Abstract
The peptide hormone gastrin has been identified as a major regulator of acid secretion and a potent mitogen for normal and malignant gastrointestinal cells. The importance of gastric acid in the absorption of dietary iron first became evident 50 years ago when iron-deficiency anemia was recognised as a long-term consequence of partial gastrectomy. This review summarises the connections between circulating gastrins, iron status and colorectal cancer. Gastrins bind two ferric ions with micromolar affinity and, in the case of non-amidated forms of the hormone, iron-binding is essential for biological activity in vitro and in vivo. The demonstration of an interaction between gastrin and transferrin by biochemical techniques led to the proposal that gastrins catalyse the loading of transferrin with iron. Several lines of evidence, including the facts that the concentrations of circulating gastrins are increased in mice and humans with the iron-overload disease hemochromatosis and that transferrin saturation positively correlates with circulating gastrin concentration, suggest the potential involvement of gastrins in iron homeostasis. Conversely, recognition that ferric ions play an unexpected role in the biological activity of gastrins may assist in the development of useful therapies for colorectal carcinoma and other disorders of mucosal proliferation in the gastrointestinal tract.
Introduction
The peptide hormone gastrin was first identified as a stimulant of gastric acid secretion [1]. The recognition that iron-deficiency anemia was a long-term consequence of partial gastrectomy indicated the importance of gastric acid in the absorption of dietary iron [2, 3]. However over the past decade evidence has been accumulating that gastrin and its precursors may be directly involved in iron homeostasis [4–6]. Since gastrin precursors also act as growth factors for the colorectal mucosa [7, 8], this review will explore the connections between gastrins and iron homeostasis, and the implications of those connections for the development of colorectal cancer.
Gastrins and Gastrin Receptors
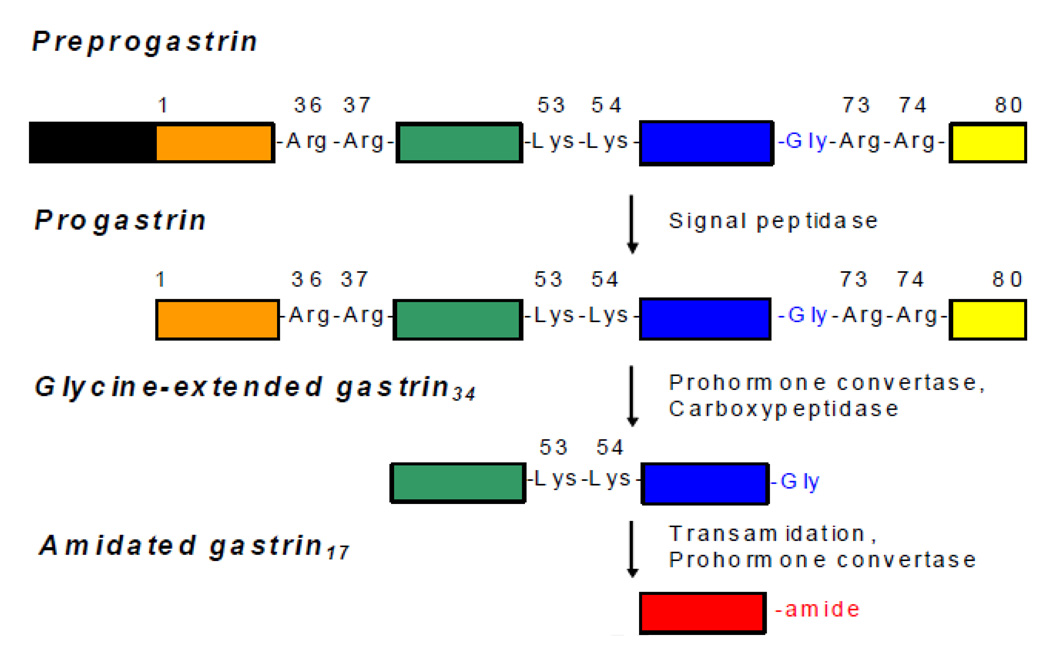
In 1905 the British physiologist John Edkins postulated the existence of gastrin as a hormonal regulator responsible for stimulating gastric acid secretion [1]. Fifty years later Gregory and Tracy purified sufficient gastrin to determine its amino acid sequence [9, 10]. Cloning of gastrin mRNA subsequently revealed that all forms of gastrin are generated from an 80 amino acid precursor, progastrin [11], which is cleaved at dibasic sequences by prohormone convertases [12, 13] (Figure 1). Subsequent removal of Arg73 and Arg74 by carboxypeptidase E yields glycine-extended gastrin34 and glycine-extended gastrin17 (Ggly), and oxidative cleavage by peptidylglycine α-amidating monooxygenase results in C-terminally amidated gastrin17 (Gamide) [12]. Many of the physiological effects of Gamide are mediated by the cholecystokinin2 (CCK2) receptor [14], which recognises the amidated C-terminal tetrapeptide of gastrin [15]. Gamide was recently shown to increase expression of the gastrin gene via a CCK2 receptor-dependent autocrine loop [16].
Figure 1. Gastrin Processing.
Preprogastrin (101 amino acids) is converted to progastrin (80 amino acids) by removal of its signal peptide (black box). The sequential action of prohormone convertases and carboxypeptidase E then converts the prohormone to glycine-extended forms such as glycine-extended gastrin34 (progastrin38–72) and glycine-extended gastrin17 (progastrin55–72, Ggly). The extent of transamidation of glycine-extended gastrin34 to amidated gastrin34 by peptidyl α–amidating monooxygenase is dependent on the tissue. Finally amidated gastrin34 is cleaved to amidated gastrin17 (Gamide) by prohormone convertases. The amino acid sequence of Gamide is ZGPWLEEEEEAYGWMDFamide, where Z represents a pyroglutamate residue. Both non-amidated and amidated forms of gastrin are independently active via different receptors. The CCK2 receptor recognises the amidated C-terminal tetrapeptide of gastrin [15], while the Ggly receptor recognises the ferric ion complexes of the central heptapeptides LEEEEEA and EEEEEAY [77].
