Abstract
Purpose
Pre-clinical in vivo studies can help guide the selection of agents and regimens for clinical testing. However, one of the challenges in screening anti-cancer therapies is the assessment of off-target human toxicity. There is a need for in vivo models that can simulate efficacy and toxicities of promising therapeutic regimens. For example, hematopoietic cells of human origin are particularly sensitive to a variety of chemotherapeutic regimens but in vivo models to assess potential toxicities have not been developed. In this study, a xenograft model containing humanized bone marrow is utilized as an in vivo assay to monitor hematotoxicity.
Experimental Design
A proof-of-concept, temozolomide-based regimen was developed that inhibits tumor xenograft growth. This regimen was selected for testing since it has been previously shown to cause myelosuppression in mice and humans. The dose-intensive regimen was administered to NOD/SCID/γchainnull mice reconstituted with human hematopoietic cells and the impact of treatment on human hematopoiesis was evaluated.
Results
The dose-intensive regimen resulted in significant decreases in growth of human-glioblastoma xenografts. When this regimen was administered to mice containing humanized bone marrow, flow cytometric analyses indicated that the human bone-marrow cells were significantly more sensitive to treatment than the murine bone-marrow cells, and that the regimen was highly toxic to human-derived hematopoietic cells of all lineages (progenitor, lymphoid, and myeloid).
Conclusions
The humanized bone-marrow xenograft model described has the potential to be used as a platform for monitoring the impact of anti-cancer therapies on human hematopoiesis and could lead to subsequent refinement of therapies prior to clinical evaluation.
Keywords: human xenograft model, myelosuppression, stem-cell
INTRODUCTION
Murine xenograft models are one of the primary tools used for screening of new therapeutic compounds and regimens (1). One major limitation of using murine xenograft studies to determine therapeutic efficacy, however, is that significant inter-species differences in drug sensitivity can exist between mouse- and human-derived cells. It is possible that the levels of a therapeutic compound reached in a mouse xenograft model may not be achievable in humans due to differential profiles of absorption, distribution, metabolism, excretion (ADME), and toxicity. Therefore, while a regimen may exhibit an acceptable toxicity and efficacy profile in mice, this may not necessarily be the case in patients.
Statement of Translational Relevance (150 words max).
A variety of human tumor xenograft models are currently used to evaluate the efficacy of novel compounds and regimens to kill tumor cells in vivo. However, in vivo models to screen for potential toxicity to normal human cells in their appropriate microenvironment, early in the drug discovery, need development. Since bone-marrow toxicity can be a major life-threatening side effect of treatment, models to screen for the impact of treatment on human hematopoiesis would improve our ability to select compounds with decreased off-target toxicities. A humanized bone-marrow xenograft model was developed to assess toxicity to human hematopoietic cells in vivo. As the first step towards validation of this model on a broader scale, we used a temozolomide-based regimen already known to exhibit dose-limiting toxicities in the clinic. This model holds promise as a new approach for in vivo hematotoxicity screening of new compounds and regimens.
Furthermore, in vitro clonogenic assays have demonstrated that human and mouse hematopoietic cells show a wide diversity in sensitivity to many anti-cancer drugs; mouse hematopoietic cells in many cases exhibit increased resistance to compound exposure compared to human hematopoietic cells (2-5). Masubuchi et al previously demonstrated differential sensitivities of mice, dog, and human bone-marrow cells exposed to camptothecin derivatives. For example, camptothecin-like compounds-SN-38 and topotecan-exhibited differential inter-species resistance with mouse CFU being the most resistant to treatment (4). However, inter-species differences in sensitivity were not always observed. Three other compounds (DX-8951f, 9-aminocamptothecin, and camptothecin) demonstrated fairly similar sensitivities amongst all three species. Studies by Kurtzberg et al (3) indicated significant differences in sensitivities for tubulin-binding agents; the IC90 values for vincristine and paclitaxel were 30 and 27 nM and for mouse CFU-GM and 3 and 9 nM for human CFU-GM respectively. In addition, IC90 values for tasidotin treatment were > 300 nM for mouse CFU-GM and 65 nM for human CFU-GM.
Evaluation of human hematopoietic toxicity in immunodeficient mice could represent an additional benchmark in the final screening and selection of new therapeutics. This in vivo screening approach would take into account the influence of the bone-marrow microenvironment on damaged cells or the cycling kinetics of hematopoietic cells following treatment. An unexplored use of the NOD.Cg-PrkdcscidIL2rgtm1Wjl/Sz (NOD/SCID/γchainnull) mouse strain is to treat mice reconstituted with human hematopoietic cells with a regimen related to clinical practice, and determine the impact of in vivo treatment on human hematopoiesis (6). As a proof-of-concept, we chose to evaluate the impact of a combination therapy consisting of O6-benzylguanine and temozolomide (O6-BG/TMZ) on human hematopoiesis in vivo since it is currently being evaluated in clinical trials and the main dose-limiting toxicity in these patients is myelosuppression (7-10). We first developed a dosing regimen consisting of O6-benzylguanine and temozolomide (O6-BG/TMZ) followed by stem-cell rescue that would significantly inhibit the growth of TMZ-resistant human xenografts in NOD.Cg-Prkdcscid IL2rgtm1Wjl/Sz mice. The underlying strategy of this regimen is to prevent repair of TMZ-mediated DNA damage by inhibiting the DNA repair protein, O6-methylguanine DNA- methyltransferase (MGMT) (11-14). Previous studies have demonstrated that numerous cancers can express high levels of MGMT, and therefore can efficiently repair TMZ-mediated DNA damage, decreasing the efficacy of tumor-cell kill (15). In addition, a variety of tumors can be sensitized to alkylating agents both in vitro and in xenograft studies with the addition of O6-BG, a direct inhibitor of MGMT activity (11, 13-14, 16). The down side of this approach is that both immature and mature hematopoietic cells can be extremely sensitive to this regimen due to low levels of endogenous DNA repair activity (17).
In this study, the delivery of two cycles of a high-dose regimen of O6-BG/TMZ in combination with stem-cell rescue significantly inhibited the growth of a TMZ-resistant glioma. This course of therapy was then used to test the hypothesis that administration of the regimen would be toxic to human hematopoietic cells in vivo. NOD/SCID/γchainnull mice were transplanted with human CD34+ cells and reconstitution confirmed one month post-transplantation by monitoring the peripheral blood (PB) for human-cell chimerism in the transplanted mice. Treatment with O6-BG/TMZ resulted in significant loss of human-derived hematopoietic cells in the bone-marrow and enumeration of total number of human and mouse cells in vehicle versus treated mice indicated that the human bone-marrow cells were significantly more sensitive to treatment compared to the murine-derived bone-marrow cells. This proof-of-concept study indicates that use of NOD/SCID/γchainnull mice with humanized bone marrow can be used as an in vivo toxicity measure of human hematopoiesis following drug treatment and holds merit as a model for improving screening strategies used to develop and test novel compounds and dosing regimens.
Materials and Methods
Isolation of umbilical cord blood (UCB) CD34+ cells
All protocols were approved by Indiana University School of Medicine's Institutional Review Board (IRB) and St. Vincent Hospital's IRB (Indianapolis, IN). Samples of UCB were collected from normal, full-term infants delivered by cesarean section and the CD34+ cells isolated using the CD34 MicroBead kit and VarioMACS Separator™ (Miltenyi Biotech Inc., Auburn, CA) according to the manufacturer's instructions. Following magnetic bead separation, the viability of the isolated CD34+ cells was routinely 95%. The quality of the isolated CD34+ cells was analyzed by a Colony-forming Unit (CFU) assay as described below on an aliquot of the CD34+ cells used for transplantation. The frequency of clonogenic cells in UCB products used for transplant in experiments I and II was similar with 60-65 colonies per 2 × 103 CD34+ cells plated.
