Abstract
The intestine is the home to a vast diversity of microbiota and a complex of mucosal immune system. Multiple regulatory mechanisms control host immune responses to microbiota and maintain intestinal immune homeostasis. This mini review will provide evidence indicating a Treg cell-IgA axis and such axis playing a major role in maintenance of intestinal homeostasis.
The intestine harbors and is in constant contact with a vast diversity of microbiota, which consists of about 1014 bacteria with hundreds of different strains (1). Despite this enormous bacterial challenge, the host lives in harmony with the microbiota, in part due to the interactions between some members of the microbiota and the host to maintain intestinal homeostasis (2-4). Great progress has been made in recent years in our understanding of how the host maintains tolerance to the microbiota yet can mount vigorous responses to invading pathogens. To achieve tolerance to commensal flora, intestinal homeostasis relies on a network of different regulatory components. Multiple strategies are present to confine commensal bacteria to the intestinal lumen while preserving their number and composition through combinations of physiological and immunological mechanisms including mucus, lysozyme, lactoferrin, defensins, and induced specific immunity based on T cells and the production of secretory immunoglobulin A (5-7). Both regulatory T (Treg) cells and secretory IgA are enriched in the intestine, and play a crucial role in the maintenance of immune homeostasis. In this brief review, we will discuss the evidence which supports a major role for the Treg cell-IgA axis in controlling host responses to the microbiota.
Compartmentalization of mucosal immune responses to the microbiota
There is a significant immune response to the enteric microbiota, however, considering that more than 500 commensal bacterial species and millions of microbial genes are present in the intestinal environment, the immunological challenge represented by these microbes is even greater (8-10). With this in mind, it is inconceivable that there is active immunity against the entire span of microbiota antigens. It has been widely believed that the immune system is immunologically tolerant to enteric bacterial antigens in normal hosts and there is a loss of such tolerance in chronic intestinal inflammation (11). However, this concept has been challenged by recent studies.
Elegant work done by MacPherson's group (2) demonstrates that when delivered intragastrically, Enterobacter cloacae (the main aerobic commensal in the Zürich colony of specific pathogen-free (SPF) mice) could be detected in dendritic cells from Peyer's patches and mesenteric lymph nodes (MLN), but not from the spleen, indicating that the MLN excludes commensal bacteria from systemic immune system. Interestingly, strong Enterobacter cloacae-specific IgA responses in the intestine were detected, however, there were no serum IgG responses against Enterobacter cloacae antigens in unmanipulated mice. Injection of bacteria into the tail vein induced a specific IgG response against Enterobacter cloacae, indicating that this is ignorance rather than tolerance in the host response to commensal bacterial antigens. The evidence highlights that the mucosal immune system presents a strong IgA response to intestinal commensal bacteria, but that this is separate from the systemic immune response (2).
To further define how the host responds to microbiota antigens, we randomly generated 20 recombinant intestinal bacterial proteins (rIB) from the intestinal microbiota of normal SPF mice to probe in a systematic fashion the immune response in systemic and mucosal compartments. Because these are random clones, they are likely to be from the most abundant species among the microbiota. Neither serum IgG responses, nor splenic T cell responses could be detected against any of these 20 antigens in normal SPF mice (12). However, when mice were immunized with each rIB , both systemic B cell and T cell responses to rIB were at a level comparable to their responses to OVA, a classic exogenous antigen. Thus, each rIB is immunogenic yet does not stimulate any systemic immune response in normal, nonimmunized mice. The lack of systemic B cell and T cell responses in normal hosts to enteric bacteria is not a result of tolerance, rather, the systemic immune system is naive or “innocent” to these antigens. In contrast, despite the lack of concomitant serum IgG or splenic T cell responses, a strong intestinal IgA response to half of the rIB was identified. All mice also demonstrated a strong intestinal IgA response to CBir1 and Fla-X flagellins, immunodominant commensal bacterial antigens present in the intestinal lumen (13). Intestinal IgA responses to these flagellins were detected in almost every individual mouse, and at a higher titer compared with intestinal IgA responses to cloned rIB. However, despite the strong mucosal immunogenicity and pathogenic potential of these flagellins, there was still no detectable serum IgG or systemic T cell response to them in normal mice. These data demonstrate that there is tight compartmentation of immune responses to microbiota antigens, which is restricted to the mucosal but not systemic immune system in normal mice. The systemic immune response remains naive to microbiota antigens rather than immunologically tolerant.
