Abstract
An abnormally high ankle brachial index (ABI) is associated with increased all-cause and cardiovascular mortality. The relationship of obesity to incident high-ABI has not been characterized. We investigated the hypothesis that increased obesity—quantified by body weight, BMI, waist circumference, and waist-to-hip-ratio—is positively associated with a high-ABI (ABI ≥ 1.3) and with mean ABI increases over a four year follow-up. Prevalence and incidence ratios for a high-ABI were obtained for 6540 and 5045 participants respectively in the Multi-Ethnic Study of Atherosclerosis (MESA), using log-binomial regression models adjusted for demographic, cardiovascular, and inflammatory/novel risk factors. Linear regression was used to analyze mean ABI change. Both prevalence and incidence of a high-ABI were significantly higher for the highest versus the lowest quartile of every baseline measure of obesity, with weight and BMI demonstrating the highest incidence ratios (2.7 and 2.4, respectively). All prevalence and incidence ratios were positive and graded across obesity quartiles, and were persistent in the subpopulation without diabetes. Among those with normal baseline ABI values, one MESA-standard deviation increase in every baseline measure of obesity was associated with significant increases in mean ABI values. In conclusion, we observed an independent, positive and graded association of increasing obesity to both prevalent and incident high-ABI, and to mean increases in ABI values over time. Weight and BMI seemed to be at least as strongly, if not more strongly, associated with a high-ABI than were measures of abdominal obesity.
Keywords: obesity, anthropometric measures, peripheral vascular disease, ankle-brachial index, epidemiology
INTRODUCTION
An abnormally elevated ankle brachial index (ABI) has recently been associated with increased cardiovascular disease (CVD) risk 1-9. The relationship of obesity to high-ABI development is unclear. We aimed to examine the longitudinal relationships of various measures of obesity with an abnormally high-ABI. As abdominal obesity is particularly strongly associated with CVD risk, including insulin resistance and vascular stiffness 10-12, we hypothesized that waist circumference and waist-to-hip ratio (WHR) would demonstrate stronger positive associations with high-ABI than would more general measures of obesity.
METHODS
The Multi-Ethnic Study of Atherosclerosis (MESA) aims to investigate the prevalence, correlates, and progression of subclinical cardiovascular disease. Details of its design have been reported 13. The cohort includes 6814 men and women aged 45-84 years, free of clinical cardiovascular disease, and recruited from six US field centers. Approximately 53% of the cohort is female, and the ethnic distribution is 38% Caucasian, 12% Chinese, 28% African American and 22% Hispanic. A baseline exam was performed in 2000-2002, and a follow-up exam performed approximately four years later.
Participants completed a self-administered questionnaire on demographics and medical history, and anthropometric measures were performed in light clothing during the baseline exam. Body mass index (BMI) was calculated as weight (kg) divided by height (m)2. Waist circumference was measured horizontally at the level of the umbilicus. Hip girth was measured at the maximum circumference of the buttocks. Systolic blood pressure was measured in a seated position three times with a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, Waukesha, Wisconsin); the final two measurements’ average was used for analysis. Total cholesterol, high density lipoprotein cholesterol (HDL), serum glucose and insulin were measured from blood samples after a 12 hour fast.
ABI was calculated from measurements of bilateral brachial, posterior tibial and dorsalis pedis arteries performed at baseline and follow-up exams. After a 5 minute rest in the supine position, systolic blood pressures were obtained using appropriate sized cuffs and a 5-mHz Doppler probe (Nicolet Vascular, Golden, CO). The average of the two brachial arteries was used as the denominator; if the two brachial blood pressures differed by >10mmHg, the higher value was used. The ABI numerator was defined as the higher of the dorsalis pedis or posterior tibial pressures in each ankle, and was used to calculate left and right sided ABIs. The lower of the left and right-sided ABI was defined as the “index-ABI” for each individual. Values between ABI > 1.3 to ABI > 1.5 have been used previously to define an abnormally elevated ABI 3, 7, 14, 15. The optimal upper limit of a normal ABI is unknown. Some have suggested that above an ABI ≥ 1.3 other modalities should be used to evaluate peripheral atherosclerotic disease 16. Thus in this study we defined a “high-ABI” to be a left or right sided ABI ≥ 1.3. Incident high-ABI was defined as a right or left ABI ≥ 1.3 on follow-up among participants with bilateral ABI > 0.9 and < 1.3 at baseline. ABI changes were calculated by subtracting baseline from follow-up index-ABI values. All ABI measurements have an intra-class correlation coefficient of >0.9 and technical error of measurement <5%.
