Abstract
The ionotropic glutamate receptor subunit, GluK1 (GluR5), is expressed in many regions of nervous system related to sensory transmission. Recently, a selective ligand for the GluK1 receptor, MSVIII-19 (8,9-dideoxy-neodysiherbaine), was synthesized as a derivative of dysiherbaine, a toxin isolated from the marine sponge Lendenfeldia chodrodes. MSVIII-19 potently desensitizes GluK1 receptors without channel activation, rendering it useful as a functional antagonist. Given the high selectivity for GluK1 and the proposed role for this glutamate receptor in nociception, we sought to test the analgesic potential of MSVIII-19 in a series of models of inflammatory, neuropathic, and visceral pain in mice. MSVIII-19 delivered intrathecally (i.t.) dose-dependently reduced formalin-induced spontaneous behaviors and reduced thermal hypersensitivity 3 hours after formalin injection and 24 hours after complete freund’s adjuvant-induced inflammation, but had no effect on mechanical sensitivity in the same models. I.T. MSVIII-19 significantly reduced both thermal hyperalgesia and mechanical hypersensitivity in the chronic constriction injury model of neuropathic pain, but had no effect in the acetic acid model of visceral pain. Peripheral administration of MSVIII-19 had no analgesic efficacy in any of these models. Finally, i.t. MSVIII-19 did not alter responses in tail flick tests or performance on the accelerating RotaRod. These data suggest that spinal administration of MSVIII-19 reverses hypersensitivity in several models of pain in mice, supporting the clinical potential of GluK1 antagonists for the management of pain.
Introduction
Glutamate is the primary excitatory neurotransmitter in the mammalian central nervous system and exerts its effects via activation of a large number of receptors that include both G-protein coupled metabotropic receptors and ligand-gated ion channels known as ionotropic glutamate receptors [9]. Kainate receptors represent a subset of ionotropic glutamate receptors (iGluRs) that are potently activated by the natural products kainic acid and domoic acid. The other members of the iGluR family, AMPA and NMDA receptors, play prominent roles in mediating excitatory synaptic transmission and plasticity in the brain, whereas the roles of kainite receptors in the nervous system are less well understood [3].
Kainate receptors are heteromeric channels formed by combinations of five different gene products, GluK1–5, which were formerly known as GluR5, GluR6, GluR7, KA1 and KA2 [3]. Several recent studies provided evidence that receptors primarily comprised of the GluK1 subunit are important in transmission in nociceptive pathways, particularly in the context of persistent pain states [13, 16]. GluK1 is expressed at many levels of the pain neuraxis, including the dorsal root ganglion, spinal cord dorsal horn, and in central neurons in many areas involved in pain transmission or perception. Previous studies of the role of GluK1 in rodent models of pain have utilized gene knockout mice [16] or drugs administered via systemic or intracisternal routes, making localization of the functionally important sites difficult [5, 13, 25]. Spinally-administered GluK1 receptor antagonists have been shown to reduce responses of spinothalamic tract neurons to noxious stimuli in primates [21], suggesting that GluK1 receptors expressed in the cord might be important in mediating analgesic actions of systemically applied antagonists and reduced pain in GluK1-deficient mice.
We recently determined that MSVIII-19, a synthetic derivative of the marine toxin dysiherbaine (DH), is a potent and selective inhibitor of GluK1 receptor activation through a mechanism distinct from previously characterized competitive antagonists [8, 22] Here, we sought to test if the compound exhibited antinociceptive efficacy in mouse models of inflammatory, visceral, and neuropathic pain, with a particular focus on GluK1-containing receptors expressed at the level of the spinal cord.
Materials and Methods
Animals
Experiments were performed on eight to twelve week-old male Swiss Webster mice from Taconic (Hudson, NY). Mice were group housed on a 12 h light/dark cycle with food and water available ad libitum. All animals were treated in accordance with ethical guidelines of the National Institute of Health and The International Association for the Study of Pain. Studies were approved by the Animal Care and use Committee of Washington University School of Medicine. The mice were allowed to acclimate for at least three days before any behavioral testing.
Drug administration
Intrathecal Injection
A method modified from that described [12] was used for an intrathecal (i.t.) injection of drug. Briefly, a 30½ gauge stainless needle attached a 50 µl Hamilton microsyringe was inserted between L5 and L6 vertebrae of conscious mice. A volume of 5 µl of drug solution or vehicle was injected into the subarachnoid space.
Peripheral Injection
A 30½ gauge stainless needle attached a 50 µl Hamilton microsyringe was inserted into the plantar skin of the hindpaw, and a total volume of 10 µl of drug or vehicle solution was injected.
All behavior tests were performed with the experimenter blind to treatment. All drug injections were administered 15 minutes prior to initiating behavior testing.
Behavioral analysis
Formalin Test
Mice were habituated in the behavior testing room for at least 30 minutes prior to testing. Fifteen minutes before formalin injection, drug or vehicle was injected either i.t. or intraplantar. Then, 10 µl of 5% formalin (Sigma, St. Louis, MO) was subcutaneously injected into the left hind paw plantar using a 50 µl Hamilton microsyringe with a 30½ gauge needle. Immediately after formalin injection, the mice were put into an observation chamber. The amount of time spent licking, biting or shaking the paw was measured, and data were pooled into five minute bins. The total observation time was 60 minutes.
Mechanical Sensitivity Testing
Animals were placed in individual 10 × 10 × 15 cm plastic boxes on an elevated metal mesh floor and allowed to acclimate for at least 30 min before drug or vehicle injection. Mechanical sensitivity was tested using von Frey sensory evaluator filaments (North Coast Medical, Inc. Morgan Hill, CA), in ascending order. Each von Frey filament was applied 5 times, and the threshold response was calculated as the filament at which a response (withdrawal, licking or shaking) was elicited on 3/5 trials.
Thermal Sensitivity Testing
The Hargreaves’ test was used for evaluation of thermal sensitivity. Mice were placed in individual 10 × 10 × 15 cm plastic boxes of on a glass floor of the apparatus (Model 390, IITC Life Science) and allowed to acclimate for at least 30 min before drug or vehicle injection. Each paw was tested at least three times. The average duration of the three trials was calculated as the withdrawal latency. The mice were tested with an active intensity of 22% of the apparatus; a cut-off time of 20 seconds was used to avoid tissue injury.
Complete Freund’s Adjuvant Test
Complete Freund’s adjuvant (CFA; 10 µl; Sigma, St. Louis, MO) was injected into the left paw using a 50 µl Hamilton microsyringe with a 30 ½ gauge needle. The CFA injection induced an area of localized edema and inflammation, with a progressive reduction in mechanical withdrawal threshold.
Tail flick test
The tail-flick reflex was evoked by focused, radiant heat applied to the tail. The withdrawal response is detected and timed. The apparatus (Model 33, IITC Life Science) was used at the active intensity of 90%; a cut-off time of 10 seconds was used to avoid tissue injury.
Chronic Constriction Injury (CCI)
The CCI model was produced similar to a method described previously [1]. Adult mice were deeply anesthetized with sodium pentobarbital (60 mg/kg, i.p.). The left sciatic nerve was exposed at mid-thigh level, and 2 chromic gut ligatures (6–0) were tied loosely around the nerve, 1–2 mm apart to cause chronic constrictive injury (CCI). Great care was taken to tie the ligations so that the diameter of the nerve was just barely constricted under magnified visual inspection. Testing began 2 weeks after the surgery.
