Abstract
Background
It was recently found that the development of typical patterns of prefrontal, but not posterior, cortical asymmetry is disrupted in right handed youth with attention-deficit/hyperactivity disorder (ADHD). Using longitudinal data, we tested the hypothesis that there would be a congruent disruption in the growth of the anterior corpus callosum, which contains white matter tracts connecting prefrontal cortical regions.
Methods
Areas of five subregions of the corpus callosum were quantified using a semiautomated method from 828 neuroanatomic magnetic resonance scans acquired from 236 children and adolescents with ADHD (429 scans) and 230 typically developing youth (399 scans), the majority of whom had repeated neuroimaging. Growth rates of each diagnostic group were defined using mixed-model linear regression.
Results
Right handed participants with ADHD showed a significantly higher rate of growth in the anterior-most region of the corpus callosum (estimated annual increase in area of 0.97%, SEM 0.12%) than their typically developing peers (annual increase in area of 0.32% SEM 0.13%; t=3.64, p=0.0003). No significant diagnostic differences in growth rates were found in any other regions in right handed participants, and no significant diagnostic differences were found in non-right handed participants.
Conclusions
As hypothesized, we found anomalous growth trajectories in the anterior corpus callosum in ADHD. This disrupted anterior callosal growth may reflect, or even drive, the previously reported disruption in the development of prefrontal cortex asymmetry. The finding documents the dynamic, age-dependent nature of callosal and congruent prefrontal cortical abnormalities characterizing ADHD.
Keywords: Attention-deficit/hyperactivity disorder, corpus callosum, magnetic resonance imaging, typical development, structural neuroimaging, cortical asymmetry
Introduction
There is increasing evidence that neuroanatomic anomalies in childhood neuropsychiatric disorders may be age-dependent, changing over the course of development (1-4). For example, we recently reported anomalous development of prefrontal cortical asymmetry in attention-deficit/hyperactivity disorder (ADHD) (5). In right handed, typically developing individuals, the left orbitofrontal/prefrontal cortex and right parieto-occipital cortex were relatively thicker than their homologues during early childhood, but with age this asymmetry reversed, so that by early adulthood the well-known pattern of greater right prefrontal and left occipital cortical dimensions emerged. In right handed children with ADHD, the posterior component of this changing cortical asymmetry remained largely intact, but the prefrontal cortical asymmetry was lost. This finding is complemented by earlier reports of a loss of typical frontal asymmetry owing to reduced right frontal volume in ADHD (6-8), as well as evidence for abnormal development of prefrontal lateralized processing (9-12). The finding of anomalous development of prefrontal cortical asymmetry in children and adolescents with ADHD would lead one to expect congruent developmental anomalies of other structures, such as the corpus callosum (CC).
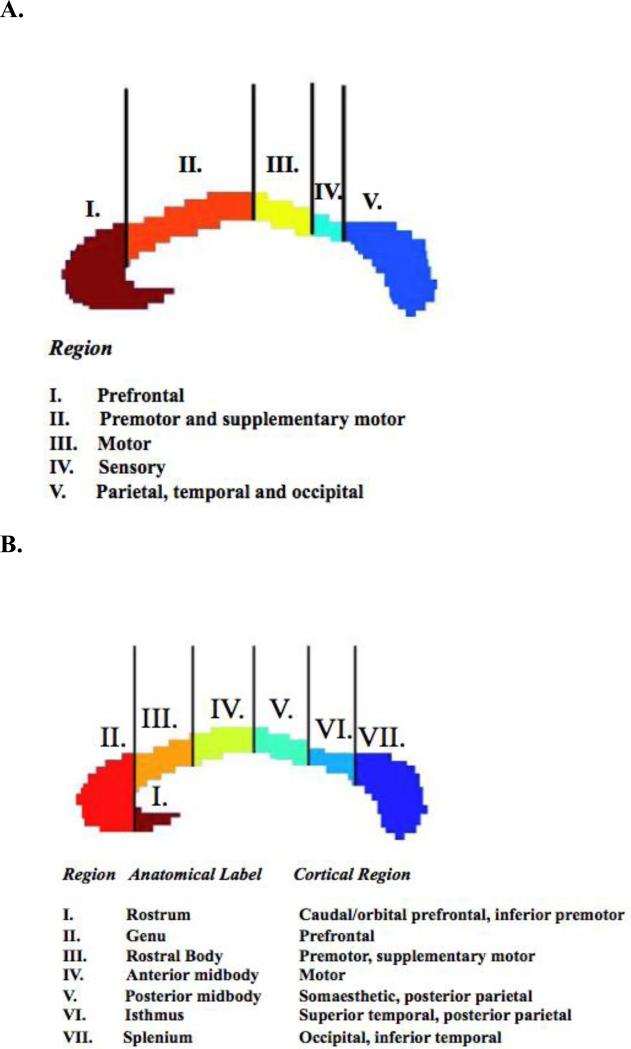
The human CC is a bundle of about two million mostly myelinated fibers connecting homologous regions of the left and right cerebral hemispheres (13, 14). Its development involves the embryonic formation of midline glial populations fusing the two hemispheres and the expression of specific molecules which guide callosal fibers as they cross the midline (15). Callosal fibers mostly take the shortest route to transverse the interhemispheric commisure, maintaining a topographic pattern (16). As shown in Figure 1, the anterior-most regions of the CC connect homologous prefrontal cortical regions. Moving rostral to caudal, the successive CC areas connect the premotor and supplementary motor cortex; then the motor and sensory cortex; followed finally by parietal, temporal and occipital cortex. The anterior-most callosal subregion is composed of smaller-diameter fibers and has the highest proportion of unmyelinated axons (17); fibers increase in diameter moving caudally. These histological differences may be functionally significant; the smaller diameter fibers of the anterior CC integrate higher-order prefrontal cortical functions, whereas larger diameter fiber of the mid-callosum are capable of higher conduction velocities (18) and connect motor and sensory cortical functions for which rapid interhemispheric integration may be particularly important (17).
Figure 1.
Topography of the corpus callosum, as devised by Witelson (bottom panel) and modified by Hofer and Frahm (top panel).