Baseline and follow-up ABI measurements were available for 6795 (99.7%) and 5885 (86.4%) cohort participants, respectively. For both prevalence and incidence high-ABI analyses, those with an ABI within the normal range (0.9<ABI<1.3) were chosen as the reference group. The presence of occlusive peripheral atherosclerotic disease may preclude accurate measurement of distally elevated ABI, and this disease process likely has a risk factor distribution that may confound the analysis of obesity and high-ABI. Thus, for the prevalence analysis, those with baseline right or left sided ABI < 0.9 were excluded, for a total population of 6540; for incidence analysis, exclusion of those with abnormal ABI at baseline (ABI < 0.9 and ABI >1.3) and those with a follow-up ABI < 0.9 yielded a total population of 5045.
Log-binomial regression models were fitted using a generalized linear model with log link and binomial error distribution 17. Linear assumptions were checked, and diabetes demonstrated the only significant interaction. Comparison among anthropometric measures was performed by calculating prevalence or incidence ratios for the highest versus the lowest quartile of each measure in separate models. Mean index-ABI change per one standard deviation of baseline difference for each anthropometric measure was calculated using linear regression. Participants missing relevant covariates were excluded from regression models. The distributions of other covariates were similar in those missing or not missing data. The only exception was missing smoking pack-years, for which imputation was performed based on current or former smoking, ethnicity, age, BMI and gender.
Regression covariates were identified a-priori to adjust for basic demographics, traditional cardiovascular risk factors and novel risk factors including markers of inflammation and dyslipidemia. Adjustment for albuminuria was based on its association with CVD and abnormal ABI 3, 18, and GFR was included for the role of kidney disease in vascular abnormality. A process of backward selection with assessment of statistical significance and model goodness of fit was used to establish covariates. All regression models included age, gender, ethnicity, smoking status (never, former, current), smoking pack-years, systolic blood-pressure, education (≥ high school, < high school), IL-6, homocysteine, high-sensitivity C-reactive protein, low density lipoprotein cholesterol (LDL), HDL, urinary albumin-creatinine ratio (mg/g), and estimated glomerular filtration rate (mL/min/1.73 m2) calculated by the Modification of Diet in Renal Disease study equation. Diabetes was defined as either a fasting glucose ≥126 mg/dl, prior diagnosis by self-report or use of anti-diabetic medication, and was included as a covariate in all regression models except the high-ABI incidence model among subjects without diabetes. In this model, the natural log of the Homeostasis Model of Insulin Resistance (HOMA-IR) = {[fasting insulin (mU/L) × fasting glucose (mg/dl)] / 405 } was included as a covariate instead of diabetes 19. Diabetes was maintained as a covariate in other models since inclusion of baseline fasting glucose, insulin or natural-log transformed HOMA-IR generally did not improve model fit. Interleukin-6, homocysteine and C-reactive protein were all skewed and thus natural-log-transformed. Inclusion of height, pulse pressure and the presence or absence of the metabolic syndrome (NCEP Adult Treatment Panel III definition) did not affect inferences, and none was significantly associated with outcomes; they were excluded from analysis. All analyses were performed using STATA 10.0 (Stata Co., College Station, TX).
RESULTS
High-ABI baseline prevalence was 8.8%. High-ABI participants were more likely to be male, Caucasian, have lower mean systolic blood pressure, total cholesterol, HDL and LDL, and higher HOMA-IR (Table 1).
Table 1.