Treg cells in host response to microbiota
Multiple levels of regulation exist to maintain the intestinal immune homeostasis and control the compartmentation of immune responses to microbiota antigens. Among the multi-mechanisms, CD25+CD4+Foxp3+ regulatory T (Treg) cells, along with Tr1 cells, play an essential role in intestinal homeostasis (6, 14). Treg cells constitutively express high levels of the transcription factor Foxp3, which is considered to confer their development as well as their regulatory activity (15, 16). CD25+Foxp3+ Treg cells are not only generated in the thymus (termed natural Treg cells) but also can be induced in periphery (termed induced Treg cells) through antigen stimulation in the presence of TGF-β. Both natural and induced Treg cells are present in intestinal lamina propria (14). As mucosal dendritic cells have been shown as efficient for the conversion of Treg cells through a mechanism depending TGF-β and retinoic acid (17, 18), the intestine with its associated lymphoid tissue appears to be an important site for induction of CD25+Foxp3+ Treg cells from naive T cell precursors. The induced Treg cells are thought as a strategy that reinforces intestinal homeostasis (19, 20).
The mice with Foxp3 deficiency develop fatal multiorgan inflammation including chronic intestinal inflammation that can be suppressed by adoptively transferred CD25+Foxp3+ Treg cells (15, 21). Intestinal inflammation, among other organs, often occurs in patients with immune dysregulation, polyendocrinopathy, enteropathy, and X-linked (IPEX) syndrome (22, 23), which is caused by germline mutations in FOXP3, highlighting the importance of Treg cells in maintaining intestinal homeostasis in human. Treg cells can regulate both T cell responses and the activity of the innate immune system. Transfer of Treg cells not only inhibits experimental colitis induced by adoptive transfer of CD4+CD45RBhi naive T cells which react to the intestinal flora into immunodeficient mice or the colitis induced by innate immune activation, but also cures the established colitis (7, 14, 24, 25). Several independent mechanisms have been demonstrated to mediate the regulatory activity of Treg cells. Mice lacking anti-inflammatory cytokine IL-10 or TGF-β in T cells develop wasting disease and colitis when they are housed with microflora (26-28). Although Treg cells of spleen or MLN produce very little IL-10 under steady-state conditions, Treg cells in the colonic LP are the main CD4+ T cell population producing IL-10, as about one-third of the IL-10-producing CD4+ T cells are Foxp3+ (29). Treg cells produce TGF-β, and a T cell–specific deletion of TGF-β in mice results in colitis after 4 months of age (26), indicating a key role of Treg cell production of TGF-β in controlling intestinal homeostasis. It is currently unknown, however, why T cell–produced TGF-β and IL-10 play such a decisive role when TGF-β and IL-10 produced by other sources are readily available in the intestinal environment. Among many possibilities, TGF-β and IL-10 produced by Treg cells could function differently from that produced by other sources or be activated differently based on the niche they are located.
Role of IgA in intestinal homeostasis and immune protection
It has been shown that IgA, the most abundant antibody isotype in our body, plays a key role in intestinal immune protection in a noninflammatory manner (30, 31). Over 75% of the total immunoglobulin produced is IgA, and most IgA is secreted across mucosal membranes. Although IgA has been shown to participate in host responses against infection, the major role of IgA is to maintain a balance between the host and its microbiota, as SPF mice that have no pathogen exposure have abundant IgA whereas the mice have highly reduced intestinal IgA levels when they are housed under germ-free conditions (32, 33). IgA provides mucosal immune protection through interactions with the antibody transporter, polymeric Ig receptor (pIgR), which is expressed on the basolateral surface of epithelial cells (34, 35). Through binding to pIgR, secreted IgA dimers translocate to the surface of epithelial cells, thereby generating secretory IgA complexes that exhibit remarkable stability in the harsh environment of the gastrointestinal tract. Secretory IgA has been shown to play multiple protective roles (5) by promoting immune exclusion and downregulating the expression of proinflammatory bacterial epitopes on commensal bacteria (36, 37).