Clonogenic survival assays
Colony forming-Unit (CFU) assays were performed using enriched human CD34+ cells (Methocult GF, H4434, Stem Cell Technologies, Inc.) and unfractionated murine-bone marrow (Methocult GF M3434), or bone marrow from NOD/SCID/γchainnull transplanted with human CD34+ cells. The cells were seeded in triplicate dishes at concentrations of 2 × 103 for human CD34+ cells and 2 × 105 for murine bone marrow to obtain 60-70 colonies per 35 mm dish. For survival assays, cells were exposed to drug combinations (O6-BG, TMZ or O6-BG/TMZ) prior to plating. For analysis of human cells from the bone marrow of NOD/SCID/γchainnull transplants, chimeric human-mouse bone marrow was plated at 2 × 105 bone-marrow cells per dish. After 10 - 14 days of incubation at 37°C in 5% CO2, colony forming units-granulocyte-macrophage (CFU-GM) and burst-forming units-erythroid (BFU-E) were enumerated using the Axiovert 25 inverted-light microscope. For assays using clonogenic SF767 cells, cells were seeded at 150 cells/well in triplicate in 6-well plates and allowed to grow overnight in IMDM medium with supplements (10% Cosmic Calf Serum, 1% Pen/Strep, and 1% Glutamine.) The cells were treated the next day and allowed to grow for 13 days at 37°C. Following incubation, the cells were rinsed with PBS, fixed with 0.05% methylene blue, and counted visually.
Animals
A breeding colony of NOD.Cg-Prkdcscid IL2rgtm1Wjl/Sz (NOD/SCID/γchainnull) mice (6) was established at the Laboratory Animal Research Center and maintained by the In Vivo Therapeutics Core at the Indiana University Simon Cancer Center (IUSCC) (Indianapolis, IN). BALB/c and C57BL/6N inbred mice were purchased from Harlan Laboratories, Inc (Indianapolis, IN). All protocols and establishment of pre-death endpoints-decreases in body weight, activity, and grooming-were approved by the Institutional Animal Care and Use Committee.
Xenograft study
The human glioblastoma cell line, SF767, was originally developed by The Brain Tumor Research Center, University of California at San Francisco and has been previously described (18). Dr. Len Erickson (Indiana University School of Medicine, Indianapolis, IN) provided this line; early-passage cells were cryopreserved in 2005 (18). Prior to use in xenograft studies, SF767 cells were tested for confirmation of human MGMT expression and sensitivity to O6-BG/TMZ. Human-cell line authentication was confirmed by the Research Animal Diagnostic Laboratory (Columbia, MO). SF767 cells (5 × 106) were implanted subcutaneously into the flank of NOD/SCID/γchainnull mice and tumors allowed to grow to 100 - 150 mm3. Tumor volume measurements were taken weekly by caliper and calculated according to the formula (α2 × β)/2, where α is the shorter and β is the longer of the two dimensions.
Transplantation of NOD/SCID/γchainnull mice with human CD34+ cells
NOD/SCID/γchainnull mice were placed on food pellets containing 0.0625% doxycycline for 5-7 days prior to irradiation. Mice were then conditioned with 300-cGy total-body irradiation using a GammaCell 40 (Nordion International Inc., Ontario Canada) equipped with two opposing Cesium-137 sources. UCB CD34+ cells were resuspended in IMDM containing 0.2 % endotoxin-free bovine serum albumin and injected into the lateral tail vein of each animal.
Chemotherapy administration
O6-BG (Sigma-Aldrich, St Louis, MO) was dissolved in 40% polyethylene glycol-400 (v/v) and 60% saline (v/v). TMZ (LKT laboratories, Inc., St. Paul, MN) was sonicated in phosphate buffered saline (PBS). The myeloablative dosing regimen consisted of 30 mg/kg O6-BG followed by 80 mg/kg TMZ one hour later and 15 mg/kg O6-BG 7 hours later (O6-BG/TMZ). One treatment cycle consisted of 3-consecutive days of treatment with this dosing regimen. Two days after a treatment cycle, 8 × 106 fresh murine bone marrow cells were injected intravenously.
Analysis of human cell engraftment
Mice were sacrificed at 6 and 11 days post-injection and the BM and spleen prepared as previously described (19). Human cell engraftment was measured by human CD45 immunostaining. The proportion of engraftment in various lineages was determined by immunostaining and flow cytometric analysis as previously described using a Becton-Dickinson FACSCalibur and CellQuest software (19). The lack of crossreactivity of human-specific antibodies with murine cells was confirmed in each experiment by staining BM from a nontransplanted mouse with each antibody combination. Cells were stained with allophycocyanin (APC)-conjugated anti-human-CD45 (anti-HLe-1; Becton Dickinson Immunocytometry, San Jose, CA) alone or in combination with phycoerthyrin (PE)-conjugated anti-human CD33 (anti-Leu-M9; Becton Dickinson). Identical aliquots were stained with APC-conjugated anti-human CD34 (clone 581; PharMingen, San Diego, CA) in combination with anti-human CD19-PE (PharMingen).
Statistical Analyses
Generalized Linear Mixed Model with mouse as the random effect was used to compare tumor volume between O6-BG /TMZ and vehicle treatments. Two-tailed t-tests were performed to determine significance of vehicle-versus drug-treated for tumor weights, clonogenic survival assays, and flow cytometric analyses.
RESULTS
Development of a proof-of-concept TMZ-based regimen that results in significant decrease in tumor-xenograft growth
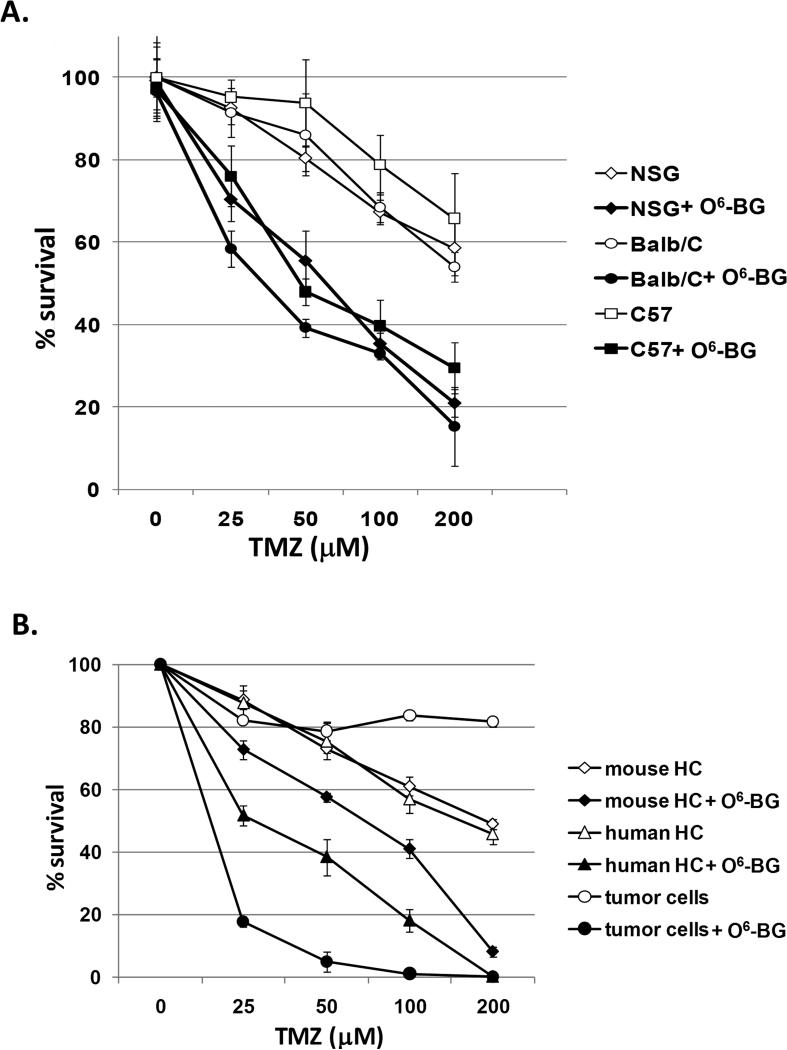
A combination treatment consisting of O6-BG and TMZ was selected to serve as the proof-of-concept treatment for development of the in vivo human toxicity model since myelosuppression is the major dose-limiting toxicity associated with this treatment in the clinic. (8-9) To gain insight into the drug sensitivity of the cell populations to be investigated (i.e. primary murine hematopoietic cells, primary human hematopoietic cells, and glioma cells), clonogenic survival assays were set up in the absence and presence of 20 μM O6-BG and increasing doses of TMZ (0 - 200 μM) (Fig. 1). Surrogate bone-marrow toxicity studies of new compounds and regimens are commonly evaluated in immunocompetent mice. Since immunodeficient mice would be required for engraftment of human CD34+ cells, it was important to compare the drug sensitivity of murine clonogenic cells derived from immunocompetent (BALB/c, C57BL/6N) and immunodeficient mice (NOD/SCID/γchainnull). Murine bone-marrow cells were set up in the absence or presence of drug combinations and sensitivity of clonogenic cells determined by CFU assays. The dose-dependent sensitivities of clonogenic cells derived from BALB/c, C57BL/6N and NOD/SCID/γchainnull mice were similar to TMZ in the presence and absence of O6-BG (Fig. 1A). Therefore, at least for the drug combination studied here, it was reasonable to assume that the sensitivity of the murine bone marrow in NOD/SCID/γchainnull can serve as an accurate read-out of normal murine hematotoxicity. In the next experiments, human CD34+ cells and NOD/SCID/γchainnull bone-marrow cells were treated with increasing doses of TMZ in the absence or presence of O6-BG. The concentrations of TMZ alone that resulted in 50% decrease in the colony number (IC50) varied slightly between mouse and human clonogenic cells (Human IC50 human = 160 μM; Mouse IC50= 190 μM TMZ). However, once O6-BG was included, the IC50 doses varied significantly: a 3-fold difference in sensitivity between mouse and human clonogenic cells was evident (IC50 human = 25 μM TMZ; mouse = 75 μM). The IC90 doses for both mouse and human were similar (IC90 human = 150 μM; mouse = 180 μM TMZ), indicating that when cells were exposed to high-doses of O6-BG/TMZ, differences in the in vitro sensitivity were less pronounced in vitro. Clonogenic assays for the human SF767 glioblastoma cell line were also set up under these same conditions. SF767 cells have been shown by us and others to constitutively express MGMT and are resistant to the methylating agent, TMZ, unless O6-BG is included (18, 20). As expected, the SF767 tumor cells were highly resistant to TMZ (Fig. 1) and exhibited significantly increased sensitivity to TMZ in the presence of O6-BG (IC90= 40 μM TMZ; Fig. 1), demonstrating the dependency of the SF767 cells on MGMT-mediated DNA repair.