Visceral Nociceptive Test
Drug effects on visceral nociception were evaluated using the acetic acid-induced writhing test. Acetic acid (0.6% v/v, 5 ml/kg) was injected intraperitoneally, and the mouse was placed in a plastic cage. The intensity of nociceptive behavior was quantified by counting the total number of writhes occurring between 0 and 20 min after acetic acid injection.
Evaluation of Motor Function
To test MSVIII-19 effects on motor function, the accelerating RotaRod (Ugo Basile, Comerio, Italy) was used. Mice were divided into drug and vehicle groups. The drug group was divided into 0.5, 1.0 and 2.0 nmol groups. The mice were not trained before the test. The mice were tested for five 5min trials with an interval of 15 minutes. Latency to fall off of the accelerating rotarod was determined.
Electrophysiology
Mice between the ages of P2 and P5 were rapidly decapitated and the spines were transferred to 10 mM HEPES-buffered saline solution. Dorsal root ganglia were removed after bisection of the spinal column and removal of the spinal cord. Ganglia were incubated in 20 units/ml papain in HEPES-buffered saline with 1 mM CaCl2 and 0.5 mM EDTA at 37°C for 30 mins, washed twice in DMEM media, and triturated with a flame-polished glass pipette. Dissociated neurons were plated on poly-D-lysine/collagen-coated glass coverslips and allowed to recover for 4–6 h in a 37°C incubator with 5% CO2.
Patch clamp recordings were made using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA). Patch electrodes were thick-walled borosilicate glass (Warner Instruments, Hamden, CT) and had a final resistance of 2 – 4 MΩ after fire-polishing. The internal solution was composed of 110 mM CsF, 30 mM CsCl, 4 mM NaCl, 0.5 mM CaCl2, 10 mM HEPES, and 5 mM EGTA (adjusted to pH 7.3 with CsOH). The external bath solution contained 150 mM NaCl, 2.8 mM KCl, 1.8 mM CaCl2, 1.0 mM MgCl2, and 10 mM HEPES (pH was adjusted to 7.3 with NaOH). Drugs were applied through three-barrel glass tubing (Vitro Dynamics, Rockaway, NJ) which had been pulled to a internal barrel diameter of ~80 µm and mounted on a piezo-ceramic bimorph. The piezo bimorph was driven by TTL pulses from pClamp 9 software (Axon Instruments) fed through a stimulation-isolation unit (S-100, Winston Electronic Co., Millbrae, CA). Data were acquired directly to a computer and were analyzed off-line using pClamp software. Inhibition-response relationships were fitted by nonlinear regression using GraphPad Prism software (La Jolla, CA) according to a one-site competition curve with constraints on the minimum and maximum percent of control amplitudes (0 and 100%, respectively).
Data presentation and statistical analysis
Results were expressed as mean ± s.e.m. Statistical comparisons were made using one way or two way analysis of variance or unpaired T-test using Graphpad Prism. In all cases, P<0.05 was considered statistically significant. * means P<0.05; ** means P<0.01; *** means P<0.001.
Results
Actions of MSVIII-19 on Native Kainate Receptors in Dorsal Root Ganglion Neurons
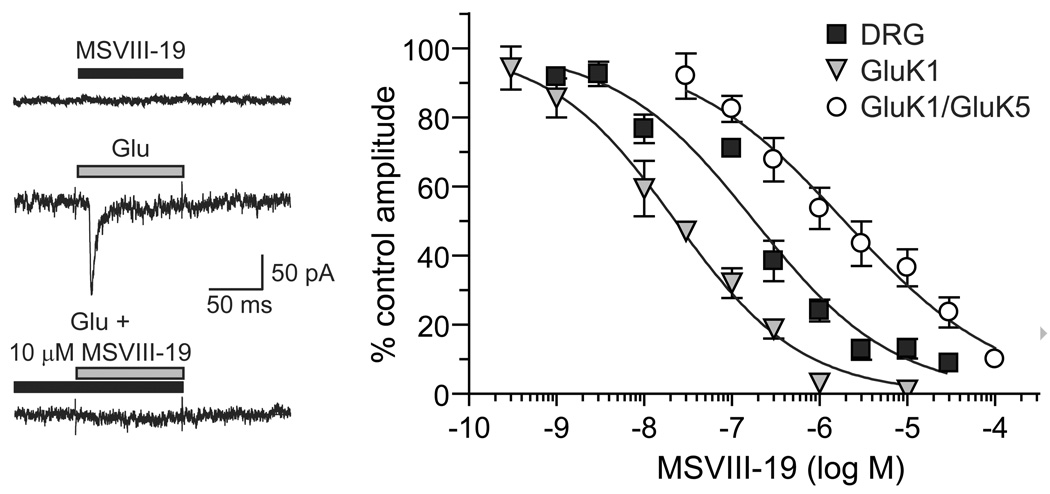
The actions of MSVIII-19 were characterized previously on recombinant kainate receptors, where it was found to be highly selective for homomeric GluK1 receptors and of lower potency on heteromeric GluK1/GluK5 or GluK2/GluK5 receptors [22]. The mechanistic basis for inhibition of GluK1 receptors by MSVIII-19 was later determined to be through exceptionally potent and complete desensitization, with very weak partial agonist activity only detected at very high concentrations of the compound [8]. Thus, it is most effective as a functional antagonist of receptor activation. To determine its potency for inhibition of a neuronal receptor population that may contribute to the behavioral actions observed in this study, we measured MSVIII-19 inhibition of kainate receptor currents in dorsal root ganglion (DRG) neurons, which are known to contain GluK1 as a critical and predominant subunit. As with recombinant kainate receptors, low micromolar concentrations of MSVIII-19 applied alone did not elicit detectable currents in whole-cell patch clamp recordings from acutely isolated mouse DRG neurons. MSVIII-19 applied in the absence of glutamate did not produce a current from DRG kainate receptors (n=4 at 30 µM, p>0.05 relative to basal current amplitude; Fig 1). Pre-application of MSVIII-19 efficiently reduced the amplitudes of currents evoked by a saturating concentration of glutamate (10 mM) (see example in Figure 1), consistent with activity as a functional antagonist. MSVIII-19 effects were reversible, consistent with its activity on recombinant GluK1-containing receptors [22]. Inhibition-response data for a range of MSVIII-19 concentrations were best-fit with a single-site logistic curve to yield an IC50 value of 179 nM (95% confidence interval: 127 – 253 nM, n = 3–4 measures at each concentration, Figure 1). This value falls in between that of recombinant homomeric GluK1 (23 nM) and heteromeric GluK1/GluK5 receptors (1.9 µM) [22], suggesting that DRG neurons contain a mixed population of homomeric and heteromeric kainate receptors. This is consistent with previous studies in which DRG neuronal kainate receptors are similar in function to homomeric GluK1 receptors in many respects (e.g., [20, 27, 28], but also likely contain a complement of heteromeric GluK5-containing receptors [14, 19].
Figure 1.
Representative neuronal kainate receptor currents evoked by glutamate in the absence and presence of 10 µM MSVIII-19 are shown in the top panel. Also shown is a representative trace showing the lack of any detectable currents induced by 30 µM MSVIII-19 alone. Glutamate (10 mM) was applied for 100 ms to a small-diameter, acutely isolated mouse DRG neuron held in voltage clamp at −70 mV. MSVIII-19 was applied for at least one minute prior to the test glutamate application. Data in the inhibition-response curve were fit to a single site logistic equation with a variable slope to derive an IC50 of 179 nM. The Hill slope was relatively shallow (0.55), as was the case with inhibition of recombinant GluK1 and GluK1/GluK5 receptors. Data and logistic fits for the latter are shown for comparative purposes and were reported in our previous publication[22].
