Abstract
Neutral sphingomyelinases (N-SMases) are considered to be key mediators of stress-induced ceramide production. The extended family of N-SMases is a subset of the DNaseI superfamily and comprises members from bacteria, yeast and mammals. In recent years, the identification and cloning of mammalian N-SMase family members has led to significant advances in understanding their physiological roles and regulation. However, there is still limited information on their regulation at the biochemical and molecular level. In this review, we summarize current knowledge about the biochemical regulation of the eukaryotic N-SMases and identify the major areas where knowledge is lacking.
Introduction
Sphingomyelinases (SMases) mediate the hydrolysis of sphingomyelin (SM) to produce ceramide and, as such, are considered major pathways of ceramide production in the cellular responses to stress and cytokines. There are three major classes of SMase divided into acid, neutral, and alkaline according to the pH optima of their activity. Of these, acid SMase exists as both a lysosomal and secreted enzyme, and deficiency in its activity causes Niemann-Pick disease whereas alkaline SMase is secreted in the gut and is thought to break down dietary sphingomyelin. In contrast, neutral SMases exist as a number of closely related isoforms.
Neutral SMase activity was first characterized in cells from Niemann-Pick patients that are deficient in acid SMase but retained a neutral activity (Schneider & Kennedy, 1967). However, it took some 20 years before the identification of the first cloned N-SMase from bacteria (Coleman et al., 1986; Yamada et al., 1988) and a decade further still before the identification of nSMase1 – the first cloned mammalian N-SMase in 1998 (Tomiuk et al., 1998). Following this, the N-SMase homologue ISC1 from S. cervisiae was identified (Sawai et al., 2000) along with its fission yeast (Schizopombe) counterpart CSS1 (Feoktistova et al., 2001). The mammalian nSMase2 was identified and cloned in 2000 (Hofmann et al., 2000) and, more recently, the mammalian nSMase3 was cloned and characterized in 2006 (Krut et al., 2006). Finally, a mitochondrial N-SMase was isolated from zebrafish in 2009 (Yabu et al., 2009) followed by the mammalian counterpart – mitochondria-associated N-SMase (MA-nSMase) (Wu et al., 2010). The majority of these cloned N-SMases belong to an extended N-SMase family – a sub-group of the DNaseI superfamily (see Clarke et al., 2006). The exception to this is nSMase3 which does not bare much significant homology to the others, but instead appears to be highly conserved between higher and lower mammals and, consequently, may comprise a distinct family of N-SMases in its own right (Krut et al., 2006). As the focus of this review is on the N-SMase family itself, nSMase3 will not be discussed.
With the identification of multiple cloned N-SMases, and the development of knockout animals for some of the cloned enzymes, research into the physiological roles and regulations of N-SMases is at a crucial juncture. However, the regulation of the cloned N-SMases at the biochemical level must also not be neglected. In this review, we will summarize current knowledge of the biochemical regulation of the eukaryotic N-SMase family members, highlighting the common traits across the family and discussing the gaps in our current knowledge.
Domain Structure
All members of the extended N-SMase family are characterized by a set of conserved catalytic core residues leading to the suggestion that the enzymes catalyze hydrolysis of SM by a common mechanism. However, beyond the common catalytic core of the N-SMases, there is surprisingly little identity between family members. For example, the yeast N-SMases ISC1 and CSS1 possess about 35% identity to each other, whereas ISC1 possesses about 28% homology with mammalian nSMase1 and nSMase2. In contrast, nSMase2 is about 20% identical to bacterial SMases and 23% homologous to nSMase1 (see Clarke et al., 2006). The conserved catalytic core of the N-SMase family defines the catalytic domains of each member (Fig. 1) which contains a P-loop-like motif that is present in all members (see section below). In addition to these domains, all eukaryotic N-SMases contain hydrophobic domains. For nSMase1 and ISC1, these are found at the C-terminus; in contrast, for nSMase2 and MA-nSMase, these are found at the N-terminus. Importantly, for nSMase2, these domains are likely not to span the membrane as evaluated by topology studies and supported by the presence of a palmitoylation site in between the two domains (Tani & Hannun, 2008). Other functional domains are also present in the eukaryotic SMases: nSMase2 contains a collagen-like domain between the N- and C-termini, and MA-nSMase contains a putative mitochondrial targeting sequence at the extreme N-terminus of the protein. Finally, a portion of the C-terminus of ISC1 serves to function as a binding domain for anionic phospholipids (see section below).