Figure 1. Determination of in vitro sensitivities to TMZ and combination O6-BG/TMZ.
(A) BALB/c, C57BL/6N, and NOD/SCID/γchainnull bone marrow cells were exposed to increasing doses of TMZ in the absence or presence of O6-BG. At days 10-12, hematopoietic colonies (human and mouse) were scored visually by inverted light microscopy. (B) Human CD34+ cells, NOD/SCID/γchainnull mice bone marrow cells, and SF767 tumor cells were exposed to increasing doses of TMZ in the absence or presence of O6-BG. At days 10-12, hematopoietic colonies (human and mouse) were enumerated. SF767 colonies were scored visually by counting methylene-blue stained colonies. p < 0.0001 for TMZ versus O6-BG/TMZ for all cell types, mouse versus human for O6-BG/TMZ, mouse versus tumor for O6-BG/TMZ at all TMZ concentrations, human versus tumor for O6-BG/TMZ at all TMZ concentrations except 200 μM TMZ. HC = hematopoietic cells.
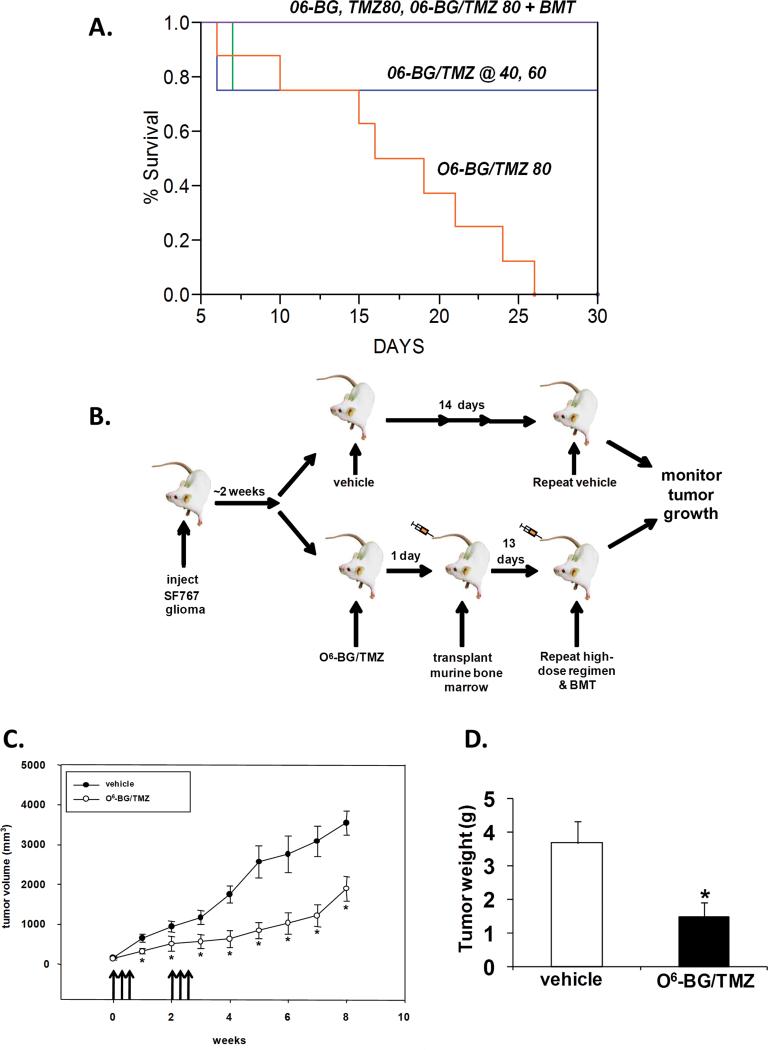
We next determined to what extent the in vitro sensitivities of the cell populations correlated with in vivo anti-tumor efficacy and toxicity to the bone-marrow compartment in NOD/SCID/γchainnull mice. To accomplish this, it was essential to develop an efficacious O6-BG/TMZ regimen that resulted in significant tumor kill in vivo. Treatment cycles containing increasing doses of TMZ were first tested for toxicity in NOD/SCID/γchainnull mice (Fig. 2A&B). Each cycle consisted of 3-consecutive days of treatment with 30 mg/kg O6-BG followed one hour later by 40, 60 or 80 mg/kg TMZ and a second bolus of O6-BG delivered seven hours later. The O6-BG-double bolus has been shown previously to deplete MGMT for at least 18 hours and prevent repair of TMZ-mediated DNA damage (21). Survival was determined using a pre-death endpoint system (see Material and Methods). Two cycles of treatment initiated a week apart resulted in morbidity in >85% of the NOD/SCID/γchainnull mice (Fig 2A). Histopathological evaluation of major organs (brain, colon, kidney, liver, lung, heart, spleen, and stomach) were performed on 2 vehicle-treated mice and 4 mice treated with O6-BG and followed by TMZ at 80 mg/kg and no gross abnormalities were noted. However, in mice treated with O6-BG and TMZ at 80 mg/kg, bone-marrow cellularity was decreased by at least 70% in the O6-BG/TMZ-treated mice compared to vehicle-treated mice when analyzed at the pre-death endpoint (data not shown). In contrast, when an autologous murine bone-marrow transplant was given 2 days after each cycle of drug administration, all mice maintained normal body weight and morbidity was prevented (Fig 2A). We next tested the efficacy of this regimen using NOD/SCID/γchainnull mice containing ectopic SF767 glioblastoma xenografts (Figure 2B). In 2 independent experiments, mice received 2 cycles of either vehicle control or O6-BG/TMZ treatment. After each 3-day treatment cycle, 8 × 106 autologous murine bone-marrow cells were transplanted via tail vein and tumor growth monitored over time. Following 2 cycles of treatment and stem-cell rescue, a significant delay in tumor progression was observed in vehicle- versus O6-BG/TMZ-treated mice (Fig. 2C). At 8 weeks post-treatment, mice were sacrificed for tumor and bone-marrow analysis. Tumors from drug-treated mice weighed significantly less than tumors from vehicle-treated mice (Fig. 2D). Bone-marrow cellularity of vehicle-treated mice versus mice receiving O6-BG/TMZ and stem-cell rescue, was not significantly different (data not shown). These data indicate that significant decreases in growth of alkylator-resistant tumor cells can be obtained with high-dose O6-BG/TMZ when combined with autologous stem-cell rescue and provided us with a relevant proof-of-concept regimen that could be used to monitor impact of the treatment on human hematopoiesis in vivo.