The effects of MSVIII-19 in models of inflammatory pain
The Formalin Test
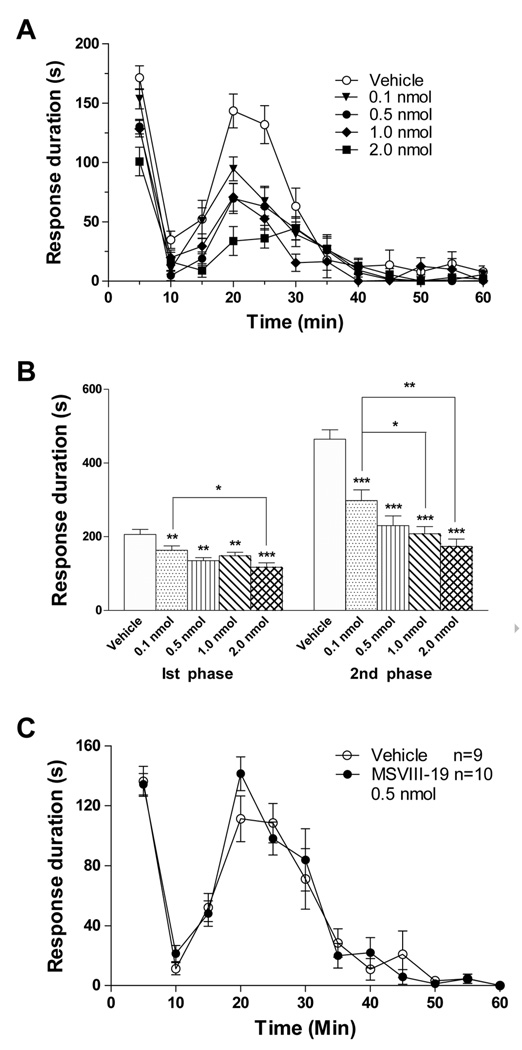
The formalin test was performed on five groups of mice after pretreatment with an intrathecal injection of vehicle or MSVIII-19 at 2 nmol, 1 nmol, 0.5 nmol and 0.1 nmol 15 min prior to formalin injection in the paw. The time spent licking, shaking and lifting the injected paw was recorded in 5 min bins (Figure 2a). MSVIII-19 resulted in a significant dose-dependent suppression of pain behaviors relative to vehicle-treated mice in both characteristic first (5–10 min time point) and second (15–60 min post-treatment) phases of the behavioral response to formalin (Figure 2B).
Figure 2. Spinally but not peripherally administered MSVIII-19 is analgesic in the formalin model.
A, Intrathecal injection of MSVIII-19 dose-dependently reduced spontaneous pain-related behaviors in both the first and second phases of the formalin test. (0.5 nmol, n=10). Vehicle n=9; 0.1 nmol, n=11; 0.5 nmol, n=7; 1 nmol, n=11; 2 nmol, n=9. At the 5 minute time point, MSVIII-19 significantly reduced responses at the 0.5 (P<0.05), 1.0 (P<0.01), and 2.0 nmol (P<0.001) doses (ANOVA with Bonferroni post-hoc test). At the 20 and 25 minute timepoints, all dose of MSVIII-19 significantly reduced responses (P<0.001). B). Summed data for the first phase (0–10min) and second phase (15–60min) showing significant dose-dependent inhibition of both the first (p<0.01) and second (p<0.001) phases by intrathecally administered MSVIII-19. C) Peripheral (intraplantar) administration of MSVIII-19 (0.5nmol) had no effect on spontaneous pain-related behaviors in the formalin test.
Previous studies suggested that peripheral GluK1 receptors located at sensory nerve endings in the skin are involved in nociceptor activation and sensitization [6]. We therefore sought to test whether blocking GluK1 receptors in the periphery reduces formalin-induced pain behaviors. Intraplantar pre-treatment with MSVIII-19 (0.5 nmol, 15 min prior to formalin injection) did not significantly reduce formalin-induced behaviors relative to vehicle treated mice (Figure 2c), supporting the interpretation that kainate receptors involved in this form of pain transmission are localized to spinal cord including dorsal horn neurons, primary afferent terminals, or both.
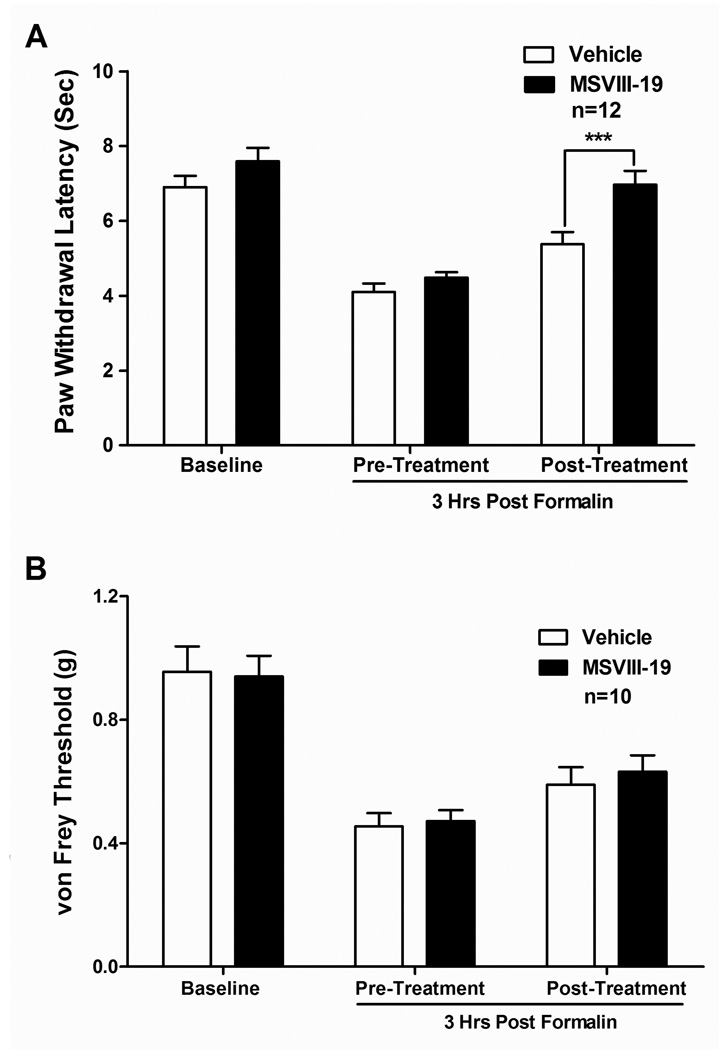
We also determined the effects of MSVIII-19 on thermal hypersensitivity induced by formalin paw injections using a Hargreaves’ test, which measures the latency to paw withdrawal upon application of heat. Baseline paw withdrawal latencies were measured prior to formalin injection. Formalin was then injected and paw withdrawal latencies were again measured 3 hr post-formalin injection to test for thermal hypersensitivity. The mice were then split into two groups: one group received MSVIII-19 (0.5 nmol) and one group received vehicle intrathecally. Thermal withdrawal latencies were measured again 15 minutes later. MSVIII-19 significantly reduced thermal hypersensitivity relative to vehicle-injected mice (p<0.001, n = 12 per group; Fig 3a), restoring the mean withdrawal latency to baseline levels.