Comparison of Characteristics Between Baseline Normal and High Ankle Brachial Index Subjects in the Multi-Ethnic Study of Atherosclerosis (2000-2002)
| Ankle Brachial Index * | |||
|---|---|---|---|
| Variable | Normal (n=5944) | High (n=596) | p-value |
| Age (year) | 61.9 SD (10.1) | 61.2 SD (10.2) | 0.117 |
| Men | 44.8% (2660) | 71.5% (426) | <0.001 |
| Ethnicity | <0.001 | ||
| Caucasian | 37.7% (2243) | 49.2% (293) | |
| Chinese | 12.5% (743) | 7.7% (46) | |
| African American | 27.8% (1650) | 18.5% (110) | |
| Hispanic | 22.0% (1308) | 24.7% (147) | |
| Body Mass Index (kg/m^2) | 28.2 SD (5.4) | 29.6 SD (5.8) | <0.001 |
| Waist Circumference (cm) | 97.6 SD (14.2) | 102.6 SD (14.9) | <0.001 |
| Diabetes Mellitus† | |||
| Impaired Fasting Glucose | 12.1% (718) | 13.6% (81) | 0.47 |
| Untreated Diabetes | 2.5% (150) | 2.9% (17) | |
| Treated Diabetes | 9.3% (553) | 10.4% (62) | |
| ln(HOMA-IR§) | 0.11 SD (0.83) | 0.21 SD (0.85) | 0.004 |
| Smoking | 0.45 | ||
| Never | 51.0% (3019) | 50.7% (301) | |
| Former | 36.1% (2139) | 40.2% (239) | |
| Current | 13.0% (768) | 9.1% (54) | |
| Pack Years Smoking | 11.0 SD (20.3) | 10.6 SD (19.0) | 0.68 |
| Education (>High School) | 81.8% (4844) | 86.9% (516) | 0.28 |
| Family History of Myocardial Infarction |
42.4% (2375) | 43.4% (243) | 0.66 |
| Prehypertension ‡ | 31.7% (1885) | 32.4% (193) | <0.001 |
| Hypertension Stage 1 | 18.1% (1074) | 13.3% (79) | |
| Hypertension Stage 2 | 7.0% (418) | 3.5% (21) | |
| Systolic Blood Pressure (mmHg) | 126.5 SD (21.2) | 121.5 SD (18.5) | <0.001 |
| Anti-hypertensive medication use | 32.8% (1949) | 28.0% (167) | 0.018 |
| Serum Creatinine (mg/dl) | 0.94 SD (0.25) | 0.99 SD (0.21) | <0.001 |
| Glomerular Filtration Rate (mL/min/1.73 m2) |
81.4 SD (18.5) | 81.2 SD (16.2) | 0.75 |
| Total Cholesterol (mg/dl) | 194.7 SD (35.8) | 189.1 SD (34.7) | <0.001 |
| High Density Lipoprotein (mg/dl) | 51.2 SD (14.9) | 48.4 SD (14.2) | <0.001 |
| Low Density Lipoprotein (mg/dl) | 117.4 SD (31.5) | 114.7 SD (30.2) | 0.05 |
| Triglycerides (mg/dl) | 131.7 SD (90.7) | 131.4 SD (76.0) | 0.93 |
| Metabolic Syndrome | 32.4% (1925) | 31.2% (186) | 0.56 |
| Homocysteine (umol/L) | 9.2 SD (3.5) | 9.8 SD (5.2) | <0.001 |
| C-Reactive Protein (mg/L) | 3.7 SD (5.7) | 3.5 SD (5.9) | 0.35 |
| Fibrinogen (mg/dl) | 345.6 SD (73.3) | 341.8 SD (72.4) | 0.23 |
| Interleukein-6 (pg/ml) | 1.5 SD (1.2) | 1.5 SD (1.3) | 0.76 |
| Urine Albumin/Creatinine (mg/g) | 24.7 SD (115.9) | 21.7 SD (79.9) | 0.64 |
Normal Ankle Brachial Index (ABI)=(0.9<ABI<1.3); High-ABI=(ABI ≥ 1.3)
(2003 ADA criteria): Normal if fasting glucose is < 100 mg/dL and not taking diabetes medication; impaired fasting glucose if fasting glucose =100-125 mg/dL and not taking diabetes medications; untreated diabetes if fasting glucose ≥ 126 mgdL and not taking diabetes medication; treated diabetes if reports taking diabetes medication.
