Abstract
Background
Human immunodeficiency virus (HIV)-infected persons are at risk for severe influenza infections. Although vaccination against the H1N1 pandemic influenza strain is recommended, currently, there are no data on the durability of post-vaccination antibody responses in this population.
Methods
HIV-infected and HIV-uninfected adults (18–50 years old) received a single dose of monovalent 2009 influenza A (H1N1) vaccine (strain A/California/7/2009H1N1). Antibody levels to the 2009 H1N1 pandemic strain were determined at day 0, day 28, and 6 months by hemagglutination-inhibition assay. A seroprotective response was a post-vaccination titer of ≥1:40 among those with a pre-vaccination level of ≤1:10. Geometric mean titers (GMT) and factors associated with higher levels were also evaluated.
Results
We studied 127 participants with a median age of 35 (interquartile range (IQR) 28, 42) years. Among the HIV-infected arm (n=63), the median CD4 count was 595 (IQR 476, 819) cells/mm3 and 83% were receiving HAART. Thirty-five percent of all participants had a pre-vaccination level of >1:10. HIV-infected compared to HIV-uninfected adults were less likely to generate a seroprotective response at day 28 (54% vs. 75%, adjusted OR 0.23, p=0.021) or have a durable response at 6 months post-vaccination (28% vs. 56%, adjusted OR 0.19, p=0.005). Additionally, although pre-vaccination GMT were similar in both arms (median 7 vs. 8, p=0.11), the GMT at 6 months was significantly lower among HIV-infected versus HIV-uninfected adults (median 20 vs. 113, p=0.003). Among HIV-infected persons, younger age (p=0.035) and receipt of HAART (p=0.028) were associated with higher GMTs at 6 months.
Conclusions
Despite vaccination, most HIV-infected adults do not have durable seroprotective antibody responses to the 2009 influenza A (H1N1) virus, and hence may remain vulnerable to infection. In addition to HAART use, more immunogenic vaccines are likely needed for improving protection against influenza in this population.
Keywords: influenza, pandemic 2009 H1N1, vaccine responses, HIV, durability, long-term immunity
Introduction
Studies have shown that a single dose of the 2009 influenza A (H1N1) monovalent vaccine is highly immunogenic among healthy adults [1–6]. Since immunocompromised persons are at higher risk for complicated influenza events [7–10], studies determining the immunogenicity of novel vaccines, including to the 2009 pandemic influenza strain, are important. After vaccination, influenza antibody levels peak in 2–4 weeks, and subsequently decline over time [11]. Studies on the short-term immunogenicity (21–28 days post-vaccination) of this new vaccine among human immunodeficiency virus (HIV)-infected persons have recently been published [12–14]; however, the durability of responses among this population remains unknown.
Durability data are important for several reasons. First, the influenza season typically lasts six months of each year (~November to April in the Northern Hemisphere). HIV-infected persons generate lower initial antibody levels after influenza vaccination compared to immunocompent hosts [12–18], but whether protective levels of antibody persist during the entire influenza season is unknown. Furthermore, data on the durability of responses could inform the optimal timing of vaccination; public health guidelines often suggest early (as soon as commercially available) influenza vaccine administration to increase opportunities for vaccination, but the ideal timing among immunosuppressed hosts is unclear. Second, since 2009 pandemic influenza infections may occur outside the typical “seasonal” months and circulate for many years with little genetic variation, information regarding durability of antibody levels over time is of clinical interest. Therefore, we evaluated HIV-infected and HIV-uninfected adults after vaccination with the monovalent 2009 influenza A (H1N1) vaccine to determine the durability of antibody responses generated against this novel strain.
Methods
Study Design
The study was designed to evaluate the immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine (strain A/California/7/2009(H1N1)pdm, Novartis Vaccines and Diagnostics Limited, Liverpool, UK); the vaccine manufacturer was not involved in the study in any capacity. The primary study objective was to compare seroconversion rates among HIV-infected and HIV-uninfected adults at day 28 [13]. Seroconversion was defined as a titer of ≥1:40 post-vaccination for the 2009 H1N1 pandemic strain among those with a pre-vaccination (day 0) level of ≤1:10 [19]. The sample size (n=132 participants) was based on the primary objective as previously described [13]. We utilized frequency matching to assure comparability between the study arms, creating four groups (each with 33 participants) stratified by HIV status (positive and negative) and age (18–34 and 35–50 years).
The current report presents data regarding the secondary study objective of durability of antibody responses at 6 months post-vaccination. Serum titers were evaluated for the primary endpoint of durability of seroprotection (titer of ≥1:40 at 6 months post-vaccination among those with a pre-vaccination level of ≤1:10). Secondary endpoints included seroprotection among all participants regardless of the pre-vaccination (day 0) antibody level and change in the geometric mean titers (GMT) between day 0 and 6 months. Additionally, day 28 results are presented to provide initial seroprotection rates and to contextualize the durability data.
In addition, we examined clinical events including influenza-like illnesses (ILI) and acute respiratory infections (ARI) during the 6-month follow-up. An ILI was defined as a temperature of >≥37.8°C and cough and/or sore throat in the absence of another known cause [20], whereas an ARI included any signs/symptoms indicative of a respiratory tract infection.
Study participants were enrolled between October 29 and December 2, 2009. HIV-infected participants had documented HIV infection (positive ELISA and Western Blot tests), while HIV-uninfected subjects had a negative HIV ELISA result ≤1 year before enrollment. Inclusion criteria for all participants were age 18–50 years, a military beneficiary, and without serious medical conditions, except for the diagnosis of HIV among the former group. Exclusion criteria included documented pregnancy or being ≤6 weeks postpartum, healthcare worker involved in direct patient care (due to higher risk of H1N1 exposure), acute febrile illness ≤30 days before vaccination, diabetes mellitus, systemic steroid or immunosuppressive medication use within four weeks of vaccination, cancer (except non-melanoma skin cancer), history of organ transplant, chronic active hepatitis B or C, current illicit drug use or alcohol abuse, blood transfusion within the last year, allergy to eggs, significant adverse reaction to a prior vaccination, receipt of another vaccination within ≤4 weeks other than seasonal influenza, or history of confirmed or suspected 2009 influenza A (H1N1) infection.
The study was approved by a central military institutional review board (IRB), conducted in accordance with the principles of the Declaration of Helsinki and standards of Good Clinical Practice (as defined by the International Conference on Harmonization), and registered with Clinicaltrials.gov (registration ID# NCT00996970).
Study and Laboratory Procedures
Vaccines were stored and administered in accordance with manufacturers’ guidelines. Subjects completed self-administered questionnaires at day 0 regarding past medical history, medication use, height and weight for body mass index (BMI), current cigarette use, history of self-reported influenza, seasonal influenza vaccinations within the last three years, and number of household members. At baseline, study coordinators also utilized medical records to collect relevant demographic and medical data including HIV history. Receipt of the seasonal 2009–2010 influenza vaccination was not part of the clinical study, but was recorded by study coordinators.