Figure 3. Spinally administered MSVIII-19 selectively reduces thermal hypersensitivity in the formalin model.
Thermal or mechanical baselines were taken, followed by paw injection with 5% formalin. Three hours after formalin injection, mice were tested for thermal (A) or mechanical (B) sensitivity. The mice then received an intrathecal injection of either vehicle or MSVIII-19 (0.5 nmol), and were then tested for post-treatment thermal or mechanical sensitivity. Formalin induced significant thermal and mechanical hypersensitivity relative to pre-formalin baselines (P < 0.01; ANOVA with Tukey’s post-hoc test). Thermal hypersensitivity was significantly reduced by MSVIII-19 relative to vehicle, whereas mechanical hypersensitivity was not affected. *** p< 0.001
Using a similar experimental design, we tested for mechanical hypersensitivity 3 hr after formalin injection with von Frey filaments of differing tensile strength. Formalin injection resulted in robust mechanical hypersensitivity (labeled as 3 hrs-Pre group, Figure 3b), as evidenced by the reduced force required to elicit paw withdrawal, but no difference was noted between the MSVIII-19- and vehicle-injected groups. Thus, the compound did not significantly reduce mechanical hypersensitivity in this model of inflammatory pain in mice (Figure 3b).
The Complete Freund's Adjuvant model of inflammatory pain
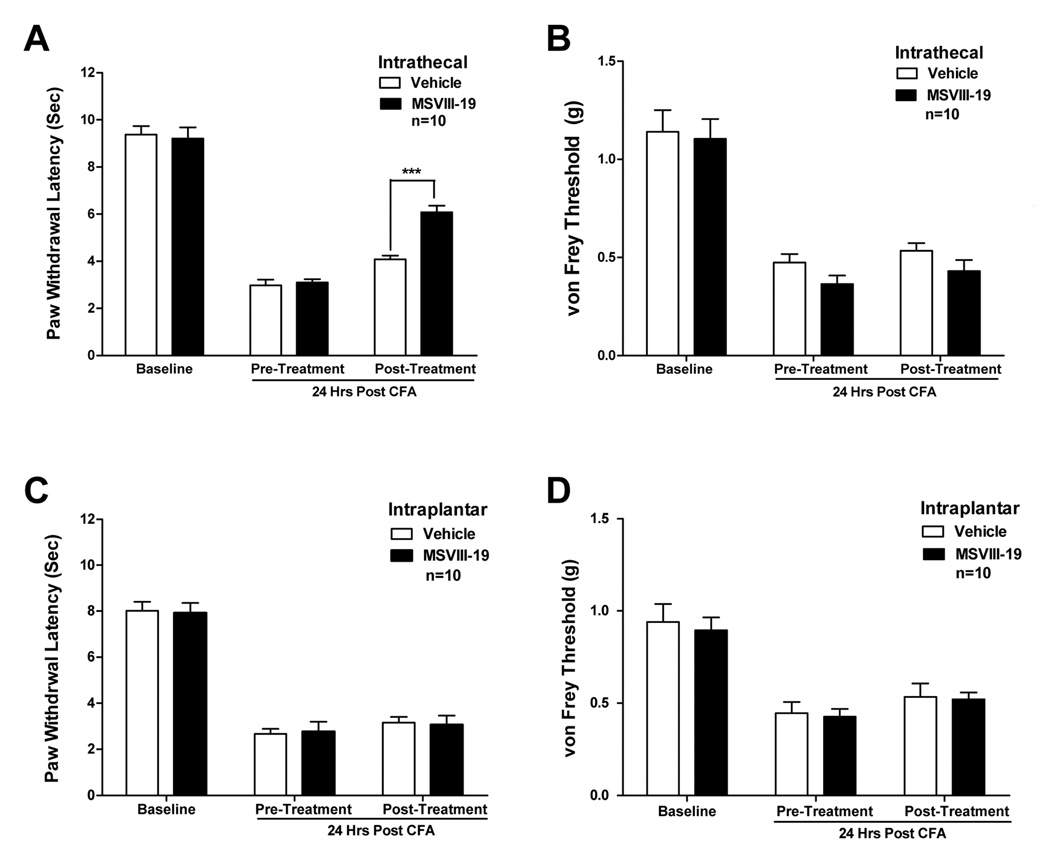
We next tested the efficacy of MSVIII-19 in reversing thermal and mechanical hypersensitivity in the more persistent inflammatory state induced by complete Freund’s adjuvant (CFA). After measuring baseline sensitivity to touch (using von Frey filaments) and heat (using the Hargreaves test), CFA (10 µl) was injected into a hindpaw of the mice. CFA consistently induced robust thermal and mechanical hypersensitivity when assessed 24 hr after CFA injection. As with the formalin-induced inflammation, MSVIII-19 (0.5 nmol, i.t.) significantly reduced thermal hypersensitivity but had no effect on mechanical hypersensitivity when compared to vehicle-injected mice (Fig 4a,b). Also consistent with results in the formalin test, intraplantar pre-treatment with MSVIII-19 (2 nmol, 15 min prior to thermal or mechanical testing) did not significantly reduce CFA-induced thermal hypersensitivity or mechanical hypersensitivity relative to vehicle treated mice (Figure 4c,d).
Figure 4. Spinally but not peripherally administered MSVIII-19 selectively reduces thermal hypersensitivity in the CFA model of inflammatory pain.
After measurement of thermal and mechanical baselines, mice received a paw injection of CFA. Twenty four hours after CFA injection, mice were tested for thermal (A) or mechanical (B) sensitivity. The mice then received an intrathecal injection of either vehicle or MSVIII-19 (0.5 nmol), and were then tested for posttreatment thermal or mechanical sensitivity. CFA induced significant thermal and mechanical hypersensitivity relative to pre-CFA baselines (P < 0.01; ANOVA with Tukey’s post-hoc test). Thermal hypersensitivity was significantly reduced by MSVIII-19 relative to vehicle, whereas mechanical hypersensitivity was not affected. *** p< 0.001. C) and D) show that intraplantar administration of MSVIII-19 (2 nmol) has no effect on thermal or mechanical sensitivity in the CFA model.