(JNC VII Guidelines): Normal BP (in mmHg): SBP <120 and DBP <80; Prehypertension BP: SBP 120-139 or DBP 80-89; Stage 1 Hypertension BP: SBP 140-159 or DBP 90-99; Stage 2 Hypertension BP: SBP ≥ 160 or DBP ≥ 100.
HOMA-IR = Homeostasis Model of Insulin Resistance
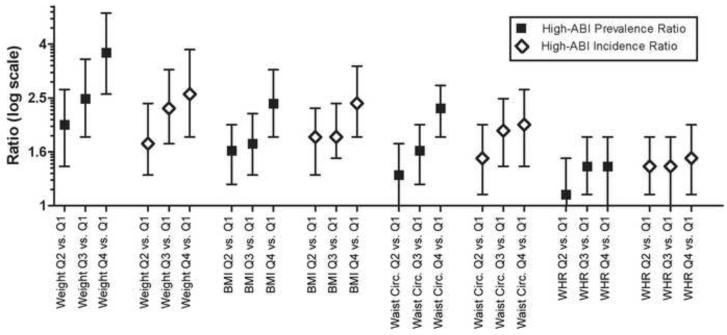
In fully adjusted models, the prevalence of a high-ABI was significantly higher for the highest compared to the lowest quartile of each baseline anthropometric measure—with body weight demonstrating the highest prevalence ratio (3.7, 95% CI 2.6-5.2) of the four measures (Table 2). Among participants with a normal ABI (0.9<ABI<1.3) at baseline, increased baseline obesity was positively associated with the development of new-onset high-ABI for all anthropometric measures—with body weight and BMI demonstrating the highest fully adjusted incidence ratios (Table 2). All high-ABI prevalence and incidence ratios were positive and graded across quartiles for all anthropometric measures (Figure 1).
Table 2.
High-Ankle Brachial Index Prevalence and Incidence Ratios for Highest to Lowest Quartile of Anthropometric Measures in the Multi-Ethnic Study of Atherosclerosis (2000-2006)
| Anthropometric Measure |
Prevalence Ratio for high-ABI (95% CI); n=6208 |
p-value | Incidence Ratio for high-ABI (95% CI); n=4805 |
p-value | Incidence Ratio for high-ABI in subjects without diabetes (95% CI); n=4303 |
p-value |
|---|---|---|---|---|---|---|
| Body Weight (kg): 89.4-158.8 vs. 32.7-66.2 |
3.7 (2.6-5.2) | <0.001 | 2.7 (1.9-3.8) | <0.001 | 2.3 (1.5-3.4) | <0.001 |
| Body Mass Index (kg/m^2): 31-62 vs. 15-25 |
2.4 (1.8-3.2) | <0.001 | 2.4 (1.8-3.3) | <0.001 | 2.1 (1.5-3.1) | 0.001 |
| Waist Circumference (cm): 107-167 vs. 59-88 |
2.3 (1.8-3.1) | <0.001 | 2.0 (1.4-2.7) | <0.001 | 1.7 (1.2-2.4) | 0.004 |
| Waist to Hip Ratio 0.99-1.30 vs. 0.65-0.87 |
1.4 (1.0-1.8) | 0.01 | 1.5 (1.1-2.0) | 0.03 | 1.3 (0.9-1.8) | 0.30 |
High-Ankle Brachial Index (ABI) is defined as ≥ 1.3
Models are fully adjusted for age, gender, ethnic background, smoking status, pack-years smoking, diabetes, systolic blood-pressure, education, IL-6, homocysteine, c-reactive protein, LDL, HDL, urinary albumin creatinine ratio, estimated glomerular filtration rate.
Incidence analysis in those without diabetes is adjusted for the natural log of the Homeostasis Model of Insulin Resistance instead of diabetes
All analyses exclude those participants missing baseline ABI measurements or relevant covariates; Incidence analyses additionally exclude those missing follow-up ABI measurements and those with abnormal ABI at baseline (see Methods).
p-values are for linear trend for all quartiles of each measure
Figure 1.
