Introduction
Sphingosine kinase (SK) and the bioactive lipid it produces, sphingosine-1-phosphate (S1P), are critical signaling components in vascular biology, tumorogenesis, inflammation, and immune function (Reviewed in (Spiegel, S. and Milstien, S., 2007)). In some respects the SK signaling system follows an orthodox paradigm; SK produces S1P, which engages G-protein linked cell surface receptors and triggers cellular responses. A deeper look, however, reveals complex and intriguing aspects of SK signaling that are unique to this and other lipid signaling systems. First, the substrate (sphingosine) and product (S1P) of SK are lipids and their hydrophobic nature restricts both their localization and their movement within the cell. Secondly, SK is a key component of the sphingolipid metabolic network. Virtually all of the elements of this network, sphingosine, S1P, the ceramides, sphingomylein, and glycosphingolipids, have potent roles in signal transduction and cellular function. Therefore, control of sphingolipid metabolism can have profound effects on cell physiology, and this aspect of SK activity may be just as important as its role in producing S1P for signaling. As illustrated in Figure 1, SK activity can, at least in theory, critically control sphingolipid metabolism at two points, quite independent of generating S1P as a lipid signaling molecule. First, S1P is the substrate for sphingosine-1-phosphate lyase. The lyase is the only enzyme in the cell capable of the irreversible degradation of the sphingosine backbone. Sphingosine is incorporated into ceramide, ceramide is incorporated into sphingomyelin and glycosphingolipids. Degradation of these products can regenerate ceramide and sphingosine, which can then be reconverted back to the downstream products. However, once the sphingosine backbone is produced, the total level of sphingolipid can only be reduced by one enzyme, the S1P lyase. S1P lyase acts as a tumor suppressor (Oskouian, B. et al., 2006) and inhibition of the lyase protects against autoimmune dysfunction (Bagdanoff, J. T. et al., 2009). These effects are generally considered to be due to changes in S1P levels, but effects on overall sphingolipid metabolism may play an important role. Therefore, by producing S1P as the obligate precursor to S1P lyase, SK is situated at a pivotal point in sphingolipid metabolism.
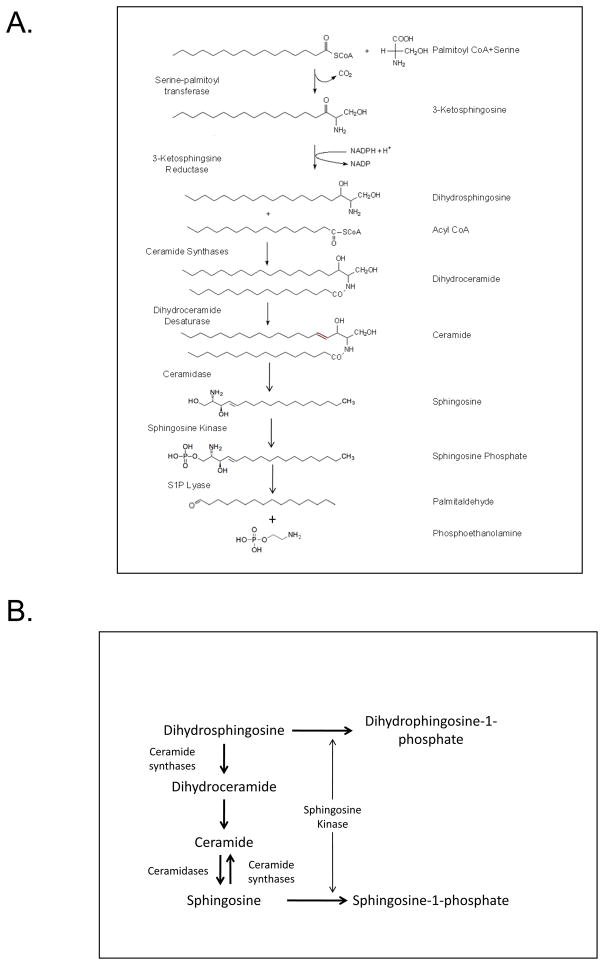
Figure 1.
A. Sphingosine/ceramide metabolism. Enzymes mediating the illustrated transformations are listed on the left, names of the products on the right. This figure highlights the biosynthetic pathway leading to sphingosine phosphate production. Not shown are the pathways that produce sphingomyelin and glycosphingolipids from ceramide and the degradative pathways that produce ceramide from sphingomyelin via sphingomyelinases, sphingosine from sphingosine-1-phosphate via sphingosine-1-phosphate phosphatases, and the degradation of sphingosine-1-phosphate by sphingosine-1-phosphate lyase. B. Sphingosine kinase substrates are used for ceramide synthesis. Illustrated are the routes of ceramide synthesis from the de novo pathway (from the top) and the salvage pathway (from the bottom) and how sphingosine kinase can potentially divert substrates from these routes of ceramide synthesis.
A second role for SK in the control of sphingolipid metabolism derives from the potential that it has to reduce levels of sphingosine, both in the de novo pathway for the production of ceramide (in which case the actual substrate would be dihydrosphingosine) and the ceramide salvage pathway (Figure 1, panel B). Ceramide is a potent bioactive lipid (Pettus, B. J. et al., 2002; Reynolds, C. P. et al., 2004). Generally it functions as a pro-apoptotic signal. Yet it is the essential precursor to sphingomyelin, a major cell surface phospholipid, as well as glycosphingolipids, which are thought to serve as cell surface signaling or recognition molecules. Therefore in non-apoptotic cells ceramide must be kept low, but be well controlled levels. During apoptosis ceramide is generated both by hydrolysis of sphingomyelin and by increased de novo and salvage pathway synthesis. SK therefore has the capacity to reduce ceramide production by blocking synthesis from either dihydrosphingosine or sphingosine by utilizing these lipids as substrates for the production of dihydrosphingosine-1-phosphate (dihydroS1P) or S1P respectively. The resulting dihydroS1P could then be degraded by the S1P lyase, dephosphorylated, or secreted from cells.
Considering that the substrates and products of SK are lipids, and therefore prone to associate with membranes, attention must be paid to the potential role of localized production and transport of S1P as a component in regulating S1P signaling and metabolism. Many of the enzymes of sphingolipid metabolism are membrane bound. The S1P-specific phosphatases, SPP1/2, are both membrane proteins localized to the endoplasmic reticulum (ER) (Le Stunff, H. et al., 2002; Ogawa, C. et al., 2003). The S1P lyase is also a membrane protein of the ER(Ikeda, M. et al., 2004). Additionally, the enzymes of ceramide biosynthesis are also restricted to the ER. On the other hand, to engage cell surface receptors, S1P must be secreted from the cell. Cell surface transporters of the ABC transporter and Spns2 families have been implicated in this secretion (Kawahara, A. et al., 2009; Kim, R. H. et al., 2009). Delivery of S1P to the ER or to the plasma membrane could therefore have substantially different outcomes in terms of degradation of S1P (in the ER) or secretion for signaling (PM). If SK exerts control over ceramide biosynthesis by diverting precursors in the ceramide biosynthetic pathway to phosphorylated derivatives, it seems likely that SK would have to access them in the ER, the site of ceramide biosynthesis. From these perspectives it can be seen that localizing SK production could potentially have a significant impact on both the utilization of S1P as either a signaling molecule or a metabolic intermediate on one hand, and on the biosynthesis of ceramide on the other. In the studies outlined here, we set out to explore these concepts.