The effects of MSVIII-19 in the CCI model of neuropathic pain
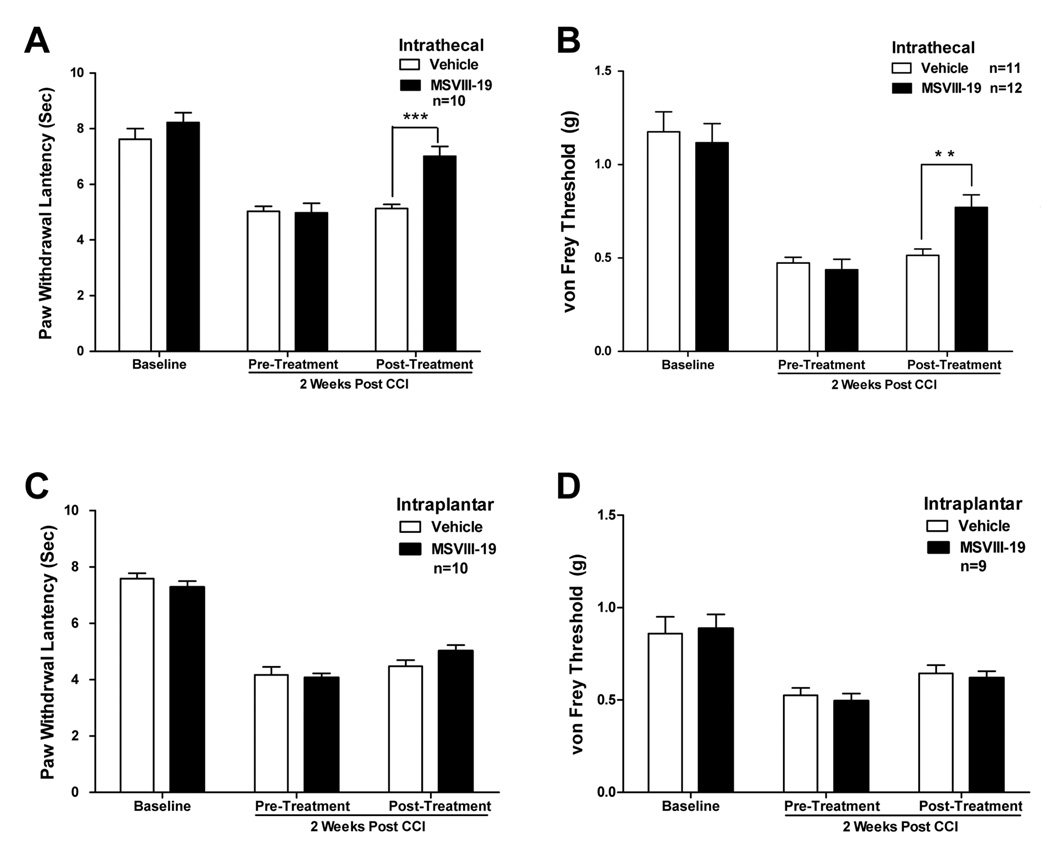
Chronic constriction injury of the sciatic nerve (CCI) is a commonly used model of neuropathic pain [1]. We performed CCI surgeries on mice and observed significant thermal and mechanical hypersensitivity that developed over a period of several days. Two weeks after surgery, mice were tested for their post-injury thresholds to thermal and mechanical stimuli, and then treated with an intrathecal injection of either vehicle or 0.5 nmol MSVIII-19. Thermal and mechanical sensitivity were then measured again 15 min after treatment. We found that MSVIII-19 significantly reduced both thermal and mechanical hypersensitivity induced by CCI; the MSVIII-19 group showed was significantly reduced hypersensitivity relative to pre-treatment values and relative to the vehicle-treated group (Fig 5).
Figure 5. Spinally but not peripherally administered MSVIII-19 reduces thermal and mechanical hypersensitivity in the CCI model of neuropathic pain.
After measurement of thermal and mechanical baselines, mice underwent surgery to induce chronic constriction injury of the sciatic nerve. Two weeks after CCI surgery, mice were tested for thermal (A) or mechanical (B) sensitivity. CCI induced both thermal and mechanical hypersensitivity (P < 0.01; ANOVA with Tukey’s post-hoc test). The mice then received an intrathecal injection of either vehicle or MSVIII-19 (0.5 nmol), and were then tested for post-treatment thermal or mechanical sensitivity. Both thermal and mechanical hypersensitivity were significantly reduced by MSVIII-19 relative to vehicle. ** p < 0.01; *** p< 0.001. C) and D) show that intraplantar administration of MSVIII-19 (2 nmol) has no effect on thermal or mechanical sensitivity in the CCI model of neuropathic pain.
As was the case for the models of inflammatory pain, intraplantar pre-treatment with MSVIII-19 (2 nmol, 15 min prior to thermal or mechanical testing) did not significantly reduce thermal hypersensitivity or mechanical hypersensitivity induced by CCI relative to vehicle treated mice (Figure 5c,d).
The effects of MSVIII-19 on Visceral Pain
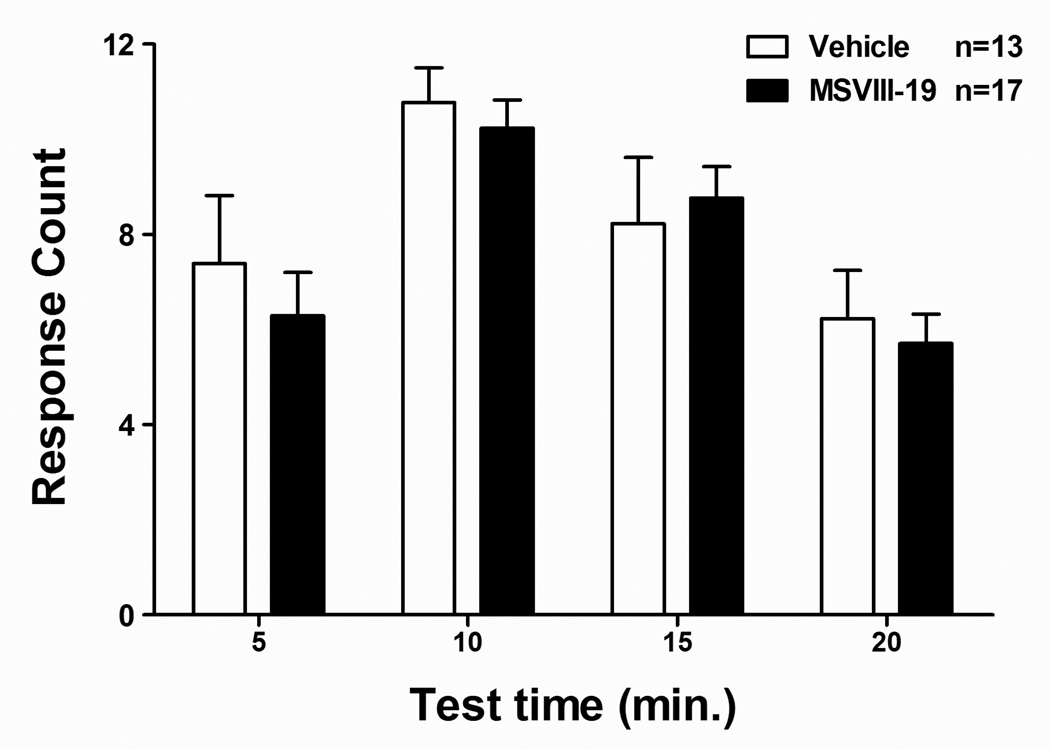
There is extensive evidence indicating that peripheral somatic pain differs substantially from mechanisms of visceral pain transmission and modulation. We therefore tested the ability of intrathecally administered MSVIII-19 to reduce pain-related behaviors following intraperitoneal administration of dilute acetic acid (the acetic acid-induced writhing test). IP administration of 0.6% acetic acid induced significant abdominal writhing in mice. When mice were pretreated with vehicle or MSVIII-19, we observed no significant effect of MSVIII-19 (0.5 nmol, i.t.) on acetic acid –induced writhing relative to vehicle-treated mice (Fig 6).
Figure 6. MSVIII-19 has no effect in the acetic acid model of visceral pain.
Following intrathecal injection of either vehicle or MSVIII-19 (0.5 nmol), mice received an intraperitoneal injection of 0.6% acetic acid, and abdominal writhes were counted for a period of 20 min, and are reported in 5 min bins. There were no significant differences between MSVIII-19 and vehicle-injected groups.