The hypothesis that there is an inverse relationship between cerebral asymmetry and CC size and connectivity (19) has much empirical support, although there are some inconsistencies and the relationship may be complicated by sex effects (20). Several neuroanatomic studies find an inverse correlation between global cerebral and more local measures of asymmetry in the perisylvian and postcentral sulcal regions with CC size and fiber number, although there are also findings of a positive correlation (17, 21, 22). Cross-species studies in non-human primates link increasing cerebral asymmetry with a smaller CC (expressed as a proportion of total neocortical volume) (23). Some find CC size to be larger in individuals with less lateralization of cognitive functions, as assessed by behavioral laterality measures such as dichotic listening tasks, although the effects are small and somewhat inconsistent (20, 24, 25). Additionally, most but not all studies find that non-right handed individuals, who have less lateralized processing in many cognitive domains, also have larger CC area (25-29). Given the importance of handedness in CC morphology, we thus report data for right and non-right handed groups separately. The right handed group is considerably larger and the focus of the current study. Data on the non-right handed group are also reported, but given the relatively small sample size, these data should be interpreted with caution and considered preliminary.
Studies of CC anomalies in ADHD have produced mixed results, with some reporting selective reduction of the anterior CC (30-32), and some the posterior CC (33-35). Still others find no difference from typically developing youth (6, 36). These inconsistent results may reflect the relatively small sample sizes of each study, as well as differences in scan acquisition parameters and methodologies used to subdivide and quantify the CC-limitations that can be overcome by a single large study using consistent methodology. No study to date has examined the possibility that callosal anomalies in ADHD may be dynamic, changing with age. Such an examination of rates of growth of the corpus callosum would inform our understanding not only of callosal development in the disorder, but also of the development of homologous cortical regions in the left and right hemispheres connected by the CC. As stated earlier, the primary focus of this study is right handed individuals, partly as this is by far the larger group, and also because our previous work has found diagnostic differences in the development of prefrontal cortical asymmetry only in right handed individuals (5).
We thus hypothesized that we would find a selective disruption in right handed individuals with ADHD in the growth of the anterior portion of the corpus callosum, which connects prefrontal cortical regions that show atypical development of asymmetry in the disorder. We predicted no difference in CC growth for posterior regions, given the evidence for relatively intact development of posterior cortical asymmetry in ADHD.
Methods
Two hundred thirty-six children and adolescents with Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)–defined ADHD participated in the present study at the Bethesda campus of the National Institutes of Health. The DSM-IV diagnosis of ADHD was based on the Parent Diagnostic Interview for Children and Adolescents, Conners’ Teacher Rating Scales, and the Teacher Report Form (threshold was ratings greater than 2 SD above age, sex specific means). Exclusion criteria were a full-scale IQ of less than 80, evidence of medical or neurological disorders on examination or by clinical history, Tourette's disorder, psychosis, drug or alcohol misuse, or any other Axis I psychiatric disorder requiring treatment with medication at study entry. Comorbidities were thus relatively mild and predominately oppositional-defiant disorder. Handedness was determined from the Physical and Neurological Examination for Soft Signs (PANESS) (37), in which right handed participants stated that they used the right hand for at least 10 of 12 everyday activities, left handed participants used the left hand for the same proportion of activities, and ambidextrous participants occupied the intermediate ground. One-hundred ninety one (81%) of the ADHD participants were right handed, 11 were left handed (5%), and 34 (14%) were ambidextrous. Left-handed and ambidextrous subjects were combined into a ‘non-right handed’ group, as separate analyses were not feasible due to small group size - for demographic details see Table S1 in the Supplement. The majority of subjects were male: the right handed group included 123 males (64.4%); the non-right handed group, 29 (64.4%). Numbers of participants at each wave of scanning and their ages are given in Table 1. IQ was assessed using age-appropriate versions of the Wechsler Intelligence scales. Treatment data are given in the Supplement.
Table 1.
Demographic and clinical details for the right handed subjects. IQ data were available on 371 of the right handed subjects. ODD=oppositional defiant disorder; CD=conduct disorder; GAD=generalized anxiety disorder.
| |
ADHD |
Typically developing |
Test of significance |
||
|---|---|---|---|---|---|
| Sex: Males N (%) |
123 (64.4%) |
130 (64.7%) |
χ2=0.003, p=0.95 |
||
| Number of subjects at each scan, mean age (SD) yr | |||||
| Scan 1 | N=191 | 10.3 (3.1) | N=201 | 10.7 (3.6) | t=1.1, p=0.27 |
| Scan 2 | N=94 | 12.9 (3.6) | N=102 | 13.3 (3.9) | t=0.5, p=0.63 |
| Scan 3 | N=50 | 15.6 (3.8) | N=46 | 14.1 (3.4) | t=2.0, p=0.05 |
| Scan 4 |
N=11 |
17.2 (3.6) |
N=9 |
16.1 (2.2) |
t=0.8, p=0.40 |
| Age range |
5 to 25.1 |
3.5 to 23.0 |
|
||
| IQ mean (SD) | 109 (15) | 111 (13) | t=1.8, p=0.06 | ||
| Range |
80 -148 |
80-142 |
|
||
| Type of ADHD | |||||
| Combined | 175 (91.6%) | na | |||
| Hyperactive/impulsive | 4 (2.1%) | na | |||
| Inattentive |
12 (6.3%) |
na |
|
||
| ODD |
70 (36.6%) |
na |
|
||
| CD |
13 (6.8%) |
na |
|
||
| Tics |
8 (4.2%) |
na |
|
||
| GAD |
4 (2.1%) |
na |
|
||
| Panic disorder |
3 (1.6%) |
na |
|
||
| Separation anxiety disorder |
7 (3.6%) |
na |
|
||
| Mood disorder |
8 (4.2%) |
na |
|
||
| Learning disability | 20 (10.5%) | na | |||
Two hundred thirty typically developing children and adolescents with no personal history of psychiatric or neurological disorders participated in the present study- a subset of a cohort reported upon previously (38, 39). Each participant underwent a structured diagnostic interview by a child psychiatrist to rule out any psychiatric or neurological diagnoses (40). Two-hundred one (87%) were right handed, 12 (5%) were left handed, and 17 (7%) were ambidextrous. Demographic and scanning details are given in Table 1 for right handed and Table S1 (in the Supplement) for non-right handed participants. After study description, assent and written informed consent were obtained from children and parents respectively.