Materials and Methods
Lipids
All lipids were purchased from Avanti Polar Lipids (Alabaster, AL)
Antibodies
Monoclonal anti-FLAG and monoclonal anit-Na+/K+ ATPase were from Sigma-Aldrich (St. Louis, MO). Anti-calnexin was from BD Transduction Laboratories (San Jose, CA).
Antisense oligonucleotides
Sphingosine-1-phosphate lyase-Applied Biosystems (Foster City, CA) #’s 118700, 118701, 214622
The following were from Qiagen (Valencia, CA):
Human S1P phosphatase 1, S102659300, S102659307
Human S1P phosphatase 2, S100716975, S104320771
Human lipid phosphate phosphatase 1/1a, S102659398, S102659391
Human lipid phosphate phosphatase 2, S102659405, S102659412
Human lipid phosphate phosphatase 3, S103043761, S103081995, S103087833
Real Time PCR
Taqman gene expression assays were purchased from Applied Biosystems (Foster City, CA).
Reagents
Unless otherwise specified, all other reagents were from Sigma Aldrich (St. Louis, MO). Lipofectamine 2000 and Lipofectamine RNAiMax were from Invitrogen (Eugene, OR). Tissue culture media and supplies were from Mediatech (Herndon, VA). Organic solvents were from Fisher Scientific (Pittsburgh, PA).
Plasmids and Transfections
All constructs used were as previously described and were transfected using Lipofectamine 2000 as previously described. (Siow, D. L. et al., 2010).
Gene expression studies
Isolation of mRNA and determination of mRNA levels by real-time PCR using the TaqMan gene expression assay was performed as previously described (Siow, D. L. et al., 2010).
Immunoblotting analysis
Quantitation of organelle markers and SK constructs in sucrose gradients used standard procedures, as previously described (Siow, D. L. et al., 2010). SK constructs were detected by probing for the FLAG epitope. Films were scanned and converted to image files for quantitation using the ImageQuant software (GE Healthcare, Piscataway, NJ).
Sucrose Density Centrifugation
Total membranes were fractionated by sucrose density centrifugation as described (Siow, D. L. et al., 2010). Briefly, cells were harvested by trypsinization, and homogenized by nitrogen cavitation. Total membranes were layered on a gradient consisting of 32%, 34%, 38%, 40%, 42%, 44%, and 60% sucrose in a 12 ml tube. Centrifugation was performed in an SW41 (Beckman-Coulter, Brea, CA) at 37,000 for 2.5 hours. Fractions (1ml) were collected from the top by hand.
Assay of S1P phosphohydrolase activity in cell extracts
Phosphohydrolase activity was determined by a modification of previously described method (Maceyka, M. et al., 2007). Total membranes were used in these assays. Membrane protein (50 μg) was incubated with 30 μM [33P]S1P in 180 μL assay buffer [100 mM HEPES (pH 7.5), 10 mM EDTA, 1 mM DTT and 1X protease inhibitor cocktail] for 30 minutes at 37°C. Reactions were quenched by adding 500 μL water saturated-butanol and 300 μL 1.5 M KCl. Samples were then extracted and the organic phase was extensively washed and then subjected to liquid scintillation counting.
Assay of S1P production by 33P-ATP labeling in intact cells
Cell culture in 48-well plates, transfections, and assays for S1P production were performed as previously described (Siow, D. L. and Wattenberg, B. W., 2007).
Determination of steady state sphingolipid content by liquid chromatography/tandem mass spectrometry
Transfection of Hela cells, lipid extractions, and LC/MS/MS were performed exactly as previously described (Siow, D. L. et al., 2010). This is a modification of an earlier method (Berdyshev, E. V. et al., 2006).
Reconstitution of (dihydro)ceramide biosynthesis in total membranes
Total Hela cell membranes were prepared by swelling trypsinized Hela cells in 10mM Tris/15mM KCl/1mM MgCl2 (pH 7.5) for 15 minutes, then adding 1M sucrose and 200mM EDTA to bring the final concentrations of sucrose to 250mM and EDTA to 2mM. Cells were broken with 7 strokes of a 7ml glass homogenizer. Nucleii and unbroken cells were removed by centrifugation (10 minutes, 600 X g). The supernatant was collected and membranes pelleted by centrifugation (20 minutes at 100,000 rpm in an TLA 100.1 rotor, Beckman). The pellet was resuspended in 25mM Tris/250mM sucrose, pH 7.5 to a concentration of 1.5mg/ml. The assay contained 50mM HEPES (pH 7.6), 1mM MgCl2, 20μM pyridoxyl-5′-phosphate, 0.5mM serine, 50μM palmitoyl CoA, 200μM ATP, 1mM dithiothreitol, 5mM glucose-6-phosphate, 200 μM NADPH, 1IU glucose-6-phosphate dehydrogenase, 35μg of total Hela cell membranes, and 1mCi 3H-serine in a total volume of 200μl. Incubations were carried out for 60 minutes at 37°C and terminated by the addition of 1.4ml CHCl3:MeOH (100:200). The phases were broken by the addition of 300μl CHCl3 and 300μl 1M KCl. The lower, organic phase was dried down, resuspended in CHCl3, 20μg of C16 ceramide was added to each tube as a standard. Samples were spotted on silica gel G thin layer chromatography plates and developed in CHCl3/MeOH/HAc (40:10:1). Ceramide spots were visualized by iodine vapor, and were scraped into scintillation vials for liquid scintillation counting.
