Abstract
We show here that the immunogenicity of the Modified Vaccinia Ankara MVA vaccine strain can be improved by deletion of the A35 gene, without diminishing the ability of the virus to replicate. Deletion of the A35 gene resulted in increased virus-specific immunoglobulin production, class switching to IgG isotypes, and virus-specific IFNγ secreting splenocytes. The MVA35 deletion virus provided excellent protective efficacy against virulent virus challenge. These results suggest that A35 deletion mutant strains will have superior vaccine performance for poxvirus vaccines as well as platform vaccines for other infectious diseases and cancer.
Keywords: poxvirus, antibody, T lymphocyte
Introduction
Vaccinia virus (VACV) was used as the live virus vaccine during the smallpox eradication campaign, and its use ultimately led to the eradication of this fatal disease [1]. Despite being phenomenally successful, vaccination of the general public was discontinued due to the high rate of adverse events, including disseminated vaccinia, progressive vaccinia, eczema vaccinatum, and encephalitis [2]. It has been estimated that 25% of the current population should not be vaccinated due to immunosuppression, inflammatory skin conditions, or heart abnormalities [3–5], and 1 out of every 450 people vaccinated will have a serious adverse reaction resulting in hospitalization [6].
Since the discontinuation of widespread poxvirus vaccination, there has been an increase in the incidence of human poxvirus infections throughout the world, including buffalopox in India [7], Cantagalo vaccinia virus in South America [8, 9] and cowpox in Europe [10]. The most dangerous emerging poxvirus is monkeypox, which is a zoonotic poxvirus that causes a smallpox-like illness and has a 10% fatality rate in humans. There is growing concern as Monkeypox incidence has increased 20 fold since the 1980’s [11]. Monkeypox is endemic to Africa, but in 2003 there was an outbreak in the United States that was associated with the importation of African rodents [12, 13]. This outbreak resulted in more than 80 cases, but because this was an attenuated strain, no deaths occurred [13, 14]. In addition to the threat of emerging poxvirus infections, there is also concern that the smallpox virus could be used as a biowarfare agent, resulting in widespread sickness and mortality in a now largely unvaccinated population. Thus, safer poxvirus vaccines are needed in the face of emerging disease and bioterrorism.
A safer alternative to the traditional poxvirus vaccine is the use of attenuated, replication-deficient strains, such as Modified Vaccinia Ankara (MVA). Attenuation of this virus occurred after >570 serial passages in chicken embryo fibroblasts (CEF), resulting in deletion of approximately 25 kb of the VACV genome [15], including genes encoding immunomodulatory proteins [16], and the inability to replicate in most mammalian cells [16]. MVA is being tested as a vaccine to protect against poxviruses [17–19], and is also being used as a platform vaccine for other infectious diseases and cancer [20–22]. Poxviruses make good platform vaccines because they are stable and easy to produce, induce robust, durable cellular and humoral immune responses, and can accommodate the insertion of large pieces of foreign DNA [23]. MVA has been shown to be safe when administered to immunodeficient mice [24], non-human primates [25] and humans [26, 27]. Recent vaccine trials have shown that MVA can be given safely to those infected with HIV [28] and those with atopic dermatitis [29], two populations that cannot receive the traditional vaccine. Thus, this attenuated VACV shows promise as a safer alternative poxvirus vaccine and also as a platform vaccine.
While MVA is clearly safer, there are concerns about its immunogenicity and efficacy [30]. In studies comparing the immunogenicity of MVA to traditional poxvirus vaccines, MVA was unable to elicit the same levels of both antibody and IFNγ-producing cells as traditional poxvirus vaccine strains, even when 10–100 times more virus was administered [17, 24, 31]. The immunogenicity of MVA may especially be a concern in populations with an already weakened immune system, such as cancer patients. Data available suggest that multiple doses of MVA may need to be administered in order to achieve the level of immunogenicity obtained with traditional replication-competent strains [31]. One strategy that can be used to enhance the immunogenicity of MVA is to identify and exclude genes that are immunosuppressive. The Copenhagen A35 gene (Western Reserve strain gene 158, MVA gene 146R) is one such gene.
We have previously shown that the poxvirus A35 gene is highly conserved in all sequenced mammalian-tropic poxviruses, including MVA [32]. It is an important virulence factor in the mouse model, increasing virulence of the Western Reserve (WR) virus by nearly 1000-fold, but A35 is not required for replication of the virus [32, 33]. In addition, we have shown that A35 in WR suppresses in vitro MHC class II-restricted antigen presentation [34], and, following intranasal (i.n.) infection in mice, the protein mediates immunosuppression, significantly decreasing virus-specific antibody production, IFNγ-secreting T cells, and CTL killing, resulting in the inability to control viral replication in target organs and thus contributing to morbidity and mortality of the host [33]. It was unknown whether A35 would have similar effects in MVA, or if some genes missing from MVA would be required for A35 functionality in MVA. Here, we characterize the A35 gene function in the background of the MVA genome and describe its immunosuppressive effects.
MATERIAL AND METHODS
Cells and Virus
VACV Western Reserve (WR) stocks were propagated using BS-C-1 cells in MEM containing 10% fetal bovine serum (FBS) as previously described [32]. MVA and MVA35Δ stocks were propagated using BHK-21. Viruses were purified using a sucrose gradient as previously described [Roper, 2006]. P815, BHK-21 cells (ATCC), and chick embryo fibroblasts (CEF) were grown in DMEM containing 10% FBS.
Immunostaining of Virus Infected Cells
Immunostaining was performed as previously described [35]. BHK-21 cells were infected with a titration of either the MVA or MVA35Δ virus for 72 h. Cells were then incubated with a 1:1000 dilution of polyclonal VACV rabbit antiserum (BEI Resources NR629) for 1 h at 4°C, followed by incubation with a 1:1000 dilution of goat anti-rabbit IgG HRP for 1 h at 4°C. 1 ml of the following substrate solution was then added to the wells to visualize virus-infected cells: 12 ul of 30% H2O2 and 240 ul of dianisidine-saturated ethanol in 12 ml of PBS.
Construction of the MVA35Δ Mutant Virus
The DNA segment containing the A35 flanking regions and the E. coli xanthine guanine phosphoribosyl transferase (gpt) gene was amplified from the existing vA35Δ mutant made from WR [32] using the primer pair TCGTGTTCATGATCTTGTTC and TTGCCTAGACCGGATACTA. The PCR product was then transfected with N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl-sulfate (DOTAP; Roche) into BHK-21 cells infected with the MVA strain. The viruses were selected in media containing mycophenolic acid (Sigma). Recombinant viruses (two independent mutant lines, designated MVA35Δ-1 and MVA35Δ-2) were plaque purified three times by using the immunostaining protocol described above and crude stocks were propagated in BHK-21 cells containing 10% FBS using previously described methods [32]. Crude virus lysates were prepped for PCR by incubation with an equal volume of 2x PCR buffer (0.9% IGEPAL, 0.9% Tween-20, 20 mM Tris-HCl, pH 8.3, 3 mM MgCl2, 100 mM KCl) and 0.3 mg/ml proteinase K for 30 m at 45°C, followed by heat-inactivation of the proteinase K for 10 m at 94°C [36]. The recombinant viruses were then PCR screened for the absence of A35 gene using the primers described above. All immunologic experiments were performed using both independently derived MVA35Δ mutant virus isolates to confirm that any difference seen compared to MVA was due to the deletion of the A35 gene.