High-ABI Prevalence and Incidence Ratios For Each Anthropometric Measure in the Multi-Ethnic Study of Atherosclerosis 2000-2006 (95% CI bars)
Caption: *ABI = Ankle brachial index; BMI = Body mass index; WHR = Waist to hip ratio. Weight (kg): Quartile 1 (Q1) = 32.7-66.2; Quartile 2 (Q2) = 66.2-77.1; Quartile 3 (Q3) = 77.1-89.4; Quartile 4 (Q4) = 89.4-158.8.
BMI (kg/m^2): Quartile 1 = 15-25; Quartile 2 = 25-28; Quartile 3 = 28-31; Quartile 4 = 31-62. Waist Circumference (cm): Quartile 1 = 59-88; Quartile 2 = 88-97; Quartile 3 = 97-107; Quartile 4 = 107-167.
WHR: Quartile 1 = 0.65-0.87; Quartile 2 = 0.87-0.93; Quartile 3 = 0.93-0.98; Quartile 4 = 0.98-1.30.
Given the central role that diabetes is often assumed to play in the development of a high-ABI, we further examined the stratum of the MESA cohort without baseline diabetes while adjusting for insulin resistance with natural-log transformed HOMA-IR. In the fully adjusted model, increased baseline weight, BMI and waist circumference remained significantly associated with incident high-ABI, with the strongest associations again seen for weight and BMI (Table 2).
To determine whether obesity is associated with changes in ankle-brachial index values over time, the index-ABI difference between baseline and follow-up was calculated for those who had a baseline ABI between 1.0 ≤ ABI ≤ 1.2, i.e., excluding those with borderline abnormally low or high ABI values at baseline, who therefore might be more likely to progress to abnormal ABI values. For all anthropometric measures, a standard deviation increase in baseline measure was associated with statistically significant, positive mean increases in index-ABI over the follow-up period (Table 3).
Table 3.
Mean Change in Index-Ankle Brachial Index* over Follow-up (2000-2006) for one MESA-Standard Deviation (SD) Increase in Baseline Anthropometric Measure, n=3850
| Anthropometric measure (1-MESA-SD) | Mean change in ABI per one SD baseline anthropometric measure increase |
p-value |
|---|---|---|
| Body Weight (17.3) | 0.0096 (0.0055-0.0137) | <0.001 |
| Body Mass Index (5.48) | 0.0098 (0.0060-0.0136) | <0.001 |
| Waist Circumference (14.34) | 0.0073 (0.0036-0.0110) | <0.001 |
| Waist to Hip Ratio (0.078) | 0.0040 (0.0030-0.0076) | 0.036 |
Index-Ankle Brachial Index (ABI) is defined as the lower of the left and right sided ABI values; see Methods
Analysis excludes those with borderline abnormal ABI values (ABI<1 or ABI>1.2) at baseline (see Methods/Results).
Model is fully adjusted for age, gender, ethnic background, smoking status, pack-years smoking, diabetes, systolic blood-pressure, education, IL-6, homocysteine, c-reactive protein, LDL, HDL, urinary albumin creatinine ratio, estimated glomerular filtration rate
Analysis excludes those participants missing baseline and follow-up ABI measurements and relevant covariates
In sensitivity analyses, we excluded the 50% of participants whose weight or BMI changed the most during follow-up, to address the possibility that obesity changes may have artifactually altered ABI measurements by affecting cuff fit. We also repeated analyses using the definition of high-ABI >1.4. Both analyses yielded results similar to those in Table 2 (data not shown). Within the MESA cohort, we also note that obesity demonstrates an independent and inverse relationship with the prevalence of abnormally low-ABI (ABI ≤ 0.9), results that are inferentially consistent with our high-ABI findings (data not shown).
DISCUSSION
Our prospective study demonstrates the novel finding that increased baseline obesity is associated with the development of new-onset high-ABI measurements over a mean four-year follow-up, as well as mean ABI increases over time. These associations are independent of cardiovascular risk factors, and are persistent among subjects without diabetes when additionally adjusting for insulin resistance. Trends of baseline characteristics from prior cross-sectional studies are largely consistent with our findings 5, 9, 14, though one study did not find a significant association between BMI and prevalent high-ABI 3.