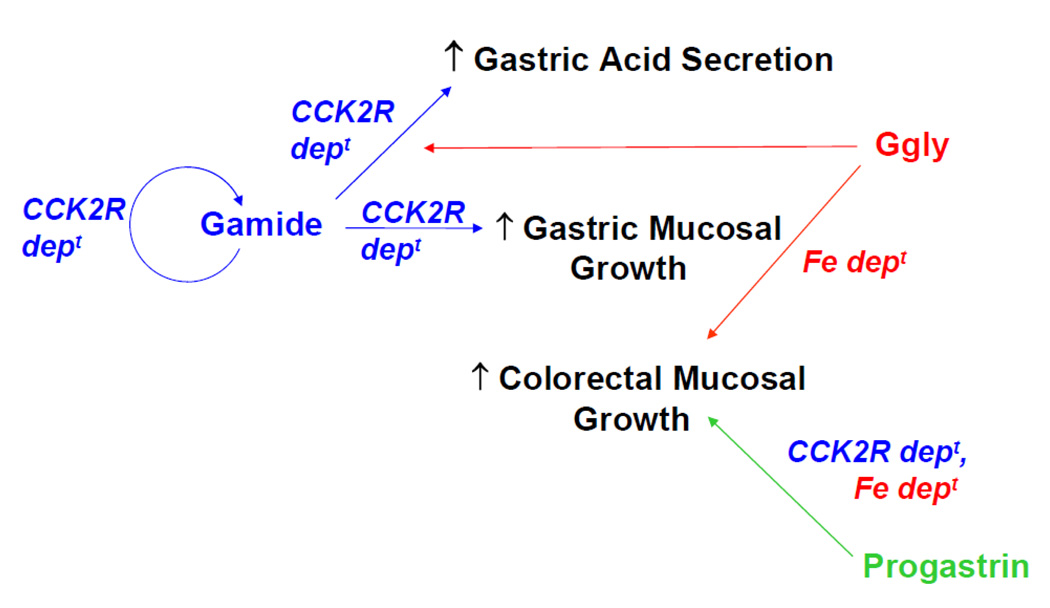
Non-amidated gastrins like progastrin and Ggly elicit biological effects in different tissues from Gamide (Figure 2). The latter targets the gastric mucosa, whereas non-amidated gastrins are principally active in the colon. Transgenic mice which overexpress progastrin (hGAS) [17] showed a two-fold increase in basal proliferation rate in the colonic mucosa, while gastrin-deficient (Gas−/−) mice showed decreased colonic proliferation [18]. Trophic effects of Ggly were demonstrated in mice overexpressing progastrin truncated at Gly72 (MTI/Ggly), with increased colonic mucosal thickness, an increased number of goblet cells per crypt and increased colonic proliferation compared with wild-type controls [19]. When circulating Ggly concentrations were increased in Gas−/− mice by continuous infusion for two weeks, increases in colonic mucosal thickness and proliferation were observed compared to saline-infused Gas−/− mice. In rats, short-term administration of Ggly after colostomy to create a defunctioned rectum caused an increase in the proliferation of the rectal mucosa [20]. Infusion of Ggly into rats also increased numbers of aberrant crypt foci in the colorectal mucosa after treatment with the carcinogen azoxymethane [20].
Figure 2. Physiological Actions of Progastrin-derived Peptides.
Gamide is now recognised as the primary stimulant of gastric acid secretion. Gamide is released from the gastric antral mucosa into the circulation, binds to CCK2 receptors (CCK2R) on ECL cells in the gastric fundic mucosa, and stimulates histamine release. Histamine together with gastrin then stimulates the release of acid from fundic parietal cells. Ggly alone is unable to stimulate acid secretion but potentiates acid release in response to Gamide. The abundant evidence that Gamide acts as a growth factor in the gastric mucosa has been reviewed previously [7]. Gamide was recently shown to increase expression of the gastrin gene via a CCK2 receptor-dependent (CCK2R dept) autocrine loop [16].
In contrast the trophic effects of non-amidated gastrins like progastrin and Ggly are principally seen in the colon. In mice overexpression of progastrin or Ggly increases the basal proliferation rate in the colonic mucosa [18]. In rats, short-term administration of Ggly after colostomy to create a defunctioned rectum caused an increase in the proliferation of the rectal mucosa [20]. The stimulation of proliferation in both models is blocked by the chelating agent desferrioxamine, presumably by removal of ferric ions [78]. Progastrin [23, 24] and Ggly [25] do not bind to the CCK2 receptor, but the stimulatory effects of progastrin on proliferation in mouse colonic mucosa are not observed in Cck2r−/− mice [91].
The identities of the receptors for non-amidated gastrins are still the subject of controversy (see [7] for a summary of early work in this area). Possible candidates include the 78 kDa gastrin-binding protein [21] and annexin II [22]. Progastrin [23, 24] and Ggly [25] do not bind to the CCK2 receptor, but more detailed binding studies of the affinites of progastrin, Ggly and Gamide for all candidate receptors for non-amidated gastrins are urgently required.
Role of Gastrins in Acid Secretion
Gamide is now recognized as the primary secretagogue for meal-stimulated acid secretion [12]. G cells in the gastric antrum are the predominant site of synthesis of progastrin and storage of Gamide which, upon luminal stimulation by amino acids or peptides, is released into the circulation and transported to the fundic mucosa where it binds to CCK2 receptors on ECL cells. The acid secretory action of Gamide is mediated via the release of histamine from ECL cells and the subsequent stimulation of parietal cells by histamine to produce H+ [26]. Gamide also directly stimulates the parietal cell [27], and CCK2 receptor antagonists have been used to confirm the involvement of Gamide in gastric acid secretion [28]. In contrast, Ggly alone does not stimulate acid secretion, but potentiates the stimulation observed in response to Gamide [29, 30].
Studies of mice genetically modified by disruption of the Gas or Cck2r genes have clarified the role of gastrin in acid secretion. Basal gastric acid secretion was abolished in Gas−/− mice, and could not be induced by subcutaneous injection of histamine, carbachol, or Gamide, probably because of defective parietal cell maturation [18, 31]. In contrast, continuous perfusion of Gas−/− mice with Gamide partially restored acid secretion [31]. The Cck2r−/− mice on the other hand had an approximately 10-fold increase in plasma Gamide concentration compared with wild-type controls, but had reduced numbers of parietal and ECL cells and an increased basal gastric pH (from 3.2 to 5.2) [32, 33]. Hence the absence of the CCK2 receptor presumably results in defective ECL and parietal cell maturation, reduced histamine secretion by ECL cells, and reduced acid secretion by parietal cells [32, 33].
Acid Secretion and Anemia
The bioavailability of non-heme iron is increased by gastric acidity by two mechanisms [34, 35]. Firstly, the high concentration of hydrogen ions at acidic pH (pH 3 = 1 mM H+) leads to efficient competition for metal ion binding sites in dietary components, and so liberates more iron from food. Secondly, the released ferric ions are only soluble in aqueous solutions at acidic pH. Hence the low pH of the stomach lumen caused by secretion of acid keeps iron soluble and therefore available for reduction to the ferrous form for rapid absorption in the small intestine [36–38]. In contrast, gastric acid is not required for the absorption of heme iron [34].
The importance of gastric acid for iron absorption became evident about 50 years ago, when Baird and others reported that iron deficiency anemia is a long-term consequence of partial gastrectomy [2, 3, 39–42]. Subsequent radioiron studies demonstrated decreased absorption of dietary non-heme iron in patients with achlorhydria [43–45], or after treatment with the histamine H2 receptor antagonist cimetidine [46]. The proton pump inhibitor omeprazole caused profound hypochlorhydria by inhibiting acid secretion, and treatment with omeprazole in rats on an iron-deficient diet caused anemia [47]. For a number of years investigators failed to demonstrate any association between omeprazole treatment and anemia in non-anemic, iron-replete individuals [38, 48]. However, a recent study in patients with established iron deficiency showed that omeprazole did in fact contribute to iron-deficiency anemia via impairment of the absorption of orally administered iron [49]. In contrast, hypergastrinemic patients with Zollinger-Ellison syndrome did not develop iron-deficiency anemia after omeprazole treatment [38].
