol. 2002;46(5):1451–1464. doi: 10.1046/j.1365-2958.2002.03268.x. [DOI] [PubMed] [Google Scholar]
- 39. Huang Y, Suyemoto M, Garner CD, Cicconi KM, Altier C. Formate acts as a diffusible signal to induce salmonella invasion. J Bacteriol. 2008;190(12):4233–4241. doi: 10.1128/JB.00205-08. **Identifies short chain fatty acids as small molecule inducers of Salmonella invasion genes.
- 40. Chavez RG, Alvarez AF, Romeo T, Georgellis D. The physiological stimulus for the bara sensor kinase. J Bacteriol. 2010;192(7):2009–2012. doi: 10.1128/JB.01685-09. **Identifies the BarA sensor kinase as the signal receptor for short chain fatty acids.