Results and Discussion
Constructs of sphingosine kinase localized to specific intracellular compartments
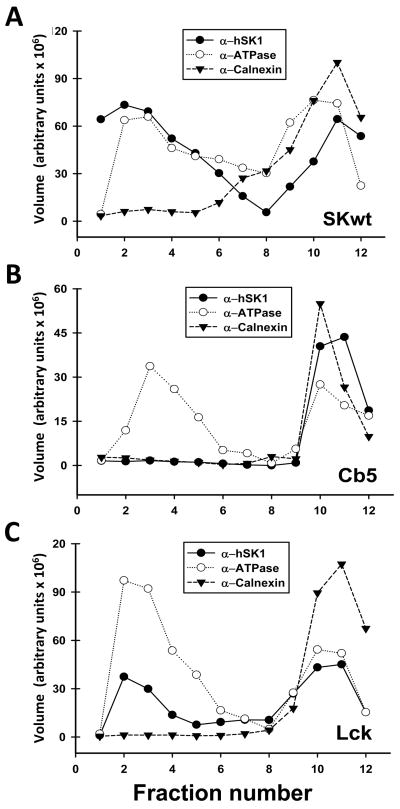
Sphingosine kinase exists as two major isoforms expressed from two genes; SK1 and SK2. SK2 is an interesting and important isoform, however SK1 is the major isoform expressed in a number of cell types and is the isoform studied here. SK1 is a cytoplasmic enzyme, lacking a membrane anchoring sequence. However, in response to agonists, SK1 translocates, at least partially, to the plasma membrane(Johnson, K. R. et al., 2002; Pitson, S. M. et al., 2003). To explore the impact of localized production of S1P we produced modified constructs of SK1 that were permanently localized either to the plasma membrane or the endoplasmic reticulum. The plasma membrane-targeted form of SK was produced by appending the myristoylation/palmitoylation sequence of Lck kinase to the amino terminus. The endoplasmic reticulum form was constructed by fusing the carboxy-terminal signal anchor of cytochrome B5 to the carboxy-terminus of SK1. Figure 2 illustrates, using sucrose density centrifugation, that these constructs are quantitatively targeted to the expected membrane sites. In these gradients the plasma membrane marker exhibits a bimodal distribution, as does the membrane targeted form of SK (Lck-SK). This localization has been confirmed by confocal microscopy (Siow, D. L. et al., 2010). In addition we have utilized the untargeted, wild-type, form of SK1.
Figure 2.
Density gradient fractionation establishes the specific localization of targeted constructs of sphingosine kinase. Hela cells were fractionated by sucrose density centrifugation as described in Materials and Methods. Levels of the plasma membrane marker Na+/K+ ATPase (open circles), the endoplasmic reticulum marker Calnexin (filled triangles), and the expressed constructs (filled circles) was determined by immunoblotting and quantitation by densitometry. A. Lck-SK. B. Cb5-SK. (Adapted from Supplementary Figure 1 of (Siow, D. L. et al., 2010) ).
A system to measure the acute production of S1P in intact cells. Use of chemical inhibitors and siRNA to deplete the enzymes of downstream metabolism of S1P
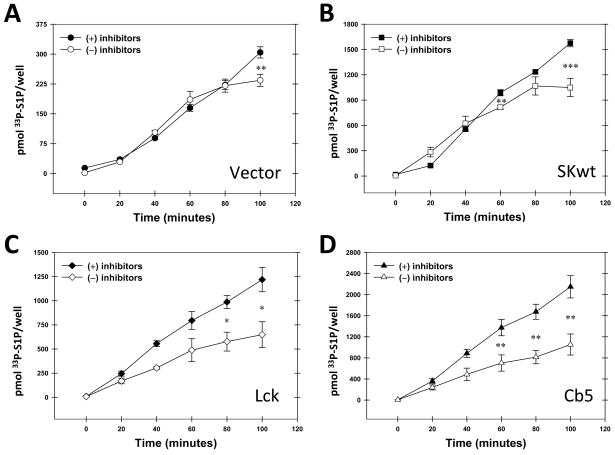
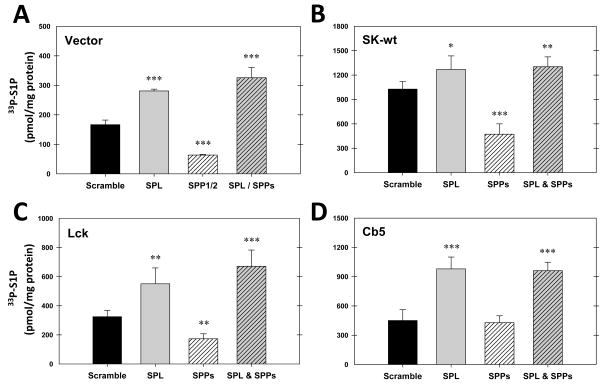
We recently established a unique system that measures the production of S1P in intact cells using labeling with 33P-ATP (Siow, D. L. et al., 2010). We have used this to test the concept that localized S1P production could affect the downstream metabolism of S1P. We expressed constructs of SK that were either not specifically localized (wild-type SK), or SK specifically localized to the plasma membrane (Lck-SK) or the endoplasmic reticulum (Cb5-SK). We also tested untransfected cells in which the only source of SK activity is endogenous SK. We initially predicted that S1P produced by Lck-SK would be relatively protected from degradation. Our reasoning was that a plasma membrane pool of S1P would be protected from the degradative enzymes localized to the endoplasmic reticulum. Conversely the ER-localized SK (Cb5-SK) would produce S1P that was rapidly degraded. To measure the degree of degradation of S1P we compared the production of S1P in the presence and absence of chemical inhibitors of the S1P phosphatases and lyase. Surprisingly, we found that these inhibitors protected S1P from degradation to a degree that was independent of the site of S1P synthesis (Figure 3). Interestingly, the S1P produced by endogenous SK activity is only slightly subject to degradation using this method.
Figure 3.
Chemical inhibitors of sphingosine-1-phosphate degradation enhance the acute production of sphingosine-1-phosphate independent of the site of sphingosine kinase localization. Hela cells were either unttransfected (panel A), or transfected with wild type sphingosine kinase (panel B), or sphingosine kinase localized to the plasma membrane (Lck-SK, panel C) or the endoplasmic reticulum (Cb5-SK, panel D). Sphingosine-1-phosphate production in intact cells was measured as described (Siow, D. L. and Wattenberg, B. W., 2007) in the presence or absence of a cocktail of inhibitors of phosphatases (10mM sodium fluoride, 1mM sodium orthovanadate) and the S1P lyase (4-deoxypyridoxine (0.5mM). Data are the means ± standard deviation of triplicate measurements and are representative of at least three independent experiments. Significance, as determined by a two-tailed, unpaired Student’s t test was (*) p≤0.05, (**)p≤0.01, and (***)p≤0.001.