The precise pathophysiologic mechanisms underlying an elevated ABI are not clearly elucidated, but several mechanisms may be relevant. Calcification of the tunica media in medium-sized muscular arteries due to medial arterial calcification (MAC) is the most commonly invoked process to explain a high-ABI, via its preferential effect on lower extremity artery stiffness 20, 21. MAC is most commonly associated with diabetes, renal failure and aging, however, and for this reason some have suggested that this may not be the predominant process underlying an elevated-ABI in the general population 14.
Through several mechanisms distinct from MAC, insulin resistance may further contribute to a high-ABI. Insulin resistance is thought to blunt the acute insulin-mediated relaxation of arterial muscular tone (within 30-60 minutes), resulting in increased arterial stiffness; the magnitude of this effect correlates with the degree of insulin resistance 12, 22-24. Insulin resistance is also likely to decrease the less acute (~2 hours) insulin-induced vasodilation of peripheral resistance arteries 11. Accordingly, when a small sample of type-2 diabetic subjects was started on insulin therapy, which is thought to increase insulin sensitivity, a decrease in a marker for arterial stiffness was observed 25. Insulin resistance also induces smooth-muscle cell proliferation and the nonenzymatic glycosylation of proteins such as collagen 26, 27. The aggregate effect of these insulin-related changes may directly increase ABI, particularly if they are shown to preferentially affect lower extremity arteries. An impact on blood pressure pulse-wave reflection 11, 28, which is altered by changes in arterial compliance and/or caliber, to increase pulse wave velocity in the leg could also elevate the ABI. Thus, mechanisms related to insulin resistance and diabetes may contribute, in part, to the positive association observed between obesity and a high-ABI.
The Strong Heart Study was the first large prospective study to indicate the potential increased all-cause and CVD mortality risk for individuals with an abnormally high-ABI 3. Its study population of American Indians had a high prevalence of diabetes of over 40%. Some, but not all, studies2 confirming an increased mortality risk associated with a high-ABI were also performed in populations in which MAC would be expected to be common—hemodialysis patients 4 and among the elderly 5. However, in studies such as our own where the prevalence of diabetes reflects that of the general population (~10%) for both normal and high-ABI groups, and where the prevalence of hypertension is actually lower in the high-ABI versus the normal-ABI group 5, 14—a finding less consistent with MAC driving an elevated ABI— other etiologies may have greater importance in the development of a high-ABI.
It has been proposed that body composition itself, rather than associated metabolic changes, may underlie a high-ABI measurement. Tabara et al. recently utilized computed tomography to measure trunk and lower extremity composition in relation to a high-ABI 29. After adjustment for cardiovascular risk factors including insulin resistance, femoral muscle cross-sectional area, but not visceral fat, femoral fat or femoral circumference, was independently associated with a high-ABI. This suggests that increased lower extremity muscle mass, not visceral fat, contributed to a high-ABI in that study population. The authors hypothesize that increased lower extremity muscle mass increases resistance to compression of lower extremity arteries, leading to a higher ABI measurement. Interestingly this study also noted higher daily physical activity in individuals with a high-ABI. Thus, patterns of body composition may significantly influence a high-ABI in certain populations. Accordingly, different anthropometric measures may be expected to vary in their association with high-ABI.
The association we demonstrate of increasing obesity with incident high-ABI likely has a multifactorial etiology. The high-ABI population in our study did have significantly higher HOMA-IR values at baseline, suggesting that insulin-mediated etiologies contribute in part to a high-ABI in our population. The persistence of this positive association among nondiabetic, obese individuals, however, after additionally adjusting for HOMA-IR, suggests that insulin-mediated mechanisms are not the only contributors to a high-ABI. The finding that measures of general obesity were stronger predictors of a high-ABI than were measures of visceral adiposity, while contrary to our hypothesis, may be consistent with the increased lower-extremity muscle mass explanation for increased ABI proposed by Tabara et al. 29. Though our study lacks the lower extremity CT data to evaluate this further, a higher femoral muscle mass may be indicative of increased generalized muscle mass, which can manifest as greater weight or BMI relative to visceral adiposity measures.