The effects of MSVIII-19 on Acute Thermal Sensation
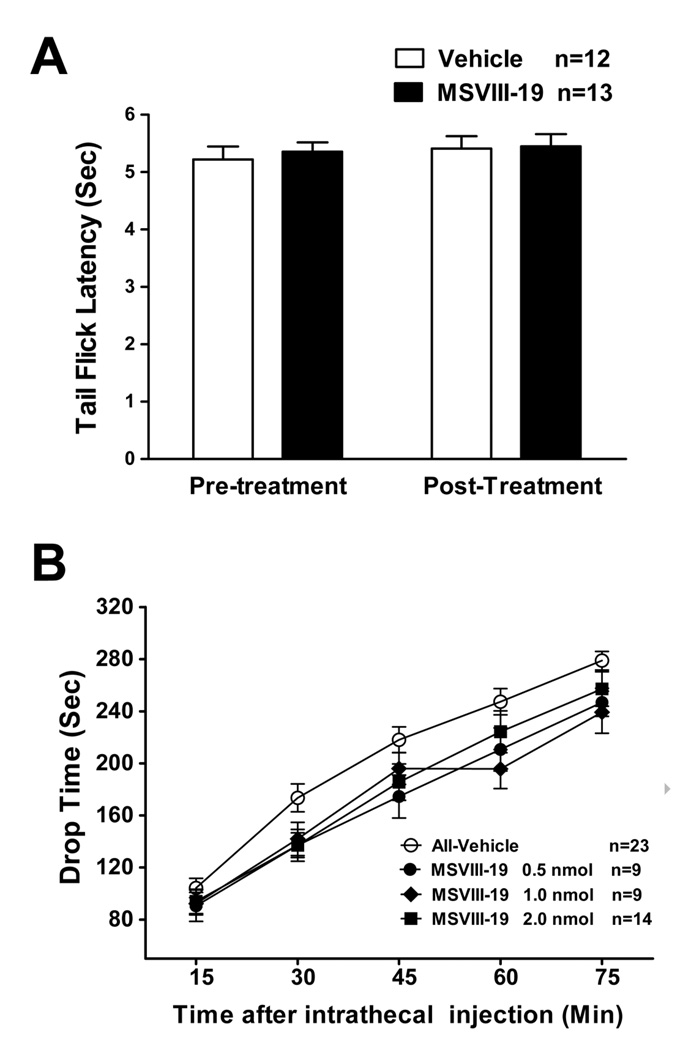
Data described above clearly indicate that GluK1 activation is important in the induction or maintenance of thermal hypersensitivity following inflammation and nerve injury. In order to test whether GluK1 receptors are involved in the detection of noxious thermal stimuli under baseline conditions, we tested the effects of MSVIII-19 in the tail flick test. Noxious heat applied to the base of the tail induced reliable and reproducible withdrawal responses. No differences were observed in tail flick latencies between mice pretreated with intrathecal MSVIII-19 (0.5 nmol) relative to vehicle-injected controls (Fig 7a).
Figure 7. MSVIII-19 has no effect in the tail-flick assay of thermal pain and has no effect on locomotor behavior in the accelerating rotarod.
A) Mice were first tested for baseline tailflick latency. (Pre-inj). They then received an intrathecal injection of MSVIII-19 (0.5nmol), and tailflick latency was then measured again (Post-inj). There was no difference between MSVIII-19 (0.5 nmol) and vehicle-injected groups. B) The effects of intrathecial MSVIII-19 (0.5–2.0nmol) were compared to vehicle-treated mice in locomotor performance on the accelerating rotarod. Mice were pretreated with an intrathecal injection of vehicle (n=23) or MSVIII-19 (0.5 nmol, n=9; 1.0 nmol, n=9; 2.0 nmol, n=14). There were no significant effects of MSVIII-19 as compared to vehicle.
Taken together, the results described above suggest that while GluK1-containing receptors are involved in sensitization in inflammatory and neuropathic pain models, they have no apparent role in acute pain transmission, temperature sensation, or pain in response to chemical activation of visceral nociceptors.
The effects of MSVIII-19 on Locomotor Function
MSVIII-19 has some antagonist activity at AMPA receptors, although the affinity for these receptors is approximately 1000-fold lower than that for GluK1 receptors [22]. To test whether some of the analgesic effects of MSVIII-19 could be due to impaired locomotor performance, we tested the effects of the drug on performance in the accelerating RotaRod. Intrathecal administration of MSVIII-19 (0.5, 1.0, or 2.0 nmol) had no effect on performance of mice in the accelerating RotaRod (Fig 7b). These data indicate that impaired motor performance does not likely contribute in a significant way to the observed effects of intrathecal MSVIII-19 on pain behaviors described above.
Discussion
In the present report, we demonstrate that MSVIII-19, which inhibits GluK1 kainate receptors through a non-conventional mechanism, reduces pain-related behaviors in mouse models of both inflammatory and neuropathic pain without degrading locomotor function. Efficacy of the compound was analyzed in several models of inflammatory pain and hypersensitivity. Intrathecal administration of MSVIII-19 dose-dependently reduced formalin-induced spontaneous pain behaviors. Thermal hypersensitivity associated with formalin- or CFA-induced inflammation was similarly reduced. The analgesic effect on inflammation-induced hypersensitivity in these models was restricted to thermal hypersensitivity, as MSVIII-19 did not alter mechanical hypersensitivity. In contrast, intrathecal administration of MSVIII-19 reduced both thermal and mechanical hypersensitivity following nerve injury in the CCI model.
As mentioned above, some previous studies have suggested that peripheral GluK1 receptors located at sensory nerve endings in the skin are involved in nociceptor activation and sensitization. It was shown using an antibody that recognizes the three low-affinity kainite receptor subunits GluK1/2/3 (GluR5/6/7) that one or more of these receptor subunits is likely expressed on approximately 28% of unmyelinated fibers in the digital nerve from normal rats, and that this increases to roughly 40% acutely following Freund’s adjuvant-induced inflammation [6]. This group further demonstrated that injection of kainate in the hindpaw induced acute mechanical hypersensitivity, and that an AMPA/Kainate receptor antagonist (CNQX) injected in the paw could reduce inflammation-induced mechanical hypersensitivity. Based on these findings, we systematically tested whether blocking GluK1 receptors in the periphery with MSVIII-19 could have analgesic effects in the mouse pain models described in this study.
In the present studies, a contribution of peripheral GluK5-containing kainate receptors was not apparent in these several models of pain behavior, because whereas intrathecal administration of MSVIII-19 showed robust analgesic actions, intraplantar administration of MSVIII-19 was ineffective. There are a number of possible explanations for the apparent discrepancy in these results relative to the above mentioned studies by Du et al. First, while there was anatomical data demonstrating possible expression of GluK1/2/3 on primary afferent neurons, the behavioral data are consistent with a peripheral sensitizing action of either AMPA receptors or kainate receptors, as the agonist used (kainate) activates both families of receptors at the concentration used (1mM), and the antagonist used (CNQX) inhibits both of these receptor families. Even amongst the kainate receptors, MSVIII-19 inhibits only GluK5-containing kainate receptors, whereas CNQX would inhibit receptors containing GluK2 or Gluk3, and thus peripheral GluK2-containing receptors could account for the actions of kainate and CNQX reported by Due et al [6]. Another possibility is that there could be species differences between rats and mice in the expression pattern or function of kainate receptors in the periphery. We have no evidence to support or refute this concept, as we have not directly tested whether activation of kainate receptors in the periphery induces sensitization in mice. Our data, rather, indicate that peripheral GluK1 receptors do not contribute to mechanical or thermal hypersensitivity in the mouse pain models we have presented here. In summary, we demonstrate a central role for spinal GluK1-containing kainate receptors in diverse modalities of mammalian nociception and show that a functional antagonist can be analgesic.