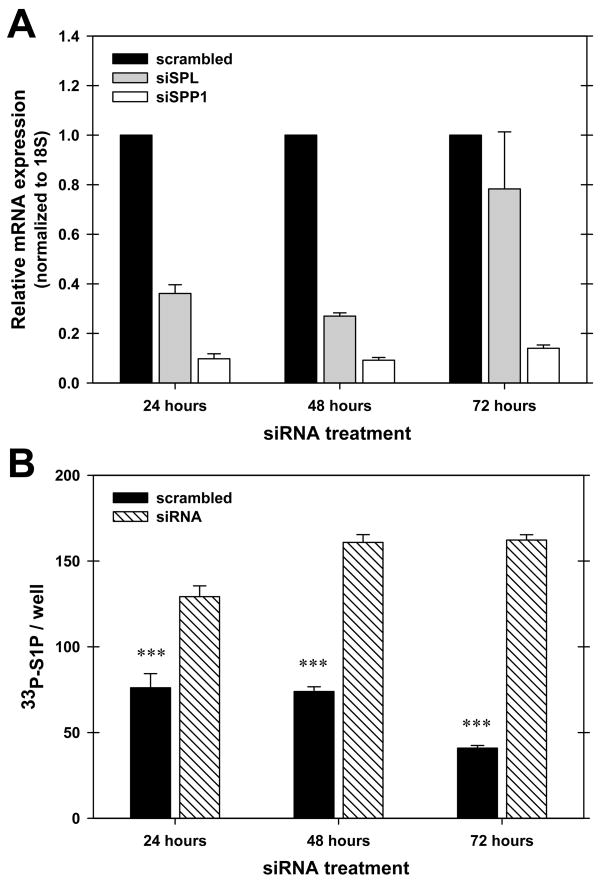
To confirm and extend the results using chemical inhibitors of the S1P-specific phosphatases and lyase, we used siRNA to knockdown these enzymes. Figure 4A demonstrates that these siRNA’s effectively deplete the mRNA of their respective targets. Measurement of protein and enzyme activity confirmed that these knockdowns were effective (Siow, D. L. et al., 2010). When S1P production was measured, the combined application of these siRNA’s clearly enhanced the production of this lipid (Figure 4B). We then tested the siRNAs for the SPPs and the lyase individually and in combination. The effect of this depletion on S1P produced at different sites was examined (Figure 5). Two surprising results were generated by these experiments. First, similar to what we had seen with the chemical inhibitors, we observed that the localization of the production of S1P had little effect on the degree to which the S1P was susceptible to degradation. Most surprisingly, the protection of S1P production by the combined effects of the SPP and lyase siRNAs was equivalent between the plasma membrane and endoplasmic-reticulum targeted SK constructs (Figure 5, panels C and D). We conclude from this result that S1P rapidly moves between membrane compartments. Our data indicate that specific intracellular pools, at least as generated by these constructs, are not stable and that a vigorous intercompartmental transport mechanism must exist for S1P.
Figure 4.
siRNA efficiently depletes mRNA of the S1P-specific phosphatase SPP1 and S1P-specific lyase and enhances S1P production. Hela cells were transfected with either a control (scrambled) siRNA or siRNA directed against SPP1 or S1P lyase as described in Materials and Methods. Panel A. At the indicated times total RNA was isolated and mRNA levels were measured by real-time PCR using Taqman primers as described in Materials and Methods. Expression was normalized to ribosomal 18S RNA and levels were normalized to levels in the control sample. The comparative CT method was used (2−−ΔΔCT). Data are the means ± standard deviation of triplicate measurements and are representative of at least three independent experiments. Panel B. Hela cells were transfected either with a control, scrambled siRNA oligonucleotide (filled bars), or siRNAs directed against SPP1, SPP2, and S1P lyase in combination (hatched bars). S1P production was measured in intact cells after the indicated times of incubation after siRNA transfection using 33P-ATP as described in Materials and Methods.
Figure 5.
Localization of S1P production does not strongly affect access of S1P to degradative enzymes. HeLa cells were depleted of the S1P lyase (SPL), the S1P-specific phosphatases (SPP), a combination of both (SPL+SPP) by siRNA transfection as described in Materials and Methods. Cells were either untransfected (Panel A), transfected with wild-type SK (Panel B), transfected with the plasma membrane targeted SK (Panel C) or transfected with the ER-targeted SK (Panel D). 24 hours later the rate of S1P production was measured for 90 minutes as described in Materials and Methods. Total protein was used to normalize lipid values. Shown are values plus or minus standard deviation of 4 samples per data point. These results are representative of at least 3 independent determinations. Significance, as determined by a two-tailed, unpaired Student’s t test was (*) p≤0.05, (**)p≤0.01, and (***)p≤0.001. (Modified from Figure 9 of (Siow, D. L. et al., 2010)).
A second surprising result was that siRNA knockdown of the S1P-specific phosphatases reduces rather than enhances S1P production. While this is counterintuitive, we believe that it may result from a recycling mechanism for S1P. Our model (Figure 6) is as follows: S1P is produced and may either be subject to degradation by the S1P lyase or by the S1P phosphatase. Lyase action is irreversible. However dephosphorylation by the S1P phosphatase diverts S1P from lyase degradation and allows the resulting sphingosine to by re-phosphorylated by SK to regenerate S1P. Knocking down the S1P phosphatases eliminates the recycling pathway and therefore promotes irreversible degradation by the S1P lyase. This notion is supported by the observation that siRNA knockdown of the lyase completely reverses the reduced levels of S1P resulting from SPP1/2 knockdown (Figure 5). It is important to note that contrary to our measurements of acute S1P production, long-term depletion of SPP1/2 (and of the more promiscuous lipid phosphate phosphatases LPP1–3, see below) results in increased mass levels of S1P, as would be expected (Zhao, Y. et al., 2007). We believe this discrepancy can be attributed to the different types of assays being used. In short-term labeling experiments, such as the ones illustrated here, the recycling pathway would be evident. However longer term accumulation studies, such as those of Natarajan and colleagues (Zhao, Y. et al., 2007), the role of the SPPs and LPPs in the steady state levels of S1P would predominate.
Figure 6.
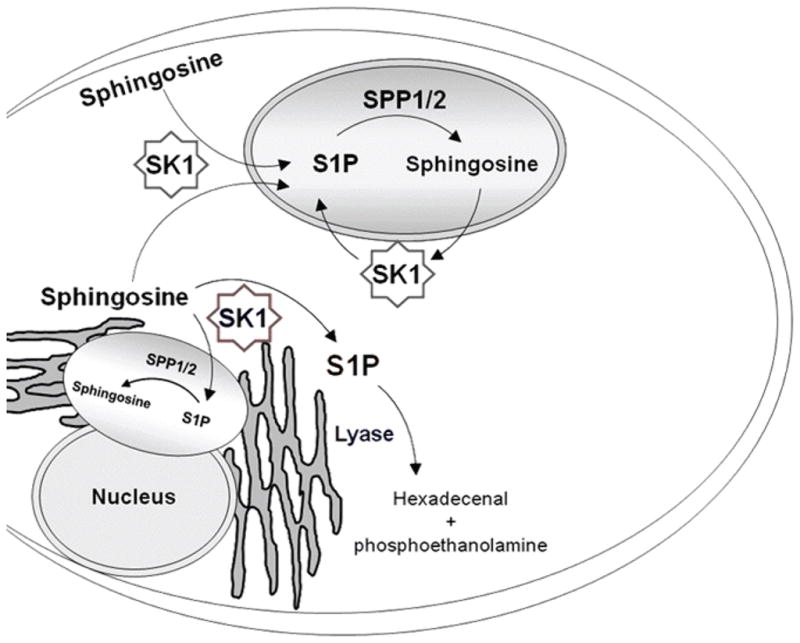
Model of role of sphingosine-1-phosphate phosphatase in directing sphingosine-1-phosphate into a recycling compartment. As demonstrated in Figure 5, we find that depletion of sphingosine-1-phosphate phosphatases reduces the accumulation of S1P in an acute measurement of S1P production. We speculate that dephosphorylation of S1P directs the resulting sphingosine into a compartment in which it can be re-phosphorylated by sphingosine kinase. This compartment directs S1P away from S1P lyase. Depletion of the S1P phosphatases therefore subjects S1P to S1P lyase degradation. This model is supported by the observation that depleting the S1P lyase completely reverses the effects of S1P phosphatase depletion (Figure 5).