On a monthly basis during the study, coordinators contacted each participant via phone calls and reviewed subjects’ computerized records for new medical conditions (including ILI or ARI events) and medications. In addition, among HIV participants, CD4 cell counts and plasma HIV RNA levels were drawn on days 0 and 28 as part of the study; values obtained as part of clinical practice from day 28 to 6 months also were recorded.
Subjects with an ILI during the study were instructed to immediately present to study coordinators and have nasopharyngeal swabs obtained. Swabs were evaluated using rapid influenza antigen testing and real time reverse-transcriptase-polymerase chain reaction testing (rRT-PCR) for influenza (2009 H1N1 and seasonal strains) [21]. In addition, PCRs were performed for other select pathogens, including adenovirus, metapneumovirus, Mycoplasma pneumoniae, Streptococcus pneumoniae, Bordetella pertussis, Legionella pneumophilia, Chlamydophila pneumoniae, and respiratory syncytial virus (RSV). Samples were also tested for RNaseP to assure that adequate samples were obtained from each participant with an ILI [21]. All PCR testing was performed at the Naval Health Research Center, San Diego, CA. Medical records were also reviewed for ILI events for which the subject failed to present for study evaluation, and data were recorded regarding the diagnostic testing performed as part of standard clinical practice.
Serum samples for influenza-specific antibody responses were collected at day 0, day 28 (±4 days), and 6 months (± 4 weeks). Antibody responses were measured by hemagglutination-inhibition assay (HAI) [22, 23] using 1:2 serial dilutions, as previously described [24, 25]. Of note, HAI performed for a prior report [13] were repeated for this study such that all three study time points were performed simultaneously, and each subject’s samples were run on the same assay plate. In brief, HAI assays were performed at the Naval Medical Research Center, Silver Spring, MD, and conducted using 0.5% turkey erythrocytes. The swine origin A/California/7/2009 X-179A virus was obtained from the U.S. FDA and the reference antisera from the CDC. Sera were treated with receptor destroying enzyme, heme-adsorbed, and tested in duplicates in two independent assays, with the GMT of the four determinations reported as the final titer. For computational purposes, titers of <1:10 were assigned a value of 1:5 and those >1:1280 a value of 1:1280.
Statistical Methods
Descriptive statistics (medians with interquartile ranges, IQR, or counts with proportions) and unadjusted group comparisons (two-sample t-tests or Fisher’s exact tests) are presented. For the primary and secondary objectives, logistic regression for binary outcomes and linear regression for continuous outcomes were used. Adjusted models included age, race, number of seasonal influenza vaccinations in past three years, and number of household members. Similar analyses were performed post-hoc for those <35 years, those ≥35 years, and those who received the inactivated 2009–2010 seasonal influenza vaccine.
Exploratory analyses examining predictors of seroconversion among all subjects, HIV-infected subjects, and HIV-uninfected subjects were performed using simple logistic or linear regression models, as appropriate. Predictors considered included HIV status (among all subjects), demographics, BMI, number of household members, number of seasonal influenza vaccinations in past three years, and self-reported prior influenza illness. Among HIV-infected participants, additional variables included HIV duration (defined as the number of years from the first HIV positive date to study enrollment), baseline and nadir CD4 cell counts, HIV RNA level, and receipt of HAART. Reported p-values are two-sided, and values <0.05 were considered as statistically significant. All analyses were conducted using R (version 2.11.1, R Development Core Team).
Results
Study Population Characteristics
A total of 132 participants were enrolled (66 HIV-infected and 66 HIV-uninfected subjects), and 127 (96%) completed both the day 28 and month 6 follow-up visits. Loss to follow-up was due to single occurrences of military deployment, leaving the military system, incarceration, noncompliance with follow-up visit, and inadvertent receipt of a second H1N1 vaccine during study follow-up. There were no significant differences in sociodemographic factors or HIV status among those with and without follow-up visits.
Subjects with complete follow-up data had a median age of 35 (IQR 28, 42) years; 91% were male; 59% were Caucasian, 25% African American, and 16% other ethnicities (Table 1). Ninety-five percent had received the 2009–2010 seasonal influenza vaccine before or at enrollment and 69% had received seasonal vaccinations each year during the past three years. Thirteen percent self-reported a diagnosis of influenza during their lifetime. Among HIV-infected persons, the median duration of HIV infection was 7 years (IQR 2, 14), median CD4 cell count was 595 cells/mm3 (IQR 476, 819), 57% had an undetectable plasma HIV RNA level (<50 copies/ml), and 83% were receiving HAART at the time of vaccination.
Table 1.
Baseline Study Population Characteristics by HIV Status
| Factor | Total Cohort (N=127) | HIV-Infected Arm (n=63) | HIV-Uninfected Arm (n=64) | p-value1 |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (IQR) years | 35 (28, 42) | 36 (26, 45) | 35 (28,40) | 0.44 |
| Gender, N (%) male | 115 (91%) | 58 (92%) | 57 (89%) | 0.76 |
| Ethnicity, N (%) | 0.11 | |||
| Caucasian | 75 (59%) | 33 (52%) | 42 (66%) | |
| African American | 32 (25%) | 21 (33%) | 11 (17%) | |
| Other | 20 (16%) | 9 (14%) | 11 (17%) | |
| Clinical History | ||||
| BMI, median (IQR) kg/m2 | 27 (24, 29) | 26 (24, 29) | 27 (24, 28) | 0.86 |
| Current cigarette use, N (%) | 23 (18%) | 14 (22%) | 9 (14%) | 0.26 |
| History of influenza infection (self-reported), N (%) | 17 (13%) | 10 (16%) | 7 (11%) | 0.45 |
| Receipt of 2009–2010 seasonal influenza vaccine, N (%) | 121 (95%) | 58 (92%) | 63 (98%) | 0.12 |
| Type of 2009–2010 seasonal vaccine | <0.01 | |||
| Inactivated, N (%) | 103 (85%) | 57 (98%) | 46 (73%) | |
| Live, N (%) | 18 (15%) | 1 (2%) | 17 (27%) | |
| Number of seasonal influenza vaccines in past 3 years | <0.01 | |||
| <3 | 39 (31%) | 28 (44%) | 11 (17%) | |
| 3 | 88 (69%) | 35 (56%) | 53 (83%) | |
| Number of other household members | <0.01 | |||
| 0 | 35 (28%) | 24 (38%) | 11 (17%) | |
| 1 | 35 (28%) | 20 (32%) | 15 (23%) | |
| ≥2 | 57 (45%) | 19 (30%) | 38 (59%) | |
| Number of school-attendees in household | <0.01 | |||
| 0 | 85 (67%) | 53 (84%) | 32 (50%) | |
| ≥1 | 42 (33%) | 10 (16%) | 32 (50%) | |
| HIV History | ||||
| HIV duration, median (IQR) years | --- | 7.1 (2.0, 13.7) | --- | --- |
| CDC Stage, N (%) | --- | --- | --- | |
| A | 47 (75%) | |||
| B | 11 (17%) | |||
| C | 5 (8%) | |||
| Nadir CD4 cell count, median (IQR) cells/mm3 | --- | 299 (214, 434) | --- | --- |
| Nadir categories, N (%) | ||||
| <200 | --- | 12 (19%) | --- | --- |
| 200–349 | 26 (41%) | |||
| ≥350 | 25 (40%) | |||
| CD4 cell count at vaccination, median (IQR) cells/mm3 | --- | 595 (476, 819) | --- | --- |
| CD4 categories, N (%) | ||||
| <350 | --- | 6 (10%) | --- | --- |
| 350–499 | 14 (22%) | |||
| 500–749 | 23 (37%) | |||
| ≥750 | 20 (32%) | |||
| Plasma HIV RNA at vaccination, median (IQR) log10 copies/ml | --- | 1.7 (1.7, 2.4) | --- | --- |
| Plasma HIV RNA <50 copies/ml, N (%) | --- | 36 (57%) | --- | --- |
| Currently taking HAART, N (%) | --- | 52 (83%) | --- | --- |
Comparison of the HIV-infected and -uninfected subjects, from two-sample t-test or Fisher’s exact test, as appropriate
BMI, body mass index; GMT, geometric mean titer; HAART, highly active antiretroviral therapy; IQR, interquartile range; N, number
HIV-infected (n=63) and HIV-uninfected (n=64) subjects were similar in terms of demographics and influenza illness histories (Table 1). Although similar percentages received the 2009–2010 seasonal vaccination, HIV-infected persons were less likely to have received the live attenuated formulation and received fewer seasonal influenza vaccines in the last three years. In addition, HIV participants had fewer household members.