Western Blotting
Western blots were performed as previously described using polyclonal rabbit anti-A35 sera [32].
Isolation of Chick Embryo Fibroblasts (CEF)
Primary fibroblasts were isolated from a 10-day old chick embryo. Embryos were minced with a 10 cc syringe and then trypsinized two times for 5 minutes at 37°C. Cells were then washed, treated with ammonium chloride for 5 minutes on ice, washed again, and then counted in preparation for use in a one-step growth curve.
One-Step Growth Curve
A one-step growth curve was performed to measure viral replication in BHK-21 and CEF as previously described [32]. Virus foci were enumerated on BHK-21 cells using the immunostaining protocol described above.
Mouse Vaccinations
Female BALB/c mice (n=5) were infected intramuscularly (i.m.) with 107 fu sucrose-gradient purified MVA, MVA35Δ–1, or MVA35Δ-2 virus in 25 ul, or mock infected with PBS. Titers were confirmed on the day of infection with the virus dilution used to infect the animals. For challenge experiments, mice were vaccinated i.m. as described above and then infected intranasally (i.n.) 4 weeks later with 500 × LD50 of virulent WR [33]. Mice were weighed and monitored daily for signs of illness, and were humanely euthanized using isoflurane overdose if 20% weight loss occurred. For experiments assessing the immune response, five mice from each virus group were sacrificed and the spleens were collected in ice cold RPMI. Splenocytes were obtained using previously described methods [33, 37]. Blood samples (500 μl) were collected into microcentrifuge tubes using a cardiac stick, followed by centrifugation at 14,000 g for 30 min to separate out the antibody-containing sera from the red blood cells. Sera were stored at −20° until use. All experimental protocols were approved by the Animal Care and Use Committee of East Carolina University.
Enzyme-Linked Immunosorbent Assay (ELISA)
Antibody response was measured similar to our previous work [33]. Ninety six-well ELISA plates (Immulon H2B Thermo Electron) were coated overnight with 0.1 ul/well (100 ul) crude WR virus in ELISA coating buffer (1x 1M Tris-HCl, pH 9.5) at 4°C. Plates were blocked with 1% FBS/PBS at room temperature for 30 min. Plates were washed with ELISA wash buffer (1x PBS, 0.02% Tween 20, 0.1% NaN3) and a titration of mouse sera ranging from 1:10–1:2560 (1:2 dilutions) from MVA-, MVA35Δ-1-, or MVA35Δ-2-vaccinated mice (n=5) (and PBS treated mice as control) was added. Plates were incubated at room temperature for 2 h and washed. Alkaline phosphatase (AP)-conjugated goat anti-mouse Ig, IgM, IgG, IgG1, or IgG2a (Southern Biotech) was added and incubated at room temperature for 1 h. Plates were washed 3 times, developed (BioRad Alkaline Phosphate Substrate Kit) and absorbance was read at 405 nm.
IFNγ Elispot
Numbers of IFNγ secreting spleen cells were enumerated similar to our previous work [33]. Ninety six-well plates (Immulon H2B Thermo Electron) were coated overnight with 0.2 ul anti-mouse IFN-γ (1 mg/ml Pharmingen) at 4°C. Plates were washed with blocking buffer before adding a titration of murine splenocytes in RPMI 1640. Stimulation of splenocytes was achieved by either the addition of WR virus only (multiplicity of infection (MOI)=2) or with the use of 50,000 WR-infected (MOI=3) P815 stimulator cells, followed by incubation for 40 h at 37°C. Plates were then washed and incubated with 0.4 ul biotinylated rat anti-mouse IFN-γ (Pharmingen 0.5 mg/ml) for 2 h at 37°C. Plates were washed again and incubated with streptavidin-AP for 1 h at 37°C. Plates were developed with agarose/BCIP/AMP mixture, and spots were counted using a dissection microscope or color intensity was read at an absorbance of 492 nm.
Flow Cytometry
Cell types present in spleens were analyzed by flow cytometry. Splenocytes (106 from MVA-, MVA35Δ-1-, or MVA35Δ-2-vaccinated mice (n=5) were fixed in 3% paraformaldehyde on ice for 5 min, washed, and then incubated for 15 min with an anti-Fc block (BD Pharmingen) to block non-specific binding of antibodies to Fc receptors. The following anti-mouse allophycocyanin (APC)-, Pacific Blue (PacBl)-, phycoerythrin (PE)-, or phycoerythrin-cyanine 7 (PE-Cy7)-conjugated monoclonal antibodies were then added to the samples for 45 min on ice: CD8a/Lyt-2 (clone 53–6.7, Southern Biotech); CD11b (clone M1/70, eBioscience); CD11c (clone N418, eBioscience); CD45R/B220 (clone 30-F11; eBioscience); CD49b (Dx5, NK marker; BD Pharmingen); Ly-6G/Ly-6C (granulocyte marker, clone RB6-8C5; BD Pharmingen); F480 (macrophage marker; Caltag Labs); MHC II (I-A/I-E, clone M5/114.15.2; eBioscience). Samples were run on an LSR II flow cytometer and data were analyzed using the FACS Diva software.
Statistical Analyses
Experiments were repeated at least 2 times and representative data are shown. A two-tailed Student’s t test was used to compare groups. p values < 0.05 were considered significant. Statistical analyses were performed using Excel (Microsoft Office 2007).
RESULTS
Molecular Characterization of MVA35Δ
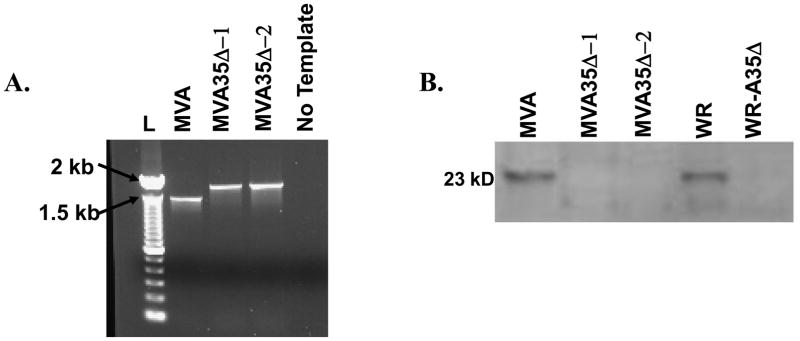
We previously showed that A35 is not required for replication in the wild type VACV WR strain, and that A35 inhibits in vitro MHC II-restricted antigen presentation [34] and suppresses both the T and B lymphocyte response in mice infected i.n. [33]. We next wished to determine if removal of A35 from an attenuated vaccine strain would affect replication or increase immunogenicity. To determine the role of A35 during infection with attenuated poxvirus strain MVA, a mutant virus missing the A35 gene was constructed. Similar to our previous A35 deletion mutant in the WR strain (designated A35Δ) [32], a PCR product containing the E. coli gpt gene with A35 flanking regions on either side was transfected into MVA-infected cells, and recombinant viruses were selected and purified. To confirm that A35 had been successfully knocked out of MVA, PCR analysis was performed using primers in the flanking regions. As shown in Fig 1a, PCR amplification of the parent MVA virus yielded a product of 1.5 kbp, and PCR analysis of two independently isolated MVA35Δ (MVA35Δ-1 and −2) mutants resulted in an approximately 400 bp larger product when compared to the product from parental MVA. This is the expected size increase as a result of the insertion of the gpt-containing PCR fragment [32]. There was no wild type A35 detected in the mutants suggesting that the A35 gene had been successfully removed and indicating that the mutants were purified from MVA parental virus. To further confirm the absence of A35 from the recombinant viruses, a Western blot was performed to analyze reactivity with rabbit anti-A35 polyclonal sera [32] using lysates from MVA and MVA35Δ infected cells (Fig 1b). A protein band of about 23 kDa (the approximate size of A35 [32]) was recognized in the lysates from the WR and MVA-infected cells, and this protein band was absent in the WR-A35Δ, MVA35Δ-1, and MVA35Δ-2 infected cells. Together, these data indicated that A35 was successfully knocked out of MVA in these two independently derived mutants.