Iron Uptake by the Intestinal Epithelium
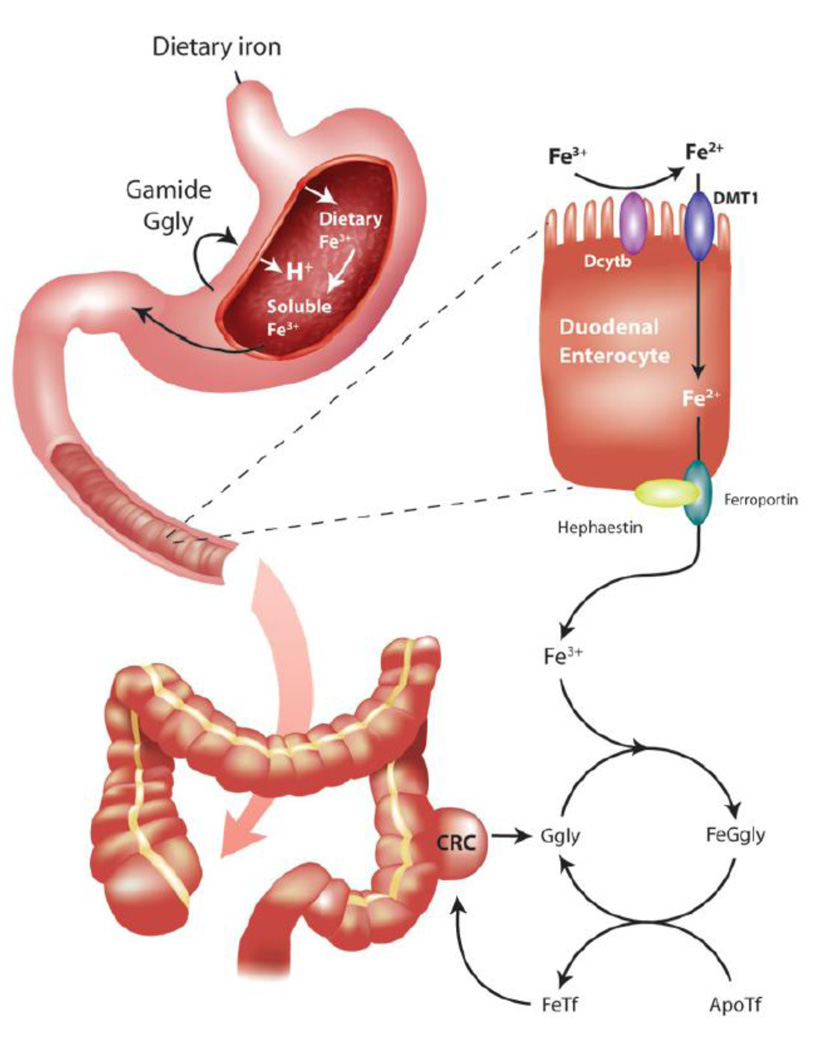
Because of the absence of excretory mechanisms, the intestinal absorption of dietary iron is a critical regulatory point of iron homeostasis (Figure 3). Although at physiological pH in the presence of oxygen iron exists in the insoluble ferric form (Fe3+), most iron transport systems take up iron in the soluble ferrous form (Fe2+). Ferric iron from food is therefore first reduced to ferrous iron by ferric reductases associated with the enterocyte apical membrane. An attractive candidate reductase is Dcytb, a cytochrome b-like ferric reductase of the duodenal mucosa, expression of which increases in response to iron deficiency and hypoxia [50]. However, other reductases may also be present in the brush border as Dcytb−/− mice do not develop iron deficiency [51]. The reduced iron is then transported into epithelial cells by divalent metal ion transporter 1 (DMT-1) [52]. The expression of DMT-1 on the apical membrane of the enterocyte is regulated by body iron status such that its level is increased in iron deficiency and decreased in iron overload [52]. The observation that targeted disruption of the mouse Dmt-1 gene leads to a profound microcytic, hypochromic anemia suggests that this transporter plays an important role in intestinal iron absorption and in erythropoiesis [51].
Figure 3. A Possible Dual Role for Gastrin in Iron Homeostasis and Colorectal Cancer.
The first role of gastrins in the stomach is well-established. Following a meal, Gamide is released from the gastric antral mucosa into the circulation and stimulates the release of histamine from ECL cells in the gastric fundic mucosa. Histamine together with gastrin then stimulates fundic parietal cells to release acid into the gastric lumen. Ggly alone is unable to stimulate acid secretion but potentiates acid release in response to Gamide. Presumably the potentiation of acid release will, like the other biological activities of Ggly, be dependent on ferric (Fe3+) ions. The acid released will compete for iron-binding sites present in dietary components, and hence increase the concentration of soluble Fe3+ ions passing into the duodenum. The cytochrome-b-like ferric reductase Dcytb, present on the villi of duodenal enterocytes, reduces Fe3+ to ferrous (Fe2+) ions, which are then transported into the enterocyte by the divalent metal ion transporter DMT-1. Fe2+ ions are exported into the circulation by ferroportin, and the iron regulator hepcidin controls iron export by formation of a complex with ferroportin. Internalisation and degradation of the complex reduces the amount of ferroportin on the cell surface, and hence reduces iron export. The exported Fe2+ ions are then re-oxidised to Fe3+ ions by hephaestin.
The second postulated role of gastrins increases the availability of iron in the circulation. Circulating Ggly and Gamide will bind Fe3+ ions, and the resultant complex will catalyse the loading of apo-transferrin with iron, and hence increase the tissue availability of iron. A like process may also occur within CRC, where the non-amidated gastrins produced by the tumour will similarly catalyse the loading of apo-transferrin (ApoTf) with iron for subsequent uptake by CRC cells. The stimulation of tumour growth by non-amidated gastrins will result from a combination of the enhanced availability of Fe-transferrin (FeTf) and of direct effects on the tumour cells, with the relative importance of the two components as yet undefined.
Iron Export from the Enterocyte
Ferrous iron in the enterocyte passes to the basolateral membrane for export into the circulation via the iron transporter ferroportin [53]. Targeted disruption of the mouse ferroportin gene demonstrated the importance of ferroportin both in intestinal iron absorption and in iron release from tissues, as Fpn−/− animals were anemic and retained excess iron in enterocytes, macrophages and hepatocytes [54]. In addition to ferroportin, the ferrous oxidases hephaestin and ceruloplasmin are required for iron release from enterocytes [55, 56]. Hephaestin, which is associated with the basolateral side of the enterocyte where it oxidises Fe2+ to Fe3+, appears to be the major ferrous oxidase in the intestine under normal conditions [55]. Circulating ceruloplasmin can also play a role in iron absorption when iron demands are particularly high [57].
Release of iron from duodenal enterocytes into the plasma is controlled by hepcidin. Hepcidin was first identified as a 25 amino acid anti-microbial peptide in urine [58], but is now recognized as a major regulator of iron homeostasis [59, 60]. Hepcidin is produced primarily by the liver [61], and its expression and circulating concentration are increased in cases of increased serum iron, iron overload [60] and inflammation [62], and decreased in response to higher erythropoietic demand, hypoxia and iron deficiency [62]. In addition, humans with mutations in the hepcidin gene develop severe iron overload [63]. The iron exporter ferroportin is the receptor for hepcidin and, as a consequence of binding, ferroportin is internalized and degraded, leading to decreased export of cellular iron [64, 65]. Thus increased hepcidin leads to reduced ferroportin expression in enterocytes, decreased intestinal iron absorption and reduced availability of iron to cells of the body. Conversely, under hypoxic conditions, coordinate downregulation of hepcidin (with a consequent increase in ferroportin) and upregulation of erythropoietin results in increased iron for biological functions including erythrocyte production [66].
Transferrin Saturation and the Sensing of Body Iron Stores
Serum transferrin plays a key role in iron metabolism by accepting newly absorbed iron from the gut and stored iron from the tissues and transporting iron to cells expressing transferrin receptors. Transferrin receptor 1 (TfR1) is the major receptor for cellular iron uptake (via the endocytic pathway) and is expressed on almost all cells, with mature red blood cells being one of the very few exceptions [67]. Transferrin receptor 2 (TfR2) on the other hand is found mainly on hepatocytes and is upregulated in response to increased transferrin saturation [68, 69]. Although both receptors bind iron-loaded transferrin with high affinity, the affinity of TfR2 for transferrin is considerably lower than that of TfR1 [70]. The observation that mutations in TfR2 cause a rare form of the iron overload disease hereditary hemochromatosis suggests that this protein plays a more important role in the regulation of iron homeostasis than in cellular iron uptake [71].