Figure 1. Domain structure of Eukaryotic N-SMase family members.
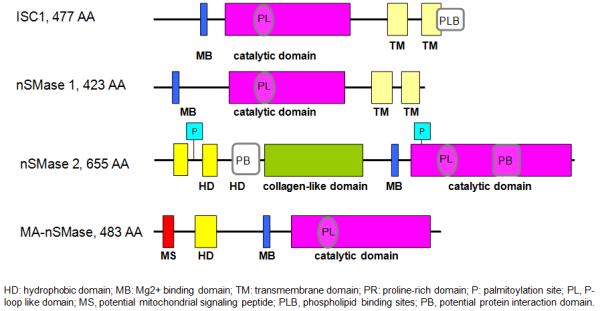
Shown is a schematic illustration of the known functional domains of ISC1 from S. cerevisiae (Genbank, P40015), human nSMase1 (O60906), human nSMase2 (Q9NY59) and murine MA-nSMase (NP_001182466).
Despite the identification of these varied domains within the N-SMases, there is still limited information on their functional roles, if any, that they play in the biochemical regulation and signaling functions of the various family members. Future studies aimed at addressing these are particularly important if we are to understand the biochemical and physiological significance of the extended N-SMase family members.
Ion Dependency
In the earliest characterization of N-SMase activity, it was found to require Mg2+ ions for activity (Gillis Rao & Spence, 1976). Subsequent to their cloning, the divalent cation dependency of N-SMase family members has been investigated for all major eukaryotic family members, in addition to their bacterial counterparts, and is a conserved characteristic across the family. As for the original activity, all enzymes have an absolute requirement for divalent cations – predominantly for Mg2+, although Mn2+ will also suffice for ISC1, nSMase1, nSMase2 and MA-nSMase; indeed, in the latter case, Mn2+ seems to work better than Mg2+. In contrast, Ca2+, Zn2+ and Cu2+ were unable to substitute for Mg2+ in activation of nSMase2 or MA-nSMase (Marchesini et al., 2003; Wu et al., 2010). For ISC1, Ca2+ could not support activity and higher concentrations of Mn2+ were found to inhibit activity (Sawai et al., 2000).
Despite the Mg2+/Mn2+ requirement for N-SMase activity, surprisingly little has been done with regards to identifying where the ions bind within the eukaryotic N-SMases. This is of particular importance for determining the localization of the active site within the enzyme structure. Some insight has been gained from studies with bacterial SMases, suggesting the presence of two Mg2+ binding sites – one low affinity, the other high affinity. Notably, both sites were essential for catalysis and Glu-53 – a highly conserved residue across the N-SMase family – was found to be a crucial Mg2+ binding ligand for the high affinity site (Ago et al., 2006; Obama et al., 2003). Importantly, the solution of the crystal structure of bacterial SMase from B. cereus served to validate these findings. However, as yet, these studies have not been carried over for any of the mammalian N-SMases, and, in the absence of crystral structures of these enzymes, such studies are important for widening our understanding of the biochemical regulation of the N-SMase family.
Regulation by Anionic Phospholipids
For many enzymes involved in signal transduction and/or lipid metabolism, protein-lipid interactions can serve as a mechanism of cellular targeting or as a means to regulate enzymatic activity (Cho & Stahelin, 2005). One such example is the effect of diacylglycerol on the classical and novel protein kinase C isoforms – the lipid serving to relieve pseudosubstrate inhibition and allowing PKC to translocate to the plasma membrane where it can bind phosphatidylserine (PS), a characteristic that can be mimicked by tumor promoting phorbol esters. With respect to N-SMase family members, the regulation by anionic phospholipids (APLs) appears to be a largely conserved characteristic across family members.