Figure 2. In vivo efficacy of alkylator-resistant SF767 tumor cells to myeloablative O6-BG/TMZ therapy in conjunction with stem-cell rescue.
(A) Development of O6-BG/TMZ myeloablative dosing-regimen. Mice (n = 4-8 mice per group) were injected ip with vehicle, O6-BG (30 mg/kg double bolus), TMZ (80 mg/kg) or O6-BG/TMZ (40-60 mg/kg). Kaplan-Meier survival plot of data compiled from 2-independent experiments. (B) Schema showing how NOD/SCID/γchainnull mice with SF767 xenografts (n = 8-10 mice per group) were treated with double-bolus O6-BG/TMZ and stem-cell rescue. Two cycles of treatment were given 2 weeks apart. (C) Tumor volumes were determined via caliper measurements. (D) Mice were euthanized and harvested tumors weighed. These data are representative of 2-independent experiments with similar results. *p < 0.05, vehicle versus drug-treated (tumor volumes and tumor weight).
In vivo bio-toxicity assay to evaluate impact of anti-cancer therapies on normal human-hematopoietic cell function
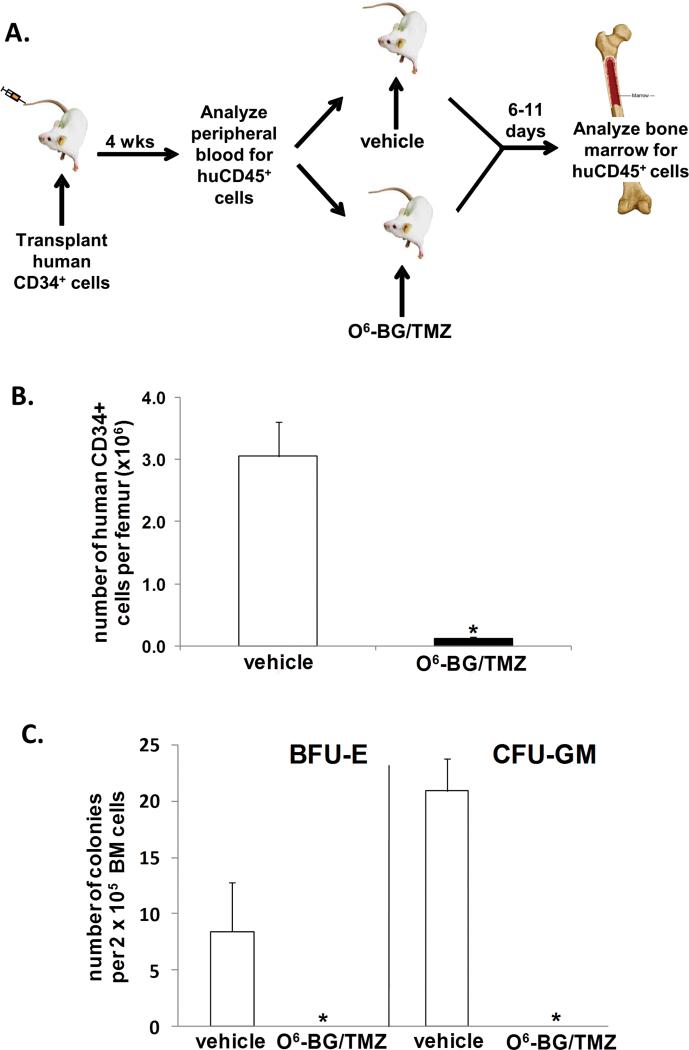
Our next objective was to determine to what extent the high-dose O6-BG/TMZ regimen hat effectively kills tumor cells may affect human hematopoiesis in vivo. NOD/SCID/γchainnull mice were sublethally irradiated and transplanted with 1 × 105 human CD34+ cells (Fig 3A). At 4 weeks post-transplant, a sample of PB was analyzed for the presence of human CD45+ cells to confirm human cell engraftment (Fig. 3A and Table 1). Next, cohorts of mice were treated with vehicle or one cycle of O6-BG/TMZ. Bone marrow was harvested at 6 or 11 days post-treatment, and analyzed for the percent and total number of human versus mouse cells (Table 1). Our objective was to determine the maximum impact of the treatment regimen on human hematopoiesis. Therefore, we analyzed the bone marrow at 6 days post-treatment since we have previously found that the nadir in human myeloid progenitor numbers is typically reached at day 6 following TMZ treatment in vitro (data not shown). In addition, the bone marrow was also analyzed at 11 days post-treatment in one experiment to assess marrow function and potential recovery after the nadir period. For both Experiments I and II, the fold decrease in the number of human CD45+ cells in vehicle versus O6-BG/TMZ treatment and for human versus mouse chimerism following treatment, was statistically significant (Table 1, p < 0.001). Although different base-line levels of human engraftment in the bone marrow were observed in Experiment I and II (see vehicle treated mice, Table 1), 2 × 105 CD34+ cells were transplanted per animal in both experiments. It has been our experience that the frequency of SCID-repopulating cells can vary widely amongst different cord blood products. In either situation (high or low human-cell engraftment), the drug sensitivity of the human bone-marrow cells was similar. In Experiment I, a 9-fold decrease in the number of human cells in the bone marrow was observed following O6-BG/TMZ treatment. In contrast, only a 1.5-fold decrease in the number of murine bone-marrow cells was observed in these same mice (Table 1). In addition, due to high levels of human engraftment in Experiment I, the spleen was also analyzed for human hematopoietic cells; significant decreases in human cells was observed in O6-BG/TMZ-treated mice compared to vehicle-treated mice (data not shown). In Experiment II, total human engraftment pre-treatment was lower than in experiment I, which is not unexpected, since different cord blood products were used in each experiment (Table I, see %huCD45+ cells in the PB pre-treatment). This also enabled us to see if lower levels of human chimerism would result in a different toxicity profile (human versus mouse) from that observed in Experiment I. Differential sensitivity of human and mouse bone-marrow cells was observed at 6 and 11 days post-drug treatment, with the human cells still exhibiting a higher sensitivity to the drug treatment than the mouse cells at both time points. A 13-fold decrease in human cell chimerism and a 1.5 fold-decrease in mouse cell chimerism were observed at 6 days post-O6-BG/TMZ treatment. At day 11 post-treatment, a 3-fold decrease and a 1.2 fold-decrease was observed for human and mouse cell chimerism respectively (Table 1). In Experiment II, it is important to note that the level of human cells in the PB pre-treatment differed from the two cohorts used for the day 11 analysis (Table 1, see Experiment II-%huCD45+ cells in the PB pre-treatment at day 11). Human cells were detected at higher levels in the mouse cohort selected for drug treatment versus the cohort selected for vehicle treatment. Even with higher percentage of human cells in the PB of the cohort selected for O6-BG/TMZ treatment, the fold-decrease in human versus mouse bone-marrow cells was still evident and statistically significant. These data collectively demonstrate that the human hematopoietic cells, even at different levels of overall human cell chimerism in the bone marrow, were significantly more sensitive to O6-BG/TMZ treatment in vivo than mouse bone-marrow cells.
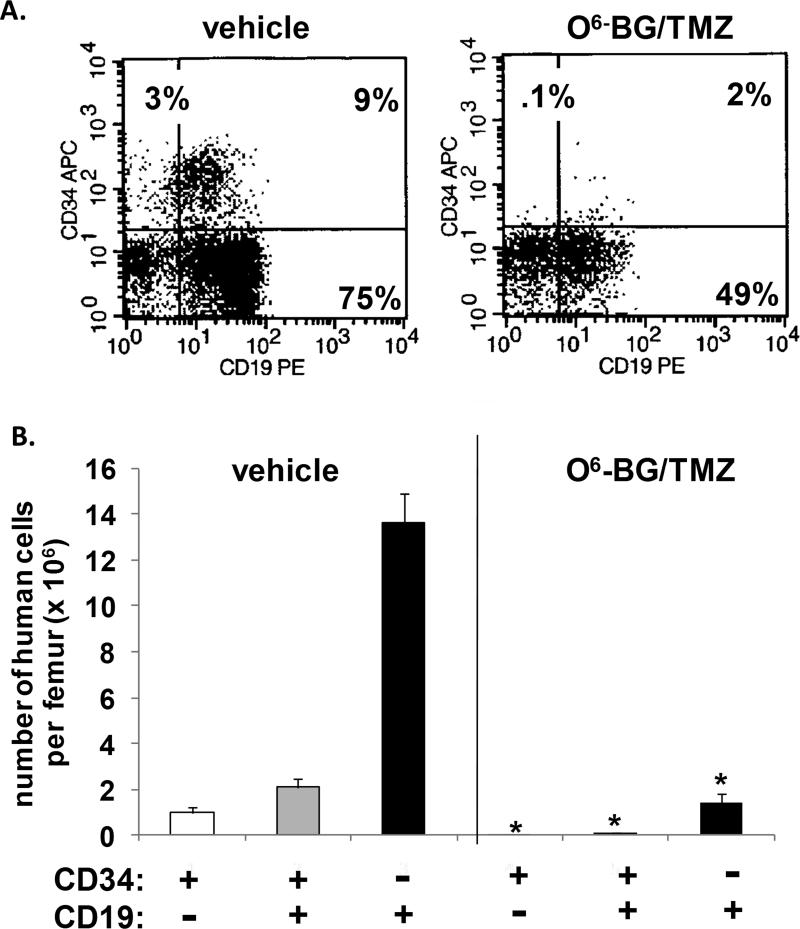
Figure 3. Human toxicity model to assess impact of treatment on human bone-marrow cells.