It is now generally agreed that diferric transferrin acts as a sensor for body iron requirements and is an important signal to alter hepcidin expression [72]. How transferrin achieves this is incompletely understood, but two models have been proposed [73, 74]. Both models suggest that in basal and low iron states, TfR1 forms a complex with HFE (the protein commonly mutated in hemochromatosis), and that diferric Tf competes with HFE for binding of TfR1. In the Frazer/Anderson model [73], the displaced HFE on the surface of hepatocytes stimulates a signalling cascade that results in increased production of hepcidin, and the plentiful supply of diferric transferrin also allows it to bind to TfR2 further stimulating hepcidin expression. In the Goswami/Andrews model [74], dissociation of the HFE/TfR1 complex by diferric transferrin allows HFE to bind to TfR2, with subsequent stimulation of hepcidin expression. Thus the two models differ in whether hecidin production is increased by HFE acting alone [73] or as a complex with TfR2 [74]. Since mice lacking both Hfe and Tfr2 have a more severe phenotype than mice lacking either gene alone [75], at least some of the effects of Hfe and Tfr2 on hepcidin expression are probably independent. Irrespective of the mechanism, in each of these models transferrin saturation controls expression of the iron homeostasis regulator hepcidin.
Gastrins and Iron: Structural Studies
Both Ggly and Gamide bind two ferric ions in aqueous solution [4]. Fluorescence quenching experiments with peptides derived from the Ggly sequence indicated that one or more of the five glutamic acid residues were necessary for iron binding [4]. The solution structure of Ggly was determined by nuclear magnetic resonance (NMR) spectroscopy, and the identity of the iron-binding ligands by NMR spectroscopy and by mutation. Glutamate7 was a ligand at the first ferric iron binding site, and glutamate8 and glutamate9 were ligands at the second ferric iron binding site [76]. The role of ferric ion binding in the stimulation of proliferation and migration by Ggly was then investigated in the mouse gastric cell line IMGE-5. The observations that the iron chelator desferrioxamine inhibited the biological action of Ggly, and that the GglyE7A mutant, which has impaired iron binding, was biologically inactive, suggested that binding of ferric ions to Ggly was essential for the peptide’s biological activity [76]. Further studies of gastrin fragments revealed that the heptapeptides LE5A and E5AY each bound two ferric ions, and confirmed that iron binding was essential for proliferative activity [77]. Ferric ions are also essential for the biological effects of Ggly and progastrin in vivo, since desferrioxamine blocked the stimulation of proliferation by non-amidated gastrins in rat and mouse colonic mucosa [78]. The simplest conclusion consistent with all of the above data is that the Ggly receptor recognises only the ferric ion complex of Ggly. In contrast, the observations that desferrioxamine had no effect on the binding of Gamide to the CCK2 receptor, or on the biological activity of Gamide, clearly indicate that ferric ions were not essential in this case and hence confirm that the CCK2 receptor and the Ggly receptor are different [79]. The latter conclusion is consistent with the early observation that the minimum requirement for binding to the CCK2 receptor is the amidated C-terminal tetrapeptide of gastrin [15].
Both Gamide and Ggly have also been shown to interact with the iron transport protein transferrin. The interaction of Gamide with transferrin was first detected in extracts of porcine gastric mucosa using covalent cross-linking assays, which demonstrated that the concentration of Gamide required to reduce cross-linking by 50% was approximately 100 µM [80]. A more detailed ultracentrifugal study subsequently revealed that apo-transferrin (iron-free transferrin) bound 2 molecules of Gamide with a Kd of 6.4 µM at pH 7.4 [81]. No significant binding of Gamide to diferric-transferrin was detected under the same conditions. Interaction between apo-transferrin and Ggly was later detected by surface plasmon resonance [82]. The fact that no interaction between apo-transferrin and either Gamide or Ggly was observed in the presence of the chelator EDTA suggested that the gastrin-ferric ion complex was the interacting species [82].
Gastrins and Iron: Biological Connections
In addition to the strong evidence that a gastrin-ferric ion complex forms in solution and interacts with apo-transferrin, accumulating data support the existence of a more general connection between iron status and gastrins. Gamide and Ggly concentrations were increased in the gastric mucosa and plasma of Hfe−/− mice, and in the sera of patients with HFE-related hemochromatosis [5]. The observed changes in gastrin in Hfe−/− mice were not due to structural changes in the gastric mucosa, which contained normal numbers of parietal cells, or to reduced gastric acid production, as the pH of the luminal contents was lower in Hfe−/− mice than in wild-type animals. Precisely how HFE deficiency and gastrins are linked remains unresolved. Conversely, modulation of circulating gastrin concentrations was also shown to alter iron homeostasis. The concentration of hepcidin mRNA in the gastric mucosa of Gas−/− mice was only 40% of the value in wild-type mice, but returned to 130% of the wild-type value after subcutaneous infusion of Gamide for 1 week [83]. In juvenile Gas−/− mice, intestinal iron absorption measured by 59Fe uptake after oral gavage was increased sixfold, and concentrations of Dmt-1 mRNA were increased fourfold, compared with age-matched wild-type mice [6]. The increased absorption probably reflects systemic iron deficiency in these animals and the reduction in hepatic hepcidin expression supports this proposal [6]. Although there was no significant change in iron absorption in hypergastrinemic Cck2r−/− mice, Dmt-1 mRNA was 5.4-fold higher than in age-matched wild-type mice. Importantly, transferrin saturation was reduced by 20% in Gas−/− mice, and increased by 50% in Cck2r−/− mice, compared to age-matched wild-type mice. Similarly, in humans hypergastrinemic because of multiple endocrine neoplasia type I, transferrin saturation correlated positively with circulating Gamide concentrations [6].
A mechanism has been proposed [6] for the connection between changes in circulating Gamide concentrations and changes in transferrin saturation (Figure 3). The model proposes that, after export of ferrous ions from the enterocyte by ferroportin and their oxidation to ferric ions by hephaestin, circulating Gamide or Ggly may act as chaperones for the uptake of ferric ions by apo-transferrin. The failure to detect significant binding of Gamide or Ggly to diferric-transferrin [81] suggests that both Gamide and Ggly dissociate after iron transfer has occurred, and hence play a catalytic role consistent with the difference in the circulating concentrations of transferrin and Gamide or Ggly. Changes in the circulating concentration of diferric-transferrin may in turn cause significant alterations in iron traffic into and throughout the body by altering the production of the regulatory peptide hepcidin by the liver [73]. The model predicts that increases in circulating gastrins would lead to an increase in iron stores, as was observed in hypergastrinaemic Cck2r−/− mice [6].
Relevance to Colorectal Cancer
There is now abundant evidence that non-amidated gastrins accelerate the development of colorectal carcinoma (CRC) [7, 84]. Colorectal cancers express progastrin in greater amounts than normal colorectal mucosa [85], and patients with colorectal adenocarcinoma have increased circulating concentrations of Gamide and total gastrins [86]. A retrospective study by Thorburn and co-workers provided additional evidence that increased circulating Gamide concentrations contribute to the increased risk of colon cancer [87].
Both progastrin and Ggly are also active in mouse models of colon cancer. In the Min+/− mouse model of intestinal polyposis, infusion of Ggly increased the number of polyps [88] and increased numbers of polyps were also observed when Min+/− mice were crossed with MTI/Ggly mice which overexpressed Ggly [19]. Transgenic mice that overexpress progastrin developed more aberrant crypt foci [89] and colon cancers [90] in response to the mutagen azoxymethane than wild-type mice. The observation that the stimulatory effects of progastrin on CRC development are not apparent in Cck2r−/− mice is rather surprising [91], since progastrin does not bind to the CCK2 receptor [23, 24]. In vitro, Ggly stimulated proliferation and migration of the mouse colon cell line YAMC [92] and invasion and migration of the human CRC cell line LoVo [93]. In other cell lines Ggly also stimulated proliferation [94] and the stimulation could be blocked with gastrin antisense constructs [95] or by immunization with a gastrin-diphtheria toxin conjugate (gastrimmune) [96].