Among the different N-SMases, the yeast Isc1p is the best characterized with regards to APL activation. The N-SMase activity of Isc1p is activated by several APLs in a dose-dependent manner, including PS, phosphatidylglycerol (PG) and cardiolipin (CL) (Okamoto et al., 2002) with studies utilizing PS suggesting that the lipid enhances the Vmax with no effect on the Km of Isc1p for its substrate (Sawai et al., 2000). Importantly, the interactions between Isc1p and APLs have been characterized using lipid-protein overlay assays in combination with mutagenesis studies (Okamoto et al., 2002). These studies identified an APL binding domain within the C terminus of Isc1p fitting the consensus sequence of FXFXLKXXXKXR that was necessary and sufficient to confer binding of Isc1p to anionic phospholipids and impart PS-dependent stimulation of activity. This sequence is also present in several PS binding proteins such as PKC-alpha, -beta and –gamma (Okamoto et al., 2002). Importantly, when separate fragments containing the N terminus catalytic domain and the C terminus APL binding domain were expressed heterologously, the activity was partially reconstituted, demonstrating that an interaction between the N terminus and the C terminus may exist and is required for activation of Isc1p. Based on these results, a “tether-and-pull” model was proposed: in the presence of APLs (PS, CL or PG), the APL-binding domain in C-terminal functions as the “tether” and pulls the N-terminal catalytic domain toward the membrane where it can interact efficiently with lipid substrates, resulting in the activation of Isc1p.
Besides Isc1p, APL stimulation of activity has been observed for all the mammalian nSMases with the exception of nSMase1, whose activity appears to be independent of APLs (Tomiuk et al., 2000). In vitro, nSMase2 is activated by several anionic phospholipids in a dose-dependent manner, most notably by PS (Marchesini et al., 2003). Notably, the activated nSMase is localized to the inner leaflet of the PM (Tani & Hannun, 2008), which is also enriched with PS (Vance & Steenbergen, 2005), suggesting PS is a relevant activator for nSMase2. In addition, the recently identified MA-nSMase and mitochondrial SMase from Zebrafish both show activation by APLs, including CL, PS and PG (Wu et al., 2010; Yabu et al., 2009). Notably, befitting the mitochondrial localization of the enzyme, CL was the most potent activator of MA-nSMase in vitro, suggesting that CL may be the primary in vivo regulator of MA-nSMase activity or localization.
Although considerable progress has been made regarding the regulation of N-SMases by APLs, there remain several important unanswered questions. First, the localization of the APL binding domains within proteins other than Isc1 has yet to be determined as this will shed important light on the direct mechanisms of regulation in vivo. Also, the structural features involved in binding are not known. Finally, as the structures for Isc1p and mammalian nSMases are unknown, the “tether-and-pull” activation model awaits further verification from the crystal structures of the enzymes. Hopefully, in understanding how APL-nSMase interactions are able to regulate N-SMase activity in vitro, this will begin to help us understand more about regulation and activation of N-SMase/ceramide signaling in vivo.
The P-loop-like Domain
Many nucleotide binding proteins contain a P-loop motif, also known as a Walker-A motif, that bears the consensus sequence GxxGxGK[T/S]. In a number of proteins, the P-loop motif is crucial for nucleotide binding through the essential conserve lysine and can also be important for substrate recognition, catalysis and regulation of activity (Prasad, 2001). For the eukaryotic N-SMases, the P-loop domains became of interest with the identification of a P-loop-like motif in the yeast N-SMase ISC1 (Okamoto et al., 2003). A functional analysis of the ISC1 domain by site-directed mutagenesis confirmed it was important in enzymatic catalysis of SM in vitro. Interestingly, the conserved motif across the N-SMase family did not match the canonical P-loop motif and therefore was dubbed a P-loop-like (PLL) motif. Functionally, as mutation of these domains altered the Vmax but not the Km, it suggested that, at least, substrate binding was not affected (Okamoto et al., 2003). Interestingly, some of the PLL mutants tested possessed increased affinity for Mg2+ implying the PLL domain may also be important for coordination of the crucial divalent cation in catalysis (Okamoto et al., 2003). Indeed, studies on other P-loop domain containing proteins have found they are important for ion binding (Prasad, 2001). However, further studies focused on the PLL domains of other N-SMase family members are required before this question can be answered.