Given the various etiologies that may contribute to increased ABI values, any interpretation of a high-ABI should consider the population in which it was measured. For those in whom MAC or insulin-resistance is thought to be the predominant etiology, the high-ABI population may share increased CVD risk factor profiles with the diabetic population. In other populations, a high-ABI may not be a marker for increased cardiac or vascular risk. In the general population, a high-ABI is most likely measured in a heterogeneous population and would have multifactorial etiologies. This may explain some of the heterogeneity of risk factor associations and mortality findings among studies of high-ABI 1, 3, 5, 7, 14, 30. Additional studies utilizing data on lower extremity composition by CT or direct measures of arterial stiffness and waveforms may further characterize the predominant contributors to a high-ABI in specific populations.
Strengths of this study include the large sample size, longitudinal data, its geographically and ethnically diverse population, measurement of lower extremity pressures in both legs, allowing for the most inclusive definition of abnormal ABI, and rigorous quality control. Limitations of our study include the possibility that, by excluding participants with clinical cardiovascular disease at baseline, MESA may have differentially excluded individuals at the extremes of both ABI values and obesity. However, participants in all quartiles of anthropometric measures had similar ABI distribution shapes (data not shown), suggesting this bias was not likely. Other potential limitations concern the loss to follow-up, measurement error associated with ABI and anthropometric measures, and lack of visceral adiposity imaging in MESA. In addition, we recognize that inferring relative strengths of association by the comparison of quartiles should be performed cautiously, as these values are population-specific.
Acknowledgement
This research was supported by contracts N01-HC-95159 through N01-HC-95167 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesanhlbi.org.
FINANCIAL SUPPORT: Geoffrey H. Tison was supported by the Johns Hopkins Predoctoral Clinical Research Training Program grant number 5T32RR-023253 from the National Center for Research Resources – Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST: The authors declare no conflict of interest
REFERENCES
- 1.Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, Ix JH, Burke GL, Liu K, Shea S. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;56:1506–1512. doi: 10.1016/j.jacc.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ankle Brachial Index Collaboration. Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Fowkes FG, Lee AJ, Price JF, d’Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodriguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J, McDermott MM. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, Howard BV. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–739. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 4.Ono K, Tsuchida A, Kawai H, Matsuo H, Wakamatsu R, Maezawa A, Yano S, Kawada T, Nojima Y. Ankle-brachial blood pressure index predicts all-cause and cardiovascular mortality in hemodialysis patients. J Am Soc Nephrol. 2003;14:1591–1598. doi: 10.1097/01.asn.0000065547.98258.3d. [DOI] [PubMed] [Google Scholar]
- 5.O’Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle-arm index spectrum: results from the Cardiovascular Health Study. Circulation. 2006;113:388–393. doi: 10.1161/CIRCULATIONAHA.105.570903. [DOI] [PubMed] [Google Scholar]
- 6.Fowkes FG, Criqui M, Murray GD, ABI Collaboration Abstract 4201: Ankle Brachial Index Improves Prediction of Mortality Determined by Framingham Risk Score in 48,115 Healthy Subjects in Sixteen Studies Worldwide. Circulation. 2006;114:II_907. [Google Scholar]