(A) Human CD34+ cells were isolated from umbilical cord blood and transplanted into sublethally irradiated NOD/SCID/γchainnull mice. At 4 weeks post-transplant, a sample of PB was analyzed from each mouse to confirm that all mice were similarly engrafted. Cohorts of mice received vehicle or one cycle of the O6-BG/TMZ regimen. At 6-11 days post-treatment, the bone marrow was harvested and analyzed via flow cytometry for the presence of human cells. (B) At 6 days post-treatment, the bone marrow was harvested, counted, and analyzed via flow cytometry to determine the number of human CD34+ cells in the bone marrow from vehicle and O6-BG/TMZ-treated mice. *p < 0.001 for vehicle versus drug-treated mice. (C) The bone marrow from vehicle and O6-BG/TMZ-treated mice was analyzed for the presence of human colony-forming unit cells and analyzed 12 days after plating. *p < 0.001 for vehicle versus drug-treated mice.
Table 1.
Analysis of human versus mouse bone-marrow cells post-treatment.
| Expt | Treatment | %huCD45 in PB pre-treatment* | Analysis day post-treatment | %huCD45 in the bone marrow† | total number of bone marrow cells‡ | number of cells per femur (×106)§ | fold decrease after treatment** | ||
|---|---|---|---|---|---|---|---|---|---|
| human | mouse | human | mouse | ||||||
| I | Vehicle | 63.5±13.9 | 6 | 85±3 | 21±3 | 18 ±2 | 3 ±1 | ||
| O6-BG/TMZ | 63±6.5 | 6 | 53±0.2 | 4±1 | 2±1 | 2±1 | 9 | 1.5 | |
| II | Vehicle | 8.8±2.4 | 6 | 69±16 | 19±0.3 | 13±3 | 6±3 | ||
| O6-BG/TMZ | 11.5±5.6 | 6 | 20±14 | 5±0.6 | 1±1 | 4±1 | 13 | 1.5 | |
| Vehicle | 5.2±0.8 | 11 | 15±2 | 20±0.2 | 3±1 | 17±1 | |||
| O6-BG/TMZ | 9.8±2.8 | 11 | 6±4 | 15±2 | 1±1 | 14±3 | 3 | 1.2 | |
Mice were transplanted with human CD34+ cells and the peripheral blood (PB) analyzed at one month post-transplantation for the presence of human CD45+ cells. This analysis was done prior to O6-BG/TMZ treatment.
At 6-11 days post-O6-BG/TMZ treatment, the bone marrow was harvested and the percent human-cell chimerism (huCD45+) was determined by flow cytometry.
The total number of human and mouse bone marrow cells in vehicle- and O6-BG/TMZ-treated mice was determined using a coulter counter.
Total number of human cells = total number bone marrow cells × % human cells. Total number of murine cells = total number bone marrow cells – total number human cells.
The fold decrease between vehicle-treated and drug-treated human or mouse bone marrow cells = total number of human or mouse in vehicle-treated mice ÷ total number of human or mouse cells remaining in O6-BG/TMZ-treated mice.
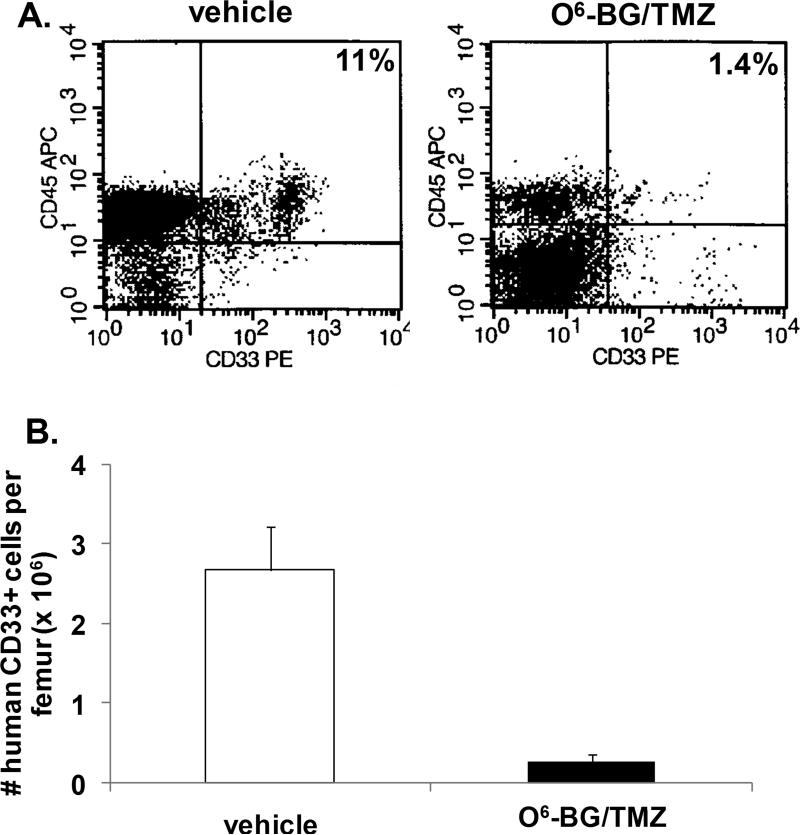
The impact of treatment on multi-lineage differentiation typically found in the bone marrow of mice reconstituted with human cells was also evaluated (Figs. 3-5). The number of CD34+ cells significantly decreased following treatment (Fig. 3b). In contrast to vehicle-treated mice, CFU assays indicated that human erythroid and myeloid progenitor cells were no longer present in the bone marrow from drug-treated mice (Fig. 3c). The CD34+ cell population was further analyzed for different B-cell CD19+ subsets typically found in humanized bone marrow (Fig. 4a & 4b). There was a significant decrease in the most primitive cells (CD34+, CD19-), B-cell progenitors (CD34+, CD19+), and more mature B-lymphoid cells (CD34-, CD19+). In addition, a significant decrease in human CD33+ myeloid cells was also evident (Fig. 5a & 5b). These studies indicate that differential sensitivities of mouse and human cells to cytotoxic dosing regimens are apparent both in vivo and in vitro, and that the regimen sensitivities of human versus mouse may be even more pronounced in the bone marrow.
Figure 5. Analysis of human myeloid cells in the bone marrow of NOD/SCID/γchainnull mice following O6-BG/TMZ treatment.
(A) At 6 days post-treatment, bone marrow from vehicle and O6-BG/TMZ-treated mice was analyzed for the presence of human CD45+ and CD33+ cells via flow cytometry. (B) Based on flow cytometry and bone-marrow cell numbers, the number of human CD33+ myeloid cells was determined. These data are representative examples of data from Experiment I. Data are similar from Experiment II except the level of overall human chimerism was lower in vehicle and O6-BG/TMZ-treated mice. *p < 0.0001 for vehicle versus drug-treated mice.
Figure 4. Analysis of human B-lymphoid cells in the bone marrow of NOD/SCID/γchainnull mice following O6-BG/TMZ treatment.