Despite the identification of PLL domains in all N-SMase family members, the specific role of the PLL domain in enzyme activity has yet to be firmly established. Notably, the PLL domains of all N-SMases are located around two highly conserved residues – Asp-428 and Lys-433 in nSMase2 – that are both essential residues for activity leading to the speculation that the P-loop-like domain serves a crucial purpose for the catalytic mechanism. Indeed, analysis of the two available crystal structure of the bacterial SMases from B. cereus and L. ivanovii suggests that the PLL domain is in a region of high structural variability (Ago et al., 2006; Clarke et al., 2006; Openshaw et al., 2005). Thus, the PLL domain may function as a ‘lid’ on the active site that when ‘open’ allows access of the substrate to the catalytic site. Alternatively, this may suggest a role for the PLL in substrate recognition – serving to allow only SM or other substrates (such as IPC in yeast) into the active site. A third possibility is that the PLL domain may serve as part of the mechanism that transduces APL binding to their domains into regulation of catalytic activity. Overall, it is certainly clear that further research is required to understand the role of the PLL domain in biochemical regulation of N-SMases.
Regulation by Phosphorylation
The phosphorylation of enzymes on serine, threonine, or tyrosine residues is a well-established mechanism of protein activation, formation of docking sites for interacting proteins, and can also lead to enzyme inhibition. The ubiquitous nature of phosphorylation as a regulatory mechanism is further emphasized by the large number of protein kinases that exist in mammalian genomes – the so-called ‘kinome’ (reviewed in Manning et al., 2002). With a number of studies reporting post-translational regulation of mammalian N-SMases by factors such as oxidative stress, cytokines and chemotherapeutic agents (see Wu et al., 2010), this raised the possibility that phosphorylation of eukaryotic N-SMases could represent one possible mechanism of post-translational regulation. However, until recently, this had not been addressed.
Following on from their earlier work investigating nSMase2 activation in response to hydrogen peroxide (H2O2) and cigarette smoke-induced oxidative stress, the Goldkorn group reported that nSMase2 exists as a phosphoprotein (Filosto et al., 2010). Surprisingly, nSMase2 was found to be constitutively phosphorylated, likely on serine residues, and that phosphorylation levels were regulated by stimulation of A549 cells with H2O2, anisomycin and phorbol esters (Filosto et al., 2010). In the latter two cases, this implies p38 MAPK and protein kinase C respectively as upstream regulators of nSMase2 phosphorylation. Notably, this is consistent with two earlier studies in A549 cells identifying p38 MAPK and protein kinase C-delta as regulators of nSMase2 in the response to TNF-α (Clarke et al., 2008; 2007). Finally, the protein phosphatase calcineurin (also known as protein phosphatase 2B) was identified as a negative regulator of nSMase2 phosphorylation (Filosto et al., 2010).
The discovery of nSMase2 as a phosphoprotein naturally raises the question of the functional roles of these phosphorylation sites. In their study, Filosto et al. posit that phosphorylation of nSMase2 functions to regulate activity based on two observations: 1) H2O2 stimulation enhanced phosphorylation and increased activity; 2) a mutant nSMase2 that was unable to interact with calcineurin had higher constitutive phosphorylation and higher activity. However, as Filosto et al. also observed that phorbol esters increased phosphorylation of nSMase2 yet have previously been reported to suppress nSMase2 activity (Clarke et al., 2008), the situation is clearly more complex than anticipated. Indeed, this raises the possibility that nSMase2 may have multiple phosphorylation sites that can regulate its activity in opposite directions. Ultimately, the functional role of nSMase2 phosphorylation will best be determined by identification of the phosphorylated residues, site-directed mutagenesis and in vitro biochemical analysis.