The importance of iron in colorectal carcinogenesis is becoming increasingly recognised [97]. Dietary iron has been identified as a risk factor for CRC [98, 99], and mutations in the HFE gene in hemochromatotic patients are associated with increased risk of CRC [100, 101]. Examination of resected polyps and CRC showed that progression to CRC was associated with increased expression of iron import proteins and decreased expression of iron export proteins [102]. The resultant increase in intracellular iron may act to stimulate growth of the tumour cells and may also initiate/promote carcinogenesis through production of reactive oxygen species and DNA damage. Alternatively, since progastrin and Ggly act as colonic growth factors, the increased circulating Ggly concentrations in the plasma of hemochromatotic patients and Hfe−/− mice may contribute to the development of CRC [5]. Although the precise mechanisms underlying the involvement of gastrins in iron homeostasis are still unknown, non-amidated gastrins and iron may contribute synergistically to the development of CRC (Figure 3).
Future Perspectives
The recognition that ferric ions are essential for the biological activities of non-amidated gastrins suggests several new possibilities for the treatment of CRC. Removal of ferric ions with chelating agents blocked Ggly activity in vitro [76] and in vivo [78]. Occupation of the Ggly metal ion binding site by bismuth ions prevented the binding of ferric ions, and so rendered the peptide inactive [79]. The major advantage of both approaches is the specificity of inactivation, since neither has any effect on the activity of amidated gastrins [103]. On the other hand a degree of caution would be required if treating CRC patients with chelating agents as many also present with iron-deficiency anaemia [104]. Since Ggly potentiates the stimulation of acid secretion by amidated gastrins [29, 30], such inhibitors may also be useful for the treatment of excessive acid production in patients with gastrointestinal ulcers.
In addition, the demonstration that plasma gastrin concentrations were increased in mice with defects in iron homeostasis [5] opens a completely new perspective for understanding the control of iron metabolism in vivo. Further investigation of the mechanisms involved may lead to novel therapies for aberrant iron homeostasis in humans. In this context it is worth remembering that iron overload is not an uncommon disease. A recent population study from Australia reported that 0.65% of the study group were homozygous for the HFE mutation C282Y, and of these homozygotes documented iron-overload-related disease developed in 28% of men and 1.2% of women [105]. The significance of this observation is that liver fibrosis, cirrhosis and hepatocellular carcinoma were common sequelae of iron overload [105].
Acknowledgement
This work was supported in part by grants from the National Health and Medical Research Council of Australia (400062, 454322) and the National Institutes of Health (5RO1GM065926).
Abbreviations
- CCK
cholecystokinin
- CRC
colorectal carcinoma
- Dcytb
a duodenal cytochrome b-like ferric reductase
- DMT-1
divalent metal transporter 1
- ECL
enterochromaffin-like
- Fpn
ferroportin
- Ggly
glycine-extended gastrin17
- Gamide
amidated gastrin17
- HFE
the protein commonly mutated in hereditary hemachromatosis
- NMR
nuclear magnetic resonance
- TfR
transferring receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edkins JS. On the chemical mechanism of gastric secretion. Proc. Roy. Soc. B. 1905;76:376. [Google Scholar]
- 2.Baird IM, Blackburn EK, Wilson GM. The pathogenesis of anaemia after partial gastrectomy. I. Development of anaemia in relation to time after operation, blood loss, and diet. Q J Med. 1959;28:21–34. [PubMed] [Google Scholar]
- 3.Baird IM, Wilson GM. The pathogenesis of anaemia after partial gastrectomy. II. Iron absorption after partial gastrectomy. Q J Med. 1959;28:35–41. [PubMed] [Google Scholar]
- 4.Baldwin GS, Curtain CC, Sawyer WH. Selective, high-affinity binding of ferric ions by glycine-extended gastrin(17) Biochemistry. 2001;40:10741–10746. doi: 10.1021/bi010016h. [DOI] [PubMed] [Google Scholar]
- 5.Smith KA, Kovac S, Anderson GJ, Shulkes A, Baldwin GS. Circulating gastrin is increased in hemochromatosis. FEBS Lett. 2006;580:6195–6198. doi: 10.1016/j.febslet.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovac S, Smith K, Anderson GJ, Burgess JR, Shulkes A, Baldwin GS. Interrelationships between circulating gastrin and iron status in mice and humans. Am J Physiol Gastrointest Liver Physiol. 2008;295:G855–G861. doi: 10.1152/ajpgi.90359.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aly A, Shulkes A, Baldwin GS. Gastrins, cholecystokinins and gastrointestinal cancer. Biochim Biophys Acta. 2004;1704:1–10. doi: 10.1016/j.bbcan.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Ferrand A, Wang TC. Gastrin and cancer: a review. Cancer Lett. 2006;238:15–29. doi: 10.1016/j.canlet.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Gregory RA, Tracy HJ. The Constitution and Properties of Two Gastrins Extracted from Hog Antral Mucosa. Gut. 1964;5:103–114. [PMC free article] [PubMed] [Google Scholar]
- 10.Gregory H, Hardy PM, Jones DS, Kenner GW, Sheppard RC. The Antral Hormone Gastrin. Structure of Gastrin, Nature. 1964;204:931–933. doi: 10.1038/204931a0. [DOI] [PubMed] [Google Scholar]
- 11.Yoo OJ, Powell CT, Agarwal KL. Molecular cloning and nucleotide sequence of full-length of cDNA coding for porcine gastrin. Proc Natl Acad Sci U S A. 1982;79:1049–1053. doi: 10.1073/pnas.79.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dockray GJ, Varro A, Dimaline R, Wang T. The gastrins: their production and biological activities. Annu Rev Physiol. 2001;63:119–139. doi: 10.1146/annurev.physiol.63.1.119. [DOI] [PubMed] [Google Scholar]
- 13.Kovac S, Shulkes A, Baldwin GS. Peptide processing and biology in human disease. Curr Opin Endocrinol Diabetes Obes. 2009;16:79–85. doi: 10.1097/MED.0b013e3283202555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shulkes A, Baldwin GS. Biology of gut cholecystokinin and gastrin receptors. Clin Exp Pharmacol Physiol. 1997;24:209–216. doi: 10.1111/j.1440-1681.1997.tb01809.x. [DOI] [PubMed] [Google Scholar]
- 15.Morley JS, Tracy HJ, Gregory RA. Structure-function relationships in the active C-terminal tetrapeptide sequence of gastrin. Nature. 1965;207:1356–1359. doi: 10.1038/2071356a0. [DOI] [PubMed] [Google Scholar]