Although many studies on the functional importance of secretory IgA have been focused on its role in protection against mucosal infection, there is evidence indicating that IgA has a role in preventing commensal bacterial penetration or limiting the growth of bacteria and their densities in the lumen of the intestine. In IgA−/− mice and other strains which have low levels of IgA, there are serum IgG responses against commensal proteins, which does not occur in wild-type mice unless the commensal bacteria or their antigens are injected systemically, indicating that IgA is integral in limiting commensal bacterial penetration in the intestinal lumen (2, 31, 38). When germ-free wild-type mice and germ-free antibody-deficient JH−/− mice were colonized with commensal bacteria, both wild-type and JH−/− mice acquired the commensal bacteria within days. Notably, JH−/− mice showed increased penetration of commensals compared to wild-type mice. IgA secretion can also affect the composition of luminal bacteria, as supported by the overgrowth of anaerobic species in activation-induced deaminase (AID)-deficient mice, which lack class-switching recombination to IgA. Repletion of AID deficient animals with wild-type B cells, which are capable of producing IgA, could restore the flora composition (39).
IgA is not only protective against penetration of luminal bacteria, but also regulates systemic T cell responses to commensal bacterial antigens (38). Adoptively transferred CBir1 TCR transgenic (CBir1 Tg) T cells, which are specific for the immunodominant commensal bacterial antigen CBir1 flagellin, did not respond to CBir1 flagellin delivered mucosally, but did respond to systemic CBir1 stimulation in wild-type mice. In contrast, OT II T cells specific for OVA, which is not present in intestinal lumen, responded very well to gavaged OVA. High levels of intestinal IgA but not systemic IgG responses against CBir1 flagellin were detected in wild-type mice. When CFSE-labeled CBir1 Tg CD4+ T cells were adoptively transferred into IgA−/− mice followed by oral CBir1 flagellin challenge, CBir1 Tg CD4+ T cells proliferated well in IgA−/− mice but not in control wild-type mice, indicating that antigen-specific intestinal IgA plays a critical role in regulating CD4+ T cell responses to commensal bacterial antigens (38).
Treg cell regulation of intestinal IgA response
Although secretory IgA is crucial in host responses to commensal bacteria and their antigens, it is well known that selective IgA deficiency is extremely common in humans with only mild phenotypes (40), and the phenotype of IgA−/− mice is also very mild (41), suggesting that other levels of regulation exist for the prevention of inflammation in IgA−/− humans and mice. Among various subsets of T cells in the intestine, Treg cells are enriched in the intestinal lamina propria (6, 29, 42). Although both intestinal Treg cells and IgA are involved in intestinal homeostasis, and considering speculation that TGF-ß production by Treg cells might stimulate IgA responses, it is still largely unknown how Treg cell and secretory IgA pathways interact in the intestine in regulation of the host response to microbiota antigens.
Both T cell-dependent and independent IgA induction against commensal bacteria occurs in the intestine (2, 43). However, nude mice with very limited T cell numbers have reduced IgA levels. In TCRβ×δ−/− mice lacking both αβ and γδ T cells, the total levels of secretory IgA, as well as CBir1-specific intestinal IgA, are reduced to about one-fourth of the wild-type levels, even though the same anti-commensal specificities are generated (2, 38). These data argue that the T cell-dependent IgA response is likely predominant in the intestine. Multiple mechanisms regulate IgA production, including environmental factors, costimulation, and cytokines (3, 44). IL-2, IL-4, IL-5, IL-6 and IL-10 have been shown to make contributions to IgA production in in vitro cell culture systems (3). TGF-β is probably the most important cytokine that promotes IgA induction, as TGF-β−/− mice have low levels of IgA and there is almost a complete absence of IgA in mice deficient for TGF-β receptor II (45, 46). However, the cellular resources of TGF-β in promotion of intestinal IgA production are unclear.