During the 6-month follow-up period, there were no differences in the number of medications initiated by study arm (p=0.56). Immunosuppressive agents were begun in two HIV-infected persons and one HIV-uninfected person (p=0.62), which consisted of oral prednisone for the diagnoses of thrombocytopenia, questionable autoimmune-related hearing loss, and cluster headaches, respectively. Among HIV-infected participants, four newly initiated HAART during the study follow-up period. Among both arms, four participants developed a medical condition which was part of the study’s exclusion criteria: immunosuppressant use (n=3) as described above, and a new diagnosis of alcoholism in an HIV-infected participant (n=1).
Durability of Antibody Responses to the 2009 H1N1 Pandemic Strain in HIV-Infected and HIV-Uninfected Adults
At baseline (day 0), 45 subjects (35%) had antibody titers of >1:10 by HAI, with marginally significant differences between HIV-infected (n=17, 27%) and HIV-uninfected (n=28, 44%) groups (p=0.06). A seroprotective antibody level (≥1:40) at day 28 among those with a baseline titer of ≤1:10 was achieved by significantly fewer HIV-infected (54%) compared to HIV-uninfected (75%) adults (adjusted OR 0.23, p=0.021, Table 2a). Likewise, seroprotection at 6 months post-vaccination was 81% less likely among HIV-infected (28%) compared to HIV-uninfected (56%) arms (adjusted OR 0.19, p=0.005).
Table 2a.
Antibody Responses to the Monovalent 2009 Influenza A (H1N1) Vaccine among HIV-Infected and HIV-Uninfected Persons
| Antibody Level | Total Cohort | HIV+ | HIV- | Unadjuste d p-value1 | Adjusted OR2 (95% CI) | Adjusted p-value2 |
|---|---|---|---|---|---|---|
|
Baseline/Day 0 | ||||||
| GMT ≤1:10 | 82/127 (65%) | 46/63 (73%) | 36/64 (56%) | 0.064 | --- | --- |
| GMT >1:10 | 45/127 (35%) | 17/63 (27%) | 28/64 (44%) | |||
|
Participants with Baseline Titer ≤1:10 (Seroconversion) | ||||||
| Day 28: Titer ≥1:40 | 52/82 (63%) | 25/46 (54%) | 27/36 (75%) | 0.067 | 0.23 (0.06, 0.76) | 0.021 |
| Month 6: Titer ≥1:40 | 33/82 (40%) | 13/46 (28%) | 20/36 (56%) | 0.023 | 0.19 (0.06, 0.59) | 0.005 |
|
All Participants Regardless of Baseline Titer (Seroprotection) | ||||||
| Day 28: Titer ≥1:40 | 95/127 (75%) | 42/63 (67%) | 53/64 (83%) | 0.042 | 0.23 (0.08, 0.62) | 0.005 |
| Month 6: Titer ≥1:40 | 74/127 (58%) | 27/63 (43%) | 47/64 (73%) | 0.001 | 0.18 (0.07, 0.42) | <0.001 |
|
All Participants with a Day 28 Titer ≥1:40 | ||||||
| Month 6: Titer ≥1:40 | 72/95 (76%) | 26/42 (62%) | 46/53 (87%) | 0.007 | 0.20 (0.06, 0.61) | 0.007 |
Seroprotection among all participants regardless of the baseline titer was noted among 67% of HIV-infected and 83% of HIV-uninfected adults at day 28 (adjusted OR 0.23, p=0.005), and among 43% and 73%, respectively, at 6 months post-vaccination (adjusted OR 0.18, p<0.001, Table 2a). Finally, for those who had an antibody titer of ≥1:40 at day 28, HIV-infected participants were less likely than HIV-uninfected participants to maintain a seroprotective antibody level (≥1:40) at 6 months (62% vs. 87%, adjusted OR 0.20, p=0.007).
Data were stratified by age and HIV arm for additional post-hoc analyses (Tables 2b and 2c). Among participants <35 years old, HIV-infected persons were less likely to have a seroprotective response at 6 months among all participants (adjusted OR 0.22, p=0.02) and among those with a day 28 seroprotective response (adjusted OR 0.12, p=0.01, Table 2b). Significant differences in seroprotection at 6 months were also seen among participants ≥35 years old (Table 2c).
Table 2b.
Antibody Responses to the Monovalent 2009 Influenza A (H1N1) Vaccine among Participants <35 Years Old
| Antibody Level | Total Cohort | HIV+ | HIV- | Unadjusted p-value1 | Adjusted OR2 (95% CI) | Adjusted p-value2 |
|---|---|---|---|---|---|---|
|
Baseline/Day 0 | ||||||
| GMT ≤1:10 | 39/62 (63%) | 23/31 (74%) | 16/31 (52%) | 0.114 | --- | --- |
| GMT >1:10 | 23/62 (37%) | 8/31 (26%) | 15/31 (48%) | |||
|
Participants with Baseline Titer ≤1:10 (Seroconversion) | ||||||
| Day 28: Titer ≥1:40 | 28/39 (72%) | 16/23 (70%) | 12/16 (75%) | 1.0 | 0.88 (0.14, 5.51) | 0.888 |
| Month 6: Titer ≥1:40 | 16/39 (41%) | 8/23 (35%) | 8/16 (50%) | 0.509 | 0.33 (0.06, 1.52) | 0.168 |
|
All Participants Regardless of Baseline Titer (Seroprotection) | ||||||
| Day 28: Titer ≥1:40 | 51/62 (82%) | 24/31 (77%) | 27/31 (87%) | 0.508 | 0.47 (0.08, 2.40) | 0.373 |
| Month 6: Titer ≥1:40 | 38/62 (61%) | 15/31 (48%) | 23/31 (74%) | 0.067 | 0.22 (0.06, 0.75) | 0.020 |
|
All Participants with a Day 28 Titer ≥1:40 | ||||||
| Month 6: Titer ≥1:40 | 37/51 (73%) | 14/24 (58%) | 23/27 (85%) | 0.058 | 0.12 (0.02, 0.57) | 0.013 |
Table 2c.