Degradation of S1P preferentially affects the secreted pool of S1P
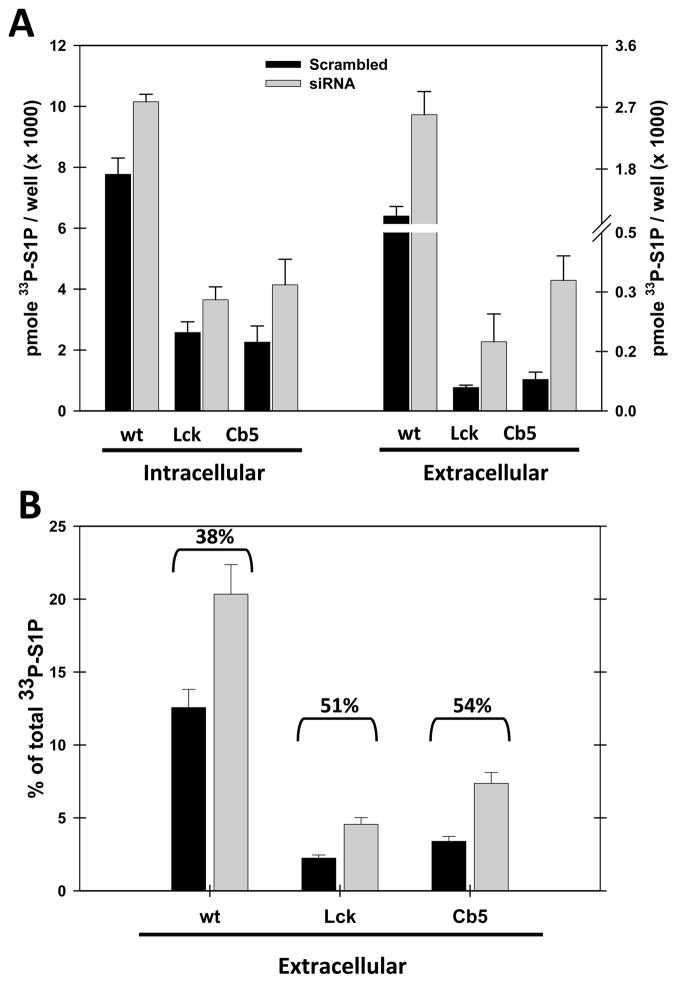
S1P is generated intracellularly, but is secreted from cells in order to interact with cell surface S1P receptors. The mechanism is thought to involve specific transporters, although the exact identity of the transporter(s) remains a matter of controversy(Kawahara, A. et al., 2009; Kim, R. H. et al., 2009). We sought to examine whether the the degradation of S1P differentially affects the secreted pool of S1P (Figure 7). Intracellular and extracellular S1P were measured after 60 minutes of S1P production in cells transfected either with wild-type, plasma membrane targeted (Lck-SK), or ER-targeted (Cb5-SK) SK. Incubations were performed with cells pre-incubated either with control siRNA or siRNA’s directed against S1P phosphatases and lyase. We find under control conditions that between 2 and 12% of total S1P is secreted under these conditions. Surprisingly, the untargeted SK generates S1P that is more efficiently secreted than S1P generated by membrane targeted SK constructs. In all cases, however, inhibition of S1P degradation preferentially enhances the S1P that is secreted from cells (Figure 7, panels A and B.) When calculated as the percentage of S1P secreted from the cells, inhibition of S1P degradation enhances the proportion secreted by over 50% in cells expressing the membrane-targeted forms of SK. This suggests that these constructs produce a pool of S1P that is both bound for secretion, but specifically sensitive to degradation.
Figure 7.
Inhibition of S1P degradation preferentially enhances the secretion of S1P. The S1P specific phosphatases and lyase were depleted in combination by siRNA treatment as described in Figure 5. Membrane-localized constructs of SK were then expressed for 24 hours and the acute production of S1P was measured for 100 minutes at 37°C. Cell supernatants, containing secreted S1P, were measured separately from S1P in the cell monolayers. S1P accumulation was increased in both intracellular and extracellular compartments, as expected (Panel A). Quantitation of the percentage of S1P secreted (Panel B) demonstrated that secreted S1P was preferentially protected by siRNA depletion of the degradative enzymes. Shown are means plus or minus standard deviation of 4 samples. Data shown are representative of 3 independent experiments. In panel B the percentage secretion was determined by the formula % secreted=(secreted/(cell associated + secreted)) × 100). Numbers above the bars depicts the percentage increase in secretion resulting from phosphatase and lyase depletion.
The non-specific lipid phosphatases preferentially utilize S1P generated at the plasma membrane
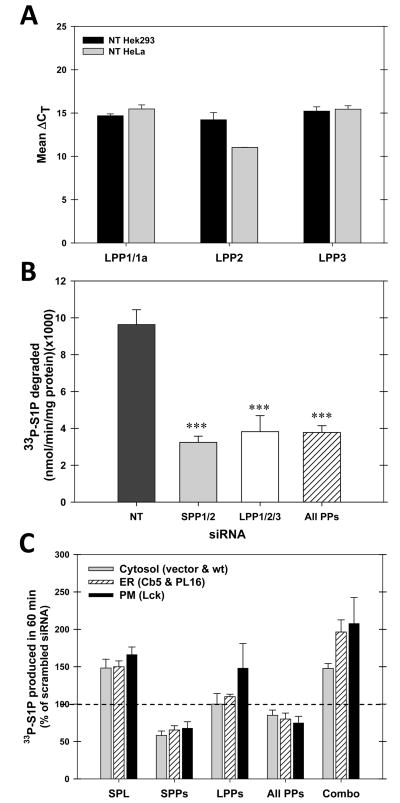
In addition to the S1P-specific phosphatases, there are lipid phosphatases with a broader substrate specificity (reviewed in (Sigal, Y. J. et al., 2005). These are known as the LPPs (lipid phosphate phosphatases), and there are three major isoforms, LPP1–3. Hela cells express all three isoforms at comparable levels (Figure 8, panel A). We employed siRNA technology to deplete all three isoforms. This substantially reduces mRNA levels (data not shown) and activity (Figure 8, panel B.). The effect of depleting these enzymes was tested on generation of S1P from both untargeted and membrane targeted SK constructs (Figure 8, panel C). Depletion of the LPP’s had no effect on the generation of S1P by endogenous SK, overexpressed wild-type SK, or overexpressed ER-targeted SK. However, depleting the LPPs significantly enhanced the production of S1P by the plasma membrane targeted form of SK. This indicates that S1P generated at the plasma membrane is preferentially degraded by one of these phosphatases. This is consistent with the plasma membrane localization of these enzymes (Sigal, Y. J. et al., 2005). Therefore, although S1P appears to rapidly move from the plasma membrane to the endoplasmic reticulum (see above), the plasma membrane localized phosphatases appear to have a kinetic advantage in the degradation of S1P localized to that site.