The phosphorylation of nSMase2 also raises the question of whether other N-SMase family members are also regulated by phosphorylation – something that has not been addressed. Within this context, it should be noted that the low identity between N-SMase family members outside of the catalytic core residues makes it unlikely that phosphorylation residues identified in nSMase2 will be conserved across the family. Nonetheless, the possibility that mammalian N-SMases may possess multiple phosphorylation sites certainly increases the chance that one of these is a family wide regulatory site. Nonetheless, significant further study is required before the full extent of N-SMase phosphorylation can be appreciated. However, this would significantly enhance our biochemical understanding of the N-SMases in addition to providing wider insight into their possible physiological roles.
Regulation by Interacting Proteins
In addition to phosphorylation, the stimulated interaction of proteins with select protein partners can also be a crucial mechanism for regulating intrinsic protein activity and can also serve to bring enzymes in closer proximity to their substrates. A number of studies have reported protein-protein interactions of other sphingolipid metabolizing enzymes such as sphingosine kinase 1. In comparison, relatively little is known about interactions of N-SMase family members with other proteins. However, two recent studies have begun to make headway into this area identifying two regulated protein-protein interactions with nSMase2.
Activation of N-SMase is well-established as a component of the response to TNF and other cytokines. Research into regulation of N-SMase by TNF identified a C-terminal region of the p55 TNF receptor that was crucial for N-SMase activation; thus, the domain was termed the NSD domain. Subsequent studies identified a WD repeat protein, FAN (Factor associated with N-SMase activation), as directly binding to the NSD and further demonstrated interactions of the protein RACK1 with the receptor-FAN complex (Tcherkaskowa et al., 2002). However, as neither FAN nor RACK1 appeared to directly interact with any of the identified N-SMases, a missing link in the chain was evident. Recently, this missing link was identified as the protein EED (embryonic ectodermal development) (Phillip et al., 2010). EED was found to interact with both nSMase2 and RACK1 in a timeframe consistent with TNF effects on nSMase2. Importantly, inhibition of EED abrogated nSMase2 activation by TNF, thus confirming that EED serves to bridge activation of the TNF receptor to increases in N-SMase activity. Finally, consistent with its effects on N-SMase activity, EED was found to interact with the C-terminal catalytic domain of nSMase2 (Phillip et al., 2010).
A second interacting protein was also identified recently. In the study by Filosto et al. on regulation of nSMase2 by calcineurin, they also demonstrated a direct interaction of calcineurin with nSMase2 in A549 cells (Filosto et al., 2010). Surprisingly, calcineurin was reported to constitutively interact with nSMase2 suggesting it may serve to ‘check’ basal nSMase2 activity. Indeed, when calcineurin levels were decreased by H2O2 stimulation or siRNA treatment, N-SMase activity was enhanced (Filosto et al., 2010). Analysis of the nSMase2 amino acid sequence identified the likely region where calcineurin interacts – a sequence PQIKIY towards the N-terminus of nSMase2. Mutagenesis analysis confirmed this with a mutant nSMase2 lacking this sequence being unable to bind to calcineurin. Importantly, consistent with the effects of H2O2 noted above, the mutant nSMase2 displayed higher constitutive activity, thus confirming that calcineurin functions as a negative regulator of nSMase2.
The possibility of protein-protein interactions of other N-SMase family members must certainly be kept in mind for the future. In addition to furthering our knowledge of the biochemical regulations of the enzymes, they are crucial for opening up new areas of study with regards to the physiological and pathological roles of the N-SMases. However, aside from demonstrating effects on N-SMase activity, it must also be emphasized that demonstration of physiological importance and relevance for these interactions within the context of N-SMase-mediated signaling is a critical next step.