Kainate receptors are of interest as antinociceptive targets because they are critical modulators transmission in pain transmission pathways [29]. MSVIII-19 most potently inhibits GluK1 receptors [22] and the specificity for this subunit likely accounts for its analgesic action. They are the predominant type of ionotropic glutamate receptor expressed by the small-diameter sensory neurons in dorsal root ganglia (and therefore particularly sensitive to inhibition by MSVIII-19, Figure 1), where they increase neuronal excitability and regulate presynaptic release of glutamate at the first synapse in the spinal cord [11, 15, 17, 24, 28]. Additionally, both GluK1 and GluK2-containing receptors function in pre- and post-synaptic roles in a subset of projection and intrinsic neurons of the dorsal horn [14, 18], although some these activities may be developmentally down-regulated [4, 31]. GluK1 receptors also are engaged in supraspinal nociceptive centers such as the thalamus and anterior cingulate cortex [2, 29, 30]. The dose-dependent antinociceptive action of MSVIII-19 supports a critical role for GluK1 receptors at the level of the spinal cord in mediating certain forms of injury-induced pain sensitization. The GluK1 receptors in question could be localized in central terminals of primary afferent neurons, in dorsal horn neurons, or both. This conclusion is consistent with the absence of formalin-induced pain behaviors in GluK1 knockout mice [16]. We did not observe a similarly complete diminution of the formalin-induced spontaneous pain behaviors at the highest dose of MSVIII-19 used in this study (2.0 nmol), but it remains possible that higher doses would effectively eliminate pain behaviors without significant motor impairment arising from low-affinity antagonism of AMPA receptors [22].
Inhibition of spinal kainate receptors provides relief from pain during conditions of enhanced nociception, but is less effective in dampening acute, physiological reactions to thermal or mechanical stimuli. Abundant pharmacological and genetic data demonstrates that kainate receptor inhibition is analgesic in a variety of short- and long-term inflammatory and neuropathic states [29], including animal models of migraine [7]. In addition to the attenuated responses to formalin, GluK1 knockout mice exhibited significant reductions in pain behaviors in response to capsaicin injection [16]. Similarly, GluK1 antagonists, including MSVIII-19, reduce pain sensitivity in these and similar models [5, 25]. While neither GluK1 knockout mice nor MSVIII-19 treatment exhibit altered mechanical hypersensitivity following a more prolonged inflammation induced by CFA, both MSVIII-19 and other antagonists clearly reduce thermal hypersensitivity 24 hours after CFA injection (Figure 4)[10]. Thus, GluK1 kainate receptors appear to contribute to a subset of pain modalities in the presence of inflammatory processes. This conclusion is underscored by the efficacy of MSVIII-19 in both thermal and mechanical hypersensitivity associated with neuropathic pain in the CCI model, as was the case in a previous study with the nonselective desensitizing agonist 2S,4R-4-methylglutamate [26]. Similarly, the competitive GluK1 antagonist LY382884 effectively reduced neuropathy-associated wind-up of spinothalamic neurons following ligature of a spinal nerve in primates [21]. The efficacy of GluK1 antagonism extends to chronic pain studies in humans as well [23], and this pharmacological approach is under clinical investigation for treatment of migraine and neuropathic pain. The success of these preclinical studies and early clinical trials provide an impetus for pursuing compounds related to or based on the novel MSVIII-19 template structure as analgesics.
As well as having a chemical structure unlike other candidate analgesics acting through inhibition of kainate receptors, the mechanism of action of MSVIII-19 differs substantially from conventional antagonists tested previously, such as LY382884. We initially characterized MSVIII-19 as a competitive antagonist, but a more rigorous pharmacological analysis and molecular resolution of the compound in complex with the GluK1 ligand-binding domain revealed that it instead potently desensitized the receptors without gating currents [8]. It therefore has some mechanistic similarity to 2S,4R-4-methylglutamate, which also has been used as a functional antagonist due to the profound desensitization that it induces, but 2S,4R-4-methylglutamate also evokes transient currents from kainate receptors and thus behaves more as a conventional high-affinity agonist. Our results demonstrate that MSVIII-19 reduces activation of neuronal GluK1-containing kainate receptors in the absence of efficacy as an agonist, recapitulating its activity on recombinant receptors. It is likely, therefore, that MSVIII-19 acts through analogous mechanism on neuronal receptors and through potent desensitization has analgesic efficacy similar to that of conventional competitive antagonists. MSVIII-19 therefore could be a viable template for additional structural modification to enhance its in vivo activity.
In summary, our data provide support for the idea that GluK1-containing receptors represent a promising target for the development of novel agents for the treatment of injury-induced pain. Further, these findings support the idea that GluK1 antagonists will need to target spinally-expressed receptors in order to be effective as analgesics. MSVIII-19 has a marked selectivity for GluK1 receptors relative to other kainate receptor subunits, similar to newer decahydroisoquinolines such as LY382884, and therefore we suggest that MSVIII-19 represents a promising lead compound for the development of novel analgesic agents that target GluK1.
Acknowledgments
These studies were supported by NIH Grants R01NS42595 and R01NS48602 to RWG and R01NS44322 to GTS. The authors wish to thank ZQ Zhao and CS Zhao for assistance with some of the behavioral models, and Dr. Sonia Bhangoo for assistance with some DRG cultures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References Cited
- 1.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 2.Binns KE, Turner JP, Salt TE. Kainate receptor (GluR5)-mediated disinhibition of responses in rat ventrobasal thalamus allows a novel sensory processing mechanism. J Physiol. 2003;551:525–537. doi: 10.1113/jphysiol.2003.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Contractor A, Swanson GT. Kainate Receptors. In: Gereau RW, Swanson GT, editors. The Glutamate Receptors. Totawa, NJ: Humana Press; 2008. pp. 99–158. [Google Scholar]
- 4.Dahlhaus A, Ruscheweyh R, Sandkuhler J. Synaptic input of rat spinal lamina I projection and unidentified neurones in vitro. J Physiol. 2005;566:355–368. doi: 10.1113/jphysiol.2005.088567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominguez E, Iyengar S, Shannon HE, Bleakman D, Alt A, Arnold BM, Bell MG, Bleisch TJ, Buckmaster JL, Castano AM, Del Prado M, Escribano A, Filla SA, Ho KH, Hudziak KJ, Jones CK, Martinez-Perez JA, Mateo A, Mathes BM, Mattiuz EL, Ogden AM, Simmons RM, Stack DR, Stratford RE, Winter MA, Wu Z, Ornstein PL. Two prodrugs of potent and selective GluR5 kainate receptor antagonists actives in three animal models of pain. J Med Chem. 2005;48:4200–4203. doi: 10.1021/jm0491952. [DOI] [PubMed] [Google Scholar]