Neuroimaging
T1-weighted sagittal magnetic resonance images were acquired using a 1.5-T scanner (GE Signa; GE Medical Systems, Milwaukee, Wisconsin) with contiguous 1.5-mm axial slices (echo time=5 milliseconds, repetition time=24 milliseconds, acquisition matrix=256 × 192, flip angle= 45°, number of excitations= 1, and field of view=24 cm). Images were manually re-aligned in the axial plane such that the interhemispheric fissure was aligned with the y-axis and in the coronal plane such that the interhemispheric fissure was aligned with the z-axis. In the sagittal plane, the line connecting the anterior-most and posterior-most points of the callosum was aligned with the y-axis. To quantify the CC, the midsagittal slice was designated as the slice which contains the maximum upward extent curvature of the rostrum and the septum pellucidum (29). The corpus callosum was then segmented automatically using the Medical Image Processing, Analysis and Visualization tool (http://mipav.cit.nih.gov/), manually outlined by a rater (M.G.) blind to diagnosis, and split into five subdivisions according to a protocol devised by Witelson (29) and modified by Hofer and Frahm (41). This recent modification incorporates data from diffusion tensor imaging which better reflect the origins of the fibers (see Figure 1). All measurements were conducted by one rater (MG). In the training phase for the study, this rater showed high inter-rater reliabilities with two other raters (intra-class correlation coefficients >0.9). Intra-rater reliabilities for the study rater were determined from repeated measurement in separate sessions of 48 randomly selected scans, blind to prior measurement. Intra-class correlation coefficients were high: Region I, ICC=0.98; Region II, ICC=0.98; Region III, ICC=0.93; Region IV, ICC=0.94; Region V, ICC=0.99.
Statistical Analyses
Linear mixed model regression was used for longitudinal analyses, as our data contain both multiple observations per participant measured at different and irregular time periods and single observations per participant. Such unbalanced longitudinal data can be explored statistically by applying mixed effect models (23). A model including linear effects of age best fit the data. We tested for effects of higher order age terms (quadratic and cubic age), and these did not contribute significantly. A random effect for each individual was also included in the model to account for within-person dependence. Thus, the jth area measure of the ith individual was modeled as
where di is a random effect modeling within-person dependence; the intercept and β terms are fixed effects, and eij represents the residual error. Group differences in growth rates were determined by the value and significance of the interaction term (β3), which gives an estimate of how the relationship between callosal area and age varies as a function of diagnostic group. Growth rates were expressed as the percentage change in the area of each region per year (taking the mean values for each area at the baseline scan as the denominator). In the primary analyses, trajectories were determined for five subregions and we thus set a level of significance of p=0.01 (p of 0.05 divided by 5). In other exploratory analyses of the effects of sex and handedness, we used an unadjusted p=0.05. To study medication effects, we compared areas and rates of growth in ADHD participants who were treated with psychostimulants against their unmedicated counterparts.
Results
Typically developing participants
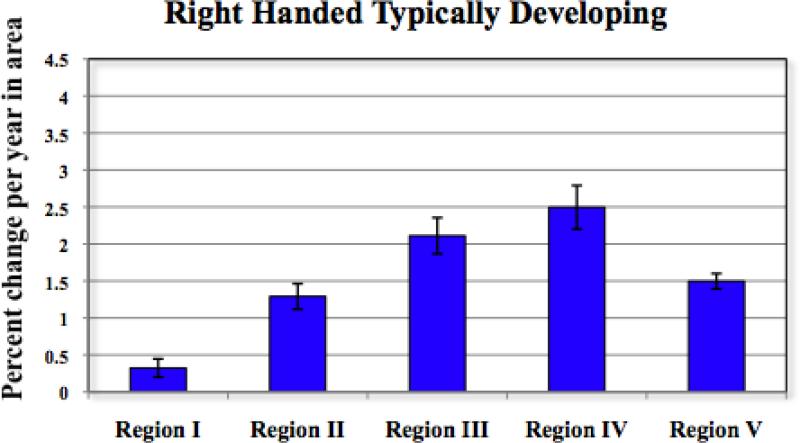
Estimated rates of callosal growth increased for right handed typically developing subjects moving rostral to caudal, with growth rates slowest in Region I (estimated annual increase in area 0.32%, SE 0.12%), and increasing in Region II (estimated annual increase 1.29% SE 0.17%) and Region III (annual increase 2.11%, SE 0.22%), peaking in Region IV (annual increase of 2.50%, SEM 0.30%), and declining slightly in region V (annual increase 1.50%, SEM 0.10%) (see Figure 2). For the entire corpus callosum, the estimated annual growth rate was 1.1%, SE 0.1%. A similar pattern of results was found for the non-right handed group (see Figure S1 and Table S2 in the Supplement).