Cellular Compartmentalization
In recent years, the lipid signaling field has begun to focus on developing the concept of topology and compartmentalization with a view to lipid metabolism and signaling functions. Indeed, the identification of many sphingolipid metabolizing enzymes and their cellular localizations has set the scene for researchers to begin to attribute specific physiological and pathological roles to the local metabolism of lipids within cellular compartments.
With this in mind, it is of particular interest to consider the eukaryotic N-SMases. Of the mammalian N-SMases cloned, all seem to possess a somewhat unique cellular distribution. Thus, nSMase1 appears to localize to the ER and nucleus (Tomiuk et al., 2000), nSMase2 localizes to the Golgi, plasma membrane and recycling compartments (Milhas et al., 2010) while MA-nSMase, as its name indicates, is found within the mitochondria and associated membranes (Wu et al., 2010). This suggests that each SMase is important for local regulation of sphingolipids, and the functional roles of these enzymes may be dictated by their immediate environment. For example, MA-nSMase could play a possible role in cell death, given that previous studies have suggested that ceramide production within the mitochondria is important for the commitment and execution of the cell to apoptosis (Birbes et al., 2001). In contrast, as ceramide accumulation in the ER is reported to activate the ER stress response (Spassieva et al., 2009), nSMase1 could play a role there. As research continues into the specific roles of the specific N-SMase isoforms, we are certainly of the opinion that researchers must also consider the cellular localizations and topologies of the enzyme.
Summary
In recent years, research into the roles and regulation of N-SMases has moved in great strides with the cloning and characterization of multiple N-SMase isoforms and the development of knockout mice. However, as researchers continue to move forward in understanding the physiological functions of these various N-SMase isoforms, it has become exceedingly important to define how these isoforms are regulated at the biochemical and molecular level. This is crucial for the development of future tools to study N-SMase signaling such as, for example, phospho-specific antibodies designating activation states. This is also an important part of identifying novel roles of N-SMases in physiological and pathological states. Finally, only by obtaining a more complete understanding of the workings of these enzymes at the molecular level, will investigators be able to design appropriate compounds that can target and inhibit their activity both efficiently and specifically. Certainly, the last of these is crucial when considering the potential of N-SMases as therapeutic targets. With this in mind, we sincerely hope that the next decade of research will even surpass the last ten years in advancing our understanding of the eukaryotic N-SMase family.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ago H, Oda M, Takahashi M, Tsuge H, Ochi S, Katunuma N, Miyano M, Sakurai J. Structural Basis of the sphingomyelin phosphodiesterase activity in neutral sphingomyelinase from Bacillus cereus. J. Biol. Chem. 2006;281:16157–16167. doi: 10.1074/jbc.M601089200. [DOI] [PubMed] [Google Scholar]
- Birbes H, El Bawab S, Hannun YA, Obeid LM. Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. FASEB J. 2001;15(14):2669–2679. doi: 10.1096/fj.01-0539com. [DOI] [PubMed] [Google Scholar]
- Cho W, Stahelin RV. Membrane-protein interactions in cell signaling and membrane trafficking. Annu. Rev. Biophy. Biomol. Struct. 2005;34:119–151. doi: 10.1146/annurev.biophys.33.110502.133337. [DOI] [PubMed] [Google Scholar]