- 6.Du J, Zhou S, Carlton SM. Kainate-induced excitation and sensitization of nociceptors in normal and inflamed rat glabrous skin. Neuroscience. 2006;137:999–1013. doi: 10.1016/j.neuroscience.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Filla SA, Winter MA, Johnson KW, Bleakman D, Bell MG, Bleisch TJ, Castano AM, Clemens-Smith A, del Prado M, Dieckman DK, Dominguez E, Escribano A, Ho KH, Hudziak KJ, Katofiasc MA, Martinez-Perez JA, Mateo A, Mathes BM, Mattiuz EL, Ogden AM, Phebus LA, Stack DR, Stratford RE, Ornstein PL. Ethyl (3S,4aR,6S,8aR)-6-(4-ethoxycar- bonylimidazol-1-ylmethyl)decahydroiso-quinoline-3-carboxylic ester: a prodrug of a GluR5 kainate receptor antagonist active in two animal models of acute migraine. J Med Chem. 2002;45:4383–4386. doi: 10.1021/jm025548q. [DOI] [PubMed] [Google Scholar]
- 8.Frydenvang K, Lash LL, Naur P, Postila PA, Pickering DS, Smith CM, Gajhede M, Sasaki M, Sakai R, Pentikainen OT, Swanson GT, Kastrup JS. Full domain closure of the ligand-binding core of the ionotropic glutamate receptor iGluR5 induced by the high affinity agonist dysiherbaine and the functional antagonist 8,9-dideoxyneodysiherbaine. J Biol Chem. 2009;284:14219–14229. doi: 10.1074/jbc.M808547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gereau R, Swanson GT. The Glutamate Receptors. Humana Press; 2008. [Google Scholar]
- 10.Guo W, Zou S, Tal M, Ren K. Activation of spinal kainate receptors after inflammation: behavioral hyperalgesia and subunit gene expression. Eur J Pharmacol. 2002;452:309–318. doi: 10.1016/s0014-2999(02)02333-6. [DOI] [PubMed] [Google Scholar]
- 11.Hwang SJ, Pagliardini S, Rustioni A, Valtschanoff JG. Presynaptic kainite receptors in primary afferents to the superficial laminae of the rat spinal cord. J Comp Neurol. 2001;436:275–289. [PubMed] [Google Scholar]
- 12.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. European Journal of Pharmacology. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 13.Jones CK, Alt A, Ogden AM, Bleakman D, Simmons RM, Iyengar S, Dominguez E, Ornstein PL, Shannon HE. Antiallodynic and antihyperalgesic effects of selective competitive GLUK5 (GluR5) ionotropic glutamate receptor antagonists in the capsaicin and carrageenan models in rats. J Pharmacol Exp Ther. 2006;319:396–404. doi: 10.1124/jpet.106.105601. [DOI] [PubMed] [Google Scholar]
- 14.Kerchner GA, Wilding TJ, Huettner JE, Zhuo M. Kainate receptor subunits underlying presynaptic regulation of transmitter release in the dorsal horn. J Neurosci. 2002;22:8010–8017. doi: 10.1523/JNEUROSCI.22-18-08010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerchner GA, Wilding TJ, Li P, Zhuo M, Huettner JE. Presynaptic kainite receptors regulate spinal sensory transmission. J Neurosci. 2001;21:59–66. doi: 10.1523/JNEUROSCI.21-01-00059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko S, Zhao MG, Toyoda H, Qiu CS, Zhuo M. Altered behavioral responses to noxious stimuli and fear in glutamate receptor 5 (GluR5)- or GluR6-deficient mice. J Neurosci. 2005;25:977–984. doi: 10.1523/JNEUROSCI.4059-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CJ, Kong H, Manzini MC, Albuquerque C, Chao MV, MacDermott AB. Kainate receptors expressed by a subpopulation of developing nociceptors rapidly switch from high to low Ca2+ permeability. J Neurosci. 2001;21:4572–4581. doi: 10.1523/JNEUROSCI.21-13-04572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li P, Wilding TJ, Kim SJ, Calejesan AA, Huettner JE, Zhuo M. Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature. 1999;397:161–164. doi: 10.1038/16469. [DOI] [PubMed] [Google Scholar]
- 19.Lucifora S, Willcockson HH, Lu CR, Darstein M, Phend KD, Valtschanoff JG, Rustioni A. Presynaptic low- and high-affinity kainate receptors in nociceptive spinal afferents. Pain. 2006;120:97–105. doi: 10.1016/j.pain.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Mulle C, Sailer A, Swanson GT, Brana C, O'Gorman S, Bettler B, Heinemann SF. Subunit composition of kainate receptors in hippocampal interneurons. Neuron. 2000;28:475–484. doi: 10.1016/s0896-6273(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 21.Palecek J, Neugebauer V, Carlton SM, Iyengar S, Willis WD. The effect of a kainate GluR5 receptor antagonist on responses of spinothalamic tract neurons in a model of peripheral neuropathy in primates. Pain. 2004;111:151–161. doi: 10.1016/j.pain.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Sanders JM, Ito K, Settimo L, Pentikainen OT, Shoji M, Sasaki M, Johnson MS, Sakai R, Swanson GT. Divergent pharmacological activity of novel marine-derived excitatory amino acids on glutamate receptors. J Pharmacol Exp Ther. 2005;314:1068–1078. doi: 10.1124/jpet.105.086389. [DOI] [PubMed] [Google Scholar]
- 23.Sang CN, Hostetter MP, Gracely RH, Chappell AS, Schoepp DD, Lee G, Whitcup S, Caruso R, Max MB. AMPA/kainate antagonist LY293558 reduces capsaicin-evoked hyperalgesia but not pain in normal skin in humans. Anesthesiology. 1998;89:1060–1067. doi: 10.1097/00000542-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Sato K, Kiyama H, Park HT, Tohyama M. AMPA, KA and NMDA receptors are expressed in the rat DRG neurones. Neuroreport. 1993;4:1263–1265. doi: 10.1097/00001756-199309000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Simmons RM, Li DL, Hoo KH, Deverill M, Ornstein PL, Iyengar S. Kainate GluR5 receptor subtype mediates the nociceptive response to formalin in the rat. Neuropharmacology. 1998;37:25–36. doi: 10.1016/s0028-3908(97)00188-3. [DOI] [PubMed] [Google Scholar]
- 26.Sutton JL, Maccecchini ML, Kajander KC. The kainate receptor antagonist 2S,4R-4-methylglutamate attenuates mechanical allodynia and thermal hyperalgesia in a rat model of nerve injury. Neuroscience. 1999;91:283–292. doi: 10.1016/s0306-4522(98)00621-6. [DOI] [PubMed] [Google Scholar]
- 27.Swanson GT, Green T, Heinemann SF. Kainate receptors exhibit differential sensitivities to (S)-5-iodowillardiine. Mol Pharmacol. 1998;53:942–949. [PubMed] [Google Scholar]
- 28.Wilding TJ, Huettner JE. Functional diversity and developmental changes in rat neuronal kainate receptors. J Physiol. 2001;532:411–421. doi: 10.1111/j.1469-7793.2001.0411f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu LJ, Ko SW, Zhuo M. Kainate receptors and pain: from dorsal root ganglion to the anterior cingulate cortex. Curr Pharm Des. 2007;13:1597–1605. doi: 10.2174/138161207780765864. [DOI] [PubMed] [Google Scholar]
- 30.Wu LJ, Zhao MG, Toyoda H, Ko SW, Zhuo M. Kainate receptor-mediated synaptic transmission in the adult anterior cingulate cortex. J Neurophysiol. 2005;94:1805–1813. doi: 10.1152/jn.00091.2005. [DOI] [PubMed] [Google Scholar]
- 31.Youn DH, Randic M. Modulation of excitatory synaptic transmission in the spinal substantia gelatinosa of mice deficient in the kainate receptor GluR5 and/or GluR6 subunit. J Physiol. 2004;555:683–698. doi: 10.1113/jphysiol.2003.057570. [DOI] [PMC free article] [PubMed] [Google Scholar]