Figure 2.
Estimated growth rates in right handed, typically developing participants. Bar indicates mean percent change per year in area and lines indicate ± SE.
No significant sex differences in growth rates emerged in the right handed typically developing group (see Table 2). At baseline, males had larger callosal area in Regions I, II, and III. However, when adjustment was made for ICV, these differences were abolished or reversed.
Table 2.
Mean baseline area (in mm2) in right-handed, typically developing participants by sex.
| Unadjusted | |||
|---|---|---|---|
| Males | Females | Difference | |
| Mean (SD) | Mean (SD) | t (p) | |
| Region I | 160.7 (28.2) | 151.5 (20.6) | 2.40 (0.02) |
| Region II | 147.4 (26.8) | 139.7 (22.8) | 2.04 (0.04) |
| Region III | 56.4 (12.8) | 53.0 (11.3) | 1.89 (0.06) |
| Region IV | 25.9 (7.4) | 25.3 (6.2) | 0.56 (0.58) |
| Region V | 174.0 (33.2) | 170.8 (24.4) | 0.71 (0.48) |
| Total CC | 564.3 (93.3) | 540.3 (68.2) | 1.91 (0.06) |
| Adjusted for intracranial volume | |||
|---|---|---|---|
| Males | Females | Difference | |
| Mean (SEM) | Mean (SEM) | F{1,194}, p | |
| Region I | 156.8 (2.07) | 158.8 (2.84) | F=0.31, p=0.58 |
| Region II | 145.6 (2.03) | 146.2 (2.79) | F=0.35, p=0.56 |
| Region III | 55.4 (1.01) | 55.7 (1.38) | F=0.21, p=0.65 |
| Region IV | 25.8 (.59) | 26.8 (.81) | F=2.55, p=0.11 |
| Region V | 170.5 (2.30) | 180.0 (3.16) | F=8.19, p=0.01 |
| Total CC | 549.3 (6.30) | 567.5 (8.70) | F=2.65, p=0.11 |
Contrast with ADHD
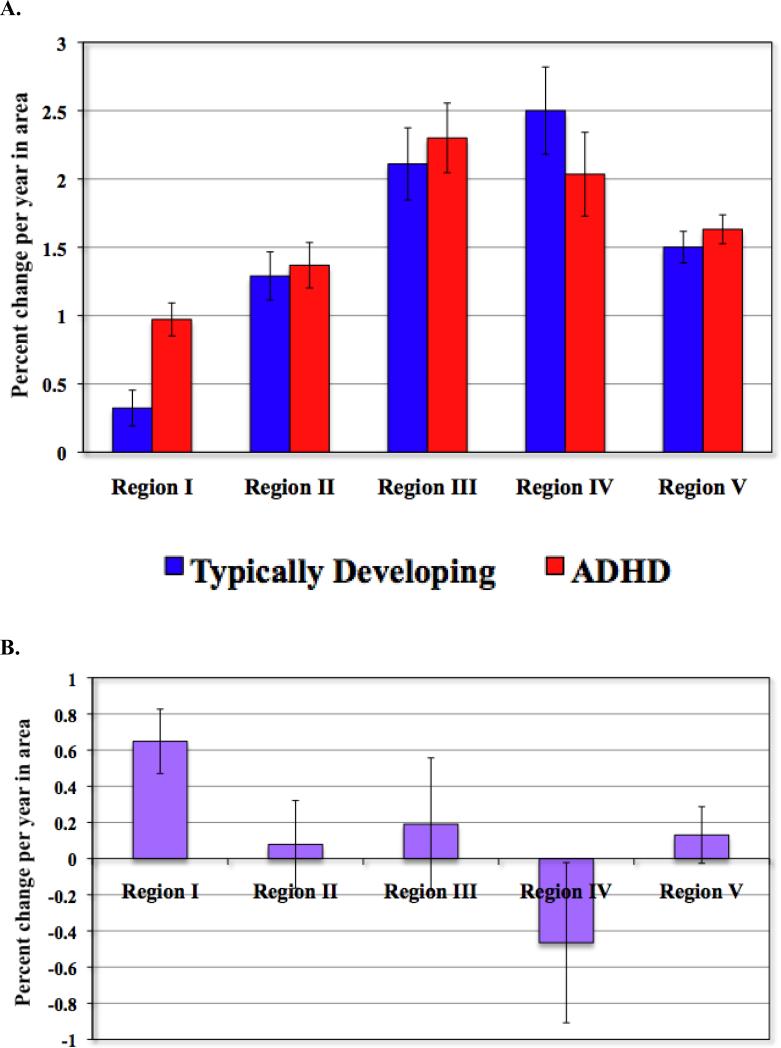
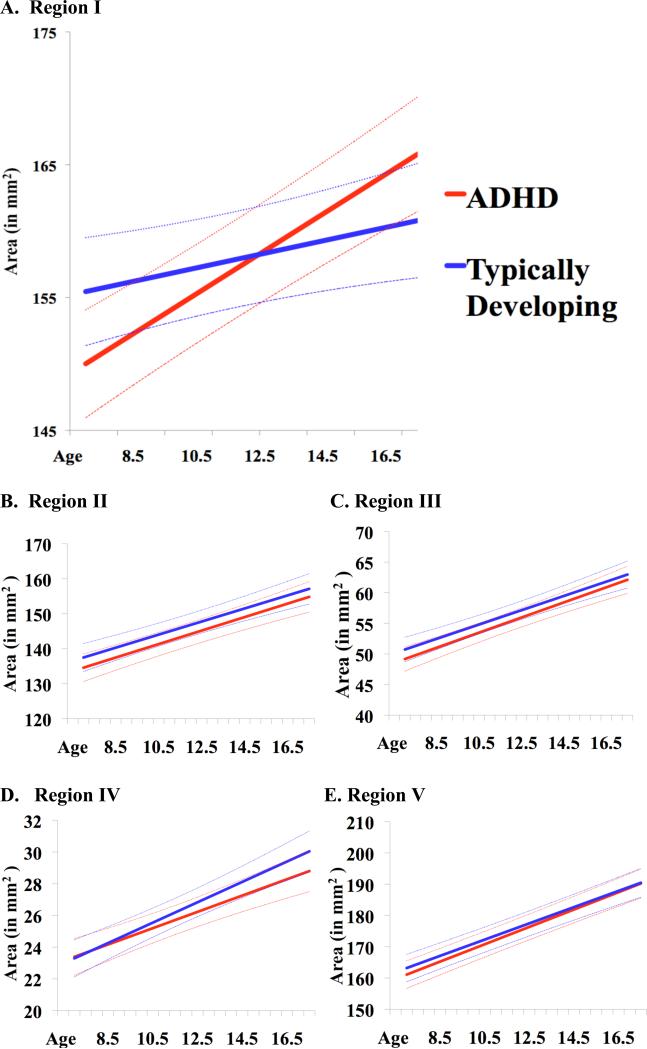
Our main hypothesis predicted different growth rates in the anterior corpus callosum in right handed ADHD compared to typically developing participants. The hypothesis was confirmed: the model term indicating the interaction of age and diagnosis in the determination of callosal area (i.e. β3) was significant for Region I only. The right handed ADHD group had a higher estimated annual increase in Region I of 0.97% (SE 0.12%) compared to the typically developing group (0.32%, SE 0.13%; t=3.64, p=0.0003) (see Table 3 and Figure 3). No other callosal subdivisions showed significant diagnostic differences in growth rates (for Region II: t=0.32, p=0.75; for Region III: t=0.52, p=0.60; for Region IV: t=1.05, p=0.29; for Region V: t=0.83, p=0.41). The pattern of results held when adjustment was made for intracranial volume (difference in estimated growth rates for Region I of 0.61% [SE 0.18%] t=3.3, p=0.001; for Region II, difference in rates of 0.00009% [SE 0.002], t=0.04, p=0.96; for Region III, difference in rates of 0.003% [SE0.003], t=0.7, p=0.48; for Region IV, difference in rates of -0.003% [SE0.004], t=0.67, p=0.49; for Region V, difference in rates of 0.002% [SE 0.001], t=1.1, p=0.29). The estimated midsagittal areas (with 95% confidence intervals for the estimate) for each callosal subregion over the course of development are shown in Figure 4. There were also no significant diagnostic differences in baseline areas in right handed individuals, either unadjusted or after adjustment for ICV (see Table 4).
Table 3.
Estimated growth rates, in estimated percent change per year in area, with standard error of the estimate, in right-handed typically developing and ADHD participants.
| Estimated value (SE) | Difference | ||
|---|---|---|---|
| Typically Developing | ADHD | t (p) | |
| Region I | 0.32 (0.13) | 0.97 (0.12) | 3.64 (0.0003) |
| Region II | 1.29 (0.18) | 1.37 (0.17) | 0.32 (0.75) |
| Region III | 2.11 (0.27) | 2.30 (0.26) | 0.52 (0.60) |
| Region IV | 2.50 (0.32) | 2.03 (0.31) | -1.05 (0.29) |
| Region V | 1.50 (0.12) | 1.63 (0.11) | 0.83 (0.41) |
| Total CC | 1.12 (0.01) | 1.39 (0.009) | 1.55 (0.12) |
Figure 3.