Antibody Responses to the Monovalent 2009 Influenza A (H1N1) Vaccine among Participants ≥35 Years Old
| Antibody Level | Total Cohort | HIV+ | HIV- | Unadjusted p-value1 | Adjusted OR2 (95% CI) | Adjusted p-value2 |
|---|---|---|---|---|---|---|
|
Baseline/Day 0 | ||||||
| GMT ≤1:10 | 43/65 (66%) | 23/32 (72%) | 20/33 (61%) | 0.434 | ||
| GMT >1:10 | 22/65 (34%) | 9/32 (28%) | 13/33 (39%) | |||
|
Participants with Baseline Titer ≤1:10 (Seroconversion) | ||||||
| Day 28: Titer ≥1:40 | 24/43 (56%) | 9/23 (39%) | 15/20 (75%) | 0.031 | 0.10 (0.01, 0.89) | 0.055 |
| Month 6: Titer ≥1:40 | 17/43 (40%) | 5/23 (22%) | 12/20 (60%) | 0.014 | 0.07 (0.01, 0.65) | 0.029 |
|
All Participants Regardless of Baseline Titer (Seroprotection) | ||||||
| Day 28: Titer ≥1:40 | 44/65 (68%) | 18/32 (56%) | 26/33 (79%) | 0.066 | 0.24 (0.05, 1.07) | 0.072 |
| Month 6: Titer ≥1:40 | 36/65 (55%) | 12/32 (38%) | 24/33 (73%) | 0.006 | 0.16 (0.03, 0.62) | 0.013 |
|
All Participants with a Day 28 Titer ≥1:40 | ||||||
| Month 6: Titer ≥1:40 | 35/44 (80%) | 12/18 (67%) | 23/26 (88%) | 0.128 | 0.36 (0.05, 2.25) | 0.287 |
Unadjusted p-value from Fisher’s exact test or T-test comparing HIV-infected with HIV-uninfected subjects.
Adjusted p-value from logistic regression comparing HIV-infected with HIV-uninfected subjects. Adjusted for age, race, number of seasonal influenza vaccinations in past three years, and number of household members.
CI, confidence interval; N, number; OR, odds ratio.
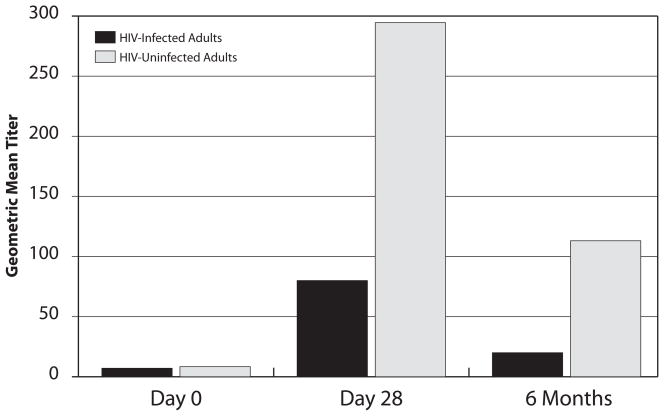
GMT data are shown in Table 3 for the three study time points. GMT were similar at baseline (day 0) among HIV-infected and HIV-uninfected participants. HIV-infected persons achieved significantly lower responses than HIV-uninfected persons at day 28 (median 80 vs. 295, p=0.003) and 6 months (median 20 vs. 113, p=0.003), respectively (Figure 1, Table 3). GMT responses post-vaccination among participants <35 years old were lower among HIV-infected vs. HIV-uninfected persons at day 28 (median 160 vs. 320, p=0.076) and at 6 months (median 28 vs. 113, p=0.026). Larger differences by HIV status were seen among those ≥35 years with median GMT among HIV-infected and HIV-uninfected adults of 57 and 226 (p=0.019) at day 28, and 18 and 80 (p=0.049) at 6 months, respectively (data not shown).
Table 3.
Geometric Mean Titers1 Generated to the 2009 Pandemic Influenza A (H1N1) Post-Vaccination among All Participants
| Time | Total Cohort | HIV-Infected Adults | HIV-Uninfected Adults | Unadjusted p-value2 |
|---|---|---|---|---|
| Day 0 | 7.1 (5.0, 20.0) | 7.1 (5.0, 13.0) | 8.4 (5.0, 29.6) | 0.11 |
| Day 28 | 160.0 (36.8, 589.1) | 80.0 (14.1, 320.0) | 294.5 (80.9, 670.3) | 0.003 |
| 6 Months | 56.6 (14.4, 160.0) | 20.0 (7.1, 80.0) | 113.1 (33.6, 190.3) | 0.003 |
Values shown are medians (interquartile ranges)
Unadjusted p-value from T test comparing HIV-infected with HIV-uninfected subjects; value is one-sided as per study design.
Figure 1.
Geometric Mean Titers to 2009 H1N1 Pandemic Strain after Vaccination among HIV-Infected and HIV-Uninfected Participants
We repeated all analyses examining only those participants who concurrently received the inactivated 2009–2010 seasonal influenza vaccine (n=103) and found similar results. We also performed an analysis (n=123) excluding those meeting one of the study’s exclusion criteria with similar results.
Influenza-Like Illnesses (ILI)
There were 16 ILI events in 13 subjects during the 6 month post-vaccination period, 11 of which occurred among HIV-uninfected persons (n = 9) and five among HIV-infected subjects (n = 4) (p=0.24). Testing for seasonal and 2009 H1N1 influenza viruses was conducted for 11 of the ILI events with negative results. Of note, all specimens had detectable RNaseP demonstrating that adequate specimens were obtained. Evaluation for other respiratory pathogens found one participant had RSV (HIV-infected) and one had metapneumovirus (HIV-uninfected); all other tests were negative for any pathogen. For five ILI events (3 in the HIV-infected and 2 in the HIV-uninfected arm), the participant did not present for nasopharyngeal testing.
The duration of ILI symptoms was a median of 8 days (IQR 5, 11) and the total numbers of days with ILI symptoms was not significantly different among HIV-infected and HIV-uninfected persons (p=0.69). All ILI events resolved, with four cases receiving empiric antibiotic agents and the remainder treated symptomatically; no subject received an antiviral medication. Among those with an ILI, nine subjects (six in the HIV-uninfected and three in the HIV-infected arms) had post-vaccination antibody levels of ≥1:40 at day 28, and six (five in the HIV-uninfected and one in the HIV-infected arms) at 6 months. We also examined ARI events: 75% of the HIV-uninfected group and 62% of the HIV-infected group reported an ARI during the study (p=0.13).