Figure 8.
Non-specific lipid phosphatases preferentially degrade a plasma membrane produced pool of sphingosine-1-phosphate. A. Real-time PCR establishes that all three isoforms of the non-specific lipid phosphatases (LPP’s) are present in Hek293 and Hela cells). Real time PCR was performed as described in Materials and Methods. B. siRNA depletion of lipid phosphatases depletes S1P phosphatase activity in cell extracts. Lysates were prepared from cells transfected with siRNA directed against the indicated enzymes, or a combination (“All PPs”). S1P phosphatase activity was measured as described in Materials and Methods. C. Depleting LPP’s enhances S1P accumulation when S1P is produced at the plasma membrane. Cells were depleted of the S1P lyase, SPPs, LPPs, a combination of the SPPs and the LPPs (“All PPs”) and a combination of the lyase, SPPs, and LPPs (“Combo”), transfected with cytsolic, plasma membrane, or ER-targeted forms of SK, and then S1P production was measured in intact cells by incorporation of 33P from 33P-ATP as described in Materials and Methods. The data is normalized to the values for scrambled siRNAs at the same concentration. The data for cytosolic SK is pooled data for endogenous SK and wild-type SK, and for ER-targeted SK the data is pooled for two ER-targeted forms, Cb5 and PL16. Means plus and minus standard deviations are shown.
Localization of sphingosine kinase dictates utilization of dihydrosphingosine as a substrate
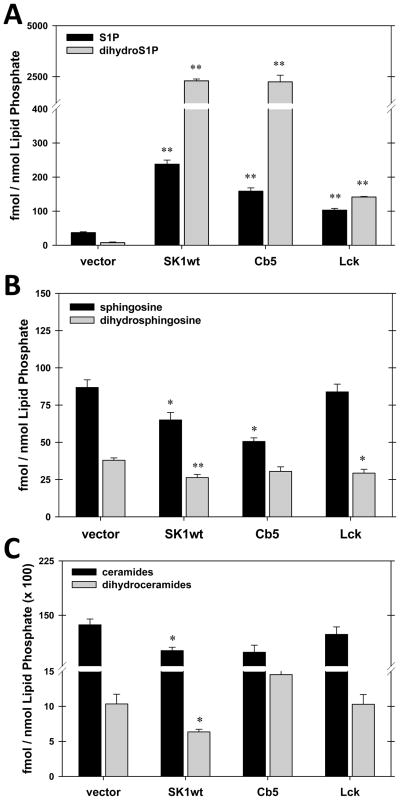
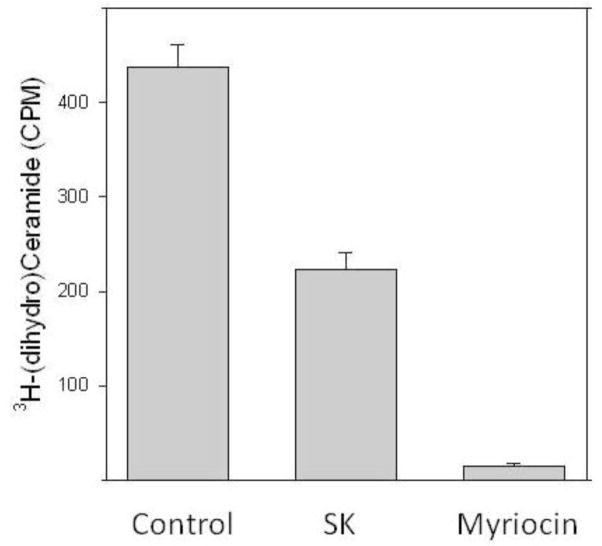
We utilized our targeted SK constructs to determine if localization of SK affects the steady-state accumulation of sphingolipids (Figure 9). SK constructs were expressed in Hela cells for 24 hours and lipid extracts were examined for sphingolipid content by mass spectroscopy. A striking observation was that high level expression of SK results in very high levels of dihydrosphingosine phosphate accumulation. A similar result has been reported by others (Berdyshev, E. V. et al., 2006). This result has two important implications. First, this result illustrates that sphingosine kinase can divert dihydrosphingosine from the ceramide biosynthetic pathway. This highlights a potential role of sphingosine kinase in the control of ceramide levels. Ceramide can be generated both by de novo synthesis and by degradation of higher level sphingolipids (Figure 1). Under apoptotic conditions, in which ceramide levels are elevated, both pathways come into play. However Hannun and colleagues have demonstrated that increased ceramide biosynthesis is pivotal in the ability of particular chemotherapeutic agents to induce increases in ceramide levels(Perry, D. K. et al., 2000). The role of sphingosine kinase in regulating ceramide levels under these conditions has yet to be tested. Secondly, it is notable that dihydrosphingosine levels are quite low at steady state, and do not appreciably change with SK overexpression (Figure 9, panel B). So the increase in dihydrosphingosine phosphate levels does not come at the expense of a static pool of dihydrosphingosine. Increased SK activity diminishes ceramide levels slightly (Figure 9, panel C), but this is far exceeded by the amount of dihydrosphingosine phosphate that is produced. How are ceramide levels maintained in the face of this large consumption of the precursor? Our data suggests that production of dihydroceramide is greatly increased to compensate for its utilization by SK. If this is correct, there is clearly an important feed-back mechanism that senses sphingolipid levels and modulates the biosynthetic pathway. The most likely target for this mechanism is the rate limiting step in sphingolipid biosynthesis, serine-palmitoyltransferase (reviewed in (Hanada, K., 2003)). As these observations were based on the levels of sphingolipids in intact cells resulting from 24 hours of SK overexpression, we wished to directly test whether SK could access dihydrosphingosine generated during ceramide biosynthesis. To accomplish this we reconstituted in a cell-free system the de novo synthesis of (dihydro)ceramide (our thin layer chromatography system does not distinguish ceramide from dihydroceramide). Total membranes were prepared from Hela cells and incubated with 3H-serine and co-factors for serine palmityoltransferase, 3-ketosphingosine reductase, and ceramide synthase (Figure 10). This generates (dihydro)ceramide. (Dihydro)ceramide production in this system is sensitive to the serine palmitoyltransferase inhibitor myriocin (Figure 10), confirming the specificity of the reaction. When cytosol from cells overexpressing wild-type SK is included in the reaction we observe a marked decrease in the levels of (dihydro)ceramide produced. Thus we have confirmed in a cell free system that SK can serve as a modulator of ceramide de novo synthesis.
Figure 9.