(A) Bone marrow from vehicle and O6-BG/TMZ-treated mice was analyzed for the presence of CD34+ and CD19+ cells via flow cytometry. (B) Based on flow cytometry and bone-marrow cell numbers, the number of human CD34+CD19- (progenitors), CD34+CD19+ (B-lymphoid progenitors), and CD34-CD19+ (more mature B-lymphoid cells) were determined by flow cytometry. These data are representative examples of data from Experiment I. Data are similar from Experiment II except the level of overall human chimerism was lower in vehicle and O6-BG/TMZ-treated mice. *p < 0.0001 for vehicle versus drug-treated mice.
DISCUSSION
Development of human xenograft models that focus on treatment efficacy as well as potential hematopoietic toxicities in vivo could yield crucial insights into how to best translate basic research findings to the clinic. The main dose-limiting toxicity in >50% of anti-cancer drugs used in the past 15 years is therapy-mediated myelosuppression (22). Therefore, pre-clinical predictive models of this adverse event in humans could help guide the selection of new and safer therapeutic agents for clinical testing. While the use of in vitro clonogenic assays and mice for initial testing and regimen development is a logical starting point, incorporation of models that mimic to a larger degree human response to multiple cycles of therapy could aid in screening for safer and effective lead compounds. It is well recognized that doses of a compound that prevent growth of tumor xenografts in mice may not be achievable in humans due to compound-induced myelotoxicity (2-5). Our data collectively demonstrate that the human hematopoietic cells were significantly more sensitive in vivo than mouse bone-marrow cells when mice previously transplanted with human CD34+ cells received a high-dose regimen that can inhibit growth of human glioma xenografts. Although humanizing the bone-marrow compartment of NOD/SCID/γchainnull mice cannot necessarily take into account all pharmacological and inter-species differences in human and mouse, it can provide improved correlations of compound levels obtained in vivo versus regimen efficacy and human hematotoxicity.
We built our study based on the scientific questions observed from clinical observations in an effort to research improvements in therapy (e.g. from the bedside back to the bench). Middleton et al originally determined the pharmacokinetic profile of TMZ in plasma from adult patients diagnosed with malignant melanoma. In patients that received TMZ starting at 200 mg/m2/day, the range of peak TMZ levels (Cmax) was 0.1-10.0 μg/ml or approximately 0.5-50 μM in the plasma. (23) Similar TMZ plasma levels were also obtained in an adult glioma clinical study by Osterman et al. (24) Additionally, when 267 mg/m2/day was administered to pediatric brain tumor patients, levels of TMZ detected in the plasma ranged from 2.7-19.5 μg/ml or 14.0-100 μM which is similar to TMZ peak levels obtained in adults. (25) These TMZ dosing schedules have been incorporated into the standard-of-care protocols for GBM.(26) In the clonogenic assays performed in Figure 1, we used clinically relevant doses of TMZ (25-100 μM) that could be obtained in the plasma of patients, and demonstrated that human hematopoietic progenitor cells were significantly more sensitive than murine hematopoietic progenitors to O6-BG in combination with TMZ.
We used a O6-BG/TMZ regimen clinically known to be toxic to the bone marrow, confirmed that it did indeed block tumor growth of ectopic human xenografts; and importantly simulated therapy-mediated toxicity in the bone marrow. An O6-BG/TMZ treatment regimen was recently tested in Phase II clinical trials in the absence of stem-cell rescue (9). In that study, 5 of 32 patients with TMZ-resistant anaplastic glioma responded to O6-BG in combination with TMZ. For TMZ-resistant glioblastoma multiforme, only one of 34 patients exhibited a response to treatment. The reason for lack of response in most patients is likely a consequence of the necessity to reduce the levels of TMZ administered due to myelosuppression; a 25% reduction in TMZ dose was necessary in 48% of patients due to therapy-induced grade-4 neutropenia and thrombocytopenia. To increase doses of O6-BG/TMZ in these patients, myelosuppression will need to be overcome and this is an area of active investigation. Strategies that protect hematopoietic cells from high-dose O6-BG/TMZ such as viral vector-mediated gene transfer and expression of O6-BG-resistant mutant MGMT in autologous hematopoietic stem and progenitor cells (27), or strategies that incorporate stem-cell rescue following administration of O6-BG/TMZ, may be the only treatment strategies to alleviate O6-BG/TMZ-associated bone-marrow toxicity.
When NOD/SCID/γchainnull mice transplanted with human CD34+ cells were treated with one cycle of O6-BG/TMZ, significant differential toxicity of human versus mouse hematopoietic cells was observed. The reason for the differential sensitivities between mouse- and human-hematopoietic cells could be due in part to subtle differences in expression or in the amino acid structure of murine and human MGMT in the bone marrow cells. While the active site of murine and human MGMT is identical (Pro-Cys-His-Arg), differences in the MGMT amino acid sequence are evident between the two species. Gerson and colleagues demonstrated that, in contrast to human MGMT, murine MGMT has a leucine at residue 180. This amino acid residue is located just outside the active site of MGMT, and correlated with increased resistance of murine MGMT to O6-BG (28).
A variety of human cancers and diseases are currently being modeled in immunodeficient mouse strains such as NOD/SCID(29), NOD/SCID mice transgenic for human Stem-cell Factor, Granulocyte-macrophage-colony-stimulating factor, and Interleukin-3 (30-31), NOD/SCID/γchainnull (32-38) and Rag2-/-γc-/- (39). Due to increased and consistent levels of human hematopoietic cell engraftment and an increased life span compared to NOD/SCID mice, NOD/SCID/γchainnull mice are beginning to replace NOD/SCID mice in studies that are testing transfer-vector designs and strategies for hematopoietic gene therapy (38). Additionally, development of pre-clinical humanized models that will allow for reconstitution of a mature human immune system will be instrumental in gaining a better understanding of in vivo human cell-mediated immune responses and for testing of novel immune therapy or chemotherapy that target the tumor and its microenvironment. Shultz et al recently demonstrated that HLA-restricted human immune responses can now be studied in vivo using NOD/SCID/γchainnull mice that have been engineered to constitutively express HLA class I heavy and light chains.(40) The use of immunodeficient mouse models to study cancer progression and testing of anti-cancer therapies is still in its infancy. Integrated modeling approaches that investigate human tumor-cell growth in mice with humanized bone marrow capable of producing mature human immune cells will be instrumental in understanding the interplay between tumor progression, the tumor microenvironment, and the hematopoietic and immune systems. For example, our research team recently demonstrated that human circulating progenitor cells that are phenotypically and functionally distinct from circulating endothelial cells can promote growth of human melanoma xenografts in NOD/SCID and NOD/SCID/γchainnull mice. (Mund, Case, and Pollok, unpublished observations and (41)
The engrafted human hematopoietic CD34+ cells and progeny derived from the initial transplanted CD34+ cells interact at least to some extent with stromal cells and cytokines located in the murine bone-marrow microenvironment since the transplanted human CD34+ cells differentiate into B-lymphoid and myeloid cells in vivo. Murine stromal feeder cells clearly interact with human hematopoietic cells, since numerous studies have used stroma of murine origin for in vitro expansion of primitive human hematopoietic cells. (42-45) Additionally, it is important to note that in vivo models in which recapitulation of the complete spectrum of human hematopoiesis can be accomplished have been developed. For example, implantation of human fetal thymus and fetal liver into SCID mice provided a human microenvironment which allowed for differentiation of human hematopoietic cells into multiple cell lineages.(46) An expansion of this model was done by Fraser et al in which SCID mice were transplanted subcutaneously with human fetal bone, thymus, and spleen fragments; this allowed for differentiation of human hematopoietic cells into multiple lineages and homing of cells to the thymus for further differentiation into mature T-cell subsets.(47) More recently, it has been demonstrated that human marrow mesenchymal stem/stromal cells (MSC) can organize a hematopoietic microenvironment with murine hematopoietic cells after transplantation into NOD/SCID mice. (48) It is possible that the greater drug susceptibility of the engrafted human hematopoietic cells compared to the mouse hematopoietic cells is that the mouse bone-marrow microenvironment may not completely support human hematopoietic cell survival particularly when under chemotherapy-mediated stress. However, in vitro clonogenic data also indicated striking differences in drug sensitivity of murine and human progenitor cells. Interactions between human MSC and hematopoietic stem and progenitor cells are important for survival and differentiation in vivo for others have demonstrated that co-transplantation of human MSC and CD34+ cells into NOD/SCID mice enhanced engraftment of human myeloid cells in the bone marrow. (49) In addition, it is clear that the marrow microenvironment and MSC, as well as the hematopoietic stem and progenitor cells, are adversely affected by chemotherapy. (50) At the present time, however, it is not known to what extent the implantation of human MSC into the bone marrow of mice engrafted with human hematopoietic cells would increase or decrease the sensitivity of human hematopoietic cells to myelotoxic therapy. The humanized bone-marrow model described here serves as a starting point for these types of investigations. Our data indicate that differential sensitivities of mouse versus hematopoietic cells to O6-BG in combination with TMZ exist in vitro (Fig 1) and that these differences in drug sensitivity could be recapitulated in the in vivo environment (Table 1 and Figs 3-5). The humanized bone-marrow model described in our study could be used on a much larger scale for drug and regimen screening since only transplant of reasonable numbers of human CD34+ cells into sublethally irradiated NOD/SCID/γchainnull is required, and confirmation of human engraftment in the bone marrow can be done by analyzing a small sample of peripheral blood from the transplanted animals.