- 16.Kovac S, Xiao L, Shulkes A, Patel O, Baldwin GS. Gastrin increases its own synthesis in gastrointestinal cancer cells via the CCK2 receptor. FEBS Lett. 2010;584:4413–4418. doi: 10.1016/j.febslet.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 17.Wang TC, Koh TJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Dockray GJ. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest. 1996;98:1918–1929. doi: 10.1172/JCI118993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koh TJ, Goldenring JR, Ito S, Mashimo H, Kopin AS, Varro A, Dockray GJ, Wang TC. Gastrin deficiency results in altered gastric differentiation and decreased colonic proliferation in mice. Gastroenterology. 1997;113:1015–1025. doi: 10.1016/s0016-5085(97)70199-9. [DOI] [PubMed] [Google Scholar]
- 19.Koh TJ, Dockray GJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Wang TC. Overexpression of glycine-extended gastrin in transgenic mice results in increased colonic proliferation. J Clin Invest. 1999;103:1119–1126. doi: 10.1172/JCI4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aly A, Shulkes A, Baldwin GS. Short term infusion of glycine-extended gastrin(17) stimulates both proliferation and formation of aberrant crypt foci in rat colonic mucosa. Int J Cancer. 2001;94:307–313. doi: 10.1002/ijc.1483. [DOI] [PubMed] [Google Scholar]
- 21.Baldwin GS. Antiproliferative gastrin/cholecystokinin receptor antagonists target the 78-kDa gastrin-binding protein. Proc Natl Acad Sci U S A. 1994;91:7593–7597. doi: 10.1073/pnas.91.16.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh P, Wu H, Clark C, Owlia A. Annexin II binds progastrin and gastrin-like peptides, and mediates growth factor effects of autocrine and exogenous gastrins on colon cancer and intestinal epithelial cells. Oncogene. 2007;26:425–440. doi: 10.1038/sj.onc.1209798. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin GS, Hollande F, Yang Z, Karelina Y, Paterson A, Strang R, Fourmy D, Neumann G, Shulkes A. Biologically active recombinant human progastrin(6–80) contains a tightly bound calcium ion. J Biol Chem. 2001;276:7791–7796. doi: 10.1074/jbc.M009985200. [DOI] [PubMed] [Google Scholar]
- 24.Dubeykovskiy A, Nguyen T, Dubeykovskaya Z, Lei S, Wang TC. Flow cytometric detection of progastrin interaction with gastrointestinal cells. Regul Pept. 2008;151:106–114. doi: 10.1016/j.regpep.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seva C, Dickinson CJ, Yamada T. Growth-promoting effects of glycine-extended progastrin. Science. 1994;265:410–412. doi: 10.1126/science.8023165. [DOI] [PubMed] [Google Scholar]
- 26.Obrink KJ. Histamine and gastric acid secretion. A review. Scand J Gastroenterol Suppl. 1991;180:4–8. [PubMed] [Google Scholar]
- 27.Chuang CN, Chen MC, Soll AH. The pathways regulating acid secretion: the view from the isolated cell. Yale J Biol Med. 1994;67:107–112. [PMC free article] [PubMed] [Google Scholar]
- 28.Hakanson R, Ding XQ, Norlen P, Lindstrom E. CCK2 receptor antagonists: pharmacological tools to study the gastrin-ECL cell-parietal cell axis. Regul Pept. 1999;80:1–12. doi: 10.1016/s0167-0115(99)00008-7. [DOI] [PubMed] [Google Scholar]
- 29.Higashide S, Gomez G, Greeley GH, Jr, Townsend CM, Jr, Thompson JC. Glycine-extended gastrin potentiates gastrin-stimulated gastric acid secretion in rats. Am J Physiol. 1996;270:G220–G224. doi: 10.1152/ajpgi.1996.270.1.G220. [DOI] [PubMed] [Google Scholar]
- 30.Cui G, Koh TJ, Chen D, Zhao CM, Takaishi S, Dockray GJ, Varro A, Rogers AB, Fox JG, Wang TC. Overexpression of glycine-extended gastrin inhibits parietal cell loss and atrophy in the mouse stomach. Cancer Res. 2004;64:8160–8166. doi: 10.1158/0008-5472.CAN-04-0876. [DOI] [PubMed] [Google Scholar]
- 31.Friis-Hansen L, Sundler F, Li Y, Gillespie PJ, Saunders TL, Greenson JK, Owyang C, Rehfeld JF, Samuelson LC. Impaired gastric acid secretion in gastrin-deficient mice. Am J Physiol. 1998;274:G561–G568. doi: 10.1152/ajpgi.1998.274.3.G561. [DOI] [PubMed] [Google Scholar]
- 32.Nagata A, Ito M, Iwata N, Kuno J, Takano H, Minowa O, Chihara K, Matsui T, Noda T. G protein-coupled cholecystokinin-B/gastrin receptors are responsible for physiological cell growth of the stomach mucosa in vivo. Proc Natl Acad Sci U S A. 1996;93:11825–11830. doi: 10.1073/pnas.93.21.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langhans N, Rindi G, Chiu M, Rehfeld JF, Ardman B, Beinborn M, Kopin AS. Abnormal gastric histology and decreased acid production in cholecystokinin-B/gastrin receptor-deficient mice. Gastroenterology. 1997;112:280–286. doi: 10.1016/s0016-5085(97)90000-7. [DOI] [PubMed] [Google Scholar]
- 34.Schade SG, Cohen RJ, Conrad ME. Effect of hydrochloric acid on iron absorption. N Engl J Med. 1968;279:672–674. doi: 10.1056/NEJM196809262791302. [DOI] [PubMed] [Google Scholar]
- 35.Conrad ME, Schade SG. Ascorbic acid chelates in iron absorption: a role for hydrochloric acid and bile. Gastroenterology. 1968;55:35–45. [PubMed] [Google Scholar]
- 36.Bezwoda W, Charlton R, Bothwell T, Torrance J, Mayet F. The importance of gastric hydrochloric acid in the absorption of nonheme food iron. J Lab Clin Med. 1978;92:108–116. [PubMed] [Google Scholar]
- 37.Conrad ME, Parmley RT, Osterloh K. Small intestinal regulation of iron absorption in the rat. J Lab Clin Med. 1987;110:418–426. [PubMed] [Google Scholar]
- 38.Stewart CA, Termanini B, Sutliff VE, Serrano J, Yu F, Gibril F, Jensen RT. Iron absorption in patients with Zollinger-Ellison syndrome treated with long-term gastric acid antisecretory therapy. Aliment Pharmacol Ther. 1998;12:83–98. doi: 10.1046/j.1365-2036.1998.00274.x. [DOI] [PubMed] [Google Scholar]
- 39.Hobbs JR. Iron deficiency after partial gastrectomy. Gut. 1961;2:141–149. doi: 10.1136/gut.2.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turnberg LA. The absorption of iron after partial gastrectomy. Q J Med. 1966;35:107–118. [PubMed] [Google Scholar]
- 41.Hines JD, Hoffbrand AV, Mollin DL. The hematologic complications following partial gastrectomy. A study of 292 patients. Am J Med. 1967;43:555–569. doi: 10.1016/0002-9343(67)90179-9. [DOI] [PubMed] [Google Scholar]
- 42.Wheldon EJ, Venables CW, Johnston ID. The relationship of gastric secretion to anemia over 15 years following vagotomy and gastroenterostomy. Gut. 1970;11:1056. [PubMed] [Google Scholar]
- 43.Goldberg A, Lochhead AC, Dagg JH. Histamine-fast achlorhydria and iron absorption. Lancet. 1963;1:848–850. doi: 10.1016/s0140-6736(63)91623-4. [DOI] [PubMed] [Google Scholar]