Figure 1. Molecular characterization of MVA35Δ.
a) PCR. MVA-infected cells were transfected with a recombinant PCR fragment containing the E. coli gpt gene inserted between the A35 flanking regions and recombinant viruses were selected in mycophenolic acid-containing media. Virus crude stocks were PCR analyzed using primers in the A35 flanking regions. Wild type A35 locus yields a product of 1400 kbp size and the mutants with gpt inserted yield a size of approx 1900 kbp. L=ladder, b) Western blot showing that A35 is not expressed in MVA35Δ-infected cells. BHK-21 cells were infected with listed viruses at an MOI of 20 for 2 h and analyzed by SDS-PAGE. Blots were incubated with rabbit anti-A35 antibody at a 1:1000 dilution and developed with an anti-rabbit alkaline phosphatase-conjugated secondary antibody.
MVA35Δ Virus Replicates Normally
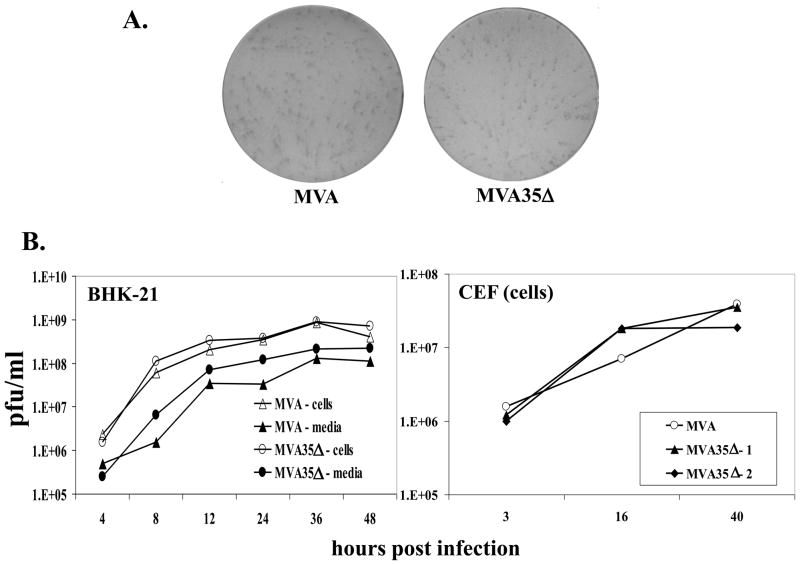
We previously reported that an A35Δ mutant virus made from WR formed normal size plaques on the BS-C-1 African green monkey kidney cells and replicated normally in several cell lines and mouse tissues [32–34], but it was possible that the loss of A35 in WR was being compensated for by another VACV protein. Since MVA is missing ~ 25 kb of the genome of WR, we assessed whether A35 might be required for MVA replication. The MVA35Δ virus formed normal, MVA-sized foci on BHK-21 cells (Fig 2a), indicating that the loss of A35 had no apparent effect on normal viral replication and spread in these cells. To compare the kinetics of replication of the MVA35Δ virus with the parental MVA virus, we performed a one-step growth curve (Fig 2b). BHK-21 cells were infected (MOI=10) with either MVA35Δ or MVA for 2 h, and then inocula were removed. At various times post infection, cells and media were collected and the amount of virus in each was determined by titration on BHK-21 cells. For virus produced in the cells, there was <2-fold difference between the amount of virus produced by MVA and MVA35Δ at all time points tested. Similarly, for virus in the supernatants, MVA35Δ showed no decrease in virus production. A one step growth curve was also used to compare the growth of MVA to MVA35Δ in primary CEF, and at no time point tested was A35 required for viral replication (Fig 2b). Similar results were seen using both MVA35Δ-1 and MVA35Δ-2 viruses. Together, these data indicate that A35 is not required for the normal replication of MVA in BHK-21 cells and primary CEF and further our conclusion that A35 is not required for VACV replication or spread from cell to cell.
Figure 2. A35 is not required for replication of MVA.
a) MVA and MVA35Δ mutant viruses form similarly sized foci on BHK-21 monolayers. Virus-infected cells were visualized by immunostaining. b) One step growth curve. BHK-21 cells or CEF were infected (MOI=10) with MVA, MVA35Δ–1, or MVA35Δ-2, and the amount of virus associated with cells and in the supernatants was titered on BHK-21 cells at various times post infection.
Infection of mice with MVA and MVA35Δ
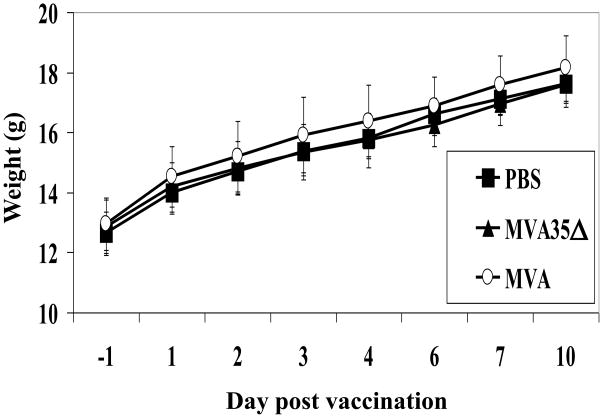
The loss of A35 in virulent WR results in a 1000-fold decrease in virulence in the mouse model [33]. To determine whether the deletion of A35 had any effect on the virulence of MVA, mice were infected intramuscularly (i.m.) with 107pfu purified MVA or one of the two MVA35Δ mutant viruses, a typical route of administration of MVA at a dose that has been shown to elicit a measurable immune response [17, 24, 31], or mock-infected with PBS as a control. Mice were weighed and monitored daily for lesions or signs of illness. As shown in Fig 3, mice infected with either MVA or MVA35Δ gained weight similar to PBS control and had no signs of illness. Similar results were seen with infection no matter which MVA35Δ virus was used. In contrast, intranasal (i.n.) vaccination of mice with 103 pfu virulent WR results in significant weight loss and death [32, 33]. These data confirm that MVA is not virulent and its virulence is not altered by the loss of A35 gene from the genome.