The humanized bone-marrow mouse model described here represents a feasible and physiologically relevant tool in which to determine if a balance between treatment efficacy and toxicity is achievable. In contrast to many other immunodeficient mouse strains, previous studies have shown that NOD/SCID/γchainnull mice can be readily and consistently reconstituted with human hematopoietic cells that have the capacity to differentiate into various blood-cell lineages (6). In addition, we have found that human tumors grow consistently in NOD/SCID/γchainnull mice, and represent a reliable model for testing anti-cancer strategies. In future studies, it will be essential to utilize orthotopic xenografts when new therapeutic regimens are tested, since the microenvironment in which the tumor resides could affect tumor sensitivity to treatment. (51) Integration of humanized hematopoietic and tumor models may be a way for anti-cancer therapies to be tested earlier in the drug development process which will ultimately lead to more efficient translation of therapies into clinical practice. While this model has the potential to be used as an in vivo screening tool that can aid in testing and refinement of treatment regimens, the validation of this model will require testing of an additional number of compounds and regimens to establish the model's predictive value. We are currently in the process of organizing a consortium of scientists to evaluate a broader set of therapeutics to determine the predictive clinical value of this modeling approach.
Acknowledgements
The authors wish to thank the expert technical assistance of Jayne Silver in the In Vivo Therapeutics Core. We also thank Mary Murray, Michael Lahn, Helmut Hanenberg, and Lana Christian for their guidance and critical reading of this manuscript. We thank the nursing staff at the St. Vincent Hospital for donation of their time for collection of umbilical cord blood products. We also acknowledge the continuing support of the Riley Childrens’ Foundation and the Indiana University Simon Cancer Center.
Grant support: Hope Street Kids Foundation, RO1 CA138798 (KEP); KO8 HL75253 (WSG); the Riley Children's Foundation (HW, LDH, WSG, and KEP).
REFERENCES
- 1.Hollingshead MG. Antitumor efficacy testing in rodents. Journal of the National Cancer Institute. 2008;100:1500–10. doi: 10.1093/jnci/djn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erickson-Miller CL, May RD, Tomaszewski J, Osborn B, Murphy MJ, Page JG, et al. Differential toxicity of camptothecin, topotecan and 9-aminocamptothecin to human, canine, and murine myeloid progenitors (CFU-GM) in vitro. Cancer chemotherapy and pharmacology. 1997;39:467–72. doi: 10.1007/s002800050600. [DOI] [PubMed] [Google Scholar]
- 3.Kurtzberg LS, Roth SD, Bagley RG, Rouleau C, Yao M, Crawford JL, et al. Bone marrow CFU-GM and human tumor xenograft efficacy of three tubulin binding agents. Cancer chemotherapy and pharmacology. 2009;64:1029–38. doi: 10.1007/s00280-009-0959-z. [DOI] [PubMed] [Google Scholar]
- 4.Masubuchi N, May RD, Atsumi R. A predictive model of human myelotoxicity using five camptothecin derivatives and the in vitro colony-forming unit granulocyte/macrophage assay. Clin Cancer Res. 2004;10:6722–31. doi: 10.1158/1078-0432.CCR-04-0721. [DOI] [PubMed] [Google Scholar]
- 5.Pessina A, Albella B, Bayo M, Bueren J, Brantom P, Casati S, et al. Application of the CFU-GM assay to predict acute drug-induced neutropenia: an international blind trial to validate a prediction model for the maximum tolerated dose (MTD) of myelosuppressive xenobiotics. Toxicol Sci. 2003;75:355–67. doi: 10.1093/toxsci/kfg188. [DOI] [PubMed] [Google Scholar]
- 6.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–89. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 7.Friedman HS, Pluda J, Quinn JA, Ewesuedo RB, Long L, Friedman AH, et al. Phase I trial of carmustine plus O6-benzylguanine for patients with recurrent or progressive malignant glioma. J Clin Oncol. 2000;18:3522–8. doi: 10.1200/JCO.2000.18.20.3522. [DOI] [PubMed] [Google Scholar]
- 8.Quinn JA, Desjardins A, Weingart J, Brem H, Dolan ME, Delaney SM, et al. Phase I trial of temozolomide plus O6-benzylguanine for patients with recurrent or progressive malignant glioma. J Clin Oncol. 2005;23:7178–87. doi: 10.1200/JCO.2005.06.502. [DOI] [PubMed] [Google Scholar]
- 9.Quinn JA, Jiang SX, Reardon DA, Desjardins A, Vredenburgh JJ, Rich JN, et al. Phase II trial of temozolomide plus o6-benzylguanine in adults with recurrent, temozolomide-resistant malignant glioma. J Clin Oncol. 2009;27:1262–7. doi: 10.1200/JCO.2008.18.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quinn JA, Pluda J, Dolan ME, Delaney S, Kaplan R, Rich JN, et al. Phase II trial of carmustine plus O(6)-benzylguanine for patients with nitrosourea-resistant recurrent or progressive malignant glioma. J Clin Oncol. 2002;20:2277–83. doi: 10.1200/JCO.2002.09.084. [DOI] [PubMed] [Google Scholar]
- 11.Dolan ME, Chae MY, Pegg AE, Mullen JH, Friedman HS, Moschel RC. Metabolism of O6-Benzylguanine, an Inactivator of O6-Alkylguanine-DNA Alkyltransferase. Cancer Research. 1994;54:5123–30. [PubMed] [Google Scholar]
- 12.Dolan P, Moschel, Vishnuvajjala, Flora, Grever, Firedman Biodistribution of O6-benzylguanine and its effectiveness against human brain tumor xenografts when given in polyethylene glycol or cremophor-EL. Cancer Chemotherpay Pharmacology. 1994;35:121–6. doi: 10.1007/BF00686633. [DOI] [PubMed] [Google Scholar]
- 13.Dolan ME, Moschel RC, Pegg AE. Depletion of mammalian O6-alkylguanine-DNA alkyltransferase activity by O6-benzylguanine provides a means to evaluate the role of this protein in protection against carcinogenic and therapeutic alkylating agents. Proc Natl Acad Sci U S A. 1990;87:5368–72. doi: 10.1073/pnas.87.14.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolan ME, Stine L, Mitchell RB, Moschel RC, Pegg AE. Modulation of mammalian O6-alkylguanine-DNA alkyltransferase in vivo by O6-benzylguanine and its effect on the sensitivity of a human glioma tumor to 1-(2-chloroethyl)-3-(4-methylcyclohexyl)-1-nitrosourea. Cancer Commun. 1990;2:371–7. doi: 10.3727/095535490820873985. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Gerson SL. Targeted modulation of MGMT: clinical implications. Clin Cancer Res. 2006;12:328–31. doi: 10.1158/1078-0432.CCR-05-2543. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell RB, Moschel RC, Dolan ME. Effect of O6-benzylguanine on the sensitivity of human tumor xenografts to 1,3-bis(2-chloroethyl)-1-nitrosourea and on DNA interstrand cross-link formation. Cancer Res. 1992;52:1171–5. [PubMed] [Google Scholar]
- 17.Buschfort-Papewalis C, Moritz T, Liedert B, Thomale J. Down-regulation of DNA repair in human CD34(+) progenitor cells corresponds to increased drug sensitivity and apoptotic response. Blood. 2002;100:845–53. doi: 10.1182/blood-2002-01-0022. [DOI] [PubMed] [Google Scholar]
- 18.Kreklau EL, Pollok KE, Bailey BJ, Liu N, Hartwell JR, Williams DA, et al. Hematopoietic expression of O(6)-methylguanine DNA methyltransferase-P140K allows intensive treatment of human glioma xenografts with combination O(6)-benzylguanine and 1,3-bis-(2-chloroethyl)-1-nitrosourea. Mol Cancer Ther. 2003;2:1321–9. [PubMed] [Google Scholar]