To determine whether there is a role for CD25+ Treg cells in intestinal IgA responses, we depleted CD25+ Treg cells by injection of anti-CD25 monoclonal antibody (47). Depletion of CD25+ Treg cells resulted in a decrease of lamina propria IgA+ B cells as well as total commensal bacterial antigen-specific secretory IgA production, and anti-CBir1 flagellin-specific IgA. Consequently, adoptively transferred CBir1 Tg CD4+ T cells proliferated in response to gavaged CBir1 flagellin in mice administered with anti-CD25 mAb, which had much lower levels of CBir1-specific intestinal IgA, but not in mice administered with control antibody, which had normal levels of CBir1-specific IgA. Furthermore, adoptive transfer of CD25+ Treg cells or CD4+ Foxp3+ Treg cells restored CBir1-specific IgA production in TCRβ×δ−/− mice, which otherwise have low levels of intestinal IgA (38). These data indicate that CD25+ Treg cells promote intestinal IgA production, and both Treg cells and IgA contribute to control host responses to microbiota antigens. To define the mechanisms of how Treg cells promote intestinal IgA production, we cultured splenic IgD+ B cells with Foxp3+ Treg cells or Foxp3- T cells. Foxp3+ Treg cells stimulated IgA production and expression of mRNA encoding AID, which were blocked by anti-TGF-ß mAb. In contrast, culture of B cells with Foxp3− T cells only slightly upregulated B cell IgA and AID expression unless TGF-ß was added to the cultures (38), indicating that Treg cells promote B cell AID expression and IgA production through production of TGF-ß.
Concluding remarks
Recent studies demonstrate a tight compartmentation of immune responses to microbiota antigens, specifically that the systemic immune response remains naive to microbiota antigens rather than immunologically tolerant. Both Treg cells and IgA pathways regulate host responses to microbiota antigens. However, intestinal memory IgA responses show additive increases after each challenge but not a synergistic increase in strength (prime-boost effect), which is different from systemic memory IgG responses (48, 49). Intestinal IgA responses require the help of Treg cells which are usually involved in the negative regulation of other immune responses. The signals from Treg cells that help intestinal B cell IgA production may be qualitatively different from those signals delivered by helper T cells to B cells in nonintestinal follicles during systemic IgG responses. Therefore, the Treg cell-IgA axis could play a unique role in the maintenance of intestinal immune homeostasis.
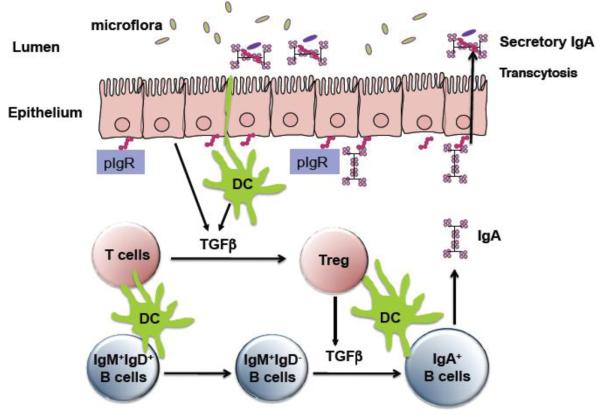
Figure 1. Treg cell-IgA axis maintains intestinal homeostasis to microbiota and protect the mice from intestinal inflammation.
Upon activation, Treg cells produce TGF-β which promotes B cell IgA class switching and IgA production in mucosa. IgA then binds polymeric Ig receptor (pIgR) on epithelial cells to be transported into intestinal lumen. The major role of IgA is to maintain a balance between the host and its microbiota.