Figure 3. Infection of mice with MVA and MVA35Δ.
Groups of mice (n=5) were infected i.m. with 107 pfu/mouse of MVA or MVA35Δ virus, or mock-vaccinated with PBS and weighed (g +SD) for 4 weeks. No mice lost weight or showed signs of illness.
Antibody Response to Infection with MVA35Δ
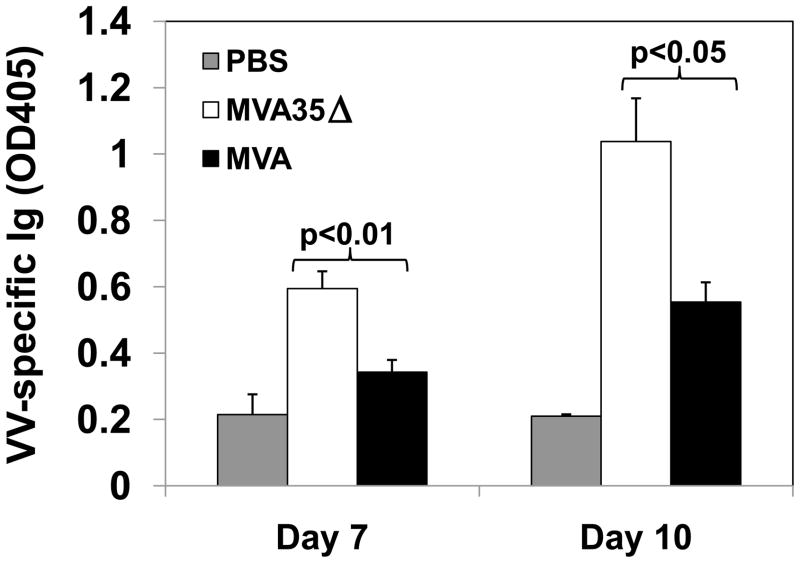
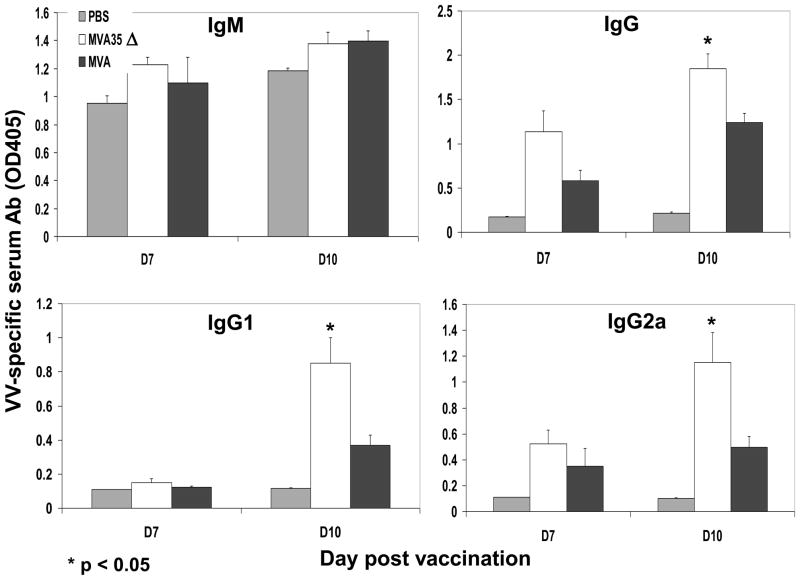
An effective poxvirus vaccine should elicit both a humoral and a cellular immune response, as both arms of the immune system have been shown to mediate aspects of protection against poxvirus infections [31, 38, 39]. Our previous data with WR indicated that removal of the A35 gene results in an increase in both cellular and humoral immunity [33], so we hypothesized that its removal from MVA would boost its immunogenicity. In WR it was possible that A35 protein interacted with another viral protein required for its function, and since MVA has several large deletions, this interacting protein may have been deleted, rendering A35 nonfunctional. To begin to understand the effects of A35 in MVA on the immune response, we compared the production of anti-VACV specific Ig in the sera of mice infected with either MVA or one of the two MVA35Δ viruses (Fig 4). Infected mice had significantly greater amounts of anti-VACV antibody than PBS treated control mice, indicating that the response seen was VACV-specific. Mice infected with MVA35Δ had significantly greater amounts of total VACV-specific serum Ig than those infected with MVA on days 7 and 10 post infection (pi), indicating that A35 is immunosuppressive in MVA. We also analyzed the production of IgG and its subclasses on days 7 and 10 pi (Fig 5), as antibody isotype switching is important for antibody functions. By day 10, mice infected with MVA35Δ virus produced significantly more VACV-specific IgG, IgG1, and IgG2a than those infected with MVA. There was no significant difference in IgM production on these days. Infection with either mutant MVA35Δ virus yielded similar results, and representative data are shown. These data indicate that A35 in MVA decreases VACV-specific antibody production and inhibits isotype switching to IgG and subclasses IgG1 and IgG2a.
Figure 4. VACV-specific antibody response.
Mice (PBS n=3 day 7, n=4 day 10; MVA35Δ n=4; MVA n=4) were infected i.m. with MVA or MVA35Δ virus, and blood was collected via cardiac stick on various days pi. Sera were titrated 1:2, dilutions ranging from 1:20–1:2560. Total VACV-Ig was measured by ELISA on VACV coated plates for day 7 and 10. Data show the average absorbance (day 7 – 1:80, day 10 – 1:20) at 405 nm (+/− SEM).
Figure 5. VACV-specific antibody isotype response.
Mice (n=3–5) were infected i.m. with MVA or MVA35Δ virus, and blood was collected on various days pi. Sera were diluted 1:2, resulting in dilutions ranging from 1:10–1:1280. VACV-specific IgM, IgG, IgG1, and IgG2a were measured by ELISA on VACV coated plates for day 7 and 10. Data show the average absorbance (1:20 dilution) at 405 nm (+/− SEM).