Factors Associated with Antibody Responses to the 2009 Influenza A (H1N1) Monovalent Vaccine
We performed exploratory analyses of factors associated with a durable antibody response, defined as a titer of ≥1:40 at 6 months among all participants with a baseline titer of ≤1:10. HIV-positive status was associated with not generating a durable response in the adjusted regression models (OR 0.19, 95% CI 0.06, 0.59, p=0.003). Age, race, history of influenza, household contacts, and number of prior seasonal vaccinations in the past three years were not predictive (Table 4). In addition, we examined factors in separate models for 1) a titer ≥1:40 at 6 months among all participants and 2) the difference in GMT between day 0 and 6 months, and found similar results (data not shown).
Table 4.
Factors Associated with Achieving Antibody Level >1:40 at 6 Months among All Participants with a Prevaccination level of <1:10
| Factor1 | Endpoint achieved (n=33) | Endpoint not achieved (n=49) | Adjusted OR (95% CI)2 | Adjusted p- value2 |
|---|---|---|---|---|
| HIV status | 0.003 | |||
| Positive | 13/33 (39%) | 16/49 (33%) | 0.19 (0.06, 0.59) | |
| Negative | 20/33 (61%) | 33/49 (67%) | 1.0 | |
| Age, years | 35 (30–40) | 35 (26–45) | 0.99 (0.93, 1.04 | 0.64 |
| Ethnicity | 0.27 | |||
| Caucasian | 21/33 (64%) | 28/49 (57%) | 1.0 | |
| African American | 8/33 (24%) | 11/49 (22%) | 1.81 (0.52, 6.48) | |
| Other | 4/33 (12%) | 10/49 (20%) | 0.47 (0.1, 1.82) | |
| Number of influenza vaccines in last three years | 0.29 | |||
| <3 | 10/33 (30%) | 13/49 (27%) | 1.0 | |
| 3 | 20/33 (70%) | 36/49 (73%) | 0.56 (0.18, 1.65) | |
| Other household members | 0.45 | |||
| 0–1 | 17/33 (52%) | 27/49 (55%) | 1.0 | |
| ≥2 | 16/33 (48%) | 22/49 (45%) | 0.67 (0.23, 1.86) |
Factors are number (percentages) or medians (interquartile ranges)
Adjusted p-values are from a multiple logistic regression model and adjusted for all the variables included in the table
CI, confidence interval; OR, odds ratio
Separate analyses were performed among HIV-infected persons for predictors of a higher change in GMT from day 0 to 6 months post-vaccination. Significant predictors for a greater change in GMT included younger age (regression coefficient of −6.9, p=0.04), a self-reported history of influenza before vaccination (regression coefficient 136, p=0.01), and receipt of HAART (regression coefficient 155.1, p=0.03). No other specific HIV factor was associated with change in GMT including duration of HIV infection, CD4 counts, or detectable HIV RNA level (Table 5). We also examined predictors for the GMT change among HIV-uninfected persons, but found no significant predictor after examining age, race, history of influenza, number of prior influenza vaccines, type seasonal influenza vaccine received in 2009–2010, or number of household members (data not shown).
Table 5.
Factors Associated with a Higher Change in GMT from Baseline to Month 6 Post-vaccination among HIV-Infected Adults
| Factor | Regression Coefficient (95% CI) | Adjusted p-value1 |
|---|---|---|
| Age, years | −6.86 (−13.21, −0.52) | 0.035 |
| Gender, female | 66.44 (−107.85, 240.73) | 0.447 |
| Ethnicity | 0.987 | |
| Caucasian | --- | |
| African American | 1.61 (−85.02, 88.25) | |
| Other | −8.02 (−120.92, 104.87) | |
| BMI | 0.840 | |
| Normal | --- | |
| Overweight | −21.72 (−103.51, 60.08) | |
| Obese | 1.51 (−110.94, 113.97) | |
| Self-reported history of prior influenza | 135.87 (33.46, 238.29) | 0.010 |
| Number of seasonal influenza vaccines in last 3 years | ||
| <3 | --- | |
| 3 | −68.21 (−140.77, 4.36) | 0.065 |
| Number of other household members | ||
| 0–1 | --- | |
| ≥2 | −27.33 (−114.42, 59.96) | 0.533 |
| HIV duration, years | 6.7 (−1.74, 15.14) | 0.117 |
| Nadir CD4 cell count, cells/mm3 | 0.19 (−0.17, 0.56) | 0.286 |
| CD4 cell count at vaccination, cells/mm3 | −0.04 (−0.22, 0.15) | 0.693 |
| Plasma HIV RNA level ≥50 copies/ml | 73.41 (−21.94, 168.76) | 0.128 |
| Receipt of HAART post-vaccination | 155.14 (17.39, 292.89) | 0.028 |
Adjusted p-values are from a multiple linear regression model and adjusted for all the variables included in the table
CI, confidence interval; HAART, highly active antiretroviral therapy
Effect of Vaccination on Plasma HIV RNA Level and CD4 Cell Count among HIV-infected Persons
All HIV-infected participants had CD4 and HIV RNA levels drawn at days 0 and 28, and 55 (87%) had at least one follow-up HIV RNA and CD4 count between day 28 and 6 months. Of note, four patients initiated HAART during the study period and were excluded from these analyses. The median change in the plasma log10 HIV RNA level was 0.0 copies/ml (IQR −0.1, 0; p=0.96) and median change in CD4 cell count was 12 cells/mm3 (IQR −92, 88; p=0.82) between days 0 and 28. Changes were also not significant between day 28 and the remainder of the study follow-up time (up to 6 months post-vaccination): HIV RNA level (0 copies/ml/month, IQR 0, 0; p=0.28) and CD4 cell count (−0.7 cells/mm3/month, IQR −31, 29; p=0.42). Finally, there were also no significant differences in the proportions of participants with a detectable HIV RNA level (defined as ≥50 copies/ml) between day 0 and day 28 (41% vs. 35%, p=0.37) or between day 28 and 6 months (35% vs. 31%, p=0.41).
Tolerability
No known long-term adverse events were ascribed to vaccination in either arm. A single case of angioedema (HIV-negative participant) occurred, but all symptoms resolved without sequelae [13]. Two additional events among HIV-infected subjects were reported to the Vaccine Adverse Event Reporting System (VAERS), although both were deemed unrelated to vaccination upon review by the Healthcare Vaccine Center. These included unilateral sensorineural hearing loss of unclear etiology, which occurred 47 days post-vaccination and was treated with intra-ear steroid injections, and transient speech difficulties occurring 56 days post-vaccination, which self-resolved and were later diagnosed as a conversion disorder. There were eight hospitalizations during follow-up (seven in HIV-infected and one in HIV-uninfected subjects), but none related to vaccination.
Discussion
Our study found that HIV-infected adults are significantly less likely to generate and maintain antibody responses to 2009 influenza A (H1N1) vaccine compared to HIV-uninfected adults. Only 28% of HIV-infected persons with baseline seronegative titers had an antibody level ≥1:40 by 6 months post-vaccination. These data suggest that HIV-infected persons may remain susceptible to the 2009 H1N1 pandemic influenza virus despite vaccination, suggesting that novel strategies to protect this population may be needed.