- 44.Cook JD, Brown GM, Valberg LS. The Effect of Achylia Gastrica on Iron Absorption. J Clin Invest. 1964;43:1185–1191. doi: 10.1172/JCI105002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacobs A, Rhodes J, Peters DK, Campbell H, Eakins JD. Gastric acidity and iron absorption. Br J Haematol. 1966;12:728–736. doi: 10.1111/j.1365-2141.1966.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 46.Skikne BS, Lynch SR, Cook JD. Role of gastric acid in food iron absorption. Gastroenterology. 1981;81:1068–1071. [PubMed] [Google Scholar]
- 47.Golubov J, Flanagan P, Adams P. Inhibition of iron absorption by omeprazole in rat model. Dig Dis Sci. 1991;36:405–408. doi: 10.1007/BF01298866. [DOI] [PubMed] [Google Scholar]
- 48.Koop H. Review article: metabolic consequences of long-term inhibition of acid secretion by omeprazole. Aliment Pharmacol Ther. 1992;6:399–406. doi: 10.1111/j.1365-2036.1992.tb00553.x. [DOI] [PubMed] [Google Scholar]
- 49.Sharma VR, Brannon MA, Carloss EA. Effect of omeprazole on oral iron replacement in patients with iron deficiency anemia. South Med J. 2004;97:887–889. doi: 10.1097/01.SMJ.0000110405.63179.69. [DOI] [PubMed] [Google Scholar]
- 50.McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, Bomford A, Peters TJ, Raja KB, Shirali S, Hediger MA, Farzaneh F, Simpson RJ. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science. 2001;291:1755–1759. doi: 10.1126/science.1057206. [DOI] [PubMed] [Google Scholar]
- 51.Gunshin H, Starr CN, Direnzo C, Fleming MD, Jin J, Greer EL, Sellers VM, Galica SM, Andrews NC. Cybrd1 (duodenal cytochrome b) is not necessary for dietary iron absorption in mice. Blood. 2005;106:2879–2883. doi: 10.1182/blood-2005-02-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 53.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, Simpson RJ. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 54.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet. 1999;21:195–199. doi: 10.1038/5979. [DOI] [PubMed] [Google Scholar]
- 56.Harris ZL, Durley AP, Man TK, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci U S A. 1999;96:10812–10817. doi: 10.1073/pnas.96.19.10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cherukuri S, Potla R, Sarkar J, Nurko S, Harris ZL, Fox PL. Unexpected role of ceruloplasmin in intestinal iron absorption. Cell Metab. 2005;2:309–319. doi: 10.1016/j.cmet.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 58.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 59.Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loreal O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 61.Ilyin G, Courselaud B, Troadec MB, Pigeon C, Alizadeh M, Leroyer P, Brissot P, Loreal O. Comparative analysis of mouse hepcidin 1 and 2 genes: evidence for different patterns of expression and co-inducibility during iron overload. FEBS Lett. 2003;542:22–26. doi: 10.1016/s0014-5793(03)00329-6. [DOI] [PubMed] [Google Scholar]
- 62.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roetto A, Papanikolaou G, Politou M, Alberti F, Girelli D, Christakis J, Loukopoulos D, Camaschella C. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2003;33:21–22. doi: 10.1038/ng1053. [DOI] [PubMed] [Google Scholar]
- 64.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 65.Papanikolaou G, Tzilianos M, Christakis JI, Bogdanos D, Tsimirika K, MacFarlane J, Goldberg YP, Sakellaropoulos N, Ganz T, Nemeth E. Hepcidin in iron overload disorders. Blood. 2005;105:4103–4105. doi: 10.1182/blood-2004-12-4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117:1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wessling-Resnick M. Iron transport. Annu Rev Nutr. 2000;20:129–151. doi: 10.1146/annurev.nutr.20.1.129. [DOI] [PubMed] [Google Scholar]
- 68.Robb A, Wessling-Resnick M. Regulation of transferrin receptor 2 protein levels by transferrin. Blood. 2004;104:4294–4299. doi: 10.1182/blood-2004-06-2481. [DOI] [PubMed] [Google Scholar]
- 69.Johnson MB, Enns CA. Diferric transferrin regulates transferrin receptor 2 protein stability. Blood. 2004;104:4287–4293. doi: 10.1182/blood-2004-06-2477. [DOI] [PubMed] [Google Scholar]
- 70.Kawabata H, Germain RS, Vuong PT, Nakamaki T, Said JW, Koeffler HP. Transferrin receptor 2-alpha supports cell growth both in iron-chelated cultured cells and in vivo. J Biol Chem. 2000;275:16618–16625. doi: 10.1074/jbc.M908846199. [DOI] [PubMed] [Google Scholar]
- 71.Camaschella C, Roetto A, Cali A, De Gobbi M, Garozzo G, Carella M, Majorano N, Totaro A, Gasparini P. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet. 2000;25:14–15. doi: 10.1038/75534. [DOI] [PubMed] [Google Scholar]
- 72.Knutson MD. Iron-sensing proteins that regulate hepcidin and enteric iron absorption. Annu Rev Nutr. 2010;30:149–171. doi: 10.1146/annurev.nutr.012809.104801. [DOI] [PubMed] [Google Scholar]
- 73.Frazer DM, Anderson GJ. The orchestration of body iron intake: how and where do enterocytes receive their cues? Blood Cells Mol Dis. 2003;30:288–297. doi: 10.1016/s1079-9796(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 74.Goswami T, Andrews NC. Hereditary hemochromatosis protein, HFE, interaction with transferrin receptor 2 suggests a molecular mechanism for mammalian iron sensing. J Biol Chem. 2006;281:28494–28498. doi: 10.1074/jbc.C600197200. [DOI] [PubMed] [Google Scholar]
- 75.Wallace DF, Summerville L, Crampton EM, Frazer DM, Anderson GJ, Subramaniam VN. Combined deletion of Hfe and transferrin receptor 2 in mice leads to marked dysregulation of hepcidin and iron overload. Hepatology. 2009;50:1992–2000. doi: 10.1002/hep.23198. [DOI] [PubMed] [Google Scholar]
- 76.Pannequin J, Barnham KJ, Hollande F, Shulkes A, Norton RS, Baldwin GS. Ferric ions are essential for the biological activity of the hormone glycine-extended gastrin. J Biol Chem. 2002;277:48602–48609. doi: 10.1074/jbc.M208440200. [DOI] [PubMed] [Google Scholar]
- 77.He H, Shehan BP, Barnham KJ, Norton RS, Shulkes A, Baldwin GS. Biological activity and ferric ion binding of fragments of glycine-extended gastrin. Biochemistry. 2004;43:11853–11861. doi: 10.1021/bi0495984. [DOI] [PubMed] [Google Scholar]
- 78.Ferrand A, Lachal S, Bramante G, Kovac S, Shulkes A, Baldwin GS. Stimulation of proliferation in the colorectal mucosa by gastrin precursors is blocked by desferrioxamine. Am J Physiol Gastrointest Liver Physiol. 2010;299:G220–G227. doi: 10.1152/ajpgi.00046.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pannequin J, Kovac S, Tantiongco JP, Norton RS, Shulkes A, Barnham KJ, Baldwin GS. A novel effect of bismuth ions: selective inhibition of the biological activity of glycine-extended gastrin. J Biol Chem. 2004;279:2453–2460. doi: 10.1074/jbc.M309806200. [DOI] [PubMed] [Google Scholar]
- 80.Baldwin GS, Chandler R, Weinstock J. Binding of gastrin to gastric transferrin. FEBS Lett. 1986;205:147–149. doi: 10.1016/0014-5793(86)80883-3. [DOI] [PubMed] [Google Scholar]