T Lymphocyte Response to Infection with MVA35Δ
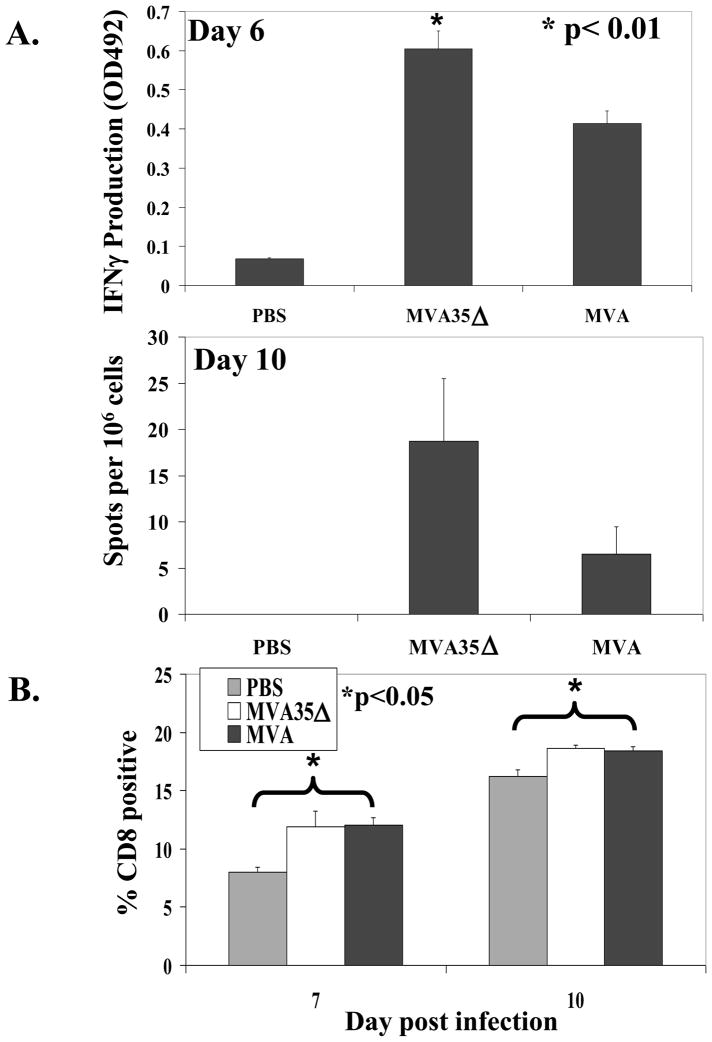
In order to measure the effect of A35 in MVA on the cellular immune response to infection, we used the ELISPOT assay to measure IFNγ production as a result of stimulation with VACV antigens presented in MHC [33, 40–42]. Mice were infected and on day six pi splenocytes were harvested and assayed for VACV specific IFNγ production. There was significantly more VACV-specific IFNγ production in the cells of mice infected with MVA35Δ compared to the mice infected with the parental MVA (Fig 6a). On day 10 pi, there was a three-fold increase in the number of IFNγ–producing spleen cells in the MVA35Δ group compared to the MVA group, but this difference did not reach statistical significance. Similar results were seen with both MVA35Δ viruses and when splenocytes were stimulated with VACV-infected P815 cells. No IFNγ was produced by splenocytes from PBS control mice or in any of the groups without addition of virus or infected P815 cells, thus demonstrating the specificity of the response. Since CD8+ T cells produce most of the IFNγ following a VACV infection [39], we also measured the percentage of CD8+ T cells in the splenocytes of infected mice on days 7 and 10 pi (Fig 6b). We found that MVA infection significantly increased the percentage of CD8+ T cells in the spleens, but there was no difference between the MVA and MVA35Δ-infected groups.
Figure 6. VACV-specific IFNγ-producing cells.
a) IFNγ production. On days 6 and 10 pi (i.m.), the spleens from 5 mice/group were harvested and splenocytes were analyzed by ELISPOT for virus-specific IFNγ production 48 h after stimulation with VACV-WR virus. b) CD8+ cells. On days 7 and 10 pi, spleens from vaccinated mice were analyzed by flow cytometry for the percentage of CD8+ T cells. Data show the average (+/− SEM).
Measurement of Cellular Subsets in the Spleens
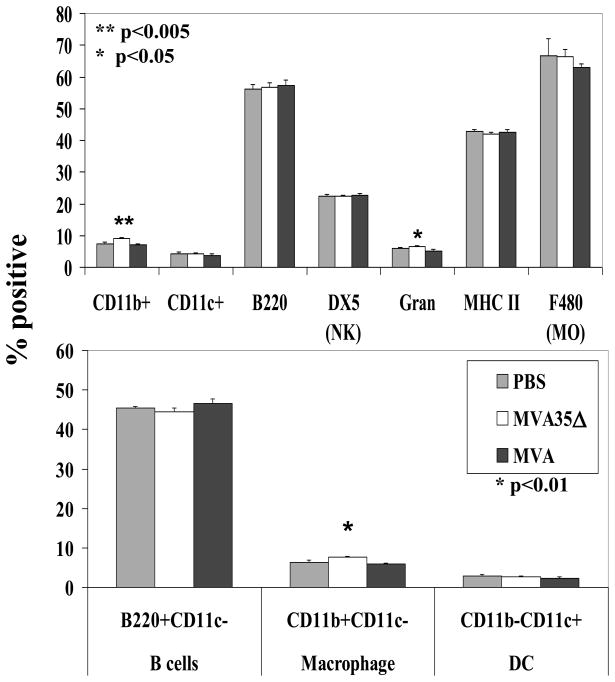
We had previously noted that VACV WR infection induces splenomegaly, presumably as a systemic specific immune response develops, and that spleen size increased most when mice were infected with a virus that is missing the immunosuppressive A35 gene [33]. To understand more about the mechanism of A35-mediated immunosuppression during infection with MVA, we performed flow cytometry to measure the percentage of cellular subsets within the spleens of MVA and MVA35Δ-infected mice. On day 6 pi, there was little change in splenocyte populations (Fig 7). There was a small but significant increase in the percentage of cells expressing CD11b and the Ly6 marker for granulocytes in the splenocytes of the MVA35Δ-infected mice compared to MVA-infected mice (Fig 7a). The CD11b difference was maintained on day 8 pi (data not shown). Also, on day 8 pi, there was a small but significant increase in the percent of DX5+ natural killer (NK) cells found in the MVA35Δ group compared to the MVA group (data not shown). Since our in vitro data indicated that A35 in WR specifically affects the APC [34], we also looked at APC subsets within the spleen, including B cells (B220+CD11c−), macrophages (CD11b+CD11c−), and dendritic cells (CD11b-CD11c+), and found that on day 6 pi, there was a small but significant increase in the percent of macrophages in the spleens of the MVA35Δ-infected mice compared to those infected with MVA (Fig 7b). There was no significant difference between the groups in the percentage of total MHC class II expressing cells (Fig 7a). Thus, infection with MVA results in a small but significant increase in cells expressing CD8 (Fig 6b), and A35 modestly reduces an infection-induced increase in the percentage of granulocytes and CD11b+ expressing cells in spleens (Fig 7).
Figure 7. Cellular subsets in spleens.
On day 6 pi, spleens from MVA and MVA35Δ-infected mice (n=5) were stained for various cell surface markers to enumerate percentage of different cell types. Data show average percentage (+/− SEM). Gran, granulocytes; NK, natural killer; MO, macrophage; DC, dendritic cell.