Dihydrosphingosine phosphate is produced at high levels by overexpression of cytosolic and ER-targeted, but not PM-targeted, forms of SK. The indicated SK constructs were transfected into HeLa cells and 24 hours later lipids were extracted and analyzed by mass spectroscopy as described in Materials and Methods. All lipid levels are normalized to total phospholipid content. Panel A. S1P (filled bars) and dihydro S1P (grey bars). Panel B. Sphingosine (filled bars) and dihydrosphingosine (grey bars). Panel C. Total ceramides. Ceramides (filled bars) and dihydroceramides (grey bars). Determinations are the mean plus or minus standard deviations of 3 independent experiments. Statistical significance of measurements differing from the vector control was determined by a two-tailed Student’s t Test. (*) p≤0.05, (**)p≤0.01 (Modified from Figure 3 of (Siow, D. L. et al., 2010)).
Figure 10.
Reconstitution of (dihydro)ceramide biosynthesis from 3H-serine and palmitoyl CoA using crude membranes. Total Hela cell membranes were incubated with 3H-serine, palmitoyl CoA and co-factors for 60 minutes, then extracted and analyzed by thin layer chromatography as described in Materials and Methods. The (dihydro)ceramide spot was visualized by iodine staining and scraped into scintillation vials. Shown are the total counts incorporated into the ceramide region of the TLC plate. Incubations also included control cytosol (“Control”) cytosol from cells overexpressing sphingosine kinase (“SK”) or the serine-palmitoyl transferase inhibitor myriocin (40μM). Shown are means plus or minus standard deviation of triplicate assays.
Another striking finding of these studies is that overexpression of the plasma membrane-, targeted form of SK, Lck-SK, does not lead to a substantial increase in dihydrosphingosine phosphate levels (Figure 9, panel A). In one sense, this is not unexpected. All of the enzymes involved in ceramide biosynthesis are found in the endoplasmic reticulum. Therefore SK that is restricted to the plasma membrane might not be expected to have access to the dihydrosphingosine generated in the endoplasmic reticulum. This indicates that, in contrast to S1P, dihydroS1P is present in pools that do not readily translocate between membranes. This illustrates that production of phosphorylated sphingosines by SK is regulated at the level of access of SK to distinct pools of substrates.
Conclusions
The study of the effects of SK localization on sphingolipid metabolism was prompted by the observation that localization of SK to the plasma membrane was critical for the ability of this enzyme to produce the transformed phenotype in mouse fibroblasts (Pitson, S. M. et al., 2005). We reasoned that S1P produced at the plasma membrane would be protected from the degradative enzymes, the S1P-specific phosphatases and S1P lyase, which are localized to the endoplasmic reticulum. Surprisingly, we find that localization of S1P production does not have a measureable effect on downstream metabolism. The explanation for this observation is that S1P must move rapidly between membrane compartments. Is this simply because S1P is a relatively soluble lipid due to its charged headgroup? We find that virtually all S1P is localized to membranes and there are almost undetectable levels in the cell cytosol (data not shown.) Moreover, S1P is only slowly extracted from membranes by lipid binding proteins such as BSA (data not shown). Therefore our data suggest that there is a robust, potentially specific, mechanism for intermembrane transport of S1P. We are currently characterizing the S1P intermembrane transport system as we believe this may have an important role in dictating the fate and function of S1P. We were also surprised by the observation that depletion of S1P-specific phosphatases decreased, rather than increased, the rate of accumulation of S1P. We suggest that phosphatase action inserts sphingosine into a recycling pool that is protected from S1P lyase activity (Figure 6). This is consistent with other reports that combined SK/SPP activity is required for the utilization of extracellular sphingosine for ceramide synthesis in both yeast and mammalian cells (Le, S. H. et al., 2007; Mao, C. et al., 1997). Similarly, extracellular S1P results in elevated intracellular S1P by the action of LPPs to generate sphingosine in a specific pool that is used by SK(Zhao, Y. et al., 2007). Together these findings indicate that sphingosine is compartmentalized and that this compartmentalization is the result of priming by the combined action of SK and S1P phosphohydrolases. A second major observation from the studies reported here is that when SK levels are elevated, the enzyme utilizes dihydrosphingosine that would otherwise enter the ceramide biosynthetic pathway. This is remarkable given that all of the precursors in this pathway are lipophilic and the enzymes in the pathway co-localize in the endoplasmic reticulum. It might be expected that this would promote substrate channeling. To the extent that this is the case, SK is still able to interrupt the flow of precursors in the ceramide biosynthetic pathway. Increased ceramide biosynthesis is thought to mediate the action of some cancer chemotherapeutic agents. SK overexpression is known to block the action of these agents. It remains to be seen whether the ability of SK to inhibit the action of anti-cancer compounds is mediated by its effects on ceramide biosynthesis. Interestingly, the plasma membrane-targeted form of SK is unable to accomplish this, presumably because it is physically prevented from accessing the surface of the ER. SK is known to translocate to the plasma membrane during agonist stimulation (Johnson, K. R. et al., 2002; Pitson, S. M. et al., 2003). Our observations suggest that one of the functions of this translocation may be to reduce the modulation of ceramide biosynthesis promoted by cytosolic SK. Our studies highlight that localization of the substrates and products of SK, as well as the localization of SK itself, is key to understanding how this fascinating enzyme produces its profound signaling effects.
Summary
The sphingosine kinases (sphingosine kinase-1 and −2) have been implicated in a variety of physiological functions. Discerning their mechanism of action is complicated because in addition to producing the potent lipid second messenger sphingosine-1-phoshphate, sphingosine kinases, both by producing sphingosine-1-phosphate and consuming sphingosine, have profound effects on sphingolipid metabolism. Sphingosine kinase-1 translocates to the plasma membrane upon agonist stimulation and this translocation is essential for the pro-oncogenic properties of this enzyme. Many of the enzymes of sphingolipid metabolism, including the enzymes that degrade sphingosine-1-phosphate, are membrane-bound with restricted subcellular distributions. In the work describe here we explore how subcellular localization of sphingosine kinase-1 affects the downstream metabolism of sphingosine-1-phosphate and the access of sphingosine kinase to its substrates. We find, surprisingly, that restricting sphingosine kinase to either the plasma membrane or the endoplasmic reticulum has a negligible effect on the rate of degradation of the sphingosine-1-phosphate that is produced. This suggests that sphingosine-1-phosphate is rapidly transported between membranes. However we also find that cytosolic or endoplasmic-reticulum targeted sphingosine kinase expressed at elevated levels produces extremely high levels of dihydrosphingosine-1-phosphate. Dihydrosphingosine is a proximal precursor in ceramide biosynthesis. Our data indicate that sphingosine kinase can divert substrate from the ceramide de novo synthesis pathway. However plasma membrane-restricted sphingosine kinase cannot access the pool of dihydrosphingosine. Therefore whereas sphingosine kinase localization does not affect downstream metabolism of sphingosine-1-phosphate, localization has an important effect on the pools of substrate to which this key signaling enzyme has access.