- Clarke CJ, Snook CF, Tani M, Matmati N, Marchesini N, Hannun YA. The extended family of neutral sphingomyelinases. Biochemistry. 2006;45(38):11247–11256. doi: 10.1021/bi061307z. [DOI] [PubMed] [Google Scholar]
- Clarke CJ, Guthrie JM, Hannun YA. Regulation of neutral sphingomyelinase-2 (nSMase2) by tumor necrosis factor-alpha involves protein kinase C-delta in lung epithelial cells. Mol. Pharmacol. 2008;74(4):1022–1032. doi: 10.1124/mol.108.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CJ, Truong TG, Hannun YA. Role for neutral sphingomyelinase-2 in tumor necrosis factor alpha-stimulated expression of vascular cell adhesion molecule-1 (VCAM) and intercellular adhesion molecule-1 (ICAM) in lung epithelial cells: p38 MAPK is an upstream regulator of nSMase2. J. Biol. Chem. 2007;282(2):1384–1396. doi: 10.1074/jbc.M609216200. [DOI] [PubMed] [Google Scholar]
- Coleman DC, Arbuthnott JP, Pomeroy HM, Birkbeck TH. Cloning and expression in Escherichia coli and Staphylococcus aureus of the beta-lysin determinant from Staphylococcus aureus: Evidence that bacteriophage conversion of beta-lysin activity is caused by insertional inactivation of the beta-lysin determinant. Microb. Pathol. 1986;1:549–564. doi: 10.1016/0882-4010(86)90040-9. [DOI] [PubMed] [Google Scholar]
- Feoktistova A, Magnelli P, Abeijon C, Perez P, Lester RL, Dickson RC, Gould KL. Coordination between fission yeast glucan formation and growth requires a sphingolipase activity. Genetics. 2001;158:1397–1411. doi: 10.1093/genetics/158.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosto S, Fry W, Knowlton AA, Goldkorn T. Neutral sphingomyelinase-2 (nSMase2) is a phosphoprotein regulated by calcineurin (PP2B) J. Biol. Chem. 2010;285(14):10213–10222. doi: 10.1074/jbc.M109.069963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis Rao B, Spence MW. Sphingomyelinase activity at pH 7.4 in human brain and a comparison to activity at pH 5.0. J. Lipid Res. 1976;17:506–515. [PubMed] [Google Scholar]
- Hofmann K, Tomiuk S, Nix M, Zumbansen M, Stoffel W. Cloning and characterization of the mammalian brain-specific, Mg2+-dependent neutral sphingomyelinase. Proc. Nat. Acad. Sci. USA. 2000;97:5895–5900. doi: 10.1073/pnas.97.11.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krut O, Wiegmann K, Kashkar H, Yazdanpanah B, Kronke M. Novel TNF-responsive mammalian neutral sphingomyelinase-3 is a C-tail-anchored protein. J. Biol. Chem. 2006;81:13784–13793. doi: 10.1074/jbc.M511306200. [DOI] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–34. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- Marchesini N, Luberto C, Hannun YA. Biochemical properties of mammalian neutral sphingomyelinase 2 and its role in sphingolipid metabolism. J Biol. Chem. 2003;278:13775–13783. doi: 10.1074/jbc.M212262200. [DOI] [PubMed] [Google Scholar]
- Milhas D, Clarke CJ, Hannun YA. Sphingomyelin metabolism at the plasma membrane: Implications for bioactive sphingolipids. FEBS Letters. 2010;584:1887–1894. doi: 10.1016/j.febslet.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obama T, Kan Y, Ikezawa H, Imagawa M, Tsukamoto K. Glu-53 of Bacillus cereus sphingomyelinase acts as an indispensable ligand of Mg2+ essential for catalytic activity. J. Biochem. (Tokyo) 2003;133:279–286. doi: 10.1093/jb/mvg038. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Vaena de Avalos S, Hannun YA. Structural requirement for selective binding of ISC1 to anionic phospholipids. J. Biol. Chem. 2002;277:46470–46477. doi: 10.1074/jbc.M207779200. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Vaena de Avalos S, Hannun YA. Functional analysis of ISC1 by site-direct mutagenesis. Biochemistry. 2003;42:7855–7862. doi: 10.1021/bi0341354. [DOI] [PubMed] [Google Scholar]