MVA35Δ Protects Mice from Lethal Challenge
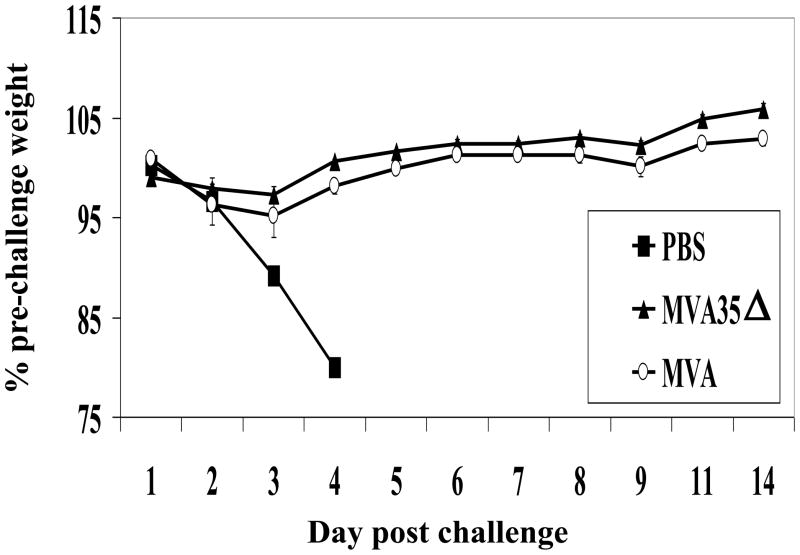
It was next determined whether the MVA35Δ virus would be an efficacious vaccine and protect mice from a lethal VACV challenge. Removal of A35 from the WR strain of VACV did not reduce vaccine efficacy [33]. Many studies have been performed to determine the protective antigenic epitopes of VACV [43, 44], but only the most recent of these studies has shown that epitopes derived from A35 can bind certain MHC class I alleles [45]. We therefore tested whether the removal of A35 from MVA would decrease vaccine efficacy due to the loss of predicted protective epitopes or perhaps increase efficacy due to enhanced immune responses. Mice were vaccinated i.m. with 107 MVA, MVA35Δ, or PBS as control, and four weeks later were challenged i.n. with 500 × LD50 virulent WR virus (Fig 8). PBS control mice quickly lost weight, and all died by 4 days post challenge. All MVA or MVA35Δ vaccinated mice survived the challenge with no significant weight loss and no signs of illness. Similar data were obtained for MVA35Δ-1 and MVA35Δ-2 virus isolates. Together, these data support the hypothesis that an MVA-based vaccine that excludes the A35 gene will be more immunogenic than and at least as efficacious as the wild-type MVA vaccine.
Figure 8. Protection from lethal challenge.
Mice (n=5) were vaccinated i.m. on day 0 with 107 pfu of MVA or MVA35Δ virus, or mock-vaccinated with PBS. Four weeks later, the mice were challenged i.n. with LD50 × 500 virulent WR virus. Data show average percent change in pre-challenge weight (+/− SEM). All mice died in the PBS mock vaccinated group.
DISCUSSION
We have previously shown in the mouse model that the VACV A35 protein in WR virus is a virulence factor, increasing virulence by nearly 1000 fold and decreasing immunogenicity [32, 33]. Since A35 is highly conserved in mammalian-tropic poxviruses, it is probably effective in many host mammals. In this manuscript, we provide a characterization of A35 in the attenuated VACV vaccine strain, MVA, and show that A35 inhibited adaptive immunity, including the generation of IFNγ secreting T cells and antibody production. These results are consistent to what we have observed for the A35 gene in the WR virus [33]. A35 was not required for replication in MVA in either BHK-21 cells or in CEF, the cells in which MVA was derived. This is fortuitous for vaccine production of MVA35Δ mutant viruses. During its >570 passages in CEFs, MVA lost many of the immunosuppressive genes found in VACV, including the secreted receptor homologs for IFNγ, IFNα, IFNβ, and TNF [16]. MVA did retain a few immunoevasion genes, including the IL-1β receptor homolog [16] and A35 [32]. Since genes are lost through random mutation in the absence of selection, the retention of certain genes is probably simply random.
Since MVA is avirulent, it is being tested as an alternative safer poxvirus vaccine and is also being used as a platform vaccine for delivering various other antigens, including those for cancer and HIV [20–22]. Unfortunately, MVA fails to elicit the same high levels of B and T cell responses as the traditional replicating poxvirus vaccines [17, 24, 31]. We therefore removed A35 from MVA in hopes of increasing its immunogenicity. The results of this experiment could not be predicted however, because MVA might have lost some required viral A35-cofactor gene through its passages and large genome deletions. We show here that A35 does indeed function to suppress the specific immune response in the background of the MVA genome. Removal of A35 resulted in increased antibody production, isotype switching to IgG and its subclasses IgG1 and IgG2a, and IFNγ secreting T cells, suggesting that removal of A35 from poxvirus-based vaccines will enhance their immunogenicity. In WR, the improved immune response in the absence of A35 correlated with improved control of virus replication in target organs [33], but since MVA does not replicate in mammalian tissues, we could not perform those experiments here. It was possible that removal of the A35 gene would diminish poxvirus vaccine efficacy if an anti-A35 immune response were protective. However, we showed that vaccination with MVA35Δ virus protects mice as well as wild type MVA from a virulent challenge.
Removal of A35 significantly increased virus-specific Ig, IgG, IgG1, and IgG2a. The latter two IgG subclasses were specifically chosen since they can be used as a measure of the Th2 and Th1 immune responses, respectively [46]. A predominantly Th1 response is associated with smaller lesion size and decreased viral replication in mice and humans [47, 48]. Following VACV infection, persons with atopic dermatitis produce higher levels of cytokines and antibodies associated with the less protective Th2 response [49], resulting in uncontrolled replication of virus in the skin. In addition, a recombinant VACV expressing IL-4 (a Th2 cytokine) was more virulent than wild-type virus [50]. However, we found no evidence that A35 was skewing the immune response towards the less protective Th2 response. Instead, A35 inhibited class switching to both IgG1 and IgG2a isotypes. These data are consistent with our in vitro data which indicate that A35 specifically inhibits the MHC class II:CD4 T cell interaction [34], required for induction of isotype switching [39]. It has been reported that splenic DCs from VACV-infected mice were deficient in presenting a model antigen to specific CD4+ T cells [51]. Our data showed that the removal of the A35 gene in WR caused elevated antibody levels at least up to one month after vaccination [33], suggesting that A35 deletion mutant viruses may give effective protection for longer time periods. Together these data support the removal of the A35 gene from poxvirus vaccine strains used for poxvirus vaccination or other infectious diseases and cancer.
Acknowledgments
We wish to acknowledge the financial support of the North Carolina Biotechnology Center, the NIH grant #U54 A1057157 from the Southeastern Regional Center of Excellence for Emerging Infections and Biodefense, and the Lineberger Comprehensive Cancer Center at University of North Carolina. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of NCBC, NIH, or the Lineberger Cancer Center. We would also like to thank Alice Tripp and Gwen Jones for technical assistance.