Acknowledgments
We would like to acknowledge Dr. Amy Massey and Dr. Christine Simmons for their cloning expertise, which was instrumental in the preparation of our targeted SK1 constructs. Funding for this work was provided by a Predoctoral Fellowship from the American Heart Association awarded to DL Siow and an NIH Project Grant (R01CA111987) awarded to BW Wattenberg and RO1 HL 079396 to V.N. E. Berdyshev was supported by the NIH grant (HL095440) and by the American Heart Association grant (0930028N). SM Pitson was supported by a Senior Research Fellowship from the National Health and Medical Research Council of Australia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagdanoff JT, Donoviel MS, Nouraldeen A, Tarver J, Fu Q, Carlsen M, Jessop TC, Zhang H, Hazelwood J, Nguyen H, Baugh SD, Gardyan M, Terranova KM, Barbosa J, Yan J, Bednarz M, Layek S, Courtney LF, Taylor J, Digeorge-Foushee AM, Gopinathan S, Bruce D, Smith T, Moran L, O’Neill E, Kramer J, Lai Z, Kimball SD, Liu Q, Sun W, Yu S, Swaffield J, Wilson A, Main A, Carson KG, Oravecz T, Augeri DJ. Inhibition of sphingosine-1-phosphate lyase for the treatment of autoimmune disorders. J Med Chem. 2009;52:3941–53. doi: 10.1021/jm900278w. [DOI] [PubMed] [Google Scholar]
- Berdyshev Evgeny V, Gorshkova Irina A, Usatyuk Peter, Zhao Yutong, Saatian Bahman, Hubbard Walter, Natarajan Viswanathan. De novo biosynthesis of dihydrosphingosine-1-phosphate by sphingosine kinase 1 in mammalian cells. Cellular Signalling. 2006;18:1779–92. doi: 10.1016/j.cellsig.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Hanada Kentaro. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2003;1632:16–30. doi: 10.1016/s1388-1981(03)00059-3. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Kihara A, Igarashi Y. Sphingosine-1-phosphate lyase SPL is an endoplasmic reticulum-resident, integral membrane protein with the pyridoxal 5′-phosphate binding domain exposed to the cytosol. Biochem Biophys Res Commun. 2004;325:338–43. doi: 10.1016/j.bbrc.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Becker KP, Facchinetti MM, Hannun YA, Obeid LM. PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. Extracellular release of sphingosine-1-phosphate induced by phorbol 12-myristate 13-acetate (PMA) J Biol Chem. 2002;277:35257–62. doi: 10.1074/jbc.M203033200. [DOI] [PubMed] [Google Scholar]
- Kawahara Atsuo, Nishi Tsuyoshi, Hisano Yu, Fukui Hajime, Yamaguchi Akihito, Mochizuki Naoki. The Sphingolipid Transporter Spns2 Functions in Migration of Zebrafish Myocardial Precursors. Science. 2009;323:524–7. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- Kim RH, Takabe K, Milstien S, Spiegel S. Export and functions of sphingosine-1-phosphate. Biochim Biophys Acta. 2009;1791:692–6. doi: 10.1016/j.bbalip.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Stunff H, Galve-Roperh I, Peterson C, Milstien S, Spiegel S. Sphingosine-1-phosphate phosphohydrolase in regulation of sphingolipid metabolism and apoptosis. J Cell Biol. 2002;158:1039–49. doi: 10.1083/jcb.200203123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Stunff H, Giussani P, Maceyka M, Lepine S, Milstien S, Spiegel S. Recycling of sphingosine is regulated by the concerted actions of sphingosine-1-phosphate phosphohydrolase 1 and sphingosine kinase 2. J Biol Chem. 2007;282:34372–80. doi: 10.1074/jbc.M703329200. [DOI] [PubMed] [Google Scholar]
- Maceyka M, Milstien S, Spiegel S. Measurement of mammalian sphingosine-1-phosphate phosphohydrolase activity in vitro and in vivo. Methods Enzymol. 2007;434:243–56. doi: 10.1016/S0076-6879(07)34013-5. [DOI] [PubMed] [Google Scholar]
- Mao Cungui, Wadleigh Martha, Jenkins Gary M, Hannun Yusuf A, Obeid Lina M. Identification and Characterization of Saccharomyces cerevisiae Dihydrosphingosine-1-phosphate Phosphatase. J Biol Chem. 1997;272:28690–4. doi: 10.1074/jbc.272.45.28690. [DOI] [PubMed] [Google Scholar]
- Ogawa Chie, Kihara Akio, Gokoh Maiko, Igarashi Yasuyuki. Identification and Characterization of a Novel Human Sphingosine-1-phosphate Phosphohydrolase, hSPP2. J Biol Chem. 2003;278:1268–72. doi: 10.1074/jbc.M209514200. [DOI] [PubMed] [Google Scholar]
- Oskouian Babak, Sooriyakumaran Prathap, Borowsky Alexander D, Crans Angelina, llard-Telm Lisa, Tam Yuen Yee, Bandhuvula Padmavathi, Saba Julie D. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is down-regulated in colon cancer. PNAS. 2006;103:17384–9. doi: 10.1073/pnas.0600050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry David K, Carton Jill, Shah Amit K, Meredith Filmore, Uhlinger David J, Hannun Yusuf A. Serine Palmitoyltransferase Regulates de NovoCeramide Generation during Etoposide-induced Apoptosis. J Biol Chem. 2000;275:9078–84. doi: 10.1074/jbc.275.12.9078. [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta. 2002;1585:114–25. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- Pitson SM, Xia P, Leclercq TM, Moretti PA, Zebol JR, Lynn HE, Wattenberg BW, Vadas MA. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitson Stuart M, Moretti Paul AB, Zebol Julia R, Lynn Helen E, Xia Pu, Vadas Mathew A, Wattenberg Binks W. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CP, Maurer BJ, Kolesnick RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004;206:169–80. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- Sigal YJ, McDermott MI, Morris AJ. Integral membrane lipid phosphatases/phosphotransferases: common structure and diverse functions. Biochem J. 2005;387:281–93. doi: 10.1042/BJ20041771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siow Deanna L, Anderson Charles D, Berdyshev Evgeny V, Skobeleva Anastasia, Pitson Stuart M, Wattenberg Binks W. Intracellular localization of sphingosine kinase 1 alters access to substrate pools but does not affect the degradative fate of sphingosine-1-phosphate. J Lipid Res. 2010 doi: 10.1194/jlr.M004374. jlr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siow Deanna L, Wattenberg Binks W. An assay system for measuring the acute production of sphingosine 1-phosphate in intact monolayers. Analytical Biochemistry. 2007;371:184–93. doi: 10.1016/j.ab.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel Sarah, Milstien Sheldon. Functions of the Multifaceted Family of Sphingosine Kinases and Some Close Relatives. J Biol Chem. 2007;282:2125–9. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Kalari SK, Usatyuk PV, Gorshkova I, He D, Watkins T, Brindley DN, Sun C, Bittman R, Garcia JG, Berdyshev EV, Natarajan V. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J Biol Chem. 2007;282:14165–77. doi: 10.1074/jbc.M701279200. [DOI] [PMC free article] [PubMed] [Google Scholar]