Acknowledgments
This work was supported by research grants from NIH DK079918, AI083484, DK071176, and a start-up fund from University of Texas Medical Branch.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Strober W. The multifaceted influence of the mucosal microflora on mucosal dendritic cell responses. Immunity. 2009;31:377–388. doi: 10.1016/j.immuni.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 3.Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 5.Mestecky J, Russell MW, Elson CO. Intestinal IgA: novel views on its function in the defence of the largest mucosal surface. Gut. 1999;44:2–5. doi: 10.1136/gut.44.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cong Y, Weaver CT, Lazenby A, Elson CO. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J Immunol. 2002;169:6112–6119. doi: 10.4049/jimmunol.169.11.6112. [DOI] [PubMed] [Google Scholar]
- 7.Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–411. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 9.Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, Gordon JI. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003;299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 10.Kraehenbuhl JP, Corbett M. Immunology. Keeping the gut microflora at bay. Science. 2004;303:1624–1625. doi: 10.1126/science.1096222. [DOI] [PubMed] [Google Scholar]
- 11.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konrad A, Cong Y, Duck W, Borlaza R, Elson CO. Tight mucosal compartmentation of the murine immune response to antigens of the enteric microbiota. Gastroenterology. 2006;130:2050–2059. doi: 10.1053/j.gastro.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 13.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 15.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 17.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 22.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 23.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 24.Maloy KJ, Antonelli LR, Lefevre M, Powrie F. Cure of innate intestinal immune pathology by CD4+CD25+ regulatory T cells. Immunol Lett. 2005;97:189–192. doi: 10.1016/j.imlet.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Roers A, Siewe L, Strittmatter E, Deckert M, Schluter D, Stenzel W, Gruber AD, Krieg T, Rajewsky K, Muller W. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med. 2004;200:1289–1297. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr., Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 30.Mestecky J, Russell MW, Elson CO. Perspectives on mucosal vaccines: is mucosal tolerance a barrier? J Immunol. 2007;179:5633–5638. doi: 10.4049/jimmunol.179.9.5633. [DOI] [PubMed] [Google Scholar]
- 31.Macpherson AJ. The immune geography of IgA induction and function. Mucosal Immunology. 2007;1:12–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 32.Benveniste J, Lespinats G, Salomon J. Serum and secretory IgA in axenic and holoxenic mice. J Immunol. 1971;107:1656–1662. [PubMed] [Google Scholar]
- 33.Moreau MC, Ducluzeau R, Guy-Grand D, Muller MC. Increase in the population of duodenal immunoglobulin A plasmocytes in axenic mice associated with different living or dead bacterial strains of intestinal origin. Infect Immun. 1978;21:532–539. doi: 10.1128/iai.21.2.532-539.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandtzaeg P, Prydz H. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature. 1984;311:71–73. doi: 10.1038/311071a0. [DOI] [PubMed] [Google Scholar]
- 35.Mostov KE, Deitcher DL. Polymeric immunoglobulin receptor expressed in MDCK cells transcytoses IgA. Cell. 1986;46:613–621. doi: 10.1016/0092-8674(86)90887-1. [DOI] [PubMed] [Google Scholar]
- 36.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu Rev Immunol. 2010;28:243–273. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- 38.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424–1427. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- 40.Cunningham-Rundles C. Physiology of IgA and IgA deficiency. J Clin Immunol. 2001;21:303–309. doi: 10.1023/a:1012241117984. [DOI] [PubMed] [Google Scholar]
- 41.Harriman GR, Bogue M, Rogers P, Finegold M, Pacheco S, Bradley A, Zhang Y, Mbawuike IN. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J Immunol. 1999;162:2521–2529. [PubMed] [Google Scholar]
- 42.Makita S, Kanai T, Oshima S, Uraushihara K, Totsuka T, Sawada T, Nakamura T, Koganei K, Fukushima T, Watanabe M. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004;173:3119–3130. doi: 10.4049/jimmunol.173.5.3119. [DOI] [PubMed] [Google Scholar]
- 43.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki K, Fagarasan S. Diverse regulatory pathways for IgA synthesis in the gut. Mucosal Immunol. 2009;2:468–471. doi: 10.1038/mi.2009.107. [DOI] [PubMed] [Google Scholar]
- 45.Cazac BB, Roes J. TGF-beta receptor controls B cell responsiveness and induction of IgA in vivo. Immunity. 2000;13:443–451. doi: 10.1016/s1074-7613(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 46.Borsutzky S, Cazac BB, Roes J, Guzman CA. TGF-beta receptor signaling is critical for mucosal IgA responses. J Immunol. 2004;173:3305–3309. doi: 10.4049/jimmunol.173.5.3305. [DOI] [PubMed] [Google Scholar]
- 47.Liaudet L, Deb A, Pacher P, Mabley JG, Murthy KG, Salzman AL, Szabo C. The Flagellin-TLR5 Axis: Therapeutic Opportunities. Drug News Perspect. 2002;15:397–409. doi: 10.1358/dnp.2002.15.7.840075. [DOI] [PubMed] [Google Scholar]
- 48.Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, Geuking MB, Curtiss R, 3rd, McCoy KD, Macpherson AJ. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cerutti A. Immunology. IgA changes the rules of memory. Science. 2010;328:1646–1647. doi: 10.1126/science.1192488. [DOI] [PMC free article] [PubMed] [Google Scholar]