- Openshaw AE, Race PR, Monzo HJ, Vazquez-Boland JA, Banfield MJ. Crystal structure of SmcL, a bacterial neutral sphingomyelinase C from Listeria. J. Biol. Chem. 2005;280:35011–35017. doi: 10.1074/jbc.M506800200. [DOI] [PubMed] [Google Scholar]
- Phillipp S, Puchert M, Adam-Klages S, Tchikov V, Winoto-Morbach S, Mathieu S, Deerberg A, Kolker L, Marchesini N, Kabelitz D, Hannun YA, Schutze S, Adam D. The polycomb group protein EED couples TNF receptor 1 to neutral sphingomyelinase. Proc. Nat. Acad. Sci. 2010;107(3):1112–1117. doi: 10.1073/pnas.0908486107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad GS. Glycine rich P-loop motif in deoxyuridine pyrophosphatase. Curr. Protein Pept. Sci. 2001;2(4):301–311. doi: 10.2174/1389203013381017. [DOI] [PubMed] [Google Scholar]
- Sawai H, Domae N, Nagan N, Hannun YA. Function of the cloned putative neutral sphingomyelinase as lyso-platelet activating factor-phospholipase C. J. Biol. Chem. 1999;274:38131–38139. doi: 10.1074/jbc.274.53.38131. [DOI] [PubMed] [Google Scholar]
- Schneider PB, Kennedy EP. Sphingomyelinase in normal human spleens and in spleens from subjects with Niemann-Pick disease. J. Lipid Res. 1967;8:202–209. [PubMed] [Google Scholar]
- Spassieva SD, Mullen TD, Townsend DM, Obeid LM. Disruption of ceramide synthesis by CerS2 downregulation leads to autophagy and the unfolded protein response. Biochem. J. 2009;424(2):273–83. doi: 10.1042/BJ20090699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani M, Hannun YA. Analysis of membrane topology of neutral sphingomyelinase 2. FEBS Letters. 2008;581(7):1323–1328. doi: 10.1016/j.febslet.2007.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkaskowa AE, Adam-Klages S, Kruse ML, Wiegmann K, Mathieu S, Kolanus K, Kronke M, Adam D. Interaction with factor associated with neutral sphingomyelinase activation, a WD motif-containing protein, identifies receptor for activated C-kinase 1 as a novel component of the signaling pathways of the p55 TNF receptor. J. Immunol. 2002;169(9):5161–5170. doi: 10.4049/jimmunol.169.9.5161. [DOI] [PubMed] [Google Scholar]
- Tomiuk S, Hofmann K, Nix M, Zumbansen M, Stoffel W. Cloned mammalian neutral sphingomyelinase: functions in sphingolipid signaling? Proc. Nat Acad. Sci. USA. 1998;95:3638–3643. doi: 10.1073/pnas.95.7.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiuk S, Zumbansen M, Stoffel W. Characterization and subcellular localization of murine and human magnesium-dependent neutral sphingomyelinase. J. Biol. Chem. 2000;275(8):5710–5717. doi: 10.1074/jbc.275.8.5710. [DOI] [PubMed] [Google Scholar]
- Vance JE, Steenbergen R. Metabolism and functions of phosphatidylserine. Prog. Lipid Res. 2005;44(4):207–234. doi: 10.1016/j.plipres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Wu BX, Clarke CJ, Hannun YA. Neutral Sphingomyelinases: Regulation and roles in cell signaling responses. Neuromolecular Med. 2010 doi: 10.1007/s12017-010-8120-z. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu BX, Rajagopalan V, Roddy PL, Clarke CJ, Hannun YA. Identification and characterization of murine mitochondrial-associated sphingomyelinase (MA-nSMase), the mammalian sphingomyelin phosphodiesterase 5. J. Biol. Chem. 2010;285(23):17993–18002. doi: 10.1074/jbc.M110.102988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabu T, Shimuzu A, Yamashita M. A novel mitochondrial sphingomyelinase in Zebrafish cells. J. Biol. Chem. 2009;284(30):20349–20363. doi: 10.1074/jbc.M109.004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A, Tsukagoshi N, Udaka S, Sasaki T, Makino S, Nakamura S, Little C, Tomita M, Ikezawa H. Nucleotide sequence and expression in Escherichia coli of the gene coding for sphingomyelinase of Bacillus cereus. Eur. J. Biochem. 1988;175:213–220. doi: 10.1111/j.1432-1033.1988.tb14186.x. [DOI] [PubMed] [Google Scholar]