The introduction of a novel H1N1 influenza strain worldwide among persons without preexisting protective antibodies resulted in a pandemic with a reported 61 million cases in the U.S. [26]. Persons with comorbidities, including those with HIV, are at higher risk of seasonal influenza-related complications [7–10]. Although it is uncertain if clinical presentation of H1N1 pandemic influenza is altered among HIV-infected persons, the presence of comorbidities has been linked to severe infections [27–29], emphasizing the importance of preventive strategies, foremost vaccination [30].
Prior studies have shown that HIV-infected adults and other immunocompromised hosts may fail to generate initial protective antibody levels to seasonal influenza vaccination [15–18, 31–35]. Regarding responses to the novel 2009 influenza A (H1N1) vaccine, recent studies found that only ~60% had an initial seroprotective response (days 21–28 post-vaccination) despite evaluating HIV adults with median CD4 counts of >500 cells/mm3 [12–14]. Since HIV patients appear to generate reduced levels of antibodies after influenza vaccination compared with healthy adults, the duration of immunity might also be limited [36].
Durability of antibody responses is important since the influenza season often spans an estimated 6 months [30]. Furthermore, 2009 pandemic H1N1 infections may occur during non-seasonal months necessitating prolonged immunity [26]. Data on the durability of influenza antibodies after seasonal vaccination among the general population suggest that protection lasts for at least one year [11, 37–43], but data among HIV-infected adults are limited, as most studies only report ~30 day follow-up [12–15, 17, 34], and no durability data currently exist regarding vaccination with the 2009 H1N1 pandemic strain.
Our study found that the majority of HIV-infected adults lacked ‘protective antibody’ responses (i.e., ≥1:40) at 6 months and were less likely to have a durable antibody response compared to HIV-uninfected adults. These findings were noted despite comparing HIV-infected and HIV-uninfected persons with similar ages, lack of comorbidities (expect for HIV), and similar sociodemographic features. Potential reasons for poorer vaccine responses among HIV-infected persons despite preserved CD4 counts are unknown, but may include unmeasured impaired cellular function, immune activation, immunosenescence, and/or altered expression of B cell homing molecules which could impair T cell-dependent B cell responses. Of note, HIV patients with a titer ≥1:40 at day 28 were more likely to have a durable antibody response, similar to data regarding seasonal influenza protection in the general population [44].
These data are informative regarding the immunogenicity of current influenza preparations for HIV-infected persons and suggest the need for novel strategies, such as the use of additional vaccine doses, higher doses, or adjuvants, to better protect this population. Recent data suggest that those ages ≥65 years should receive a “high-dose” influenza vaccine containing larger amounts of antigen [37]. Whether or not HIV-infected persons and other immunocompromised hosts should also be advised to receive higher doses of vaccine components is currently unknown. Regarding the administration of >1 vaccine dose, prior studies examining seasonal influenza vaccinations in HIV patients failed to find a benefit from this strategy [16, 31], although no published data exist regarding 2009 influenza A (H1N1) vaccine. We hope our study results will provide a framework for the future design and conduct of studies on protecting vulnerable populations from influenza.
In addition, our data may inform clinicians caring for HIV-infected persons regarding potential waning immune status during the latter months of the influenza season. Our study findings also may be important in determining the impact of expanding vaccination windows earlier in the season. While initiation of vaccinations earlier in the respiratory season may increase opportunities for vaccination, this should be balanced with what is known regarding the durability of immunity such that vaccinations for vulnerable populations are strategically timed.
Among HIV-infected persons, predictors for greater post-vaccination antibody responses included younger age, similar to other reports [3]. Furthermore, we noted that HAART use resulted in higher influenza GMTs. Prior studies have emphasized the benefit of HAART on vaccine responses [17, 45, 46], and our data demonstrate that HAART has a positive effect on maintaining higher antibody levels over time. Our finding that a history of self-reported influenza was associated with higher vaccine responses is interesting, but requires confirmation by other studies, especially since this association was only noted in the HIV-infected arm and our population consisted of persons ≤50 years of age [19].
Our study had limitations. First, antibody level thresholds may not be exact correlates of protection. For example, despite a seroprotective antibody level, immunosuppressed patients may remain at risk for influenza due to cellular or other immune deficits. On the other hand, despite low antibody levels, patients may be protected against influenza as suggested by a recent vaccine trial among HIV patients in Africa [15, 33, 47]. Our study used standard HAI methodology to define seroresponses, since it is the most consistent correlate of immunity to influenza infection [11, 41]; however, studies examining the validity of antibody levels for both pandemic and seasonal influenza strains among HIV cohorts are needed [44]. Second, we could not define the protective efficacy of the vaccination, as our study was not powered for this outcome and the incidence of H1N1 influenza cases dramatically decreased during the study period. On a positive note, the lack of influenza illnesses in our study population likely reduced confounding regarding the potential effects of intercurrent influenza infections on antibody levels. Finally, similar to other studies, the precise etiology of ILI was often unknown, despite an extensive evaluation for a number of bacterial and viral pathogens using state-of-the-art PCR technology [48, 49].
To our knowledge, this study is the first published report examining the durability of the 2009 influenza A (H1N1) vaccine among HIV-infected persons. Despite evaluating a cohort of early-diagnosed and -treated HIV-infected adults with high CD4 counts and few comorbidities, the majority of HIV patients had low antibody levels at 6 months post-vaccination. These data suggest that a significant proportion of HIV-infected adults may remain vulnerable to influenza despite vaccination, which may have important implications for both HIV care and public health.
Acknowledgments
We express our gratitude to those assisting with study conduct including Brian Agan MD, Mary Bavaro MD, Michelle Linfesty, Connor Eggleston, Barbara Nagaraj, Sara Echols RN, Sheila Medina MPH, Erin McDonough, Jean Vita, Heather Hairston, and Anna Mason. Additionally, we express gratitude to Maryna Eichelberger PhD and Kathy Hancock PhD for assistance with HAI assay development.
Support for this work (IDCRP-053) was provided by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed through the Uniformed Services University of the Health Sciences. This project has been funded in whole, or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement Y1-AI-5072. In addition, funding was provided by the Armed Forces Health Surveillance Center’s Global Emerging Infections Surveillance and Response System via project I204_10.
The authors acknowledge that research protocol (“Immunogenicity of Novel H1N1 Vaccination among HIV-Infected Compared to HIV-Uninfected Persons”, IDCRP-000-53) received applicable Institutional Review Board review and approval. We certify that all individuals who qualify as authors have been listed; each has participated in the conception and design of this work, the analysis of data, the writing of the document, and the approval of the submission of this version; that the document represents valid work; that if we used information derived from another source, we obtained all necessary approvals to use it and made appropriate acknowledgements in the document; and that each takes public responsibility for it. Nothing in the presentation implies any Federal/DOD/DON endorsement.
Footnotes
Conflict of Interest: None. The authors have no financial interest in this work or the vaccine evaluated in this clinical trial. All authors contributed to the content of the manuscript and concurred with the decision to submit it for publication.