A. Estimated growth rates in right handed participants by diagnosis. Bars indicate estimated percent change per year in area. Lines indicate ± SE.
B. Difference in annual percentage growth rates between right-handed participants with ADHD and typically developing controls. Bars indicate estimated percent change per year in area. Lines indicate ± SE.
Figure 4.
Estimated area (in mm2) by age in right-handed ADHD and typically developing participants in each callosal subregion. Solid lines represent fitted growth curves, with dashed lines for 95% confidence intervals, estimated from linear mixed model regression.
Table 4.
Mean baseline area (in mm2) in right-handed participants by diagnostic group.
| Unadjusted | |||
|---|---|---|---|
| Typically Developing | ADHD | Difference | |
|
Mean (SD) Range |
Mean (SD) Range |
t (p) | |
| Region I | 157.4 (26.1) 104.1 to 243.3 |
154.9 (28.3) 92.8 to 244.7 |
0.92 (0.36) |
| Region II | 144.7 (25.6) 98.4 to 232 |
141.3 (25.5) 94.2 to 240.5 |
1.31 (0.19) |
| Region III | 55.2 (12.3) 19.7 to 99.8 |
53.7 (12.9) 28.1 to 99.8 |
1.14 (0.25) |
| Region IV | 25.7 (7.0) 12.6 to 46.4 |
25.4 (7.6) 8.4 to 52 |
0.41 (0.68) |
| Region V | 172.9 (30.3) 99.8 to 254.5 |
170.2 (33.3) 90 to 268.6 |
0.82 (0.41) |
| Total CC | 555.8 (85.8) 385.3 to 832.5 |
545.5 (92.5) 352.9 to 884.5 |
1.15 (0.25) |
| Adjusted for intracranial volume | |||
|---|---|---|---|
| Typically Developing | ADHD | Difference | |
| Mean (SEM) | Mean (SEM) | F{1,372}, p | |
| Region I | 155.7 (1.73) | 157.8 (1.82) | F=0.70, p=0.40 |
| Region II | 143.3 (1.63) | 144.4 (1.72) | F=0.23, p=0.62 |
| Region III | 54.4 (.81) | 55.0 (.86) | F=0.27, p=0.61 |
| Region IV | 25.3 (.48) | 26.0 (.51) | F=0.95, p=0.33 |
| Region V | 170.3 (2.0) | 173.6 (2.1) | F=1.38, p=0.24 |
| Total CC | 555.8 (5.6) | 548.9 (5.3) | F=1.03, p=0.31 |
No diagnostic differences were found for growth rates nor baseline areas for the non-right handed participants (all p>0.1) (see Tables S2-S4 in the Supplement). We did not test for higher order interactions of diagnosis, handedness, and sex, given the small sample sizes.
Correlates of comorbidity, intelligence, type of ADHD and medication
Oppositional defiant disorder and conduct disorder were the major comorbidities; others were uncommon and not the focus of treatment. The pattern of results held when the 83 right handed subjects with ADHD and comorbid ODD/CD were considered separately from those with ADHD uncomplicated by ODD/CD. Thus for Region I, those with ADHD and ODD/CD had an estimated growth rate of 0.99%/year (SE 0.16%) compared to the rate of 0.96%/year (SE 0.17%) for those with ADHD uncomplicated by ODD/CD, a non-significant difference (t=0.14, p=0.89). These growth rates for Region I differed significantly from the typically developing group: for ADHD with ODD/CD versus typically developing t=3.22, p=0.001; for ADHD uncomplicated by ODD/CD versus typically developing t=2.93, p=0.004. For all other regions, the ADHD groups divided into those with and without comorbid ODD/CD did not differ significantly from each other nor from the typically developing group ( all p>0.1).
Results also held when analyses were confined to those with combined type ADHD only. For Region I, difference in growth rates remained significant at t=3.6, p<0.001; all other regions did not differ significantly. When IQ was entered as a covariate, the difference in growth rates remained significant for Region I (t=3.3, p=0.001). There were no significant differences in baseline areas or in growth rates in ADHD participants who were treated with psychostimulants compared to those who were unmedicated – see Tables S5 and S6 in the Supplement.
Discussion
We confirm our hypothesis by demonstrating a selective abnormality in developmental trajectories of the anterior CC in right handed children and adolescents with ADHD relative to their typically developing peers. This was predicted on the basis of our previous finding of anomalous development in ADHD of the asymmetry of the prefrontal cortical regions that are connected by the anterior CC (5). The anomaly of corpus callosal development we now report was dynamic: while no significant diagnostic differences in area were present at baseline in either right handed or non-right handed groups, significant diagnostic differences emerged when velocities of growth were examined. Our longitudinal analysis captured this ‘dysregulated’ anterior growth trajectory in the right handed ADHD cohort.
Interesting parallels and differences exist in other neuropsychiatric disorders. There is some diagnostic specificity to the finding: in autism and early onset schizophrenia, there appears to be a progressive loss of posterior callosal dimensions throughout childhood (42, 43). In obsessive compulsive disorder, there is a childhood increase, which is not sustained into adolescence (44). While both these patterns are distinct from those we find in ADHD, a close parallel to our findings has been reported in Tourette's syndrome (45). In this disorder, the CC was smaller in childhood but this pattern reversed by adulthood, implying an accelerated rate of CC growth similar to that we report in ADHD. However, this anomalous growth pattern was not localized to the anterior portions of the CC as in ADHD, and cannot explain our results as Tourette's syndrome was an exclusionary criterion. Nonetheless, both findings imply that a dysregulation of CC growth is an important feature of several neuropsychiatric disorders. Perhaps the partial failure to develop typical patterns of prefrontal cortical asymmetry in ADHD leads to an increased reliance on interhemispheric processing with a concomitant increase in the dimensions of the anterior CC.
There is a complex story linking atypical cerebral lateralization with ADHD. There is epidemiological evidence that non-right handed individuals, particularly those who are ambidextrous, have an increased risk of ADHD (46). Neurophysiological evidence for atypical prefrontal cortical lateralization includes abnormal left/right EEG coherence (47, 48), atypical asymmetries in cerebral blood flow and activation during cognitive tasks (10, 49), in addition to the abnormalities in tasks directly assessing interhemispheric integration noted earlier. There is also genetic evidence linking atypical cerebral lateralization with ADHD, as polymorphisms of genes that are asymmetrically expressed in the typical human cerebrum have been found to confer risk for adult ADHD (50). Clinically non-right handed children with ADHD have more severe symptoms, although most studies are biased by the use of referred samples (51). Our study adds to this work by suggesting that atypical development of the anterior CC may reflect or drive this tendency to atypical lateralization in ADHD.