- 7.McDermott MM, Liu K, Criqui MH, Ruth K, Goff D, Saad MF, Wu C, Homma S, Sharrett AR. Ankle-brachial index and subclinical cardiac and carotid disease: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 8.Allison MA, Laughlin GA, Barrett-Connor E, Langer R. Association between the ankle-brachial index and future coronary calcium (the Rancho Bernardo study) Am J Cardiol. 2006;97:181–186. doi: 10.1016/j.amjcard.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Allison MA, Hiatt WR, Hirsch AT, Coll JR, Criqui MH. A high ankle-brachial index is associated with increased cardiovascular disease morbidity and lower quality of life. J Am Coll Cardiol. 2008;51:1292–1298. doi: 10.1016/j.jacc.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 10.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P, Jr, Razak F, Sharma AM, Anand SS, INTERHEART Study Investigators Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 11.Yki-Jarvinen H, Westerbacka J. Insulin resistance, arterial stiffness and wave reflection. Adv Cardiol. 2007;44:252–260. doi: 10.1159/000096746. [DOI] [PubMed] [Google Scholar]
- 12.Sutton-Tyrrell K, Newman A, Simonsick EM, Havlik R, Pahor M, Lakatta E, Spurgeon H, Vaitkevicius P. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension. 2001;38:429–433. doi: 10.1161/01.hyp.38.3.429. [DOI] [PubMed] [Google Scholar]
- 13.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 14.Wattanakit K, Folsom AR, Duprez DA, Weatherley BD, Hirsch AT. Clinical significance of a high ankle-brachial index: insights from the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2007;190:459–464. doi: 10.1016/j.atherosclerosis.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 15.Weatherley BD, Nelson JJ, Heiss G, Chambless LE, Sharrett AR, Nieto FJ, Folsom AR, Rosamond WD. The association of the ankle-brachial index with incident coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study, 1987-2001. BMC Cardiovasc Disord. 2007;7:3. doi: 10.1186/1471-2261-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–1621. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 17.Lumley T, Kronmal R, Ma S. UW Biostatistics Working Paper Series. 2006. Relative Risk Regression in Medical Research: Models, Contrasts, Estimators, and Algorithms. [Google Scholar]
- 18.Wattanakit K, Folsom AR, Criqui MH, Kramer HJ, Cushman M, Shea S, Hirsch AT. Albuminuria and peripheral arterial disease: Results from the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 20.Young MJ, Adams JE, Anderson GF, Boulton AJ, Cavanagh PR. Medial arterial calcification in the feet of diabetic patients and matched non-diabetic control subjects. Diabetologia. 1993;36:615–621. doi: 10.1007/BF00404070. [DOI] [PubMed] [Google Scholar]
- 21.Everhart JE, Pettitt DJ, Knowler WC, Rose FA, Bennett PH. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia. 1988;31:16–23. doi: 10.1007/BF00279127. [DOI] [PubMed] [Google Scholar]
- 22.Tamminen M, Westerbacka J, Vehkavaara S, Yki-Jarvinen H. Insulin-induced decreases in aortic wave reflection and central systolic pressure are impaired in type 2 diabetes. Diabetes Care. 2002;25:2314–2319. doi: 10.2337/diacare.25.12.2314. [DOI] [PubMed] [Google Scholar]
- 23.Westerbacka J, Seppala-Lindroos A, Yki-Jarvinen H. Resistance to acute insulin induced decreases in large artery stiffness accompanies the insulin resistance syndrome. J Clin Endocrinol Metab. 2001;86:5262–5268. doi: 10.1210/jcem.86.11.8047. [DOI] [PubMed] [Google Scholar]
- 24.Scuteri A, Najjar SS, Muller DC, Andres R, Hougaku H, Metter EJ, Lakatta EG. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. J Am Coll Cardiol. 2004;43:1388–1395. doi: 10.1016/j.jacc.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 25.Tamminen MK, Westerbacka J, Vehkavaara S, Yki-Jarvinen H. Insulin therapy improves insulin actions on glucose metabolism and aortic wave reflection in type 2 diabetic patients. Eur J Clin Invest. 2003;33:855–860. doi: 10.1046/j.1365-2362.2003.01220.x. [DOI] [PubMed] [Google Scholar]
- 26.Brownlee M, Vlassara H, Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Intern Med. 1984;101:527–537. doi: 10.7326/0003-4819-101-4-527. [DOI] [PubMed] [Google Scholar]
- 27.Stout RW. Insulin as a mitogenic factor: role in the pathogenesis of cardiovascular disease. Am J Med. 1991;90:62S–65S. doi: 10.1016/0002-9343(91)90041-u. [DOI] [PubMed] [Google Scholar]
- 28.Nichols WW, O’Rourke MF. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. Oxford University Press; New York: 1998. p. 564. [Google Scholar]
- 29.Tabara Y, Igase M, Kido T, Ochi N, Miki T, Kohara K. Composition of lower extremity in relation to a high ankle-brachial index. J Hypertens. 2009;27:167–173. doi: 10.1097/HJH.0b013e328314b821. [DOI] [PubMed] [Google Scholar]
- 30.Resnick HE, Foster GL. Prevalence of elevated ankle-brachial index in the United States 1999 to 2002. Am J Med. 2005;118:676–679. doi: 10.1016/j.amjmed.2004.11.025. [DOI] [PubMed] [Google Scholar]