Abbreviations
- ACV
Vaccinia virus
- MVA
Modified Vaccinia Ankara
- WR
Western Reserve
- CEF
chick embryo fibroblasts
- MOI
multiplicity of infection
- NK
natural killer
- i.n
intranasal
- i.m
intramuscular
- pi
post infection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mahalingam S, Damon IK, Lidbury BA. 25 years since the eradication of smallpox: why poxvirus research is still relevant. Trends Immunol. 2004 Dec;25(12):636–9. doi: 10.1016/j.it.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Lederman E, Miramontes R, Openshaw J, Olson VA, Karem KL, Marcinak J, et al. Eczema vaccinatum resulting from the transmission of vaccinia virus from a smallpox vaccinee: An investigation of potential fomites in the home environment. Vaccine. 2009 Jan 14;27(3):375–7. doi: 10.1016/j.vaccine.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Eckart RE, Love SS, Atwood JE, Arness MK, Cassimatis DC, Campbell CL, et al. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J Am Coll Cardiol. 2004 Jul 7;44(1):201–5. doi: 10.1016/j.jacc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Kemper A, Davis M, Freed G. Expected Adverse Events in a Mass Smallpox Vaccination Campaign. Effective Clinical Practice. 2002 March; [PubMed] [Google Scholar]
- 5.Upfal MJ, Cinti S. Smallpox vaccination and adverse cardiac events. Emerg Infect Dis. 2004 May;10(5):961–2. doi: 10.3201/eid1005.030967. discussion 2. [DOI] [PubMed] [Google Scholar]
- 6.Casey CG, Iskander JK, Roper MH, Mast EE, Wen XJ, Torok TJ, et al. Adverse events associated with smallpox vaccination in the United States, January-October 2003. Jama. 2005 Dec 7;294(21):2734–43. doi: 10.1001/jama.294.21.2734. [DOI] [PubMed] [Google Scholar]
- 7.Kolhapure RM, Deolankar RP, Tupe CD, Raut CG, Basu A, Dama BM, et al. Investigation of buffalopox outbreaks in Maharashtra State during 1992–1996. Indian J Med Res. 1997 Nov;106:441–6. [PubMed] [Google Scholar]
- 8.Damaso CR, Esposito JJ, Condit RC, Moussatche N. An emergent poxvirus from humans and cattle in Rio de Janeiro State: Cantagalo virus may derive from Brazilian smallpox vaccine. Virology. 2000 Nov 25;277(2):439–49. doi: 10.1006/viro.2000.0603. [DOI] [PubMed] [Google Scholar]
- 9.Silva-Fernandes AT, Travassos CE, Ferreira JM, Abrahao JS, Rocha ES, Viana-Ferreira F, et al. Natural human infections with Vaccinia virus during bovine vaccinia outbreaks. J Clin Virol. 2009 Apr;44(4):308–13. doi: 10.1016/j.jcv.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Kurth A, Straube M, Kuczka A, Dunsche AJ, Meyer H, Nitsche A. Cowpox virus outbreak in banded mongooses (Mungos mungo) and jaguarundis (Herpailurus yagouaroundi) with a time-delayed infection to humans. PLoS One. 2009;4(9):e6883. doi: 10.1371/journal.pone.0006883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rimoin AW, Mulembakani PM, Johnston SC, Lloyd Smith JO, Kisalu NK, Kinkela TL, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci U S A. Sep 14;107(37):16262–7. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lederman ER, Reynolds MG, Karem K, Braden Z, Learned-Orozco LA, Wassa-Wassa D, et al. Prevalence of antibodies against orthopoxviruses among residents of Likouala region, Republic of Congo: evidence for monkeypox virus exposure. Am J Trop Med Hyg. 2007 Dec;77(6):1150–6. [PubMed] [Google Scholar]
- 13.Chen N, Li G, Liszewski MK, Atkinson JP, Jahrling PB, Feng Z, et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology. 2005 Sep 15;340(1):46–63. doi: 10.1016/j.virol.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis MW, Graham MB, Hammarlund E, Hanifin J, Slifka MK. Monkeypox without exanthem. N Engl J Med. 2007 May 17;356(20):2112–4. doi: 10.1056/NEJMc062788. [DOI] [PubMed] [Google Scholar]
- 15.Meisinger-Henschel C, Schmidt M, Lukassen S, Linke B, Krause L, Konietzny S, et al. Genomic sequence of chorioallantois vaccinia virus Ankara, the ancestor of modified vaccinia virus Ankara. J Gen Virol. 2007 Dec;88(Pt 12):3249–59. doi: 10.1099/vir.0.83156-0. [DOI] [PubMed] [Google Scholar]
- 16.Blanchard TJ, Alcami A, Andrea P, Smith GL. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J Gen Virol. 1998 May;79( Pt 5):1159–67. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- 17.Ferrier-Rembert A, Drillien R, Tournier JN, Garin D, Crance JM. Short- and long-term immunogenicity and protection induced by non-replicating smallpox vaccine candidates in mice and comparison with the traditional 1st generation vaccine. Vaccine. 2008 Mar 25;26(14):1794–804. doi: 10.1016/j.vaccine.2007.12.059. [DOI] [PubMed] [Google Scholar]
- 18.Earl PL, Americo JL, Wyatt LS, Eller LA, Whitbeck JC, Cohen GH, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004 Mar 11;428(6979):182–5. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 19.Stittelaar KJ, van Amerongen G, Kondova I, Kuiken T, van Lavieren RF, Pistoor FH, et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol. 2005 Jun;79(12):7845–51. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reyes-Sandoval A, Berthoud T, Alder N, Siani L, Gilbert SC, Nicosia A, et al. Prime-boost immunization with adenoviral and modified vaccinia virus ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infect Immun. Jan;78(1):145–53. doi: 10.1128/IAI.00740-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreijtz JH, Suezer Y, de Mutsert G, van den Brand JM, van Amerongen G, Schnierle BS, et al. Preclinical evaluation of a modified vaccinia virus Ankara (MVA) based vaccine against influenza A/H5N1 viruses. Vaccine. 2009 Oct 23;27(45):6296–9. doi: 10.1016/j.vaccine.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Earl PL, Cotter C, Moss B, VanCott T, Currier J, Eller LA, et al. Design and evaluation of multi-gene, multi-clade HIV-1 MVA vaccines. Vaccine. 2009 Sep 25;27(42):5885–95. doi: 10.1016/j.vaccine.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocha CD, Caetano BC, Machado AV, Bruna-Romero O. Recombinant viruses as tools to induce protective cellular immunity against infectious diseases. Int Microbiol. 2004 Jun;7(2):83–94. [PubMed] [Google Scholar]
- 24.Wyatt LS, Earl PL, Eller LA, Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Natl Acad Sci U S A. 2004 Mar 30;101(13):4590–5. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stittelaar KJ, Kuiken T, de Swart RL, van Amerongen G, Vos HW, Niesters HG, et al. Safety of modified vaccinia virus Ankara (MVA) in immune-suppressed macaques. Vaccine. 2001;19(27):3700–9. doi: 10.1016/s0264-410x(01)00075-5. [DOI] [PubMed] [Google Scholar]