Our findings also raise the question of whether developmental anomalies of callosal growth in ADHD reflect or drive the anomalous development in prefrontal cortical asymmetry we reported earlier (5). The primacy of an anomaly in CC structures derives support from evidence of anomalies of other midline, non-cerebral brain structures in ADHD. We recently found evidence that structural compromise of the superior portion of the vermis, a cerebellar midline structure, was a prominent structural anomaly in a cohort of children with ADHD, a finding in line with many previous studies (52-56). Interestingly, the clustering of midline anomalies, such as total or partial agenesis of the CC, along with vermis anomalies, has been linked to several cytogenetic lesions that may point to regions harboring genes that regulate CC growth (57). It is equally possible that the CC anomalies are a downstream effect of disruption in the development of prefrontal cortical asymmetry, given the evidence for regional reductions of the CC following injury to the cortex (58).
We previously reported delayed prefrontal cortical maturation in ADHD – a delay within which the development of anomalous asymmetries is nested (59). Could the CC findings be another instance of structural delay? Previous studies of typical callosal development have reported a rostro-caudal wave of peak growth rates (60, 61) with peak growth rates of the anterior CC occurring in early childhood. Our main finding could thus be driven by a delayed end to this childhood phase of relatively fast anterior CC growth. One major limitation of this explanation is that it does not however explain the persistence of rate differences into adolescence.
Additionally, this study constitutes the largest study to date of typical development of the corpus callosum. We find increasing rates of growth moving rostral to caudal, with the slowest rate in the anterior-most callosal subdivision. This pattern was similar in both sexes. At baseline, males had larger CC areas than females, but this effect disappeared when adjustment was made for ICV- a frequent finding in the study of morphometric sex differences (62, 63). The variability in growth rates was higher for Region IV and III, perhaps as these are the smallest CC regions with the lowest reliability estimates, which could inflate variability. Additionally the fibers found in these mid-callosal regions connecting sensory areas are relatively large and show more variance in size (17).
Limitations
Our phenotype included mainly those with combined type ADHD and we did not have sufficient numbers of inattentive and hyperactive/impulsive subtypes to determine if they showed a different pattern of callosal growth. We also did not collect measures of pubertal status on the ADHD group, and thus are unable to tease apart the effects of chronological and pubertal age. We found no significant baseline differences with handedness in the typically developing youth, although this finding should be regarded as preliminary since larger samples of non-right handed individuals are needed to test more fully for possible effects of handedness and its interaction with sex and diagnosis. We estimated corpus callosal area from one slice and did not attempt to define volume, and thus may have missed some anomalies in thickness which have been demonstrated in ADHD using this approach (64). While we included data concerning the correlates of psychostimulant medication, these must be interpreted with caution, given the small sample sizes involved and the observational nature of the data. There was no evidence of any cohort effect in our study on measured variables, as there was no significant correlation between age at study entry and IQ, socioeconomic status, or higher proportions of right handed subjects or males. It remains possible that there are cohort differences on unmeasured variables that might influence CC development, such as some age-related factors; for example, familial and scholastic environment.
We could not determine the cognitive significance of these findings as we did not collect longitudinal data on tests reflecting interhemispheric integration mediated by the CC- such as dichotic listening tasks (65), lateralized naming tasks (66), and alternate finger-tapping tasks (67). Recent studies suggest that children with ADHD seem to be most impaired when tasks involve lateralized processing where the right hemisphere is challenged to mediate tasks it does not typically support, such as linguistic processing and coordinating writing like movements (68). Such tasks require rapid interhemispheric integration with a recruitment of the left hemisphere, and the anterior CC anomalies may contribute to these deficits. Interestingly, the same study also found that measures of atypical interhemispheric processing correlated with measures of oppositional and defiant symptoms in these children.
This first large-scale longitudinal study of callosal growth trajectories informs our understanding of the dynamic nature of anomalous callosal development in ADHD and the disruptions in cortical asymmetry and connectivity that characterize the disorder.
Supplementary Material
Disclosures and acknowledgements
All authors report no biomedical financial interests or potential conflicts of interest. Supported by the Intramural Research Program of the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Marsh R, Gerber AJ, Peterson BS. Neuroimaging studies of normal brain development and their relevance for understanding childhood neuropsychiatric disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:1233–1251. doi: 10.1097/CHI.0b013e318185e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavuluri MN, Sweeney JA. Integrating functional brain neuroimaging and developmental cognitive neuroscience in child psychiatry research. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:1273–1288. doi: 10.1097/CHI.0b013e318185d2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention deficit/hyperactivity disorder. Archives of General Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 4.Shaw P, Gogtay N, Rapoport J. Childhood Psychiatric Disorders as Anomalies in Neurodevelopmental Trajectories. Human Brain Mapping. 2010;31:917–925. doi: 10.1002/hbm.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw P, Lalonde F, Lepage C, Rabin C, Eckstrand K, Sharp W, et al. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2009;66:888–896. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Archives of General Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- 7.Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48:589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- 8.Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2002;52:785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- 9.