The content of this publication is the sole responsibility of the authors and does not necessarily reflect the views or policies of the NIH or the Department of Health and Human Services, the DoD or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
This work is original and has not been published elsewhere.
Author Contributions: All authors have reviewed and approved this manuscript.
Nancy Crum-Cianflone, Erik Iverson, and Lynn Eberly had full access to all the data and take responsibility for the accuracy of the data.
Study concept and design: Crum-Cianflone
Acquisition of the data: Crum-Cianflone, Defang, Blair, Maguire, Ganesan, Faix, Duplessis, Lalani, Whitman, Brandt, Burgess
Study statisticians: Iverson, Eberly
Drafting of the manuscript: Crum-Cianflone, Defang, Iverson
Critical review of the manuscript: Blair, Maguire, Ganesan, Eberly, Faix, Duplessis, Lalani, Whitman, Macalino, Brandt, Millar, Burgess
Obtaining funding: Crum-Cianflone, Macalino, Millar, Defang, Burgess, Faix, Blair
Administrative or technical support: Crum-Cianflone, Macalino, Millar, Defang, Burgess, Faix, Blair
Study supervision: Crum-Cianflone
References
- 1.Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361:2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, et al. Response after one dose of a monovalent influenza A (H1N1) 2009 vaccine – preliminary report. N Engl J Med. 2009;361:2405–2413. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 3.Zhu FC, Wang H, Fang HH, Yang JG, Lin XJ, Liang XF, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med. 2009;361:2414–2423. doi: 10.1056/NEJMoa0908535. [DOI] [PubMed] [Google Scholar]; Nolan T, McVernon J, Skeljo M, Richmond P, Wadia U, Lambert S, et al. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: A randomized trial. JAMA. 2010;303:37–46. doi: 10.1001/jama.2009.1911. [DOI] [PubMed] [Google Scholar]
- 4.Liang XF, Wang HQ, Wang JZ, Fang HH, Wu J, Zhu FC, et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2010;375:56–66. doi: 10.1016/S0140-6736(09)62003-1. [DOI] [PubMed] [Google Scholar]
- 5.Plennevaux E, Sheldon E, Blatter M, Reeves-Hoché MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet. 2010;375:41–48. doi: 10.1016/S0140-6736(09)62026-2. [DOI] [PubMed] [Google Scholar]
- 6.Vajo Z, Tamas F, Sinka L, Jankovics I. Safety and immunogenicity of a 2009 pandemic influenza A H1N1 vaccine when administered alone or simultaneously with the seasonal influenza vaccine for the 2009–10 influenza season: a multicentre, randomised controlled trial. Lancet. 2010;375:49–55. doi: 10.1016/S0140-6736(09)62039-0. [DOI] [PubMed] [Google Scholar]
- 7.Neuzil KM, Reed GW, Mitchel EF, Jr, Griffin MR. Influenza-associated morbidity and mortality in young and middle-aged women. JAMA. 1999;281:901–907. doi: 10.1001/jama.281.10.901. [DOI] [PubMed] [Google Scholar]
- 8.Kunisaki K, Janoff E. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009;9:493–504. doi: 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JC, Nichol KL. Excess mortality due to pneumonia or influenza during influenza seasons among person with acquired immunodeficiency syndrome. Arch Intern Med. 2001;161:441–446. doi: 10.1001/archinte.161.3.441. [DOI] [PubMed] [Google Scholar]
- 10.Safrin S, Rush JD, Mills J. Influenza in patients with human immunodeficiency virus infection. Chest. 1990;98:33–37. doi: 10.1378/chest.98.1.33. [DOI] [PubMed] [Google Scholar]
- 11.Couch RB, Kasel JA. Immunity to influenza in man. Ann Rev Microbiol. 1983;37:529–549. doi: 10.1146/annurev.mi.37.100183.002525. [DOI] [PubMed] [Google Scholar]
- 12.Tebas P, Frank I, Lewis M, Quinn J, Zifchak L, Thomas A, et al. Center for AIDS Research and Clinical Trials Unit of the University of Pennsylvania. Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS. 2010;24:2187–2192. doi: 10.1097/QAD.0b013e32833c6d5c. [DOI] [PubMed] [Google Scholar]
- 13.Crum-Cianflone NF, Eberly L, Duplessis C, Maguire J, Ganesan A, Faix D, et al. Immunogenicity of a Monovalent 2009 Influenza A (H1N1) Vaccine in an Immune Compromised Population: A Prospective Study Comparing HIV-Infected to HIV-Uninfected Adults. Clin Infect Dis. 2010 doi: 10.1093/cid/ciq019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bickel M, Wieters I, Khaykin P, Nisius G, Haberl A, Stephan C, et al. Low rate of seroconversion after vaccination with a split virion, adjuvanted pandemic H1N1 influenza vaccine in HIV-1-infected patients. AIDS. 2010;24:F31–35. doi: 10.1097/QAD.0b013e3283398da1. [DOI] [PubMed] [Google Scholar]
- 15.Tasker SA, Treanor JJ, Paxton WB, Wallace MR. Efficacy of influenza vaccination in HIV-infected persons. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;131:430–433. doi: 10.7326/0003-4819-131-6-199909210-00006. [DOI] [PubMed] [Google Scholar]
- 16.Kroon FP, van Dissel JT, de Jong JC, Zwinderman K, van Furth R. Antibody response after influenza vaccination in HIV-infected individuals: a consecutive 3-year study. Vaccine. 2000;18:3040–3049. doi: 10.1016/s0264-410x(00)00079-7. [DOI] [PubMed] [Google Scholar]
- 17.Kroon FP, Rimmelzwaan GF, Roos MT, Osterhaus AD, Hamann D, Miedema F, et al. Restored humoral immune response to influenza vaccination in HIV-infected adults treated with highly active antiretroviral therapy. AIDS. 1998;12:F217–223. doi: 10.1097/00002030-199817000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Anema A, Mills E, Montaner J, Brownstein JS, Cooper C. Efficacy of influenza vaccination in HIV-positive patients: a systematic review and meta-analysis. HIV Medicine. 2008;9:57–61. doi: 10.1111/j.1468-1293.2008.00515.x. [DOI] [PubMed] [Google Scholar]
- 19.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) [Accessed March 16, 2010];Updated Interim Recommendations–HIV-Infected Adults and Adolescents: Considerations for Clinicians Regarding 2009 H1N1 Influenza. http://www.cdc.gov/h1n1flu/guidance_hiv.htm.
- 21. [Accessed on June 11, 2009];CDC protocol of realtime RTPCR for influenza A (H1N1) http://www.who.int/csr/resources/publications/swineflu/realtimeptpcr/en/index.html.
- 22.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972;70:767–777. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 2004;103:133–138. doi: 10.1016/j.virusres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 24.Kendal AP, Pereira MS, Skehel JJ. Concepts and procedures for laboratory-based influenza surveillance. Geneva: World Health Organization; 1982. [Google Scholar]
- 25.Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–943. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CDC. Updated CDC Estimates of 2009 H1N1 Influenza Cases, Hospitalizations and Deaths in the United States, April 2009 – April 10, 2010. [Accessed August 1, 2010]; http://www.cdc.gov/h1n1flu/estimates_2009_h1n1.htm.