- 26.Stickl H, Hochstein-Mintzel V, Mayr A, Huber HC, Schafer H, Holzner A. MVA vaccination against smallpox: clinical tests with an attenuated live vaccinia virus strain (MVA) (author’s transl) Dtsch Med Wochenschr. 1974 Nov 22;99(47):2386–92. doi: 10.1055/s-0028-1108143. [DOI] [PubMed] [Google Scholar]
- 27.Mahnel H, Mayr A. Experiences with immunization against orthopox viruses of humans and animals using vaccine strain MVA. Berl Munch Tierarztl Wochenschr. 1994 Aug;107(8):253–6. [PubMed] [Google Scholar]
- 28.Cosma A, Nagaraj R, Staib C, Diemer C, Wopfner F, Schatzl H, et al. Evaluation of modified vaccinia virus Ankara as an alternative vaccine against smallpox in chronically HIV type 1-infected individuals undergoing HAART. AIDS Res Hum Retroviruses. 2007 Jun;23(6):782–93. doi: 10.1089/aid.2006.0226. [DOI] [PubMed] [Google Scholar]
- 29.Jones T. IMVAMUNE, an attenuated modified vaccinia Ankara virus vaccine for smallpox infection. Curr Opin Mol Ther. 2008 Aug;10(4):407–17. [PubMed] [Google Scholar]
- 30.Artenstein AW. New generation smallpox vaccines: a review of preclinical and clinical data. Rev Med Virol. 2008 Jul-Aug;18(4):217–31. doi: 10.1002/rmv.571. [DOI] [PubMed] [Google Scholar]
- 31.Belyakov IM, Earl P, Dzutsev A, Kuznetsov VA, Lemon M, Wyatt LS, et al. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc Natl Acad Sci U S A. 2003 Aug 5;100(16):9458–63. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roper RL. Characterization of the Vaccinia Virus A35R Protein and its Role in Virulence. J Virol. 2006;80(1):306–13. doi: 10.1128/JVI.80.1.306-313.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rehm KE, Jones GJ, Tripp AA, Metcalf MW, Roper RL. The poxvirus A35 protein is an immunoregulator. J Virol. 2010 Jan;84(1):418–25. doi: 10.1128/JVI.01802-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rehm KE, Connor RF, Jones GJ, Yimbu K, Roper RL. Vaccinia virus A35R inhibits MHC class II antigen presentation. Virology. 2010 Feb 5;397(1):176–86. doi: 10.1016/j.virol.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roper RL, Payne LG, Moss B. Extracellular Vaccinia Virus Envelope Glycoprotein Encoded by the A33R Gene. JVirol. 1996;70:3753–62. doi: 10.1128/jvi.70.6.3753-3762.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roper RL. Rapid preparation of vaccinia virus DNA template for analysis and cloning by PCR. Methods Mol Biol. 2004;269:113–8. doi: 10.1385/1-59259-789-0:113. [DOI] [PubMed] [Google Scholar]
- 37.Clark RH, Kenyon JC, Bartlett NW, Tscharke DC, Smith GL. Deletion of gene A41L enhances vaccinia virus immunogenicity and vaccine efficacy. J Gen Virol. 2006 Jan;87(Pt 1):29–38. doi: 10.1099/vir.0.81417-0. [DOI] [PubMed] [Google Scholar]
- 38.Chaudhri G, Panchanathan V, Bluethmann H, Karupiah G. Obligatory requirement for antibody in recovery from a primary poxvirus infection. J Virol. 2006 Jul;80(13):6339–44. doi: 10.1128/JVI.00116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004 May 15;172(10):6265–71. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 40.Coulibaly S, Bruhl P, Mayrhofer J, Schmid K, Gerencer M, Falkner FG. The nonreplicating smallpox candidate vaccines defective vaccinia Lister (dVV-L) and modified vaccinia Ankara (MVA) elicit robust long-term protection. Virology. 2005 Oct 10;341(1):91–101. doi: 10.1016/j.virol.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 41.Gherardi MM, Ramirez JC, Esteban M. IL-12 and IL-18 act in synergy to clear vaccinia virus infection: involvement of innate and adaptive components of the immune system. J Gen Virol. 2003 Aug;84(Pt 8):1961–72. doi: 10.1099/vir.0.19120-0. [DOI] [PubMed] [Google Scholar]
- 42.See RH, Zakhartchouk AN, Petric M, Lawrence DJ, Mok CP, Hogan RJ, et al. Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus. J Gen Virol. 2006 Mar;87(Pt 3):641–50. doi: 10.1099/vir.0.81579-0. [DOI] [PubMed] [Google Scholar]
- 43.Jing L, Chong TM, McClurkan CL, Huang J, Story BT, Koelle DM. Diversity in the acute CD8 T cell response to vaccinia virus in humans. J Immunol. 2005 Dec 1;175(11):7550–9. doi: 10.4049/jimmunol.175.11.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones-Trower A, Garcia A, Meseda CA, He Y, Weiss C, Kumar A, et al. Identification and preliminary characterization of vaccinia virus (Dryvax) antigens recognized by vaccinia immune globulin. Virology. 2005 Sep 13; doi: 10.1016/j.virol.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Johnson KL, Ovsyannikova IG, Mason CJ, Bergen HR, 3rd, Poland GA. Discovery of naturally processed and HLA-presented class I peptides from vaccinia virus infection using mass spectrometry for vaccine development. Vaccine. 2009 Dec 10;28(1):38–47. doi: 10.1016/j.vaccine.2009.09.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin B, Wang RY, Qiu Q, Sugauchi F, Grandinetti T, Alter HJ, Shih JW. Induction of potent cellular immune response in mice by hepatitis C virus NS3 protein with double-stranded RNA. Immunology. 2007 Sep;122(1):15–27. doi: 10.1111/j.1365-2567.2007.02607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oyoshi MK, Elkhal A, Kumar L, Scott JE, Koduru S, He R, et al. Vaccinia virus inoculation in sites of allergic skin inflammation elicits a vigorous cutaneous IL-17 response. Proc Natl Acad Sci U S A. 2009 Sep 1;106(35):14954–9. doi: 10.1073/pnas.0904021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scott JE, ElKhal A, Freyschmidt EJ, MacArthur DH, McDonald D, Howell MD, et al. Impaired immune response to vaccinia virus inoculated at the site of cutaneous allergic inflammation. J Allergy Clin Immunol. 2007 Dec;120(6):1382–8. doi: 10.1016/j.jaci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Becker Y. Vaccinia virus pathogenicity in atopic dermatitis is caused by allergen-induced immune response that prevents the antiviral cellular and humoral immunity. Virus Genes. 2003 Dec;27(3):269–82. doi: 10.1023/a:1026399916888. [DOI] [PubMed] [Google Scholar]
- 50.Sharma DP, Ramsay AJ, Maguire DJ, Rolph MS, Ramshaw IA. Interleukin-4 mediates down regulation of antiviral cytokine expression and cytotoxic T-lymphocyte responses and exacerbates vaccinia virus infection in vivo. J Virol. 1996 Oct;70(10):7103–7. doi: 10.1128/jvi.70.10.7103-7107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao Y, Li P, Singh P, Thiele AT, Wilkes DS, Renukaradhya GJ, et al. Vaccinia virus infection induces dendritic cell maturation but inhibits antigen presentation by MHC class II. Cell Immunol. 2007 Apr;246(2):92–102. doi: 10.1016/j.cellimm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]