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neuroscience & Biobehavioral Reviews. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- 10.Langleben DD, Austin G, Krikorian G, Ridlehuber HW, Goris ML, Strauss HW. Interhemispheric asymmetry of regional cerebral blood flow in prepubescent boys with attention deficit hyperactivity disorder. Nuclear Medicine Communications. 2001;22:1333–1340. doi: 10.1097/00006231-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. American Journal of Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- 12.Rubia KPD, Smith ABPD, Brammer MJPD, Toone BPD, Taylor EMDPD. Abnormal Brain Activation During Inhibition and Error Detection in Medication-Naive Adolescents With ADHD. American Journal of Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- 13.Innocenti GM, Bressoud R. Callosal axons and their development. In: I M, Z E, editors. The parallel brain: The cognitive neuroscience of the corpus callosum. MIT Press; Cambridge, MA: 2003. pp. 11–26. [Google Scholar]
- 14.Ramaekers G, Njiokiktjien C. The Child's Corpus Callosum: Pediatric Behavioral Neurology. Suyi Publications; Amsterdam: 1991. [Google Scholar]
- 15.Richards LJ, Plachez C, Ren T. Mechanisms regulating the development of the corpus callosum and its agenesis in mouse and human. Clinical Genetics. 2004;66:276–289. doi: 10.1111/j.1399-0004.2004.00354.x. [DOI] [PubMed] [Google Scholar]
- 16.Pandya DN, Seltzer B. Two Hemispheres-- One Brain: Functions of the Corpus Callosum. Alan R. Liss; New York: 1996. The topography of commisural fibers. pp. 47–73. [Google Scholar]
- 17.Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Research. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- 18.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends in Neurosciences. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galaburda AM, Rosen GD, Sherman GF. Individual variability in cortical organization: its relationship to brain laterality and implications to function. Neuropsychologia. 1990;28:529–546. doi: 10.1016/0028-3932(90)90032-j. [DOI] [PubMed] [Google Scholar]
- 20.Zaidel E, Aboitiz F, Clarke J. Sexual dimorphism in interhemispheric relations: anatomical-behavioral convergence. Biological Research. 1995;28:27–43. [PubMed] [Google Scholar]
- 21.Dorion AA, Chantome M, Hasboun D, Zouaoui A, Marsault C, Capron C, et al. Hemispheric asymmetry and corpus callosum morphometry: a magnetic resonance imaging study. Neuroscience Research. 2000;36:9–13. doi: 10.1016/s0168-0102(99)00102-9. [DOI] [PubMed] [Google Scholar]
- 22.Luders E, Rex DE, Narr KL, Woods RP, Jancke L, Thompson PM, et al. Relationships between sulcal asymmetries and corpus callosum size: gender and handedness effects. Cerebral Cortex. 2003;13:1084–1093. doi: 10.1093/cercor/13.10.1084. [DOI] [PubMed] [Google Scholar]
- 23.Hopkins W, Rilling J. A Comparative MRI Study of the Relationship Between Neuroanatomical Asymmetry and Interhemispheric Connectivity in Primates: Implication for the Evolution of Functional Asymmetries. Behavioural Neuroscience. 2000;114:739–748. doi: 10.1037//0735-7044.114.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jancke L, Steinmetz H. Brain size: a possible source of interindividual variability in corpus callosum morphology. In: Zaidel E, Iacoboni M, Pascual-Leone A, editors. The parallel brain: the cognitive neuroscience of the corpus callosum. Plenum Press; New York: 2003. pp. 51–64. [Google Scholar]
- 25.Westerhausen R, Kreuder F, Dos Santos Sequeira S, Walter C, Woerner W, Wittling RA, et al. Effects of handedness and gender on macro- and microstructure of the corpus callosum and its subregions: a combined high-resolution and diffusion-tensor MRI study. Brain Research Cognitive Brain Research. 2004;21:418–426. doi: 10.1016/j.cogbrainres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Habib M, Gayraud D, Oliva A, Regis J, Salamon G, Khalil R. Effects of handedness and sex on the morphology of the corpus callosum: a study with brain magnetic resonance imaging. Brain and Cognition. 1991;16:41–61. doi: 10.1016/0278-2626(91)90084-l. [DOI] [PubMed] [Google Scholar]
- 27.Luders E, Thompson PM, Toga AW. The development of the corpus callosum in the healthy human brain. Journal of Neuroscience. 30:10985–10990. doi: 10.1523/JNEUROSCI.5122-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinmetz H, Jancke L, Kleinschmidt A, Schlaug G, Volkmann J, Huang Y. Sex but no hand difference in the isthmus of the corpus callosum. Neurology. 1992;42:749–752. doi: 10.1212/wnl.42.4.749. [DOI] [PubMed] [Google Scholar]
- 29.Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112(Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- 30.Baumgardner TL, Singer HS, Denckla MB, Rubin MA, Abrams MT, Colli MJ, et al. Corpus callosum morphology in children with Tourette syndrome and attention deficit hyperactivity disorder. Neurology. 1996;47:477–482. doi: 10.1212/wnl.47.2.477. [DOI] [PubMed] [Google Scholar]
- 31.Giedd JN, Castellanos FX, Casey BJ, Kozuch P, King AC, Hamburger SD, et al. Quantitative morphology of the corpus callosum in attention deficit hyperactivity disorder. American Journal of Psychiatry. 1994;151:665–669. doi: 10.1176/ajp.151.5.665. [see comment] [DOI] [PubMed] [Google Scholar]
- 32.Hynd GW, Semrud-Clikeman M, Lorys AR, Novey ES, Eliopulos D, Lyytinen H. Corpus callosum morphology in attention deficit-hyperactivity disorder: morphometric analysis of MRI. Journal of Learning Disabilities. 1991;24:141–146. doi: 10.1177/002221949102400302. [DOI] [PubMed] [Google Scholar]
- 33.Hill DE, Yeo RA, Campbell RA, Hart B, Vigil J, Brooks W. Magnetic resonance imaging correlates of attention-deficit/hyperactivity disorder in children. Neuropsychology. 2003;17:496–506. doi: 10.1037/0894-4105.17.3.496. [DOI] [PubMed] [Google Scholar]
- 34.Lyoo IK, Noam GG, Lee CK, Lee HK, Kennedy BP, Renshaw PF. The corpus callosum and lateral ventricles in children with attention-deficit hyperactivity disorder: a brain magnetic resonance imaging study. Biological Psychiatry. 1996;40:1060–1063. doi: 10.1016/s0006-3223(96)00349-6. [DOI] [PubMed] [Google Scholar]
- 35.Semrud-Clikeman M, Filipek PA, Biederman J, Steingard R, Kennedy D, Renshaw P, et al. Attention-deficit hyperactivity disorder: magnetic resonance imaging morphometric analysis of the corpus callosum. Journal of the American Academy of Child & Adolescent Psychiatry. 1994;33:875–881. doi: 10.1097/00004583-199407000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Overmeyer S, Simmons A, Santosh J, Andrew C, Williams SC, Taylor A, et al. Corpus callosum may be similar in children with ADHD and siblings of children with ADHD. Developmental Medicine & Child Neurology. 2000;42:8–13. doi: 10.1017/s0012162200000037. [DOI] [PubMed] [Google Scholar]