- 19.Cai S, Hartwell JR, Cooper RJ, Juliar BE, Kreklau E, Abonour R, et al. In vivo effects of myeloablative alkylator therapy on survival and differentiation of MGMTP140K-transduced human G-CSF-mobilized peripheral blood cells. Mol Ther. 2006;13:1016–26. doi: 10.1016/j.ymthe.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Chen ZP, Pan J, Huang Q, Sun ZF, Zhou LY, Wang AD. Antitumor efficacy of SarCNU in a human glioma xenograft model expressing both MGMT and extraneuronal monoamine transporter. Journal of neuro-oncology. 2001;51:19–24. doi: 10.1023/a:1006480818373. [DOI] [PubMed] [Google Scholar]
- 21.Kreklau EL, Kurpad C, Williams DA, Erickson LC. Prolonged inhibition of O(6)-methylguanine DNA methyltransferase in human tumor cells by O(6)-benzylguanine in vitro and in vivo. J Pharmacol Exp Ther. 1999;291:1269–75. [PubMed] [Google Scholar]
- 22.Parchment RE, Gordon M, Grieshaber CK, Sessa C, Volpe D, Ghielmini M. Predicting hematological toxicity (myelosuppression) of cytotoxic drug therapy from in vitro tests. Ann Oncol. 1998;9:357–64. doi: 10.1023/a:1008245906772. [DOI] [PubMed] [Google Scholar]
- 23.Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–66. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 24.Ostermann S, Csajka C, Buclin T, Leyvraz S, Lejeune F, Decosterd LA, et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10:3728–36. doi: 10.1158/1078-0432.CCR-03-0807. [DOI] [PubMed] [Google Scholar]
- 25.Broniscer A, Gururangan S, MacDonald TJ, Goldman S, Packer RJ, Stewart CF, et al. Phase I trial of single-dose temozolomide and continuous administration of o6-benzylguanine in children with brain tumors: a pediatric brain tumor consortium report. Clin Cancer Res. 2007;13:6712–8. doi: 10.1158/1078-0432.CCR-07-1016. [DOI] [PubMed] [Google Scholar]
- 26.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 27.website Cg [9.21.09. 2009];Combination Chemotherapy and Radiation Therapy in Treating Younger Patients Who are Undergoing an Autologous Stem Cell Transplant for Newly diagnosed gliomas. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00253487?term=autologous+stem+cell+transplant+for+newly+diagnosed+gliomas&rank=1.
- 28.Liu L, Lee K, Wasan E, Gerson SL. Differential sensitivity of human and mouse alkyltransferase to O6-benzylguanine using a transgenic model. Cancer research. 1996;56:1880–5. [PubMed] [Google Scholar]
- 29.Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180–91. [PubMed] [Google Scholar]
- 30.Feuring-Buske M, Gerhard B, Cashman J, Humphries RK, Eaves CJ, Hogge DE. Improved engraftment of human acute myeloid leukemia progenitor cells in beta 2-microglobulin-deficient NOD/SCID mice and in NOD/SCID mice transgenic for human growth factors. Leukemia. 2003;17:760–3. doi: 10.1038/sj.leu.2402882. [DOI] [PubMed] [Google Scholar]
- 31.Wei J, Wunderlich M, Fox C, Alvarez S, Cigudosa JC, Wilhelm JS, et al. Microenvironment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer Cell. 2008;13:483–95. doi: 10.1016/j.ccr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andre MC, Erbacher A, Gille C, Schmauke V, Goecke B, Hohberger A, et al. Long-term human CD34(+) stem cell-engrafted nonobese diabetic/SCID/IL-2Rgamma(null) mice show impaired CD8(+) T cell maintenance and a functional arrest of immature NK cells. J Immunol. 2010;185:2710–20. doi: 10.4049/jimmunol.1000583. [DOI] [PubMed] [Google Scholar]
- 33.Chiu PP, Jiang H, Dick JE. Leukemia-initiating cells in human T-lymphoblastic leukemia exhibit glucocorticoid resistance. Blood. 2010 doi: 10.1182/blood-2010-06-292300. [DOI] [PubMed] [Google Scholar]
- 34.Hayakawa J, Hsieh MM, Anderson DE, Phang O, Uchida N, Washington K, et al. The assessment of human erythroid output in NOD/SCID mice reconstituted with human hematopoietic stem cells. Cell Transplant. 2010 doi: 10.3727/096368910X314161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu C, Chen BJ, Deoliveira D, Sempowski GD, Chao NJ, Storms RW. Progenitor cell dose determines the pace and completeness of engraftment in a xenograft model for cord blood transplantation. Blood. 2010 doi: 10.1182/blood-2009-12-260810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDermott SP, Eppert K, Lechman ER, Doedens M, Dick JE. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood. 2010;116:193–200. doi: 10.1182/blood-2010-02-271841. [DOI] [PubMed] [Google Scholar]
- 37.Notta F, Doulatov S, Dick JE. Engraftment of human hematopoietic stem cells is more efficient in female NOD/SCID/IL-2Rgc-null recipients. Blood. 2010;115:3704–7. doi: 10.1182/blood-2009-10-249326. [DOI] [PubMed] [Google Scholar]
- 38.Sumiyoshi T, Holt NG, Hollis RP, Ge S, Cannon PM, Crooks GM, et al. Stable transgene expression in primitive human CD34+ hematopoietic stem/progenitor cells, using the Sleeping Beauty transposon system. Hum Gene Ther. 2009;20:1607–26. doi: 10.1089/hum.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–7. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 40.Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, Tomizawa M, et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci U S A. 2010;107:13022–7. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Estes ML, Mund JA, Mead LE, Prater DN, Cai S, Wang H, et al. Application of polychromatic flow cytometry to identify novel subsets of circulating cells with angiogenic potential. Cytometry A. 2010;77:831–9. doi: 10.1002/cyto.a.20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins LS, Dorshkind K. A stromal cell line from myeloid long-term bone marrow cultures can support myelopoiesis and B lymphopoiesis. J Immunol. 1987;138:1082–7. [PubMed] [Google Scholar]
- 43.Suzuki J, Fujita J, Taniguchi S, Sugimoto K, Mori KJ. Characterization of murine hemopoietic-supportive (MS-1 and MS-5) and non-supportive (MS-K) cell lines. Leukemia. 1992;6:452–8. [PubMed] [Google Scholar]
- 44.Takakura N, Kodama H, Nishikawa S. Preferential proliferation of murine colony-forming units in culture in a chemically defined condition with a macrophage colony-stimulating factor-negative stromal cell clone. J Exp Med. 1996;184:2301–9. doi: 10.1084/jem.184.6.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wineman J, Moore K, Lemischka I, Muller-Sieburg C. Functional heterogeneity of the hematopoietic microenvironment: rare stromal elements maintain long-term repopulating stem cells. Blood. 1996;87:4082–90. [PubMed] [Google Scholar]
- 46.Namikawa R, Weilbaecher KN, Kaneshima H, Yee EJ, McCune JM. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172:1055–63. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fraser CC, Kaneshima H, Hansteen G, Kilpatrick M, Hoffman R, Chen BP. Human allogeneic stem cell maintenance and differentiation in a long-term multilineage SCID-hu graft. Blood. 1995;86:1680–93. [PubMed] [Google Scholar]
- 48.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 49.Angelopoulou M, Novelli E, Grove JE, Rinder HM, Civin C, Cheng L, et al. Cotransplantation of human mesenchymal stem cells enhances human myelopoiesis and megakaryocytopoiesis in NOD/SCID mice. Exp Hematol. 2003;31:413–20. doi: 10.1016/s0301-472x(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 50.Cruet-Hennequart S, Prendergast AM, Barry FP, Carty MP. Human mesenchymal stem cells (hMSCs) as targets of DNA damaging agents in cancer therapy. Curr Cancer Drug Targets. 2010;10:411–21. doi: 10.2174/156800910791208553. [DOI] [PubMed] [Google Scholar]
- 51.Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs. 1999;17:343–59. doi: 10.1023/a:1006326203858. [DOI] [PubMed] [Google Scholar]