- 81.Longano SC, Knesel J, Howlett GJ, Baldwin GS. Interaction of gastrin with transferrin: effects of ferric ions. Arch Biochem Biophys. 1988;263:410–417. doi: 10.1016/0003-9861(88)90653-4. [DOI] [PubMed] [Google Scholar]
- 82.Kovac S, Ferrand A, Estève J-P, Mason A, Baldwin GS. Definition of the Residues Required for the Interaction between Glycine-extended Gastrin and Transferrin In Vitro. F.E.B.S. Journal. 2009;276:4866–4874. doi: 10.1111/j.1742-4658.2009.07186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Friis-Hansen L, Rieneck K, Nilsson HO, Wadstrom T, Rehfeld JF. Gastric inflammation, metaplasia, and tumor development in gastrin-deficient mice. Gastroenterology. 2006;131:246–258. doi: 10.1053/j.gastro.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 84.Watson SA, Grabowska AM, El-Zaatari M, Takhar A. Gastrin - active participant or bystander in gastric carcinogenesis? Nat Rev Cancer. 2006;6:936–946. doi: 10.1038/nrc2014. [DOI] [PubMed] [Google Scholar]
- 85.Van Solinge WW, Nielsen FC, Friis-Hansen L, Falkmer UG, Rehfeld JF. Expression but incomplete maturation of progastrin in colorectal carcinomas. Gastroenterology. 1993;104:1099–1107. doi: 10.1016/0016-5085(93)90279-l. [DOI] [PubMed] [Google Scholar]
- 86.Ciccotosto GD, McLeish A, Hardy KJ, Shulkes A. Expression, processing, and secretion of gastrin in patients with colorectal carcinoma. Gastroenterology. 1995;109:1142–1153. doi: 10.1016/0016-5085(95)90572-3. [DOI] [PubMed] [Google Scholar]
- 87.Thorburn CM, Friedman GD, Dickinson CJ, Vogelman JH, Orentreich N, Parsonnet J. Gastrin and colorectal cancer: a prospective study. Gastroenterology. 1998;115:275–280. doi: 10.1016/s0016-5085(98)70193-3. [DOI] [PubMed] [Google Scholar]
- 88.Koh TJ, Bulitta CJ, Fleming JV, Dockray GJ, Varro A, Wang TC. Gastrin is a target of the beta-catenin/TCF-4 growth-signaling pathway in a model of intestinal polyposis. J Clin Invest. 2000;106:533–539. doi: 10.1172/JCI9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh P, Velasco M, Given R, Wargovich M, Varro A, Wang TC. Mice overexpressing progastrin are predisposed for developing aberrant colonic crypt foci in response to AOM. Am J Physiol Gastrointest Liver Physiol. 2000;278:G390–G399. doi: 10.1152/ajpgi.2000.278.3.G390. [DOI] [PubMed] [Google Scholar]
- 90.Singh P, Velasco M, Given R, Varro A, Wang TC. Progastrin expression predisposes mice to colon carcinomas and adenomas in response to a chemical carcinogen. Gastroenterology. 2000;119:162–171. doi: 10.1053/gast.2000.8527. [DOI] [PubMed] [Google Scholar]
- 91.Jin G, Ramanathan V, Quante M, Baik GH, Yang X, Wang SS, Tu S, Gordon SA, Pritchard DM, Varro A, Shulkes A, Wang TC. Inactivating cholecystokinin-2 receptor inhibits progastrin-dependent colonic crypt fission, proliferation, and colorectal cancer in mice. J Clin Invest. 2009;119:2691–2701. doi: 10.1172/JCI38918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hollande F, Imdahl A, Mantamadiotis T, Ciccotosto GD, Shulkes A, Baldwin GS. Glycine-extended gastrin acts as an autocrine growth factor in a nontransformed colon cell line. Gastroenterology. 1997;113:1576–1588. doi: 10.1053/gast.1997.v113.pm9352860. [DOI] [PubMed] [Google Scholar]
- 93.Kermorgant S, Lehy T. Glycine-extended gastrin promotes the invasiveness of human colon cancer cells. Biochem Biophys Res Commun. 2001;285:136–141. doi: 10.1006/bbrc.2001.5132. [DOI] [PubMed] [Google Scholar]
- 94.Stepan VM, Krametter DF, Matsushima M, Todisco A, Delvalle J, Dickinson CJ. Glycine-extended gastrin regulates HEK cell growth. Am J Physiol. 1999;277:R572–R581. doi: 10.1152/ajpregu.1999.277.2.R572. [DOI] [PubMed] [Google Scholar]
- 95.Singh P, Owlia A, Varro A, Dai B, Rajaraman S, Wood T. Gastrin gene expression is required for the proliferation and tumorigenicity of human colon cancer cells. Cancer Res. 1996;56:4111–4115. [PubMed] [Google Scholar]
- 96.Watson SA, Michaeli D, Grimes S, Morris TM, Robinson G, Varro A, Justin TA, Hardcastle JD. Gastrimmune raises antibodies that neutralize amidated and glycine-extended gastrin-17 and inhibit the growth of colon cancer. Cancer Res. 1996;56:880–885. [PubMed] [Google Scholar]
- 97.Chua AC, Klopcic B, Lawrance IC, Olynyk JK, Trinder D. Iron: an emerging factor in colorectal carcinogenesis. World J Gastroenterol. 16:663–672. doi: 10.3748/wjg.v16.i6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stevens RG, Jones DY, Micozzi MS, Taylor PR. Body iron stores and the risk of cancer. N Engl J Med. 1988;319:1047–1052. doi: 10.1056/NEJM198810203191603. [DOI] [PubMed] [Google Scholar]
- 99.Knekt P, Reunanen A, Takkunen H, Aromaa A, Heliovaara M, Hakulinen T. Body iron stores and risk of cancer. Int J Cancer. 1994;56:379–382. doi: 10.1002/ijc.2910560315. [DOI] [PubMed] [Google Scholar]
- 100.Shaheen NJ, Silverman LM, Keku T, Lawrence LB, Rohlfs EM, Martin CF, Galanko J, Sandler RS. Association between hemochromatosis (HFE) gene mutation carrier status and the risk of colon cancer. J Natl Cancer Inst. 2003;95:154–159. doi: 10.1093/jnci/95.2.154. [DOI] [PubMed] [Google Scholar]
- 101.Osborne NJ, Gurrin LC, Allen KJ, Constantine CC, Delatycki MB, McLaren CE, Gertig DM, Anderson GJ, Southey MC, Olynyk JK, Powell LW, Hopper JL, Giles GG, English DR. HFE C282Y homozygotes are at increased risk of breast and colorectal cancer. Hepatology. 2010;51:1311–1318. doi: 10.1002/hep.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brookes MJ, Hughes S, Turner FE, Reynolds G, Sharma N, Ismail T, Berx G, McKie AT, Hotchin N, Anderson GJ, Iqbal T, Tselepis C. Modulation of iron transport proteins in human colorectal carcinogenesis. Gut. 2006;55:1449–1460. doi: 10.1136/gut.2006.094060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pannequin J, Tantiongco JP, Kovac S, Shulkes A, Baldwin GS. Divergent roles for ferric ions in the biological activity of amidated and non-amidated gastrins. J Endocrinol. 2004;181:315–325. doi: 10.1677/joe.0.1810315. [DOI] [PubMed] [Google Scholar]
- 104.Beale AL, Penney MD, Allison MC. The prevalence of iron deficiency among patients presenting with colorectal cancer. Colorectal Dis. 2005;7:398–402. doi: 10.1111/j.1463-1318.2005.00789.x. [DOI] [PubMed] [Google Scholar]
- 105.Allen KJ, Gurrin LC, Constantine CC, Osborne NJ, Delatycki MB, Nicoll AJ, McLaren CE, Bahlo M, Nisselle AE, Vulpe CD, Anderson GJ, Southey MC, Giles GG, English DR, Hopper JL, Olynyk JK, Powell LW, Gertig DM. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med. 2008;358:221–230. doi: 10.1056/NEJMoa073286. [DOI] [PubMed] [Google Scholar]