- 27.Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, et al. California Pandemic (H1N1) Working Group. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302:1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira W, Carmo E, Penna G, Kuchenbecker R, Santos H, Araujo W, et al. Surveillance Team for the pandemic influenza A(H1N1) 2009 in the Ministry of Health. Pandemic H1N1 influenza in Brazil: analysis of the first 34,506 notified cases of influenza-like illness with severe acute respiratory infection (SARI) Euro Surveill. 2009;14:19362. doi: 10.2807/ese.14.42.19362-en. [DOI] [PubMed] [Google Scholar]
- 29.Riera M, Payeras A, Marcos MA, Viasus D, Farinas MC, Segura F, et al. Clinical presentation and prognosis of the 2009 H1N1 influenza A infection in HIV-1-infected patients: a Spanish multicenter study. AIDS. 2010;24:2461–2467. doi: 10.1097/QAD.0b013e32833e508f. [DOI] [PubMed] [Google Scholar]
- 30.Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Centers for Disease Control and Prevention (CDC) Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. [PubMed] [Google Scholar]
- 31.Miotti PG, Nelson KE, Dallabetta GA, Farzadegan H, Margolick J, Clements ML. The influence of HIV infection on antibody responses to a two-dose regimen of influenza vaccine. JAMA. 1989;262:779–783. [PubMed] [Google Scholar]
- 32.Glesby MJ, Hoover DR, Farzadegan H, Margolick JB, Saah AJ. The effect of influenza vaccination on human immunodeficiency virus type 1 load: a randomized, double-blind, placebo-controlled study. J Infect Dis. 1996;174:1332–1336. doi: 10.1093/infdis/174.6.1332. [DOI] [PubMed] [Google Scholar]
- 33.Yamanaka H, Teruya K, Tanaka M, Kikuchi Y, Takahashi T, Kimura S, et al. HIV/Influenza Vaccine Study Team. Efficacy and immunologic responses to influenza vaccine in HIV-1-infected patients. J Acquir Immune Defic Syndr. 2005;39:167–173. [PubMed] [Google Scholar]
- 34.Fuller JD, Craven DE, Steger KA, Cox N, Heeren TC, Chernoff D. Influenza vaccination of human immunodeficiency virus (HIV)-infected adults: impact on plasma levels of HIV type 1 RNA and determinants of antibody response. Clin Infect Dis. 1999;28:541–547. doi: 10.1086/515170. [DOI] [PubMed] [Google Scholar]
- 35.Durando P, Fenoglio D, Boschini A, Ansaldi F, Icardi G, Sticchi L, et al. Safety and immunogenicity of two influenza virus subunit vaccines, with or without MF59 adjuvant, administered to human immunodeficiency virus type 1-seropositive and -seronegative adults. Clin Vaccine Immunol. 2008;15:253–259. doi: 10.1128/CVI.00316-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miotti PG, Nelson KE, Dallabetta GA, Farzadegan H, Margolick J, Clements ML. The influence of HIV infection on antibody responses to a two-dose regimen of influenza vaccine. JAMA. 1989;262:779–783. [PubMed] [Google Scholar]
- 37.Center for Disease Control and Prevention. [Access October 20, 2010];Inactivated influenza vaccine: what you need to know. 2010–2011 Available at: http://www.cdc.gov/vaccines/pubs/vis/default.htm#flu.
- 38.Foy HM, Cooney MK, McMahan R. A Hong Kong influenza immunity three years after immunization. JAMA. 1973;226:758–761. [PubMed] [Google Scholar]
- 39.Lerman SJ, Wright PF, Patil KD. Antibody decline in children following A/New Jersey/76 influenza virus immunization. J Pediatr. 1980;96:271–274. doi: 10.1016/s0022-3476(80)80823-7. [DOI] [PubMed] [Google Scholar]
- 40.Ambrose CS, Yi T, Walker RE, Connor EM. Duration of protection provided by live attenuated influenza vaccine in children. Pediatr Infect Dis J. 2008 Aug;27(8):744–748. doi: 10.1097/INF.0b013e318174e0f8. [DOI] [PubMed] [Google Scholar]
- 41.Clark A, Potter CW, Jennings R, Nicholl JP, Langrick AF, Schild GC, et al. A comparison of live and inactivated influenza A (H1N1) virus vaccines. 2. Long-term immunity. J Hyg (Lond) 1983;90:361–370. doi: 10.1017/s0022172400028990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cate TR, Couch RB, Parker D, Baxter B. Reactogenicity, immunogenicity, and antibody persistence in adults given inactivated influenza virus vaccines - 1978. Rev Infect Dis. 1983;5:737–747. doi: 10.1093/clinids/5.4.737. [DOI] [PubMed] [Google Scholar]
- 43.Noble GR, Kaye HS, O’Brien RJ, Kendal AP, Bregman DJ, Wright PF, et al. Persistence of influenza A/New Jersey/76 (Hsw1N1) antibody one year after vaccination. Dev Biol Stand. 1977;39:253–260. [PubMed] [Google Scholar]
- 44.Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis. 2008;197:490–502. doi: 10.1086/524146. [DOI] [PubMed] [Google Scholar]
- 45.Landrum ML, Huppler Hullsiek K, Ganesan A, Weintrob AC, Crum-Cianflone NF, Barthel RV, et al. Hepatitis B vaccine responses in a large U.S. military cohort of HIV-infected individuals: another benefit of HAART in those with preserved CD4 count. Vaccine. 2009;27:4731–4738. doi: 10.1016/j.vaccine.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Søgaard OS, Schønheyder HC, Bukh AR, Harboe ZB, Rasmussen TA, Ostergaard L, et al. Pneumococcal conjugate vaccination in persons with HIV: the effect of highly active antiretroviral therapy. AIDS. 2010;24:1315–1322. doi: 10.1097/QAD.0b013e328339fe0b. [DOI] [PubMed] [Google Scholar]
- 47.Madhi SA, Maskew M, Koen A, Kuwanda L, Besselaar TG, Naidoo D, et al. Trivalent inactivated influenza vaccine in African adults infected with human immunodeficient virus: double blind, randomized clinical trial of efficacy, immunogenicity, and safety. Clin Infect Dis. 2011;52:128–137. doi: 10.1093/cid/ciq004. [DOI] [PubMed] [Google Scholar]
- 48.Metzgar D, Baynes D, Myers CA, Kammerer P, Unabia M, Faix DJ, et al. Initial identification and characterization of an emerging zoonotic influenza virus prior to pandemic spread. J Clin Microbiol. 2010;48:4228–4234. doi: 10.1128/JCM.01336-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hymas WC, Mills A, Ferguson S, Langer J, She RC, Mahoney W, et al. Development of a multiplex real-time RT-PCR assay for detection of influenza A, influenza B, RSV and typing of the 2009-H1N1 influenza virus. J Virol Methods. 2010;167:113–118. doi: 10.1016/j.jviromet.2010.03.020. [DOI] [PubMed] [Google Scholar]