- 37.Denckla MB. Revised neurological examination for subtle signs. Psychopharmacological Bulletin. 1985;21:773–800. [PubMed] [Google Scholar]
- 38.Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 39.Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, et al. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1999;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- 40.Giedd JN, Rumsey JM, Castellanos FX, Rajapakse JC, Kaysen D, Vaituzis AC, et al. A quantitative MRI study of the corpus callosum in children and adolescents. Brain Research Developmental Brain Research. 1996;91:274–280. doi: 10.1016/0165-3806(95)00193-x. [DOI] [PubMed] [Google Scholar]
- 41.Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 42.Keller A, Jeffries NO, Blumenthal J, Clasen LS, Liu H, Giedd JN, et al. Corpus callosum development in childhood-onset schizophrenia. Schizophrenia Research. 2003;62:105–114. doi: 10.1016/s0920-9964(02)00354-7. [DOI] [PubMed] [Google Scholar]
- 43.Frazier TW, Hardan AY. A meta-analysis of the corpus callosum in autism. Biological Psychiatry. 2009;66:935–941. doi: 10.1016/j.biopsych.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenberg DR, Keshavan MS, Dick EL, Bagwell WW, Master FPM, Birmaher B. Corpus callosal morphology in treatment-naive pediatric obsessive compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1997;21:1269–1283. doi: 10.1016/s0278-5846(97)00163-2. [DOI] [PubMed] [Google Scholar]
- 45.Plessen KJ, Wentzel-Larsen T, Hugdahl K, Feineigle P, Klein J, Staib LH, et al. Altered interhemispheric connectivity in individuals with Tourette's disorder. American Journal of Psychiatry. 2004;161:2028–2037. doi: 10.1176/appi.ajp.161.11.2028. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez A, Kaakinen M, Moilanen I, Taanila A, McGough JJ, Loo S, et al. Mixed-handedness is linked to mental health problems in children and adolescents. Pediatrics. 2009;125:e340–348. doi: 10.1542/peds.2009-1165. [DOI] [PubMed] [Google Scholar]
- 47.Barry RJ, Clarke AR, McCarthy R, Selikowitz M, Johnstone SJ. EEG coherence adjusted for inter-electrode distance in children with attention-deficit/hyperactivity disorder. International Journal of Psychophysiology. 2005;58:12–20. doi: 10.1016/j.ijpsycho.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Chabot RJ, Serfontein G. Quantitative electroencephalographic profiles of children with attention deficit disorder. Biological Psychiatry. 1996;40:951–963. doi: 10.1016/0006-3223(95)00576-5. [DOI] [PubMed] [Google Scholar]
- 49.Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 50.Ribases M, Bosch R, Hervas A, Ramos-Quiroga JA, Sanchez-Mora C, Bielsa A, et al. Case-control study of six genes asymmetrically expressed in the two cerebral hemispheres: association of BAIAP2 with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2009;66:926–934. doi: 10.1016/j.biopsych.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 51.Biederman J, Lapey KA, Milberger S, Faraone SV, Reed ED, Seidman LJ. Motor preference, major depression and psychosocial dysfunction among children with attention deficit hyperactivity disorder. Journal of Psychiatric Research. 1994;28:171–184. doi: 10.1016/0022-3956(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 52.Bussing R, Grudnik J, Mason D, Wasiak M, Leonard C. ADHD and conduct disorder: an MRI study in a community sample. World Journal of Biological Psychiatry. 2002;3:216–220. doi: 10.3109/15622970209150624. [DOI] [PubMed] [Google Scholar]
- 53.Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF, 3rd, et al. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. American Journal of Psychiatry. 2007;164:647–655. doi: 10.1176/ajp.2007.164.4.647. [see comment] [DOI] [PubMed] [Google Scholar]
- 54.Berquin PC, Giedd JN, Jacobsen LK, Hamburger SD, Krain AL, Rapoport JL, et al. Cerebellum in attention-deficit hyperactivity disorder: a morphometric MRI study. Neurology. 1998;50:1087–1093. doi: 10.1212/wnl.50.4.1087. [DOI] [PubMed] [Google Scholar]
- 55.Mostofsky SH, Reiss AL, Lockhart P, Denckla MB. Evaluation of cerebellar size in attention-deficit hyperactivity disorder. Journal of Child Neurology. 1998;13:434–439. doi: 10.1177/088307389801300904. [DOI] [PubMed] [Google Scholar]
- 56.Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 57.Bedeschi MF, Bonaglia MC, Grasso R, Pellegri A, Garghentino RR, Battaglia MA, et al. Agenesis of the corpus callosum: clinical and genetic study in 63 young patients. Pediatric Neurology. 2006;34:186–193. doi: 10.1016/j.pediatrneurol.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Moses P, Courchesne E, Stiles J, Trauner D, Egaas B, Edwards E. Regional size reduction in the human corpus callosum following pre- and perinatal brain injury. Cerebral Cortex. 2000;10:1200–1210. doi: 10.1093/cercor/10.12.1200. [DOI] [PubMed] [Google Scholar]
- 59.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- 61.Luders E, Thompson PM, Toga AW. The development of the corpus callosum in the healthy human brain. Journal of Neuroscience. 2010;30:10985–10990. doi: 10.1523/JNEUROSCI.5122-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bishop KM, Wahlsten D. Sex differences in the human corpus callosum: myth or reality? Neuroscience & Biobehavioral Reviews. 1997;21:581–601. doi: 10.1016/s0149-7634(96)00049-8. [DOI] [PubMed] [Google Scholar]
- 63.Driesen NR, Raz N. The influence of sex, age, and handedness on corpus callosum morphology: a meta-analysis. Psychobiology. 1995;23:240–247. [Google Scholar]
- 64.Luders E, Narr KL, Hamilton LS, Phillips OR, Thompson PM, Valle JS, et al. Decreased callosal thickness in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2009;65:84–88. doi: 10.1016/j.biopsych.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bryden M. An overview of the dichotic listening procedure and its relation to cerebral organization. In: Hugdhal K, editor. Handbook of Dichotic Listening: Theory, Methods, and Research. Wiley; Chichester: 1988. pp. 1–44. [Google Scholar]
- 66.Malone M, Kershner J, Siegel L. The effects of methylphenidate on levels of processing and laterality in children with attention deficit disorder. Journal of Abnormal Child Psychology. 1998;16:379–395. doi: 10.1007/BF00914170. [DOI] [PubMed] [Google Scholar]
- 67.Pelletier J, Habib M, Lyon-Caen O, Salamon G, Poncet M, Khalil R. Functional and magnetic resonance imaging correlates of callosal involvement in multiple sclerosis. Archives of Neurology. 1993;50:1077–1082. doi: 10.1001/archneur.1993.00540100066018. [DOI] [PubMed] [Google Scholar]
- 68.Hale TS, Loo SK, Zaidel E, Hanada G, Macion J, Smalley SL. Rethinking a right hemisphere deficit in ADHD. Journal of Attention Disorders. 2009;13:3–17. doi: 10.1177/1087054708323005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.